Abstract
The development of novel, tumor-selective and boron-rich compounds as potential agents for use in boron neutron capture therapy (BNCT) represents a very important field in cancer treatment by radiation therapy. Here, we report the design and synthesis of two promising compounds that combine meta-carborane, a water-soluble monosaccharide and a linking unit, namely glycine or ethylenediamine, for facile coupling with various tumor-selective biomolecules bearing a free amino or carboxylic acid group. In this work, coupling experiments with two selected biomolecules, a coumarin derivative and folic acid, were included. The task of every component in this approach was carefully chosen: the carborane moiety supplies ten boron atoms, which is a tenfold increase in boron content compared to the l-boronophenylalanine (l-BPA) presently used in BNCT; the sugar moiety compensates for the hydrophobic character of the carborane; the linking unit, depending on the chosen biomolecule, acts as the connection between the tumor-selective component and the boron-rich moiety; and the respective tumor-selective biomolecule provides the necessary selectivity. This approach makes it possible to develop a modular and feasible strategy for the synthesis of readily obtainable boron-rich agents with optimized properties for potential applications in BNCT.
Keywords: boron neutron cancer therapy (BNCT), modular approach, carboxylic acids and amines
1. Introduction
Since boron neutron capture therapy (BNCT) was ascertained to be a very promising binary cancer treatment [1,2,3,4], research has focused on the development of potent and selective boron-containing drugs [5,6]. The main advantage of this therapy is the generation of highly cytotoxic particles comprising a high linear energy transfer (LET) (α particle and Li particle). Their free mean path lengths of about 5 to 9 µm [7,8] roughly represent the diameter of a human cell [5]; therefore, these particles can only harm the surrounding tissue within this radius. However, only the lighter isotope of boron, 10B (20% natural abundance) [9], produces high LET particles after irradiation with thermal neutrons [10]. Therefore, BNCT agents have to be enriched with 10B [11,12]. Activation of the BNCT agents is caused by irradiation with thermal neutrons [13,14] for which 10B exhibits a large capture cross section (3835 barn, 1 barn = 1 × 10−24 cm2) [9]. This renders BNCT a promising strategy to treat malignant tissue with tumor-selective boron-containing drugs [6,15,16,17,18,19,20], as the thermal neutron beam can be focused on the affected area [21,22,23,24], thus generating therapeutic particles only upon neutron irradiation. In this manner, normal tissue can be spared and severe side effects, as known from pure radiotherapy or systemic effective chemotherapy, can be reduced.
The first boron-containing compounds used in clinical trials were l-boronophenylalanine (l-BPA) and sodium borocaptate (BSH) [5,6,10], but both compounds exhibit several drawbacks. For example, BSH and BPA do not exhibit optimized selectivity towards cancer cells (especially BSH), show a limited solubility in water (especially BPA [25]) and, in the case of BSH, are not able to penetrate cells due to their anionic character. Therefore, their application follows a tailored strategy where BSH is mostly applied for glioma treatment, as the dianionic compound is able to cross the damaged blood–brain barrier adjoining the malignant tissue in the human brain, and BPA is used as its fructose complex to overcome the low water solubility [5,6,10,25]. Since May 2020, the company Stella Pharma [26] has been allowed to market Steboronine® [27] (generic name: Borofalan), which contains 10B-enriched (99%) l-BPA as its d-sorbitol complex. This BNCT agent, in comparison to the respective fructose complex, exhibits the advantage of being storable for about three years and does not have to be freshly prepared for each use with retention of its GMP grade.
Therefore, the development of novel boron-containing tumor-selective agents for application in BNCT is important to overcome these limitations [19,20]. For all compounds, the basic requirements that must be fulfilled are: sufficient water solubility, low inherent toxicity, high boron content and high tumor selectivity. Water solubility can be increased by using charged compounds [28] or introducing hydrophilic moieties [29,30]. Tumor-selectivity can be achieved by using essential biological nutrients, substrates like boronated saccharides or amino acids [19,20,31], or even tumor-selective complex compounds, like boron-containing antibodies [20,29,30,32,33,34,35,36]. A variety of different boron-containing bioconjugates are known, including nucleosides [16], carbohydrates [37,38], amino acids [39,40,41] and peptides [29,30,42,43]. One main prerequisite of BNCT especially plays an essential role in this treatment, namely the selective accumulation of sufficient amounts of 10B-containing compounds in cancerous tissues, so that the therapeutically active particles destroy only the malignant cells without destroying healthy tissue. An effective treatment requires boron concentrations of 10–30 µg 10B/g tumor, or 109 10B-atoms/cancer cell [7,10]. One approach focuses on compounds with a very large boron content [29,30,42,44,45], another on the use of very selective BNCT agents over a longer period, taking advantage of specific shuttle systems that facilitate accumulation of the compound in the cells by internalization processes [17,29,30,33,46]. We pursued a combination of both strategies by combining tumor-selective small peptides, such as highly selective G protein-coupled receptor agonists, as biomolecules with meta-carborane derivatives to increase the boron load [29,30,43,47,48]. However, very high carborane loading (more than two carboranes attached to a peptide including 36 amino acids) results in loss of solubility or aggregation in aqueous media and, therefore, decreased potency and higher EC50 values [29,30]. Carbohydrate moieties, such as galactosyl groups, can be employed to compensate the hydrophobic character of carborane clusters (up to eight modified carboranes attached to the same peptide comprising 36 amino acids).
Here, we report the development of small molecules representing potential boron-rich coupling partners for tumor-selective molecules based on a modular strategy [47,48,49] combining readily available starting materials, like meta-carborane, α-d-galactopyranose and glycine or ethylenediamine derivatives (compounds 5 and 6 in Scheme 1). Compounds bearing a primary amine or carboxylic acid group represent potentially universal coupling partners for biomolecules [48]; representative coupling experiments are also included here to demonstrate the generalizability of this approach.
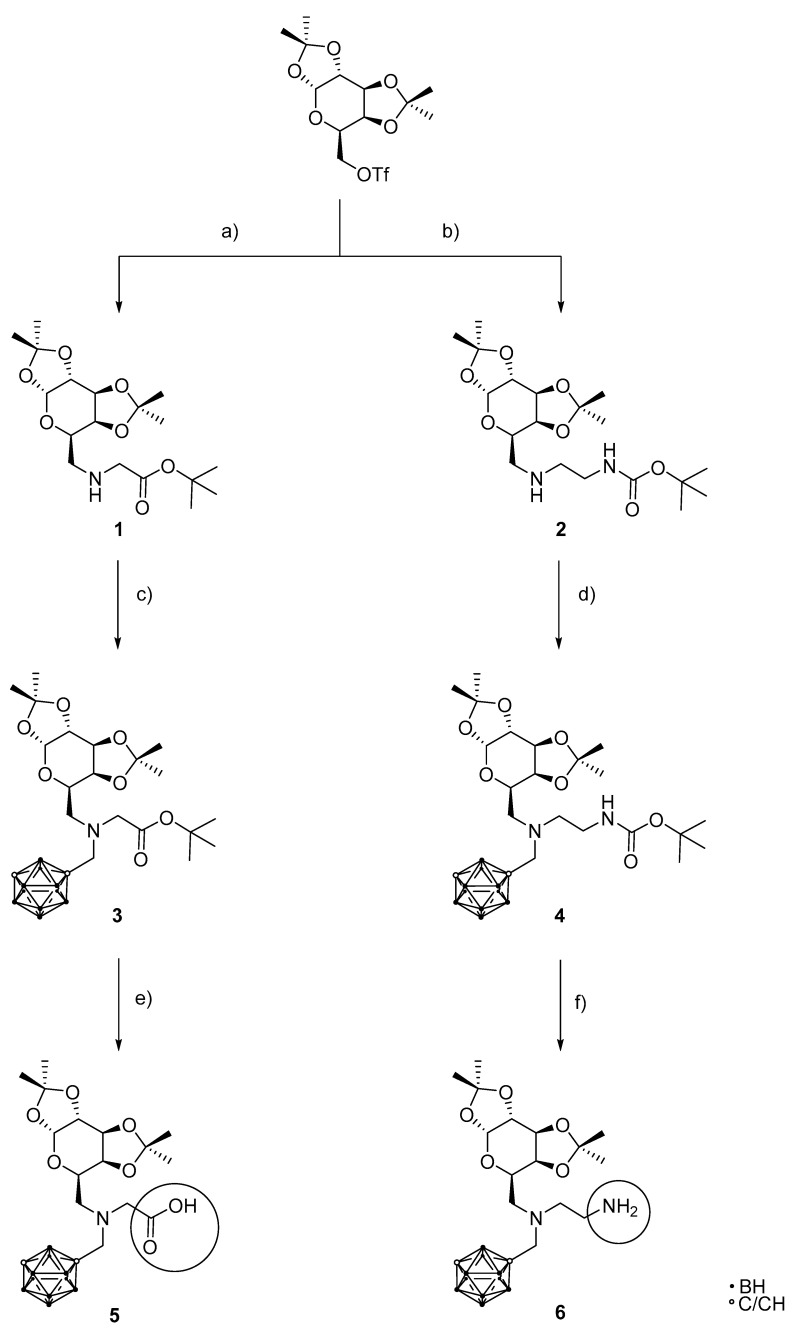
Scheme 1.
Synthesis of galactopyranosyl-modified meta-carborane-containing carboxylic acid (5) and primary amine (6) as the target compounds for modular conjugation with tumor-selective biomolecules. (a) 1.20 eq. tert-butyl glycinate hydrochloride, 2.96 eq. DIPEA, MeCN, 72 h, 45 °C, 68%; (b) 1.11 eq. N-tert-butoxycarbonyl-ethylenediamine, 1.12 eq. DIPEA, MeCN, 15 min, 0 °C, then 48 h, 40 °C, 77%; (c) 1.20 eq. 1-(trifluoromethanesulfonylmethyl)-1,7-dicarba-closo-dodeca-borane(12), 1.20 eq. K2CO3, toluene, 43 h, 95 °C, 54%; (d) 1.20 eq. 1-(trifluoromethanesulfonylmethyl)-1,7-dicarba-closo-dodecaborane(12), 1.20 eq. K2CO3, toluene, 48 h, 98 °C, 51%; (e) 55.0 eq. TFA, 3 h, rt, 65%; (f) 48.2 eq. TFA, 3 h, rt, quant.
In this regard, the synthesis of bifunctional anticancer drugs is of special interest. Several examples are known where drugs are used as theranostic compounds [50,51] or exhibit dual effects [52,53]. For example, derivatives of 7-amino-4-methylcoumarin are known for their anticancer activity [54]. Thus, combination with a carborane derivative can lead to drugs that possess anti-cancer properties and the ability to capture thermal neutrons for applications in BNCT. Furthermore, folic acid and its derivatives, which are already used as tumor-selective synthons for applications in BNCT [15,32,55], can act as diagnostic probes for some solid cancer types when combined with imaging agents [56]. Thus, conjugates of folic acid with carborane derivatives and an imaging agent using both carboxylic groups of folic acid could be used to generate highly selective BNCT agents with excellent imaging properties.
2. Results and Discussion
The starting materials 1-(trifluoromethanesulfonylmethyl)-1,7-dicarba-closo-dodecaborane(12) and 1,2:3,4-di-O-isopropylidene-6-deoxy-α-d-galactopyranosyl-6-triflate were prepared according to protocols from the literature. The synthetic procedures of Goto and co-workers for the synthesis of 1-(hydroxymethyl)-1,7-dicarba-closo-dodecaborane(12) [57] and those of Kalinin and co-workers for the preparation of 1-(trifluoromethanesulfonylmethyl)-1,7-dicarba-closo-dodecaborane(12) [58] were employed. 1,2:3,4-Di-O-isopropylidene-α-d-galactopyranose is commercially available or can be prepared in quantitative yield according to a procedure described by Saltan and co-workers [59]. 1,2:3,4-Di-O-isopropylidene-α-d-galactopyranose was converted to the triflate 1,2:3,4-di-O-isopropylidene-6-deoxy-α-d-galactopyranosyl-6-triflate following a procedure described by Brackhagen and co-workers [60].
The next step was the introduction of the respective linking units (glycine or ethylenediamine) starting from the commercially available protected derivatives, namely tert-butyl glycinate hydrochloride and N-tert-butoxycarbonyl-ethylenediamine, to prevent undesired side reactions during the synthetic steps. The reaction with the galactopyranosyl moiety was conducted under basic conditions using N,N-diisopropylethylamine (DIPEA) as a base in acetonitrile (MeCN) at elevated temperature (Scheme 1a,b). Both reactions gave the desired products, tert-butyl-N-(1,2:3,4-di-O-isopropyliden-6-deoxy-α-d-galactopyranos-6-yl)glycinate (1) and tert-butyl-{2-[(1,2:3,4-di-O-isopropylidene-6-deoxy-α-d-galactopyranos-6-yl)amino]ethyl}carbamate (2) in good to excellent yields.
Beside the desired product 2, the disubstituted compound tert-butyl-(2-{[bis(1,2:3,4-di-O-isopropylidene-6-deoxy-α-d-galactopyranos-6-yl)]amino}ethyl)-carbamate (2′) was isolated with an 8% yield (see the Supplementary Materials). This product was formed due to the increased nucleophilicity of the secondary amine in 2 in comparison to a primary amine in the starting material; the amount corresponded to the small excess of the ethylenediamine derivative employed here.
The carboranyl moiety was introduced by reacting 1 and 2 with 1-(trifluoromethanesulfonylmethyl)-1,7-dicarba-closo-dodecaborane(12) under basic conditions (potassium carbonate) in toluene (Scheme 1c,d); for optimized reaction conditions see the Supplementary Materials. The products, tert-butyl-N-[(1,7-dicarba-closo-dodecaboran-1-yl)methyl]-N-(1,2:3,4-di-O-isopropylidene-6-deoxy-α-d-galacatopyranos-6-yl)glycinate (3) and tert-butyl-{2-[(1,7-dicarba-closo-dodecaboran-1-ylmethyl)-(1,2:3,4-di-O-isopropyliden-6-deoxy-α-d-galactopyranos-6-yl)amino]ethyl}carbamat (4) were obtained in moderate yields (54 and 51%, respectively), presumably due to steric hindrance (bulky carboranyl moiety and secondary amine derivative).
The final step was the deprotection of the acid-labile protecting groups (R(CO)OtBu and RNH(CO)OtBu, where R is the organic moiety) of compounds 3 and 4 with trifluoroacetic acid (TFA), with formation of the volatile reaction products isobutene and carbon dioxide leading to very pure products N-[(1,7-dicarba-closo-dodecaboran-1-yl)methyl]-N-(1,2:3,4-di-O-isopropylidene-6-deoxy-α-d-galacatopyranos-6-yl)glycine (5) and N1-(1,7-dicarba-closo-dodecaboran-1-yl-methyl)-N1-(1,2:3,4-di-O-isopropylidene-6-deoxy-α-d-galactopyranos-6-yl)ethane-1,2-diamine (6) in high yields due to a facile purification [61]. Anhydrous conditions must be employed to prevent cleavage of the monosaccharide protecting groups [61]. Compound 5 was first purified by column chromatography, which led to a noticeable loss of product (47% yield) due to the highly polar carboxylic acid group. Therefore, compounds 5 and 6 were purified by evaporation of all volatile reaction products with dichloromethane (DCM) as an entrainer, leading to pure compounds in good to excellent yields (65% and quantitative yield, respectively; details are given in the Supplementary Materials). Apparently, deprotection of the carboxylic acid ester (tert-butyl group) is less efficient than deprotection of the carbamate (Boc group).
The new compounds 1–6 were fully characterized by NMR spectroscopy (numbering scheme given in Figure S1), mass spectrometry and infrared spectroscopy and showed a purity of at least 95%. Furthermore, we were able to demonstrate that the same procedure can also be applied for the synthesis of the corresponding ortho-carborane derivative N1-[(1,2-dicarba-closo-dodecaboran-1-yl)methyl]-N1-(1,2:3,4-di-O-isopropylidene-6-deoxy-α-d-galactopyranos-6-yl)ethane-1,2-diamine (see the Supplementary Materials for further details).
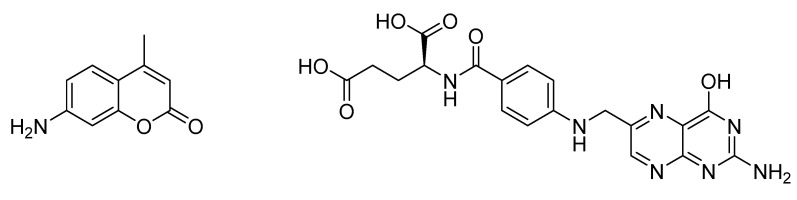
With the glycine derivative 5 and ethylenediamine derivative 6 in hand, exemplary coupling reactions with two selected biomolecules that play roles in cancer treatment were conducted. 7-Amino-4-methylcoumarin (Figure 1, left), already employed in the preparation of polyfunctional cancer therapeutics based on cisplatin derivatives [62] but not for the preparation of potential BNCT agents [63,64,65], was selected as a coupling partner for 5. As coupling partner for amine 6, folic acid (Figure 1, right) was chosen as this biomolecule has already been employed in the synthesis of potential BNCT agents [15,32,46,55,66]. Some cancer types overexpress folate receptors on their cell membrane surfaces, which can be used for selective uptake of the final BNCT agent in the respective cancer cells, increasing the efficacy of the therapy [32,67].
Figure 1.
7-Amino-4-methylcoumarin (left) and folic acid (right) as two biomolecules selected to demonstrate the coupling behavior of glycine derivative 5 and ethylenediamine derivative 6.
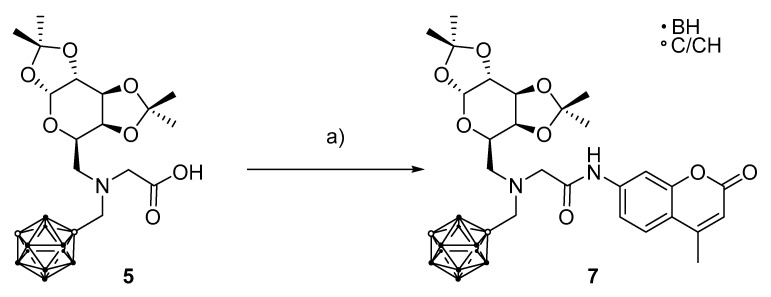
The strategy for coupling glycine derivative 5 with the weakly nucleophilic coumarin derivative followed a procedure described by Quéléver and co-workers using phosphoryl chloride and pyridine [68] and resulted in N1-[(1,7-dicarba-closo-dodecaborane-1-yl)methyl]-N1-(1,2:3,4-di-O-isopropylidene-6-deoxy-α-d-galacatopyranos-6-yl)-N2-[(4-methyl)-2-oxo-2H-chromen-7-yl)glycineamide (7), albeit in low yield (27%) (Scheme 2). Attempts to use less harsh conditions (N,N’-dicyclohexylcarbodiimide (DCC) and N-hydroxysuccinimide (NHS)) for this coupling reaction were not successful [69]. Using different coupling reagents (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDCI) and hydroxybenzotriazol (HOBt) with N,N-diisopropylethylamine (DIPEA) as base [69]) yielded the desired product; however, it was in a very low yield of only 18%, indicating that this method is inferior to the phosphoryl chloride approach and the low reactivity of the coumarin derivative is the main issue. Compound 7 was fully characterized by NMR spectroscopy, mass spectrometry and infrared spectroscopy, proving the successful synthesis (with at least 95% purity) of this bioconjugate as a proof of principle in this approach.
Scheme 2.
(a) 1.20 eq. 7-amino-4-methylcoumarin, 1.10 eq. POCl3, pyridine, 1 h, −18 °C, 27%.
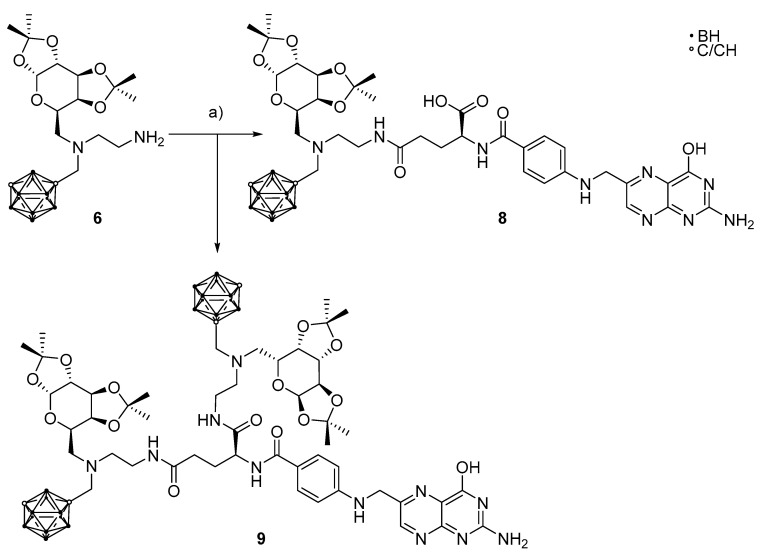
The coupling reaction between folic acid and the primary amine 6 turned out to be more complicated due to the presence of two unprotected carboxylic acid groups in the former. Similar reactions have been reported where no additional protecting group was used for the secondary carboxylic acid group [55,66,70], as the primary COOH group exhibits a higher reactivity and, therefore, is more prone to undergo coupling reactions. However, here, the reaction of 6 with folic acid, following the procedure described by Trindade and co-workers, using DCC and NHS as activation reagents [70], gave both the desired monosubstituted species, namely N2-(4-{[(2-amino-4-hydroxypteridine-6-yl)methyl]amino}benzoyl)-N5-{2-[(1,7-dicarba-closo-dodecaboran-1-yl)methyl-(1,2:3,4-di-O-isopropyliden-6-deoxy-α-d-galactopyranos-6-yl)amino]ethyl}-l-glutamine (8) or its isomer, and the disubstituted species, namely (S)-2-(4-{[(2-amino-4-hydroxypteridine-6-yl)methyl]amino}benzamido)-N1,N5-bis-{2-[(1,7-dicarba-closo-dodecaboran-1-yl)methyl-(1,2:3,4-di-O-isopropylidene-6-deoxy-α-d-galactopyranos-6-yl)amino]ethyl}amino)pentanediamide (9) (Scheme 3), also verified by high-resolution mass spectrometry (m/z 883.5151 for 8 and m/z 1323.8829 for 9). Obviously, in this case, the protocol from the literature [70] for conjugate formation with folic acid was not applicable, as we got a mixture of 8 and 9 in an unknown ratio. However, the obtained disubstituted folic acid derivative 9 has a high boron contents which could be useful for applications as a BNCT agent.
Scheme 3.
(a) 1.00 eq. folic acid, 1.00 eq. DCC, 1.00 eq. NHS, 1.10 eq. DIPEA, dimethylformamide, no yield determined.
3. Materials and Methods
All reactions were carried out under nitrogen atmosphere using Schlenk techniques, if not reported otherwise. Anhydrous diethyl ether and DCM were obtained with an MBRAUN solvent purification system MB SPS-800 (M. Braun Inertgas-Systeme GmbH, Garching, Germany). MeCN and 2,4,6-collidine were dried over calcium hydride and distilled prior to use. Anhydrous tetrahydrofuran was dried over potassium and distilled prior to use. All solvents were stored over a molecular sieve (3 Å) under nitrogen atmosphere. 1,2-Dicarba-closo-dodecaborane(12) and 1,7-dicarba-closo-dodecaborane(12) are commercially available. 1-(Hydroxymethyl)-1,2-dicarba-closo-dodecaborane(12) [71], 1-(hydroxymethyl)-1,7-dicarba-closo-dodecaborane(12) [57], 1-(trifluoromethanesulfonylmethyl)-1,2-dicarba-closo-dodecaborane(12) [58], 1-(trifluoromethanesulfonylmethyl)-1,7-dicarba-closo-dodecaborane(12) [58], 1,2:3,4-di-O-isopropylidene-6-deoxy-α-d-galactopyranos-6-yltriflat [60] and 7-amino-4-methylcoumarin [72] were synthesized according to respective protocols from the literature. All other chemicals were commercially available and were used as received.
Thin-layer chromatography (TLC) with silica gel 60 F254 on glass, available from Merck KGaA (Darmstadt, Germany), or ALUGRAM® XTRA SIL G/UV254 from Macherey-Nagel GmbH & Co. KG (Düren, Germany) on aluminum foil were used for monitoring the reactions. Carborane-containing spots were visualized with a 5% solution of PdCl2 in methanol. Non-carborane-containing spots were visualized with a basic potassium permanganate solution. For chromatography, silica gel (60 Å) with a particle diameter in the range of 0.035 to 0.070 mm was used. Prior to column chromatography, raw products were adsorbed on Celite® S from Sigma-Aldrich Chemie GmbH (Taufkirchen, Germany).
NMR measurements were carried out on a Bruker AVANCE III HD spectrometer (Bruker Corporation, Billerica, MA, United States of America) with an AscendTM 400 magnet (Bruker Corporation, Billerica, MA, United States of America) at room temperature. Tetramethylsilane was used as internal standard for 1H- and 13C{1H}-NMR spectra; 11B- and 11B{1H}-NMR spectra were referenced to the Ξ scale [73]. NMR spectra were recorded at the following frequencies: 1H: 400.16 MHz, 13C: 100.63 MHz, 11B: 128.38 MHz. All chemical shifts are reported in parts per million (ppm). Assignment of the 1H and 13C signals was based on 2D-NMR spectra (H,H-COSY, H,C-HSQC, H,C-HMQC and H,C-HMBC). NMR data were interpreted with MestReNova [74]. NMR signals that appeared as broad overlapping signals with the shape of a multiplet or singlet in either 1H-, 11B{1H}- or 11B-NMR spectra were described as “br” (broad). The numbering scheme of the compounds for assignment of NMR signals is given in the Supplementary Materials (see Figure S1).
IR data were obtained with a PerkinElmer FT-IR spectrometer Spectrum 2000 (Perkin Elmer, Inc., Waltham, MA, United States of America) as KBr pellets and with a Thermo Scientific Nicolet iS5 with an ATR unit (Thermo Fisher Scientific, Waltham, MA, United States of America) in the range from 4000 to 400 cm−1.
High-resolution electrospray ionization mass spectrometry (ESI-HRMS) was performed with an ESI ESQUIRE 3000 PLUS spectrometer (Bruker Corporation, Billerica, MA, United States of America) with an IonTrap-analyzer from Bruker Daltonics or on a MicroTOF spectrometer from Bruker Daltonics with a ToF analyzer in negative or positive mode. As solvents for the measurements, DCM, MeCN, methanol or mixtures of these solvents were used. Interpretation of the spectra was carried out using MestReNova [74].
Tert-Butyl-N-(1,2:3,4-di-O-isopropylidene-6-deoxy-α-d-galactopyranos-6-yl)glycinate (1): Diisopropylethylamine (2.55 mL, 15.0 mmol, 2.96 eq.) was added dropwise under nitrogen atmosphere at room temperature to a solution of tert-butyl glycinate hydrochloride (1.12 g, 6.12 mmol, 1.20 eq.) and 1,2:3,4-di-O-isopropylidene-6-deoxy-α-d-galactopyranos-6-trifluoromethanesulfonate (2.00 g, 5.10 mmol, 1.00 eq.) in 50 mL MeCN. The reaction mixture was stirred for 72 h at 45 °C. The reaction was stopped by evaporating all volatile components under reduced pressure at 45 °C. The crude product was dissolved in ethyl acetate (100 mL) and washed with H2O and saturated NaCl solution. Subsequently, the aqueous layer was extracted with ethyl acetate (3 × 50 mL), dried with Na2SO4, filtered and the solvent was removed under reduced pressure. The crude material was purified by column chromatography using n-hexane/ethanol (14:1 (Rf = 0.41) to 10:1 (Rf = 0.58), (v/v)) as eluent. Compound 1 was obtained as colorless oil in 68% yield (1.30 g, 3.48 mmol). 1H-NMR (400 MHz, chloroform-d1): δ [ppm] = 1.33, 1.34, 1.45 and 1.54 (s, 12H, 13, 13′, 14 and 14′CH3), 1.46 (s, 9H, 10, 10′ and 10′’CH3), 2.77 to 2.92 (m, 2H, 6CH2), 3.26 to 3.40 (m, 2H, 7CH2), 3.88 (m, 1H, 5CH), 4.22 (dd, 1H, 4CH, 3JHH = 7.9 Hz, 3JHH = 4.9 Hz), 4.31 (dd, 1H, 2CH, 3JHH = 5.1 Hz, 3JHH = 2.4 Hz), 4.60 (dd, 1H, 3CH, 3JHH = 7.9 Hz, 3JHH = 4.9 Hz), 5.54 (d, 1H, 1CH, 3JHH = 5.1 Hz). 13C{1H}-NMR (100 MHz, chloroform-d1): δ [ppm] = 24.5 (s, 13,13′,14 or 14′CH3), 24.9 (s, 13,13′,14 or 14′CH3), 26.0 (s, 13,13′,14 or 14′CH3), 26.1 (s, 13,13′,14 or 14′CH3), 28.1 (s, 10,10′ and 10′’CH3), 49.1 (s, 6CH2), 51.8 (s, 7CH2), 67.1 (s, 5CH), 70.5 (s, 2CH), 70.8 (s, 3CH), 71.9 (s, 4CH), 80.9 (s, 9Cq), 96.4 (s, 1CH), 108.4 (s, 11Cq), 109.1 (s, 12Cq), 171.4 (s, 8Cq). IR (KBr): ṽ = 2982 (m, νCH-sp3), 2935 (m, νCH-sp3), 2361 (w), 1736 (s, νCOEster), 1459 (w, δas.CH2), 1372 (m, δs.CH3), 1256 (m), 1213 (s), 1165 (s, νas.C-O-CEster), 1114 (m), 1070 (s, νC-O-CEther), 1003 (m), 918 (w), 898 (w), 854 (w), 804 (w), 771 (w), 650 (w), 512 (w) cm−1. ESI-HRMS: (m/z) calculated for [NaC18H31NO7]+ = 396.19986; observed 396.20153 [M+Na+]+; calculated for [C18H32NO7]+ = 374.21791; observed 374.21969 [M+H]+; calculated for [NaC14H23NO7]+ = 340.13726; observed 340.13844 [M-C4H8+Na]+; calculated for [C14H24NO7]+ = 318.15531; observed 318.15632 [M−C4H8+H]+.
Tert-Butyl-{2-[(1,2:3,4-di-O-isopropylidene-6-deoxy-α-d-galactopyranos-6-yl)amino]-ethyl}carbamate (2): A 250 mL round-bottom flask was charged with 3.20 g (8.16 mmol, 0.76 eq.) 1,2:3,4-di-O-isopropylidene-6-deoxy-α-d-galactopyranos-6-trifluormethanesulfonate and 60 mL MeCN. Then, 1.70 mL (1.73 g, 10.8 mmol, 1.00 eq.) tert-butyl-N-(2-aminoethyl)carbamate, dissolved in 10 mL MeCN, were added via a dropping funnel to this solution. The reaction mixture was cooled to 0 °C and, subsequently, 2.05 mL (1.56 g, 12.1 mmol, 1.12 eq.) N,N-diisopropylethylamine, dissolved in 10 mL MeCN, were added dropwise. After stirring for 15 min at 0 °C, the reaction mixture was warmed to 40 °C and stirred for two days. The reaction was stopped by cooling to room temperature and adding 30 mL saturated NH4Cl solution. All volatile components were removed under reduced pressure, and the remaining aqueous layer was extracted four times with 40 mL ethyl acetate, respectively. The combined organic layers were dried over MgSO4. The drying agent was filtered off and the solvent was removed under reduced pressure. The crude product was purified by column chromatography (a: DCM/methanol, 20:1, (v/v); b: acetone; c: n-hexane/isopropanol, 8:1 to 3:1, (v/v)). A total of 0.56 g tert-butyl-(2-{[bis(1,2:3,4-di-O-isopropylidene-6-deoxy-α-d-galactopyranos-6-yl)]amino}ethyl)-carbamate (2′) (Rf = 0.38, DCM/methanol, 20:1, (v/v), 0.87 mmol, 8%, analytical data are given in the Supplementary Materials) was obtained as a colorless solid, as well as 3.28 g tert-butyl-{2-[(1,2:3,4-di-O-isopropylidene-6-deoxy-α-d-galactopyranos-6-yl)amino]ethyl}carbamate (2) (Rf = 0.12 n-hexane/isopropanol, 5:1, (v/v), 8.27 mmol, 77%) as a colorless solid. 1H-NMR (400 MHz, acetone-d6): δ [ppm] = 1.34, 1.35, 1.43 and 1.52 (s, 21H, 13,13′,13′’,16,16′,17 and 17′CH3), 3.48 to 3.61 (m, 4H, 8,9CH2), 3.58 (m, 2H, 6CH2), 4.28 (td, 1H, 5CH, 3JHH = 6.0 Hz, 3JHH = 1.8 Hz), 4.39 (dd, 1H, 4CH, 3JHH = 7.9 Hz, 3JHH = 1.9 Hz), 4.45 (dd, 1H, 2CH, 3JHH = 5.0 Hz, 3JHH = 2.5 Hz), 4.73 (dd, 1H, 3CH, 3JHH = 7.8 Hz, 3JHH = 2.5 Hz), 5.56 (d, 1H, 1CH, 3JHH = 4.9 Hz), 6.64 (s, 1H, 7NH), 8.24 (s, 1H, 10NH). 13C{1H}-NMR (100 MHz, acetone-d6): δ [ppm] = 24.4, 25.0, 26.2, 28.5 (s, 13, 13′, 13′’16,16′,17 and 17′CH3), 38.2 (s, 6CH2), 49.3 (s, 8 or 9CH2), 50.7 (s, 8 or 9CH2), 64.6 (s, 5CH), 71.2 (s, 2CH), 71.6 (s, 3CH), 72.2 (s, 4CH), 80,6 (s, 12Cq), 97.1 (s, 1CH) 109.9 and 110.7 (s, 14, 15Cq). Carbonyl carbon atom 11C was not observed.
Tert-Butyl-N-[(1,7-dicarba-closo-dodecaboran-1-yl)methyl]-N-(1,2:3,4-di-O-isopropylidene-6-deoxy-α-d-galacatopyranos-6-yl)glycinate (3): A suspension of tert-butyl-N-(1,2:3,4-di-O-isopropylidene-6-deoxy-α-d-galactopyranos-6-yl)glycinate (1) (0.94 g, 2.52 mmol, 1.00 eq.), 1-(trifluoromethanesulfonylmethyl)-1,7-dicarba-closo-dodecaborane (0.93 g, 3.02 mmol, 1.20 eq.) and potassium carbonate (0.42 g, 3.02 mmol, 1.20 eq.) in 10 mL toluene was stirred at 95 °C for 43 h under nitrogen atmosphere. Then, the suspension was diluted with ethyl acetate and washed with 20 mL H2O and saturated NaCl solution. Subsequently, the aqueous layer was extracted with ethyl acetate (3 × 15 mL), the combined organic layers were dried over MgSO4 and filtered, and the solvent was removed under reduced pressure. The crude material was purified by column chromatography using n-hexane/ethyl acetate (20:1, (v/v), Rf = 0.50) and then n-hexane/ethanol (20:1, (v/v), Rf = 0.27) as eluent. Compound 3 was obtained as a colorless oil in 54% yield (721 mg, 1.36 mmol). 1H-NMR (400 MHz, chloroform-d1): δ [ppm] = 1.34, 1.42 and 1.56 (s, 12H, 13,13′,14 and 14′CH3), 1.44 (s, 9H, 10,10′ and 10′’CH3), 1.50 to 3.00 (br m, 10H, 10 BH), 2.83 to 2.91 (m, 1H, 6CHH), 2.89 (s, 1H, 17CH), 2.97 to 3.09 (dd, 1H, 6CHH, 2JHH = 14.1 Hz, 3JHH = 7.0 Hz), 3.13 (d, 1H, 15CHH, 2JHH = 15.7 Hz), 3.23 (d, 1H, 15CHH, 3JHH = 15.7 Hz), 3.36 (d, 1H, 7CHH, 3JHH = 17.8 Hz), 3.53 (d, 1H, 7CHH, 3JHH = 17.8 Hz), 3.84 (m, 1H, 5CH), 4.25 (dd, 1H, 4CH, 3JHH = 7.9 Hz, 3JHH = 1.9 Hz), 4.29 (dd, 1H, 2CH, 3JHH = 5.1 Hz, 3JHH = 2.4 Hz), 4.60 (dd, 1H, 3CH, 3JHH = 7.9 Hz, 3JHH = 2.4 Hz), 5.50 (d, 1H, 1CH, 3JHH = 5.1 Hz). 13C{1H}-NMR (100 MHz, chloroform-d1): δ [ppm] = 24.6, 24.9, 26.0 (s, 13,13′,14 and 14′CH3), 28.2 (s, 10,10′ and 10′’CH3), 54.8 (s, 17CH), 55.0 (s, 6CH2), 55.9 (s, 7CH2), 60.9 (s, 15CH2), 66.8 (s, 5CH), 70.4 (s, 2CH), 70.8 (s, 3CH), 71.9 (s, 4CH), 78.5 (s, 16Cq), 81.5 (s, 9Cq), 96.5 (s, 1CH), 108.5 and 109.1 (s, 11, 12Cq), 170.8 (s, 8Cq). 11B{1H}-NMR (128 MHz, chloroform-d1): δ [ppm] = −15.7 (s, 2B), −13.6 (s, 2B), −10.9 (s, 4B), −9.5 (s, 1B), −4.4 (s, 1B). ESI-HRMS: (m/z) calculated for [NaC21H43B10NO7]+ = 552.3941; observed 552.3966 [M+Na]+.
Tert-Butyl-{2-[(1,7-dicarba-closo-dodecaboran-1-yl)methyl-(1,2:3,4-di-O-isopropyliden-6-deoxy-α-d-galactopyranos-6-yl)amino]ethyl}carbamat (4): A total of 1.66 g (4.12 mmol, 1.00 eq.) tert-butyl-{2-[(1,2:3,4-di-O-isopropylidene-6-deoxy-α-d-galactopyranos-6-yl)amino]-ethyl}carbamate (2) was placed in a 50 mL round-bottom flask and dissolved in 10 mL toluene. Subsequently, 1.51 g (4.94 mmol, 1.20 eq.) 1-(trifluoromethanesulfonylmethyl)-1,7-dicarba-closo-dodecaborane(12), dissolved in 10 mL toluene, and 0.68 g (4.94 mmol, 1.20 eq.) K2CO3 were added. The suspension was heated to 98 °C and stirred for 48 h. The reaction was stopped by adding 20 mL distilled water and 4 mL saturated NaCl solution. The aqueous layer and organic layer were separated. The aqueous layer was extracted two times with 20 mL ethyl acetate. The combined organic layers were dried over MgSO4, the drying agent was filtered off and the solvent was removed under reduced pressure. The resulting yellow-brownish oil was purified by column chromatography (n-hexane/ethyl acetate, 5:1, (v/v)) and 4 (1.17 g, 2.09 mmol, 51%, Rf = 0.24 (n-hexane/ethyl acetate, 5:1, (v/v))) was isolated as a colorless foamy solid. 1H-NMR (400 MHz, chloroform-d1): δ [ppm] = 1.19 to 3.41 (m, br, 10H, 10 BH), 1.34, 1.36, 1.45 and 1.56 (s, 12H, 15,15′,16 and 16′CH3), 1.45 (s, 9H, 12, 12′ and 12′’CH3), 2.55 to 2.63 (m, 1H, 7 or 8CHH), 2.63 to 2.72 (m, 1H, 6CHH), 2.80 to 2.88 (m, 2H, 7 or 8CHH and 6CHH), 2.90 (s, 1H, 19CH), 2.94 to 3.07 (m, 2H, 17CHH and 17CHH), 3.10 to 3.25 (m, 2H, 7 and 8CHH), 3.85 (m, 1H, 5CH), 4.24 (d, 1H, 4CH, 3JHH = 7.9 Hz), 4.31 (dd, 1H, 2CH, 3JHH = 5.1 Hz, 3JHH = 2.4 Hz), 4.62 (dd, 1H, 3CH, 3JHH = 7.9 Hz, 3JHH = 2.4 Hz), 5.35 (s, br, 1H, 9NH), 5.51 (d, 1H, 1CH, 3JHH = 5.1 Hz). 13C{1H}-NMR (100 MHz, chloroform-d1): δ [ppm] = 24.4, 24.9, 26.0 (s, 15,15′,16 and 16′CH3), 28.5 (s, 12,12′ and 12′’CH3), 38.4 (s, 7 or 8CH2), 53.0 (s, 6CH2), 54.6 (s, 7 or 8CH2) 54.7 (s, 19CH), 60.7 (s, 17CH2), 65.8 (s, 5CH), 70.4 (s, 2CH), 70.9 (s, 3CH), 71.8 (s, 4CH), 77.8 (s, 18Cq), 79.0 (s, 11Cq), 96.5 (s, 1CH), 108.4 and 109.3 (s, 13 and 14Cq), 156.1 (s, 10Cq). 11B{1H}-NMR (128 MHz, chloroform-d1): δ [ppm] = −15.6 (s, 2B), −13.6 (s, 2B), −10.8 (s, 4B), −9.3 (s, 1B), −4.3 (s, 1B). IR (KBr): ṽ = 3390 (w, νNH), 2979 (w, νCH-sp3), 2933 (w, νCH-sp3), 2594 (m, νBH), 1707 (m, amide I), 1504 (m, amide II), 1455 (m, δCH), 1366 (m, δCH), 1252 (m, amide III) cm−1. ESI-HRMS: (m/z) calculated for [C22H47B10N2O7]+ = 559.4386; observed 559.4395 [M+H]+; calculated for [NaC22H46B10N2O7]+ = 581.4282; observed 581.4208 [M+Na]+.
N-[(1,7-dicarba-closo-dodecaborane-1-yl)methyl]-N-(1,2:3,4-di-O-isopropylidene-6-deoxy-α-d-galacatopyranos-6-yl)glycine (5): Method A. Anhydrous TFA (4.20 mL, 54.4 mmol, 40.0 eq.) was added dropwise under nitrogen atmosphere at 0 °C to a solution of tert-butyl-N-[(1,7-dicarba-closo-dodecaboran-1-yl)methyl]-N-(1,2:3,4-di-O-isopropylidene-6-deoxy-α-d-galacatopyranos-6-yl)glycinate (3) (0.71 g, 1.36 mmol, 1.00 eq.) in 4.20 mL DCM. The mixture was warmed to room temperature and stirred for 3 h. When the reaction was completed, TFA and DCM were removed under reduced pressure. DCM and diethyl ether were used as an entrainer to remove remaining TFA. The crude material was purified by column chromatography using n-hexane/ethyl acetate (10:1, (v/v)) as eluent. Diethyl ether was used to remove remaining solvent. Compound 5 was obtained as a colorless solid in 47% yield (300 mg, 0.63 mmol).
Method B. A Schlenk flask was charged with tert-butyl-N-[(1,7-dicarba-closo-dodecaboran-1-yl)methyl]-N-(1,2:3,4-di-O-isopropylidene-6-deoxy-α-d-galacatopyranos-6-yl)-glycinate (3) (0.50 g, 0.94 mmol, 1.00 eq.). Then, 4.0 mL anhydrous TFA (52.2 mmol, 55.6 eq.) were added and the resulting solution was stirred at room temperature for 3 h. Afterwards, about 4 mL DCM were added and both, TFA and DCM, were removed under reduced pressure. This process was repeated three more times with DCM as an entrainer to remove remaining TFA. Then, 3 mL concentrated NaHCO3 solution were added to the obtained crude product while stirring and the mixture was subsequently sonicated for 15 min, resulting in a thick, cloudy white suspension with a brownish oil-like layer on top. Upon addition of 2 mL DCM, the oil-like layer was dissolved and gas evolution was observed. Once the gas evolution had almost stopped, the solution was stirred for five more minutes. Subsequently, the aqueous layer was removed using a syringe and extracted two times with 2 mL ethyl acetate each. The combined organic layers were washed twice with 2 mL of water. Afterwards, the combined organic layers were dried over MgSO4 and filtered. Finally, the solvent was removed under reduced pressure, affording compound 5 as a yellowish foamy solid in 65% yield (290 mg, 0.612 mmol). 1H-NMR (400 MHz, chloroform-d1): δ [ppm] = 1.33, 1.34, 1.43 and 1.56 (s, 12H, 11,11′,12 and 12′CH3), 1.50 to 3.00 (m, br, 10H, 10 BH), 2.91 to 2.99 (m, 3H, 6CH2 and 15CH), 3.17 (d, 1H, 13CHH, 2JHH = 20.0 Hz), 3.21 (d, 1H, 13CHH, 2JHH = 20.0 Hz), 3.48 (d, 1H, 7CHH, 2JHH = 18.2 Hz), 3.59 (d, 1H, 7CHH, 2JHH = 18.2 Hz), 3.87 (m, 1H, 5CH), 4.15 (dd, 1H, 4CH, 3JHH = 7.9 Hz, 3JHH = 2.0 Hz), 4.35 (dd, 1H, 2CH, 3JHH = 5.2 Hz, 3JHH = 2,4 Hz), 4.62 (dd, 1H, 3CH, 3JHH = 7.9 Hz, 3JHH = 2.4 Hz), 5.55 (d, 1H, 1CH, 3JHH = 5.1 Hz). 13C{1H}-NMR (100 MHz, chloroform-d1): δ [ppm] = 24.4, 24.8, 25.95 and 26.03 (s, 11,11′,12 and 12′CH3), 54.6 (s, 6CH2), 55.3 (s, 15CH), 57.2 (s, 7CH2), 59.1 (s, 13CH2), 65.5 (s, 5CH), 70.4 (s, 2CH), 70.8 (s, 3CH), 71.6 (s, 4CH), 76.3 (s, 14Cq), 96.4 (s, 1CH), 108.8 and 109.7 (s, 9 and 10Cq), 172.4 (s, 8Cq). 11B{1H}-NMR (128 MHz, chloroform-d1): δ [ppm] = −15.7 (s, 2B), −13.5 (s, 2B), −11.3 (s, 2B), −10.6 (s, 2B), −9.0 (s, 1B), −4.5 (s, 1B). IR (KBr): ṽ = 3061 (w, νCH2-sp2), 2985 (w, νas.CH2-sp3), 2961 (w, νs.CH2-sp3), 2931 (m, νas.CH3-sp3), 2871 (w, νs.CH3-sp3), 2595 (s, νBH-sp3), 1716 (m, νC=Ocarboxylic acid), 1456 (m, δas.CH3-sp3), 13 (m, δs.CH3-sp3), 1066 (s, νC-O-Cether) cm-1. ESI-HRMS: (m/z) calculated for [C17H36B10NO7]+ = 474.3495; observed 474.3485 [M+H]+.
N1-[(1,7-Dicarba-closo-dodecaborane-1-yl)methyl]-N1-(1,2:3,4-di-O-isopropylidene-6-deoxy-α-d-galactopyranos-6-yl)ethane-1,2-diamine (6): A 25 mL round-bottom flask was charged with 0.30 g (0.54 mmol, 1.00 eq.) tert-butyl-{2-[(1,7-dicarba-closo-dodecaboran-1-yl)methyl-(1,2,:3,4-di-O-isopropylidene-6-deoxy-α-d-galactopyranos-6-yl)amino]ethyl}-carbamate (4) and 2.00 mL (26.0 mmol, 48.2 eq.) TFA were added. The reaction mixture was stirred for 3 h at room temperature. The reaction was stopped by adding 4 mL DCM with subsequent evaporation of all volatile components under reduced pressure. This procedure was repeated three times. The resulting crude product was further purified by adding 4 mL saturated NaHCO3 solution and sonication for about 15 min. Again, 3 mL DCM were added with stirring, under observation of gas evolution, and after 5 min the resulting layers were separated. The aqueous layer was extracted with 3 mL ethyl acetate. The combined organic layers were washed twice with 2 mL distilled water each. The organic layer was dried over MgSO4, the drying agents were filtered off and the solvent was removed under reduced pressure. Compound 6 (0.25 g, 0.54 mmol, quant., Rf = 0.03, n-hexane/ethyl acetate, 5:1, (v/v)) was isolated as a colorless foamy solid. 1H-NMR (400 MHz, chloroform-d1): δ [ppm] = 1.33, 1.34, 1.45, 1.54 (s, 12H, 12,12′,13 and 13′CH3), 1.40 to 3.16 (m, br, 10H, 10 BH), 2.62 to 2.69 (m, 1H, 7 or 8CHH), 2.72 to 2.77 (m, 2H, 7 or/and 8CHH), 2.77 to 2.86 (m, 3H, 7 or 8CHH and 6CH2), 2.92 (s, 1H, 16CH), 3.00 (d, 1H, 14CHH, 2JHH = 15.4 Hz), 3.11 (d, 1H, 14CHH, 2JHH = 15.4 Hz) 3.87 (m, 1H, 5CH), 4.17 (dd, 1H, 4CH, 3JHH = 7.9 Hz, 3JHH = 1.9 Hz), 4.30 (dd, 1H, 2CH, 3JHH = 5,2 Hz, 3JHH = 2.4 Hz), 4.60 (dd, 1H, 3CH, 3JHH = 7.9 Hz, 3JHH = 2.4 Hz), 5.52 (d, 1H, 1CH, 3JHH = 5.1 Hz). 13C{1H}-NMR (100 MHz, chloroform-d1): δ [ppm] = 24.5, 24.8, 25.96 and 26.0 (s, 12,12′,13 and 13′CH3), 39.8 (s, 7 or 8CH2), 53.2 (s, 6CH2), 55.0 (s, 16CH), 57.7 (s, 7 or 8CH2), 60.2 (s, 14CH2), 66.1 (s, 5CH), 70.3 (s, 2CH), 70.8 (s, 3CH), 72.0 (s, 4CH), 96.5 (s, 1CH), 108.5 and 109.3 (s, 10 and 11Cq), 15Cq was not observable (assumed at 77.7 ppm). 11B{1H}-NMR (128 MHz, chloroform-d1): δ [ppm] = −15.6 (s, 2B), −13.5 (s, 2B), −11.2 (s, 2B), −10.8 (s, 2B), −9.3 (s, 1B), −4.3 (s, 1B). IR (KBr): ṽ = 2986 (w, νCH-sp3), 2933 (w, νCH-sp3), 2593 (m, νBH-sp3), 1685 (w, δNH-sp3), 1455 (w, δCH-sp3), 1381 (m, δCH-sp3) cm−1. ESI-HRMS: calculated for [C17H39B10N2O5]+ = 459.3862; observed 459.3851 [M+H]+; calculated for [C34H77B20N4O10]+ = 918.7724; observed 918.7607 [2M+H]+.
N1-[(1,7-dicarba-closo-dodecaboran-1-yl)methyl]-N1-(1,2:3,4-di-O-isopropylidene-6-deoxy-α-d-galacatopyranos-6-yl)-N2-[(4-methyl)-2-oxo-2H-chromen-7-yl)glycineamide (7): Method A. Under nitrogen atmosphere, 1 mL (0.98 g, 12.4 mmol) of pyridine was added to a mixture of N-[(1,7-dicarba-closo-dodecaboran-1-yl)methyl]-N-(1,2:3,4-di-O-isopropylidene-6-deoxy-α-d-galacatopyranos-6-yl)glycine (5) (100 mg, 0.21 mmol, 1.00 eq.) and 7-amino-4-methylcoumarin (44.4 mg, 0.25 mmol, 1.20 eq.). After degassing the yellowish solution with nitrogen, phosphorus(V) oxychloride (20.0 µL, 0.23 mmol, 1.10 eq.) was added dropwise at −18 °C. The solution immediately changed to a red suspension and after a while back to a yellow solution. The mixture was stirred for 1 h at −18 °C. After the reaction was finished, the mixture was poured into H2O and extracted with ethyl acetate. Subsequently, the combined organic layers were washed with a NaHCO3 solution and a saturated NaCl solution and then dried over MgSO4. After filtration, the solvent was removed under reduced pressure and the crude product was purified by column chromatography using n-hexane/ethyl acetate (1:1, (v/v), Rf = 0.51) as eluent. Compound 7 was obtained as a brownish solid in 27% yield (36 mg, 5.71 µmol).
Method B. N-[(1,7-dicarba-closo-dodecaboran-1-yl)methyl]-N-(1,2:3,4-di-O-isopropylidene-6-deoxy-α-d-galacatopyranos-6-yl)glycine (86.1 mg, 0.18 mmol, 1.00 eq.) was added to a Schlenk tube and was dissolved in 5 mL absolute DCM. Subsequently, the solution was cooled to 0 °C in an ice bath and 7-amino-4-methylcoumarin (39.1 mg, 0.22 mmol, 1.23 eq.), HOBt (29.5 mg, 0.22 mmol, 1.20 eq.), EDCI (45.1 mg, 0.24 mmol, 1.30 eq.) and DIPEA (80 µL, 0.46 mmol, 2.54 eq.) were added. Afterwards, the ice bath was removed, the reaction mixture was allowed to reach room temperature and stirred at room temperature for 22 h. Subsequently, the solvent was removed under reduced pressure and the residue was redissolved in 20 mL ethyl acetate and washed with 30 mL of 1 N HCl, concentrated NaHCO3 and NaCl solution, respectively. The organic layer was dried over Na2SO4, filtered and the solvent was removed under reduced pressure. The obtained crude product was purified by column chromatography using n-hexane/ethyl acetate (1:1 (Rf = 0.51) to 1:2, (v/v)) as eluent, affording compound 7 as a sticky, brownish solid in 18% yield (20 mg, 32 µmol). 1H-NMR (400 MHz, chloroform-d1): δ [ppm] = 1.28, 1.29, 1.38, 1.59 (s, 12H, 12,12′,13 and 13′CH3), 1.50 and 3.00 (m, br, 10H, 10 BH), 2.42 (d, 3H, 23CH3, 4JHH = 1.2 Hz), 2.86 to 2.97 (m, 3H, 6CHH, 6CHH and 16CH), 3.16 (d, 1H, 14CHH, 2JHH = 15.5 Hz), 3.25 (d, 1H, 14CHH, 2JHH = 15.5 Hz), 3.43 (d, 1H, 7CHH, 2JHH = 17.5 Hz), 3.52 (d, 1H, 7CHH, 2JHH = 17.4 Hz), 3.93 (m, 1H, 5CH, JHH = 9.7 and 2.5 Hz), 4.10 (dd, 1H, 4CH, 3JHH = 7.8 Hz, 3JHH = 2.0 Hz), 4.41 (dd, 1H, 2CH, 3JHH = 5.2 Hz, 3JHH = 2.4 Hz), 4.63 (dd, 1H, 3CH, 3JHH = 7.8 Hz, 3JHH = 2.4 Hz), 5.69 (d, 1H, 1CH, 3JHH = 5.2 Hz), 6.21 (d, 1H, 21CH, 4JHH = 1.4 Hz), 7.53 to 7.58 (m, 2H, 18CH and 25CH), 7.71 (dd, 1H, 26CH, 3JHH = 8.7 Hz, 4JHH = 2.0 Hz), 9.57 (s, 1H, 9NH). 13C{1H}-NMR (100 MHz, chloroform-d1): δ [ppm] = 18.6 (s, 23CH3), 24.4, 24.7, 25.9 and 26.1 (s, 12,12′,13 or 13′CH3), 54.5 (s, 6CH2), 55.3 (s, 16CH), 58.7 (s, 14CH2), 60.0 (s, 7CH2), 64.8 (s, 5CH), 70.3 (s, 2CH), 70.8 (s, 3CH), 71.7 (s, 4CH), 75.9 (s, 15Cq), 96.5 (s, 1CH), 107.0 (s, 18CH), 108.9 and 109.6 (s, 10 and 11Cq), 113.4 (s, 21CH), 115.7 (s, 26CH), 116.2 (s, 22Cq), 125.2 (s, 25CH), 141.2 (s, 17Cq), 152.3 (s, 24Cq), 154.3 (s, 19Cq), 161.2 (s, 20Cq), 169.3 (s, 8Cq). 11B{1H}-NMR (128 MHz, chloroform-d1): δ [ppm] = −15.6 (s, 2B), −13.4 (s, 2B), −11.2 (s, 2B), −10.5 (s, 2B), −8.9 (s, 1B), −4.4 (s, 1B). IR (KBr): ṽ = 3288 (m, ν(NH, secondary amide (trans)), 3058 (w, νCH-sp2), 2985 (w, νasCH2-sp3), 2957 (w, νsCH2-sp3), 2922 (m, νasCH3-sp3), 2852 (m, νsCH3-sp3), 2595 (m, νBH-sp3), 1727 (s, νC=Olactone), 1706 (s, vC=Oamide, amide I), 1616 (νC=C, α,ß-unsaturation), 1567 (νC=C, aromatic or δNH, amide II), 1519 (vC=C, aromatic or νCN, amide II), 1454 (m, δasCH3-sp3), 1369 (m, δsCH3-sp3), 1066 (s, νC-O-CEther), 901 (m, δCH-sp2/aromatic), 853, 806 (m, δCH-sp2/aromatic) cm−1. ESI-HRMS: (m/z) calculated for [NaC27H42B10N2O8]+ = 653.3843; observed 653.3840 [M+Na]+.
N2-(4-{[(2-Amino-4-hydroxypteridine-6-yl)methyl]amino}benzoyl)-N5-{2-[(1,7-dicarba-closo-dodecaboran-1-ylmethyl)-(1,2:3,4-di-O-isopropyliden-6-deoxy-α-d-galactopyranos-6-yl)amino]ethyl}-l-glutamine (8) and (S)-2-(4-{[(2-amino-4-hydroxypteridine-6-yl)methyl]amino}benzamido)-N1,N5-bis-{2-[(1,7-dicarba-closo-dodecaborane-1-yl)methyl-(1,2:3,4-di-O-isopropylidene-6-deoxy-α-d-galactopyranos-6-yl)amino]ethyl}amino)pentanediamide (9): A 100 mL Schlenk flask was charged with 0.24 g (0.55 mmol, 1.00 eq.) folic acid and 20 mL dimethylformamide were added. The mixture was sonicated for 15 min and, subsequently, warmed to 37 °C for 15 min until a clear solution was obtained. To this mixture, 0.11 g (0.55 mmol, 1.00 eq.) DCC and 0.06 g (0.55 mmol, 1.00 eq.) NHS were added. The reaction mixture was stirred for 16 h at room temperature. Afterwards, 0.25 g (0.55 mmol, 1.00 eq.) N1-[(1,7-dicarba-closo-dodecaborane-1-yl)methyl]-N1-(1,2:3,4-di-O-isopropylidene-6-deoxy-α-d-galactopyranos-6-yl)ethane-1,2-diamine (6), dissolved in 7 mL dimethylformamide, were added to this solution and the mixture was stirred overnight at room temperature. The resulting suspension was filtered under inert conditions. Subsequently, 0.10 mL (0.61 mmol, 1.10 eq.) N,N-diisopropylethylamine were added and the mixture was stirred overnight at room temperature. The reaction was stopped by adding 50 mL ice-cold diethyl ether. Simultaneously, the mixture was cooled in an ice bath. Completion of the precipitation was achieved by storage for one additional night at −20 °C. The resulting precipitate was filtered off and washed with 10 mL ice-cold diethyl ether. The precipitate was suspended in 5 mL diethyl ether and subsequently sonicated for 15 min. Afterwards, the raw product was filtered again. The orange-red solid was washed with 5 mL diethyl ether and the previously described procedure was repeated one more time. Afterwards, the precipitate was dried in vacuo. It was not possible to isolate the desired compound 8, but mass spectrometry revealed the presence of N2-(4-{[(2-amino-4-hydroxypteridine-6-yl)methyl]amino}benzoyl)-N5-{2-[(1,7-dicarba-closo-dodecaboran-1-yl)methyl-(1,2:3,4-di-O-isopropyliden-6-deoxy-α-d-galactopyranos-6-yl)amino]ethyl}-l-glutamine (8) and (S)-2-(4-{[(2-amino-4-hydroxypteridine-6-yl)methyl]amino}benzamido)-N1,N5-bis-{2-[(1,7-dicarba-closo-dodecaborane-1-yl)methyl-(1,2:3,4-di-O-isopropylidene-6-deoxy-α-d-galactopyranos-6-yl)amino]ethyl}amino)pentanediamide (9). Due to impurities, it was not possible to determine a yield based on HRMS. 8: ESI-HRMS: (m/z) calculated for [C36H56B10N9O10]+ = 883.5139; observed 883.5151 [M+H]+; calculated for [NaC36H55B10N9O10]+ = 905.4959; observed 905.4963 [M+Na]+; calculated for [KC36H55B10N9O10]+ = 921.4700; observed 921.4715 [M+K]+. 9:1E7D IR (KBr): ṽ = 3315 (w, νNH-sp3), 2931 (w, νCH-sp3), 2850 (w, νCH-sp3), 2594 (w, νBH-sp3), 1723 (m, amide I), 1687 (m, amide II), 1605 (s, νC=Carom.), 1412 (m, δCH-sp3) cm−1. ESI-HRMS: (m/z) calculated for [C53H92B20N11O14]+ = 1323.8830; observed 1323.8829 [M+H]+; calculated for [NaC53H91B20N11O14]+ = 1345.8649; observed 1345.8625 [M+Na]+; calculated for [KC53H91B20N11O14]+ = 1361.8390; observed 1361.8349 [M+K]+.
4. Conclusions
In this work we reported the successful design of a novel modular, small-molecule-based approach to synthesizing boron-rich compounds bearing a carboxylic acid group or a primary amine group as potential coupling partners for suitable tumor-selective biomolecules. As proof of concept, conjugates with 7-amino-4-methylcoumarin and folic acid were obtained. While the present work focused on the development of a synthetic protocol, the next steps will include the deprotection of the respective galactopyranosyl protecting groups under acidic aqueous conditions [61] followed by biological investigations.
Acknowledgments
We thank Ramona Oehme, Susann Billig and Claudia Birkemeyer for measuring the mass spectra and Ines Rein, Stefanie Märcker-Recklies and Jaqueline Lewandowski for recording the infrared spectra.
Supplementary Materials
Supplementary information is available online, including the numbering scheme of the isolated compounds 1‒7, NMR spectra of compounds 5, 6 and 7 and mass spectra of 8 and 9, additional synthetic procedures and analytical data for 2′, ESI-3, ESI-3′, ESI-4 and 1-(trifluoromethanesulfonylmethyl)-1,7-dicarba-closo-dodecaborane(12), crystallographic information for compound ESI-3′, information about the exploration of the optimization for the synthesis of 3 and the deprotection protocol for 4, and the extension of the synthetic protocol to ortho-carborane derivatives.
Author Contributions
Conceptualization, M.K. and E.H.-H.; Data curation, M.K. and P.L.; formal analysis, M.K., J.-S.J.F., A.F., N.A.U., J.T. and P.L.; funding acquisition, E.H.-H.; investigation, M.K., J.-S.J.F., A.F., N.A.U. and J.T.; methodology, M.K.; project administration, M.K. and E.H.-H.; resources, E.H.-H.; supervision, E.H.-H.; validation, M.K., J.-S.J.F., A.F., N.A.U. and J.T.; visualization, M.K.; writing—original draft, M.K.; writing—review & editing, J.-S.J.F., A.F., N.A.U., J.T., P.L. and E.H.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Europäischer Fonds für regionale Entwicklung (EFRE), the Free State of Saxony (ESF) and the Leipzig School of Natural Sciences—Building with Molecules and Nano-objects (BuildMoNa).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Locher G.L. Biological Effects and Therapeutic Possibilities of Neutrons. Am. J. Roentgenol. Radi. 1936;36:1–18. [Google Scholar]
- 2.Hatanaka H. A Revised Boron-Neutron Capture Therapy for Malignant Brain Tumors. J. Neurol. 1975;209:81–94. doi: 10.1007/BF00314601. [DOI] [PubMed] [Google Scholar]
- 3.Taylor H.J., Goldhaber M. Detection of Nuclear Disintegration in a Photographic Emulsion. Nature. 1935;135:341. doi: 10.1038/135341a0. [DOI] [Google Scholar]
- 4.Chadwick J., Goldhaber M. Disintegration by Slow Neutrons. Nature. 1935;135:65. doi: 10.1038/135065a0. [DOI] [Google Scholar]
- 5.Barth R.F., Soloway A.H., Fairchild R.G. Boron Neutron Capture Therapy of Cancer. Cancer Res. 1990;50:1061–1070. doi: 10.1038/scientificamerican1090-100. [DOI] [PubMed] [Google Scholar]
- 6.Soloway A.H., Tjarks W., Barnum B.A., Rong F.-G., Barth R.F., Codogni I.M., Wilson J.G. The Chemistry of Neutron Capture Therapy. Chem. Rev. 1998;98:1515–1562. doi: 10.1021/cr941195u. [DOI] [PubMed] [Google Scholar]
- 7.Hartman T., Carlsson J. Radiation Dose Heterogeneity in Receptor and Antigen Mediated Boron Neutron Capture Therapy. Radiother. Oncol. 1994;31:61–75. doi: 10.1016/0167-8140(94)90414-6. [DOI] [PubMed] [Google Scholar]
- 8.Coderre J.A., Turcotte J.C., Riley K.J., Binns P.J., Harling O.K., Kiger W.S. Boron Neutron Capture Therapy: Cellular Targeting of High Linear Energy Transfer Radiation. Technol. Cancer Res. Treat. 2003;2:355–375. doi: 10.1177/153303460300200502. [DOI] [PubMed] [Google Scholar]
- 9.Sears V.F. Neutron Scattering Lengths and Cross Sections. Neutron News. 1992;3:26–37. doi: 10.1080/10448639208218770. [DOI] [Google Scholar]
- 10.Hawthorne M.F. The Role of Chemistry in the Development of Boron Neutron Capture Therapy of Cancer. Angew. Chem. Int. Ed. 1993;32:950–984. doi: 10.1002/anie.199309501. [DOI] [Google Scholar]
- 11.Adams L., Tomlinson S., Wang J., Hosmane S.N., Maguire J.A., Hosmane N.S. Novel Approach to Boron-10 Enriched Decaborane(14): An Important Advance in Synthetic Boron Hydride Chemistry. Inorg. Chem. Commun. 2002;5:765–767. doi: 10.1016/S1387-7003(02)00553-1. [DOI] [Google Scholar]
- 12.Yinghuai Z., Widjaja E., Lo Sia S.P., Zhan W., Carpenter K., Maguire J.A., Hosmane N.S., Hawthorne M.F. Ruthenium(0) Nanoparticle-Catalyzed Isotope Exchange between 10B and 11B Nuclei in Decaborane(14) J. Am. Chem. Soc. 2007;129:6507–6512. doi: 10.1021/ja070210c. [DOI] [PubMed] [Google Scholar]
- 13.Chadwick J. Possible Existence of a Neutron. Nature. 1932;129:312. doi: 10.1038/129312a0. [DOI] [Google Scholar]
- 14.Petry W., Neuhaus J. Neutronen nach Maß. Phys. J. 2007;6:31–37. [Google Scholar]
- 15.Kettenbach K., Schieferstein H., Grunewald C., Iffland D., Reffert L.M., Hampel G., Schütz C.L., Bings N.H., Ross T.L. Synthesis and Evaluation of Boron Folates for Boron-Neutron-Capture-Therapy (BNCT) Radiochim. Acta. 2015;103:799–809. doi: 10.1515/ract-2014-2360. [DOI] [Google Scholar]
- 16.Rong F.-G., Soloway A.H., Ikeda S., Ives D.H. Synthesis and Biochemical Activity of Hydrophilic Carborane-Containing Pyrimidine Nucleosides as Potential Agents for DNA Incorporation and BNCT. Nucleos. Nucleot. 1997;16:379–401. doi: 10.1080/07328319708001357. [DOI] [Google Scholar]
- 17.Kueffer P.J., Maitz C.A., Khan A.A., Schuster S.A., Shlyakhtina N.I., Jalisatgi S.S., Brockman J.D., Nigg D.W., Hawthorne M.F. Boron Neutron Capture Therapy Demonstrated in Mice Bearing EMT6 Tumors Following Selective Delivery of Boron by Rationally Designed Liposomes. Proc. Natl. Acad. Sci. USA. 2013;110:6512–6517. doi: 10.1073/pnas.1303437110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahrens V.M., Frank R., Boehnke S., Schütz C.L., Hampel G., Iffland D.S., Bings N.H., Hey-Hawkins E., Beck-Sickinger A.G. Receptor-mediated Uptake of Boron-rich Neuropeptide Y Analogues for Boron Neutron Capture Therapy. ChemMedChem. 2015;10:164–172. doi: 10.1002/cmdc.201402368. [DOI] [PubMed] [Google Scholar]
- 19.Calabrese G., Daou A., Barbu E., Tsibouklis J. Towards Carborane-functionalised Structures for the Treatment of Brain Cancer. Drug Discov. Today. 2018;23:63–75. doi: 10.1016/j.drudis.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Barth R.F., Mi P., Yang W. Boron Delivery Agents for Neutron Capture Therapy of Cancer. Cancer Commun. 2018;38:35. doi: 10.1186/s40880-018-0299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bleuel D.L., Donahue R.J., Ludewigt B.A., Vujic J. Designing Accelerator-based Epithermal Neutron Beams for Boron Neutron Capture Therapy. Med. Phys. 1998;25:1725–1734. doi: 10.1118/1.598353. [DOI] [PubMed] [Google Scholar]
- 22.Onishi T., Kumada H., Takada K., Naito F., Kurihara T., Sakae T. Investigation of the Neutron Spectrum Measurement Method for Dose Evaluation in Boron Neutron Capture Therapy. Appl. Radiat. Isot. 2018;140:5–11. doi: 10.1016/j.apradiso.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Durisi E., Alikaniotis K., Borla O., Bragato F., Costa M., Giannini G., Monti V., Visca L., Vivaldo G., Zanini A. Design and Simulation of an Optimized E-linac Based Neutron Source for BNCT Research. Appl. Radiat. Isot. 2015;106:63–67. doi: 10.1016/j.apradiso.2015.07.039. [DOI] [PubMed] [Google Scholar]
- 24.Kato T., Hirose K., Tanaka H., Mitsumoto T., Motoyanagi T., Arai K., Harada T., Takeuchi A., Kato R., Yajima S., et al. Design and Construction of an Accelerator-based Boron Neutron Capture Therapy (AB-BNCT) Facility with Multiple Treatment Rooms at the Southern Tohoku BNCT Research Center. Appl. Radiat. Isot. 2019;156:108961. doi: 10.1016/j.apradiso.2019.108961. [DOI] [PubMed] [Google Scholar]
- 25.Mori Y., Suzuki A., Yoshino K., Kakihana H. Complex Formation of p-Boronophenylalanine with some Monosaccharides. Pigment Cell Res. 1989;2:273–277. doi: 10.1111/j.1600-0749.1989.tb00203.x. [DOI] [PubMed] [Google Scholar]
- 26.Stella Pharma Corporation [(accessed on 6 March 2021)]; Available online: https://stella-pharma.co.jp/en.
- 27.Stella Pharma Corporation News Release: STELLA PHARMA will Launch Steboronine®, the World’s First BNCT Drug, on May 20, 2020. [(accessed on 6 March 2021)]; Available online: https://stella-pharma.co.jp/cp-bin/wordpress5/wp-content/uploads/2020/05/Steboronine-launched_ENG.pdf.
- 28.Frank R., Ahrens V.M., Boehnke S., Beck-Sickinger A.G., Hey-Hawkins E. Charge-Compensated Metallacarborane Building Blocks for Conjugation with Peptides. ChemBioChem. 2016;17:308–317. doi: 10.1002/cbic.201500569. [DOI] [PubMed] [Google Scholar]
- 29.Worm D.J., Hoppenz P., Els-Heindl S., Kellert M., Kuhnert R., Saretz S., Köbberling J., Riedl B., Hey-Hawkins E., Beck-Sickinger A.G. Selective Neuropeptide Y Conjugates with Maximized Carborane Loading as Promising Boron Delivery Agents for Boron Neutron Capture Therapy. J. Med. Chem. 2020;63:2358–2371. doi: 10.1021/acs.jmedchem.9b01136. [DOI] [PubMed] [Google Scholar]
- 30.Hoppenz P., Els-Heindl S., Kellert M., Kuhnert R., Saretz S., Lerchen H.-G., Köbberling J., Riedl B., Hey-Hawkins E., Beck-Sickinger A.G. A Selective Carborane-Functionalized Gastrin-Releasing Peptide Receptor Agonist as Boron Delivery Agent for Boron Neutron Capture Therapy. J. Org. Chem. 2020;85:1446–1457. doi: 10.1021/acs.joc.9b02406. [DOI] [PubMed] [Google Scholar]
- 31.Hattori Y., Kusaka S., Mukumoto M., Uehara K., Asano T., Suzuki M., Masunaga S., Ono K., Tanimori S., Kirihata M. Biological Evaluation of Dodecaborate-Containing L-Amino Acids for Boron Neutron Capture Therapy. J. Med. Chem. 2012;55:6980–6984. doi: 10.1021/jm300749q. [DOI] [PubMed] [Google Scholar]
- 32.Pan X.Q., Wang H., Shukla S., Sekido M., Adams D.M., Tjarks W., Barth R.F., Lee R.J. Boron-Containing Folate Receptor-Targeted Liposomes as Potential Delivery Agents for Neutron Capture Therapy. Bioconjug. Chem. 2002;13:435–442. doi: 10.1021/bc015557y. [DOI] [PubMed] [Google Scholar]
- 33.Dubey R., Kushal S., Mollard A., Vojtovich L., Oh P., Levin M.D., Schnitzer J.E., Zharov I., Olenyuk B.Z. Tumor Targeting, Trifunctional Dendritic Wedge. Bioconjug. Chem. 2015;26:78–89. doi: 10.1021/bc500436b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doi A., Kawabata S., Iida K., Yokoyama K., Kajimoto Y., Kuroiwa T., Shirakawa T., Kirihata M., Kasaoka S., Maruyama K., et al. Tumor-specific Targeting of Sodium Borocaptate (BSH) to Malignant Glioma by Transferrin-PEG Liposomes: A Modality for Boron Neutron Capture Therapy. J. Neurooncol. 2008;87:287–294. doi: 10.1007/s11060-008-9522-8. [DOI] [PubMed] [Google Scholar]
- 35.Mier W., Gabel D., Haberkorn U., Eisenhut M. Conjugation of the closo-Borane Mercaptoundecahydrododecaborate (BSH) to a Tumour Selective Peptide. Z. Anorg. Allg. Chem. 2004;630:1258–1262. doi: 10.1002/zaac.200400064. [DOI] [Google Scholar]
- 36.Liu L., Barth R.F., Adams D.M., Soloway A.H., Reisfeld R.A. Bispecific Antibodies as Targeting Agents for Boron Neutron Capture Therapy of Brain Tumors. J. Hematother. 1995;4:477–483. doi: 10.1089/scd.1.1995.4.477. [DOI] [PubMed] [Google Scholar]
- 37.Orlova A.V., Zinin A.I., Malysheva N.N., Kononov L.O., Sivaev I.B., Bregadze V.I. Conjugates of Polyhedral Boron Compounds with Carbohydrates. 1. New Approach to the Design of Selective Agents for Boron Neutron Capture Therapy of Cancer. Russ. Chem. Bull. Int. Ed. 2003;52:2766–2769. doi: 10.1023/B:RUCB.0000019902.98142.33. [DOI] [Google Scholar]
- 38.Marepally S.R., Yao M.-L., Kabalka G.W. Boronated Carbohydrate Derivatives as Potential Boron Neutron Capture Therapy Reagents. Future Med. Chem. 2013;5:693–704. doi: 10.4155/fmc.13.39. [DOI] [PubMed] [Google Scholar]
- 39.Varadarajan A., Hawthorne M.F. Novel Carboranyl Amino Acids and Peptides: Reagents for Antibody Modification and Subsequent Neutron-Capture Studies. Bioconjug. Chem. 1991;2:242–253. doi: 10.1021/bc00010a008. [DOI] [PubMed] [Google Scholar]
- 40.Timofeev S.V., Bregadze V.I., Osipov S.N., Titanyuk I.D., Petrovskii P.V., Starikova Z.A., Glukhov I.V., Beletskaya I.P. New Carborane-containing Amino Acids and Their Derivatives. Crystal Structures of N-protected Carboranylalaninates. Russ. Chem. Bull. Int. Ed. 2007;56:791–797. doi: 10.1007/s11172-007-0118-9. [DOI] [Google Scholar]
- 41.Stogniy M.Y., Zakharova M.V., Sivaev I.B., Godovikov I.A., Chizov A.O., Bregadze V.I. Synthesis of New Carborane-based Amino Acids. Polyhedron. 2013;55:117–120. doi: 10.1016/j.poly.2013.02.076. [DOI] [Google Scholar]
- 42.Michiue H., Sakurai Y., Kondo N., Kitamatsu M., Bin F., Nakajima K., Hirota Y., Kawabata S., Nishiki T., Ohmori I., et al. The Acceleration of Boron Neutron Capture Therapy Using Multi-linked Mercaptoundecahydrododecaborate (BSH) Fused Cell-penetrating Peptide. Biomaterials. 2014;35:3396–3405. doi: 10.1016/j.biomaterials.2013.12.055. [DOI] [PubMed] [Google Scholar]
- 43.Worm D.J., Els-Heindl S., Kellert M., Kuhnert R., Saretz S., Koebberling J., Riedl B., Hey-Hawkins E., Beck-Sickinger A.G. A Stable meta-Carborane Enables the Generation of Boron-rich Peptide Agonists Targeting the Ghrelin Receptor. J. Pept. Sci. 2018;32:e3119. doi: 10.1002/psc.3119. [DOI] [PubMed] [Google Scholar]
- 44.Lerouge F., Viñas C., Teixidor F., Núñez R., Abreu A., Xochitiotzi E., Santillan R., Farfán N. High Boron Content Carboranyl-functionalized Aryl Ether Derivatives Displaying Photoluminescent Properties. Dalton Trans. 2007;92:1898–1903. doi: 10.1039/B618771D. [DOI] [PubMed] [Google Scholar]
- 45.Feng B., Tomizawa K., Michiue H., Miyatake S.-I., Han X.-J., Fujimura A., Seno M., Kirihata M., Matsui H. Delivery of Sodium Borocaptate to Glioma Cells Using Immunoliposome Conjugated with Anti-EGFR Antibodies by ZZ-His. Biomaterials. 2009;30:1746–1755. doi: 10.1016/j.biomaterials.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 46.Ciofani G., Raffa V., Menciassi A., Cuschieri A. Folate Functionalized Boron Nitride Nanotubes and their Selective Uptake by Glioblastoma Multiforme Cells: Implications for their Use as Boron Carriers in Clinical Boron Neutron Capture Therapy. Nanoscale Res. Lett. 2008;4:113–121. doi: 10.1007/s11671-008-9210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kellert M., Worm D.J., Hoppenz P., Sárosi M.B., Lönnecke P., Riedl B., Koebberling J., Beck-Sickinger A.G., Hey-Hawkins E. Modular Triazine-based Carborane-containing Carboxylic Acids—Synthesis and Characterisation of Potential Boron Neutron Capture Therapy Agents Made of Readily Accessible Building Blocks. Dalton Trans. 2019;48:10834–10844. doi: 10.1039/C9DT02130B. [DOI] [PubMed] [Google Scholar]
- 48.Kellert M., Hoppenz P., Lönnecke P., Worm D.J., Riedl B., Koebberling J., Beck-Sickinger A.G., Hey-Hawkins E. Tuning a Modular System-Synthesis and Characterisation of a Boron-rich s-Triazine-based Carboxylic Acid and Amine Bearing a Galactopyranosyl Moiety. Dalton Trans. 2020;49:57–69. doi: 10.1039/C9DT04031E. [DOI] [PubMed] [Google Scholar]
- 49.Kellert M., Lönnecke P., Riedl B., Koebberling J., Hey-Hawkins E. Enlargement of a Modular System-Synthesis and Characterization of an s-Triazine-based Carboxylic Acid Ester Bearing a Galactopyranosyl Moiety and an Enormous Boron Load. Molecules. 2019;24:3288. doi: 10.3390/molecules24183288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He H., Li D.-W., Yang L.-Y., Fu L., Zhu X.-J., Wong W.-K., Jiang F.-L., Liu Y. A Novel Bifunctional Mitochondria-targeted Anticancer Agent with High Selectivity for Cancer Cells. Sci. Rep. 2015;5:13543. doi: 10.1038/srep13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bryden F., Savoie H., Rosca E.V., Boyle R.W. PET/PDT Theranostics: Synthesis and Biological Evaluation of a Peptide-targeted Gallium Porphyrin. Dalton Trans. 2015;44:4925–4932. doi: 10.1039/C4DT02949F. [DOI] [PubMed] [Google Scholar]
- 52.Griffith D., Morgan M.P., Marmion C.J. A Novel Anti-cancer Bifunctional Platinum Drug Candidate with Dual DNA Binding and Histone Deacetylase Inhibitory Activity. Chem. Comm. 2009:6735–6737. doi: 10.1039/b916715c. [DOI] [PubMed] [Google Scholar]
- 53.Chen L., Wang X., Ji F., Bao Y., Wang J., Guo L., Li Y. New Bifunctional-pullulan-based Micelles with Good Biocompatibility for Efficient Co-delivery of Cancer-suppressing p53 Gene and Doxorubicin to Cancer Cells. RSC Adv. 2015;5:94719–94731. doi: 10.1039/C5RA17139C. [DOI] [Google Scholar]
- 54.Soni R., Soman S.S. Design and Synthesis of Aminocoumarin Derivatives as DPP-IV Inhibitors and Anticancer Agents. Bioorg. Chem. 2018;79:277–284. doi: 10.1016/j.bioorg.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 55.Achilli C., Jadhav S.A., Guidetti G.F., Ciana A., Abbonante V., Malara A., Fagnoni M., Torti M., Balduini A., Balduini C., et al. Folic Acid-Conjugated 4-Amino-Phenylboronate, a Boron-Containing Compound Designed for Boron Neutron Capture Therapy, is an Unexpected Agonist for Human Neutrophils and Platelets. Chem. Biol. Drug Des. 2014;83:532–540. doi: 10.1111/cbdd.12264. [DOI] [PubMed] [Google Scholar]
- 56.Assaraf Y.G., Leamon C.P., Reddy J.A. The Folate Receptor as a Rational Therapeutic Target for Personalized Cancer Treatment. Drug Resist. Updat. 2014;17:89–95. doi: 10.1016/j.drup.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 57.Goto T., Ohta K., Suzuki T., Ohta S., Endo Y. Design and Synthesis of Novel Androgen Receptor Antagonists with Sterically Bulky Icosahedral Carboranes. Bioorgan. Med. Chem. 2005;13:6414–6424. doi: 10.1016/j.bmc.2005.06.061. [DOI] [PubMed] [Google Scholar]
- 58.Kalinin V.N., Rys E.G., Tyutyunov A.A., Starikova Z.A., Korlyukov A.A., Ol’shevskaya V.A., Sung D.D., Ponomaryov A.B., Petrovskii P.V., Hey-Hawkins E. The First Carborane Triflates: Synthesis and Reactivity of 1-Trifluoromethanesulfonylmethyl- and 1,2-Bis(trifluoromethanesulfonylmethyl)-o-carborane. Dalton Trans. 2005:903–908. doi: 10.1039/b417199c. [DOI] [PubMed] [Google Scholar]
- 59.Saltan F., Akat H. Synthesis and Thermal Degradation Kinetics of D-(+)-Galactose Containing Polymers. Polímeros. 2013;23:697–704. doi: 10.4322/polimeros.2014.012. [DOI] [Google Scholar]
- 60.Brackhagen M., Boye H., Vogel C. Synthesis of (6-2H)- and 6-Deoxy-6-fluoro-L-galactose Derivatives. J. Carbohyd. Chem. 2001;20:31–43. doi: 10.1081/CAR-100102541. [DOI] [Google Scholar]
- 61.Wuts P.G.M., Greene T.W. Greene’s Protective Groups in Organic Synthesis. 4th ed. Wiley-Interscience; Hoboken, NJ, USA: 2007. [Google Scholar]
- 62.Kokotos G., Theodorou V., Tzougraki C. Synthesis and In Vitro Cytotoxicity of Aminocoumarin Platinum(II) Complexes. Bioorg. Med. Chem. Lett. 1997;7:2165–2168. doi: 10.1016/S0960-894X(97)00384-3. [DOI] [PubMed] [Google Scholar]
- 63.Kaur M., Kohli S., Sandhu S., Bansal Y., Bansal G. Coumarin: A Promising Scaffold for Anticancer Agents. Anticancer Agents Med. Chem. 2015;15:1032–1048. doi: 10.2174/1871520615666150101125503. [DOI] [PubMed] [Google Scholar]
- 64.Klenkar J., Molnar M. Natural and Synthetic Coumarins as Potential Anticancer Agents. J. Chem. Pharm. Res. 2015;7:1223–1238. [Google Scholar]
- 65.Majnooni M.B., Fakhri S., Smeriglio A., Trombetta D., Croley C.R., Bhattacharyya P., Sobarzo-Sánchez E., Farzaei M.H., Bishayee A. Antiangiogenic Effects of Coumarins against Cancer: From Chemistry to Medicine. Molecules. 2019;24:4278. doi: 10.3390/molecules24234278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shukla S., Wu G., Chatterjee M., Yang W., Sekido M., Diop L.A., Müller R., Sudimack J.J., Lee R.J., Barth R.F., et al. Synthesis and Biological Evaluation of Folate Receptor-targeted Boronated PAMAM Dendrimers as Potential Agents for Neutron Capture Therapy. Bioconjug. Chem. 2003;14:158–167. doi: 10.1021/bc025586o. [DOI] [PubMed] [Google Scholar]
- 67.Cheung A., Bax H.J., Josephs D.H., Ilieva K.M., Pellizzari G., Opzoomer J., Bloomfield J., Fittall M., Grigoriadis A., Figini M., et al. Targeting Folate Receptor Alpha for Cancer Treatment. Oncotarget. 2016;7:52553–52574. doi: 10.18632/oncotarget.9651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Quéléver G., Burlet S., Garino C., Pietrancosta N., Laras Y., Kraus J.-L. Simple Coupling Reaction between Amino Acids and Weakly Nucleophilic Heteroaromatic Amines. J. Comb. Chem. 2004;6:695–698. doi: 10.1021/cc034069p. [DOI] [PubMed] [Google Scholar]
- 69.El-Faham A., Albericio F. Peptide Coupling Reagents, More than a Letter Soup. Chem. Rev. 2011;111:6557–6602. doi: 10.1021/cr100048w. [DOI] [PubMed] [Google Scholar]
- 70.Trindade A.F., Frade R.F.M., Maçôas E.M.S., Graça C., Rodrigues C.A.B., Martinho J.M.G., Afonso C.A.M. “Click and Go”: Simple and fast Folic Acid Conjugation. Org. Biomol. Chem. 2014;12:3181–3190. doi: 10.1039/C4OB00150H. [DOI] [PubMed] [Google Scholar]
- 71.Nakamura H., Aoyagi K., Yamamoto Y. Tetrabutylammonium Fluoride Promoted Novel Reactions of o-Carborane: Inter- and Intramolecular Additions to Aldehydes and Ketones and Annulation via Enals and Enones. J. Am. Chem. Soc. 1998;120:1167–1171. doi: 10.1021/ja973832e. [DOI] [Google Scholar]
- 72.Tiwari N., Sharma R.K., Gupta P., Misra S., Misra-Bhattacharya S., Butcher R.J., Singh K., Katiyar D. Synthesis, Structure Elucidation, Homology Modeling and Antifilarial Activity of 7-Benzamidocoumarin Derivatives. ChemistrySelect. 2019;4:3300–3307. doi: 10.1002/slct.201803549. [DOI] [Google Scholar]
- 73.Harris R.K., Becker E.D., de Menezes S.M.C., Goodfellow R., Granger P. NMR Nomenclature: Nuclear Spin Properties and Conventions for Chemical Shifts. IUPAC Recommendations 2001. Pure Appl. Chem. 2001;73:1795–1818. doi: 10.1351/pac200173111795. [DOI] [PubMed] [Google Scholar]
- 74.MestReNova. Mestrelab Research S.L.; Santiago de Compostela, Spain: 2017. v12.00-20080. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.