Abstract
Ag3PO4/g-C3N4 heterojunctions, with different g-C3N4 dosages, were synthesized using an in situ deposition method, and the photocatalytic performance of g-C3N4/Ag3PO4 heterojunctions was studied under simulated sunlight conditions. The results revealed that Ag3PO4/g-C3N4 exhibited excellent photocatalytic degradation activity for rhodamine B (Rh B) and phenol under the same light conditions. When the dosage of g-C3N4 was 30%, the degradation rate of Rh B at 9 min and phenol at 30 min was found to be 99.4% and 97.3%, respectively. After five cycles of the degradation experiment for Rh B, g-C3N4/Ag3PO4 still demonstrated stable photodegradation characteristics. The significant improvement in the photocatalytic activity and stability of g-C3N4/Ag3PO4 was attributed to the rapid charge separation between g-C3N4 and Ag3PO4 during the Z-scheme charge transfer and recombination process.
Keywords: Ag3PO4, g-C3N4, semiconductor photocatalyst, Z-scheme mechanism
1. Introduction
With the rapid development of industry, environmental pollution caused by industrial wastewater is becoming increasingly serious. Photocatalysis is an effective technology to degrade pollutants in water, which has been widely researched [1,2]. However, one-component semiconductor photocatalysts always face various defects, such as low visible-light availability and easy recombination of photogenerated charges. It has been proven that the construction of semiconductor heterostructures is an effective route to improve photocatalytic efficiency [3,4]. In recent years, an all-solid Z-scheme semiconductor composite photocatalyst has been applied in photocatalysis [5,6,7,8,9]. When Z-scheme photocatalysts are excited, h+ from the valence band (VB) at a higher energy level can combine with e− from the conduction band (CB) at a lower energy level, while e− with a stronger reducing ability in CB at a higher energy level and h+ with a stronger oxidation ability in lower VB at a lower energy level can participate in the reduction and oxidation processes during photocatalytic degradation, respectively. This method is conducive to obtain high charge separation efficiency and strong redox ability simultaneously, thus improving the photocatalytic efficiency [8,9].
In recent years, Z-scheme Ag3PO4-based photocatalysts with a high photocatalytic activity have been designed and applied in wastewater treatment and environmental control [10,11,12,13], including Ag3PO4/MoS2 [14], Bi2MoO6/Ag3PO4 [15], Ag3PO4/Bi2WO6 [16], Ag3PO4RGO/BiMoO4 [17], AgPO4/Ag/WO3−x [18], and Ag3PO4/Pd/LaPO4 [19]. Lamellar g-C3N4 nanosheets possess high surface area, suitable band gap (2.7 eV), low cost, and good thermal and chemical stability, which has attracted extensive attention in the field of photocatalysis [20,21,22,23]. When g-C3N4 is combined with Ag3PO4, the resultant g-C3N4/Ag3PO4 photocatalyst is expected to show significantly enhanced photocatalytic activity.
Among the many types of pollutants, dyes and dangerous compounds are two main pollutants in industrial wastewater. Rh B and phenol are the typical substances of the two pollutants, respectively. Rh B is very harmful to human health. It can cause redness of skin and viscera, mild congestion of cerebral vascular, rupture of myocardial fiber, and other symptoms. Phenol has a strong corrosive effect on skin and mucous membrane, inhibiting the central nervous system and damaging the function of liver and kidney, etc. In addition, phenol is more difficult to degrade than other pollutants in water. Thus, they were chosen as the degradation object in photocatalytic experiments.
In this paper, we synthesized the Ag3PO4/g-C3N4 Z-scheme heterojunction photocatalyst using the in situ deposition method and evaluated the photocatalytic activity by the degradation experiment for Rh B and phenol. The influence of g-C3N4 and Ag3PO4 on photocatalytic activity was studied in detail and the probable photocatalytic mechanism of Ag3PO4/g-C3N4 was proposed.
2. Experimental Section
2.1. Sample Preparation
Preparation of g-C3N4: A typical calcination method was used to prepare g-C3N4. Briefly, 10 g urea powder was placed in an alumina crucible with a lid. The crucible was heated in air at a heating rate of 2 °C·min−1 to 550 °C and, then held at this temperature for 2 h to obtain g-C3N4. Subsequently, the bulk g-C3N4 was thermally exfoliated into g-C3N4 nanosheets by calcination at 600 °C for 2 h in air. The light yellow product was collected and ground using an agate mortar for subsequent use.
Synthesis of Ag3PO4/g-C3N4: Fifty milligrams of g-C3N4 nanosheets were dispersed in 80 mL of deionized water by ultrasonication. Silver ammonia solution (0.1 g·L−1) was dropped into the aqueous dispersion of g-C3N4 nanosheets and, then magnetically stirred for 1 h to fully adsorb Ag(NH3)2+ ions on the surface of g-C3N4 nanosheets. Then, the KH2PO4 solution (0.1 g·L−1) was dropped into the above mixture under magnetic agitation and the mixture continued to be stirred for 1 h. The final product was collected by centrifugation, washed with deionized water and ethanol thrice, and dried at 70 °C for 1 h. Finally, the product was collected and ground with an agate mortar for further use. According to the theoretical dosage of g-C3N4, the as-prepared samples were named Ag3PO4/g-C3N4-10 wt%, Ag3PO4/g-C3N4-20 wt%, Ag3PO4/g-C3N4-30 wt%, and Ag3PO4/g-C3N4-40 wt%. The actual dosage of g-C3N4 detected by EDS were 9.2 wt%, 16.3 wt%, 27.7 wt%, and 41.8 wt%, respectively. In addition, the simple physical mixture of Ag3PO4 and 30 wt% g-C3N4 was named the Ag3PO4/g-C3N4-30% mixture.
2.2. Sample Characterization
The crystal structure was analyzed by a Bruker D8 X-ray diffractometer (XRD, Bruker, Germany), equipped with a Cu Kα irradiation light source (λ = 0.154 nm). The microstructure was observed using a Tecnai G2 F20 transmission electron microscopy (TEM, FEI, Hillsboro, OR, USA). Room-temperature transient photoluminescence (PL) spectra were recorded using an FLS1000 spectrometer (EI, UK). UV-vis diffuse reflectance spectra (UV-Vis, Hitachi, Tokyo, Japan) were measured by using a UH4150 UV-Vis near-infrared spectrophotometer. The photocurrent response was measured using a CHI 760E electrochemical workstation (Chenhua, Shanghai, China).
2.3. Photocatalytic Activity Test
The photocatalytic activity was evaluated by the pollutant degradation experiments at room temperature. A Polfilet xenon lamp (300 W) with a 320-nm filter was used as the light source. The spectra of the xenon lamp are shown in Figure S1 and detailed experimental devices are shown in Figure S2. The reaction solution consisted of 50 mL of rhodamine B (Rh B, 5 mg·L−1) or 50 mL of phenol (10 mg·L−1), and the photocatalyst was 0.03 g Ag3PO4, g-C3N4, or Ag3PO4/g-C3N4. The photocatalyst was weighed and added to the reaction solution, and the reaction solution was continuously stirred in the dark for 30 min to achieve an adsorption–desorption balance between the photocatalytic material and pollutant. Subsequently, the solution was irradiated by a full-wavelength Xenon lamp, and the absorbance of the supernatant was measured at certain intervals. In the cyclic experiments, the photocatalyst was separated from the reaction system after each degradation experiment, washed with ethanol and deionized water, and re-dispersed in the newly-prepared reaction solution to repeat the degradation experiment.
3. Results and Discussion
3.1. Structural Analysis and Microstructure
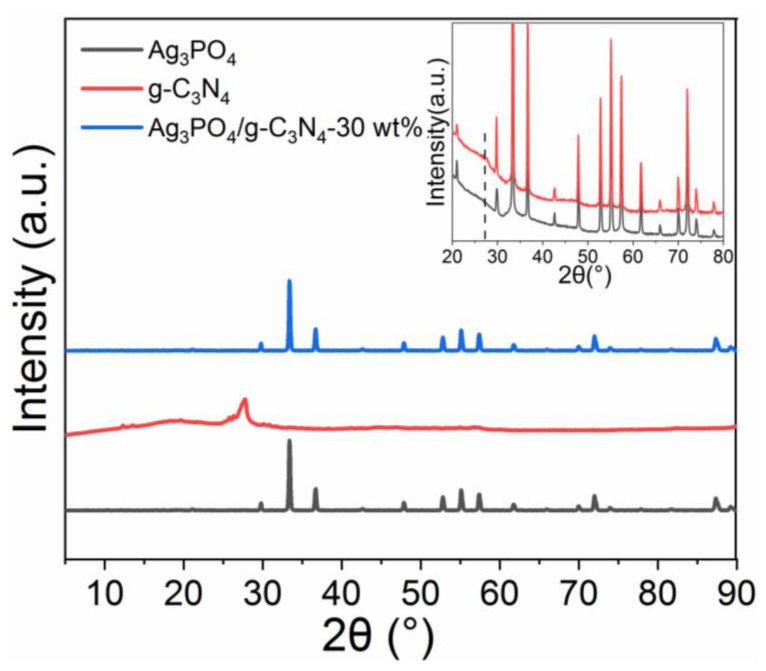
Figure 1 shows the XRD patterns of Ag3PO4, g-C3N4 and Ag3PO4/g-C3N4-30 wt%. As shown in Figure 1, a strong peak appeared in the diffraction pattern of g-C3N4 at 2θ = 26.5°, corresponding to the (002) planes of g-C3N4 (JCPDS card no. 87-1526), which is the characteristic interlayer stacking peak of g-C3N4 [24]. The Ag3PO4 and Ag3PO4/g-C3N4-30 wt% exhibited similar XRD patterns and all strong diffraction peaks corresponded to the cubic Ag3PO4 phase (JCPDS card no. 06-0505). The inset provided the refined XRD patterns of Ag3PO4 and Ag3PO4/g-C3N4-30 wt%. Compared with Ag3PO4, the XRD pattern of Ag3PO4/g-C3N4 showed the characteristic peaks of g-C3N4; however, the peak intensities were far weaker than that of Ag3PO4. This may be attributed to the inferior crystallinity and lower content of well-exfoliated g-C3N4.
Figure 1.
XRD patterns of as-prepared Ag3PO4, g-C3N4, and Ag3PO4/g-C3N4.
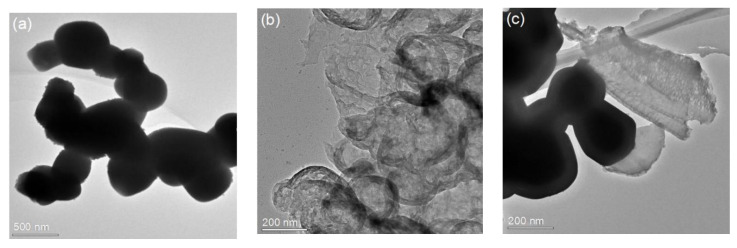
Figure 2 shows TEM images of Ag3PO4, g-C3N4, and Ag3PO4/g-C3N4 photocatalysts. Figure 2a illustrates that Ag3PO4 consisted of approximately cubic particles with a size of 200–300 nm. As shown in Figure 2b, g-C3N4 presented thin wrinkled nanosheets. After thermal exfoliation, the specific surface area of g-C3N4 increased significantly, due to morphological changes. Figure 2c shows that the small-sized Ag3PO4 particles were attached to the surface of g-C3N4, forming a stable composite.
Figure 2.
TEM images of (a) Ag3PO4, (b) g-C3N4, and (c) Ag3PO4/g-C3N4.
3.2. Optical Properties
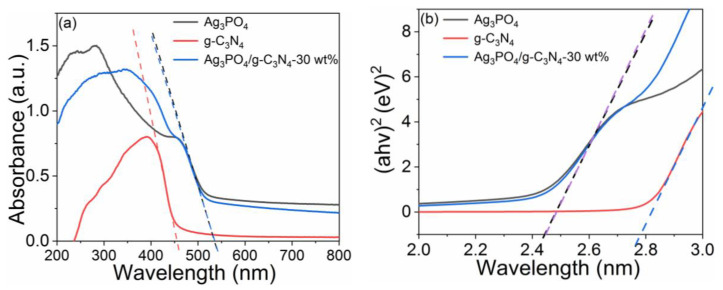
Figure 3 shows the UV-vis diffuse reflectance spectra of Ag3PO4, g-C3N4, and Ag3PO4/g-C3N4-30 wt% photocatalysts. As shown in Figure 3a, the absorption cutoff edges of Ag3PO4 and g-C3N4 were located at about 460 and 530 nm, respectively. Compared with Ag3PO4, the absorption edge of Ag3PO4/g-C3N4-30 wt% was basically unchanged. Based on the UV-vis absorption data, the bandgap width of the photocatalysts was calculated and results are shown in Figure 3b. The calculated bandgap width of g-C3N4 was about 2.78 eV, whereas the bandgap of Ag3PO4 and Ag3PO4/g-C3N4-30wt% decreased to 2.45 eV.
Figure 3.
(a) UV-vis diffuse reflectance spectra, (b) estimated bandgap of Ag3PO4, g-C3N4, and Ag3PO4/g-C3N4-30 wt%.
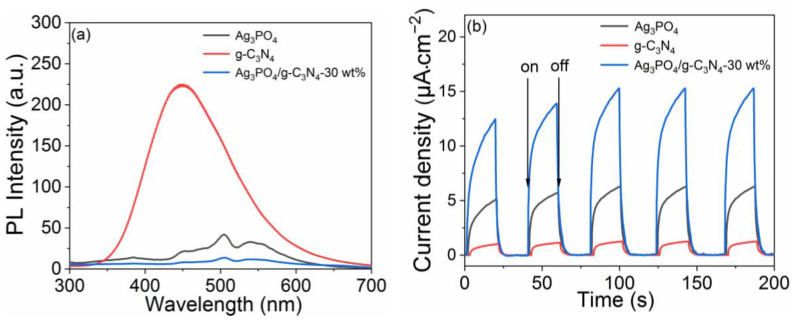
By testing the photoelectrochemical properties of Ag3PO4, g-C3N4, and Ag3PO4/g-C3N4-30 wt% photocatalysts, the separation and transfer efficiency of photogenerated electron-hole pairs were studied and results are shown in Figure 4. Figure 4a presents the photoluminescence (PL) spectra of the as-synthesized photocatalysts. The PL emission peak of g-C3N4 was located at 460 nm, showing the highest PL intensity and indicating that the photogenerated charge of g-C3N4 exhibited high recombination efficiency. The PL emission peak of Ag3PO4 was located at 460 nm, showing a far lower PL intensity than g-C3N4. When Ag3PO4 was combined with g-C3N4, the location of the PL emission peak of Ag3PO4/g-C3N4-30 wt% was basically the same as Ag3PO4, but the PL peak intensity of Ag3PO4/g-C3N4-30 wt% was significantly lower than Ag3PO4. Among Ag3PO4, g-C3N4, and Ag3PO4/g-C3N4-30 wt%, Ag3PO4/g-C3N4 exhibited the lowest PL peak intensity, which corresponded to the lowest recombination efficiency for photogenerated charges. As can be observed in Figure 4b, all photocatalyst electrodes exhibited rapid response when irradiated by a Xenon lamp (full wavelength). The Ag3PO4/g-C3N4-30 wt% showed the highest photocurrent response of about 16.35 μA·cm−2, which was 2.79 times higher than Ag3PO4 (5.87 μA·cm−2) and 21.8 times higher than g-C3N4 (0.75 μA·cm−2). These results indicate that the combination of Ag3PO4 and g-C3N4 reduced the recombination efficiency of photogenerated electrons and holes, and accelerated the charges transfer, which is beneficial for photocatalysis.
Figure 4.
(a) Photoluminescence spectra and (b) transient photocurrent response curves of Ag3PO4, g-C3N4, and Ag3PO4/g-C3N4-30 wt%.
3.3. Photocatalytic Activity
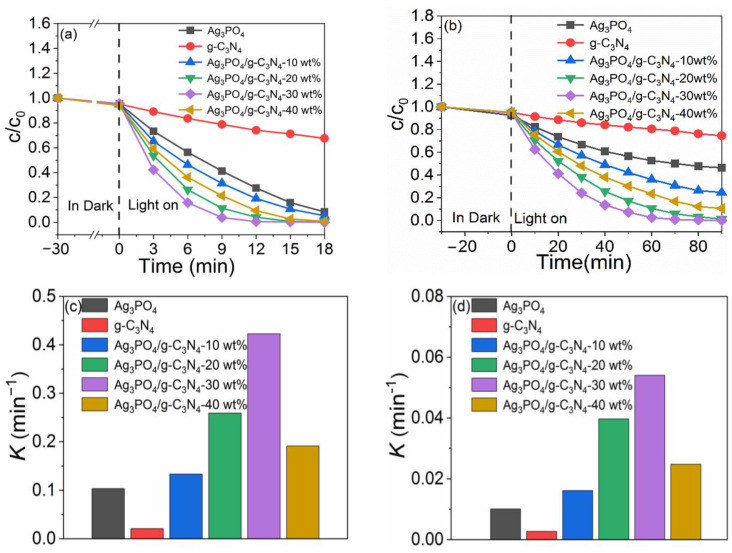
Furthermore, using Rh B and phenol as target pollutants, we simulated the photocatalytic reaction under sunlight irradiation using Xenon lamp (full wavelength) irradiation, and evaluated the photocatalytic activity, as shown in Figure 5. Figure 5a shows the photocatalytic activity of Ag3PO4/g-C3N4 with different amounts of g-C3N4 After irradiation by the Xenon lamp for 9 min, the photocatalytic degradation rate of RhB by Ag3PO4, g-C3N4, Ag3PO4/g-C3N4-10 wt%, Ag3PO4/g-C3N4-20 wt%, Ag3PO4/g-C3N4-30 wt%, and Ag3PO4/g-C3N4-40 wt% was found to be 71.1%, 22.2%, 79.8%, 95.5%, 99.4%, and 89.9%, respectively. With the increase of g-C3N4 content, the photocatalytic activity of Ag3PO4/g-C3N4 initially increased, followed by a decrease. The optimal photocatalytic activity was achieved for Ag3PO4/g-C3N4-30 wt%. The first-order kinetic model [25,26] was used to calculate the corresponding reaction rate constants (k), and the results are shown in Figure 5c. The observed reaction rate constant of Ag3PO4, g-C3N4, Ag3PO4/g-C3N4-10 wt%, Ag3PO4/g-C3N4-20 wt%, Ag3PO4/g-C3N4-30 wt%, and Ag3PO4/g-C3N4-40 wt% was found to be 0.1033, 0.0209, 0.1333, 0.2591, 0.4227, and 0.1911 min−1, respectively. The k value of Ag3PO4/g-C3N4-30 wt% (0.4227 min−1) was the highest, which was ≈4.09 and 20.24 times higher than Ag3PO4 and g-C3N4, respectively.
Figure 5.
(a,b) Photocatalytic curves, (c,d) rate constants in the degradation of Rh B and phenol, with different g-C3N4 content.
In order to further verify the superior photocatalytic activity of Ag3PO4/g-C3N4, the photocatalytic degradation experiment for phenol was also carried out and the results are shown in Figure 5b. Under Xenon lamp irradiation for 30 min, the degradation rate of phenol by Ag3PO4, g-C3N4, Ag3PO4/g-C3N4-10 wt%, Ag3PO4/g-C3N4-20 wt%, Ag3PO4/g-C3N4-30 wt%, and Ag3PO4/g-C3N4-40 wt% was found to be 43.0%, 15.8%, 63.9%, 90.9%, 99.6%, and 77.5%, respectively. Figure 5d shows that the Ag3PO4/g-C3N4-30 wt% exhibits the highest rate constant k (0.0540 min−1), which was ≈5.35 and 20.00 times higher than Ag3PO4 (0.01009 min−1) and g-C3N4 (0.0027 min−1), respectively. Hence, Ag3PO4/g-C3N4 showed obvious advantages for the degradation of pollutants.
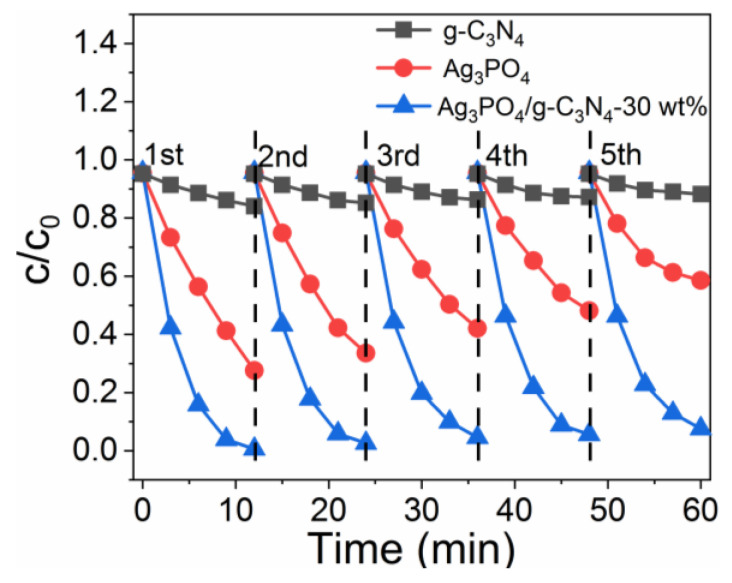
Figure 6 presents the cyclic stability of Rh B degradation by Ag3PO4, g-C3N4, and Ag3PO4/g-C3N4-30 wt% photocatalysts. Under Xenon lamp irradiation, the loss rate of Rh B degradation by Ag3PO4, g-C3N4, and Ag3PO4/g-C3N4-30 wt% during the fifth cycle, compared with the initial degradation, was 32.5%, 11.5%, and 7.3%, respectively. The presence of g-C3N4 significantly reduced the loss rate for Rh B and phenol degradation. Hence, Ag3PO4/g-C3N4 showed excellent photocatalytic stability.
Figure 6.
Recycling runs results of Ag3PO4, g-C3N4, and Ag3PO4/g-C3N4-30 wt% in degradation of Rh B .
3.4. Photocatalysis Species
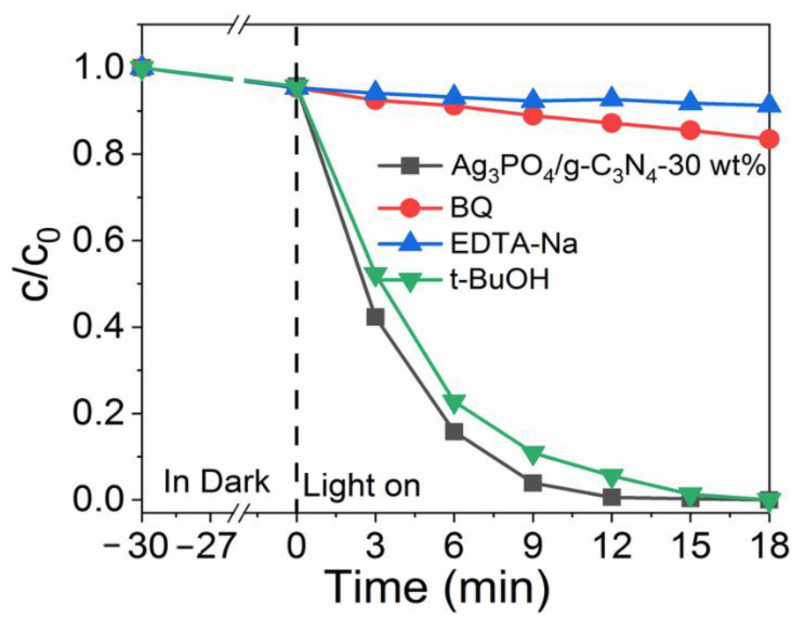
In order to identify the active species during the photocatalytic process, free radical capture experiments were carried out using Rh B as a target pollutant. EDTA-2Na, p-benzoquinone (BZQ), and tert-butanol were introduced during the photocatalytic process as h+, ·O2−, and OH− inhibitors, respectively, and the results are shown in Figure 7. The introduction of tert-butanol during the photocatalytic process of Ag3PO4/g-C3N4-30 wt% rendered no influence on the photodegradation efficiency of Rh B, whereas EDTA-2Na and BZQ both significantly reduced the degradation efficiency of Rh B with a degradation rate of 4.4% and 12.4%, respectively. These results indicate that h+ and O2− are the main active species in Ag3PO4/g-C3N4-30 wt%.
Figure 7.
Photocatalytic activities of Ag3PO4/g-C3N4-30wt% for the degradation of Rh B in the presence of different scavengers.
3.5. Energy Band Structure and Photocatalytic Mechanism
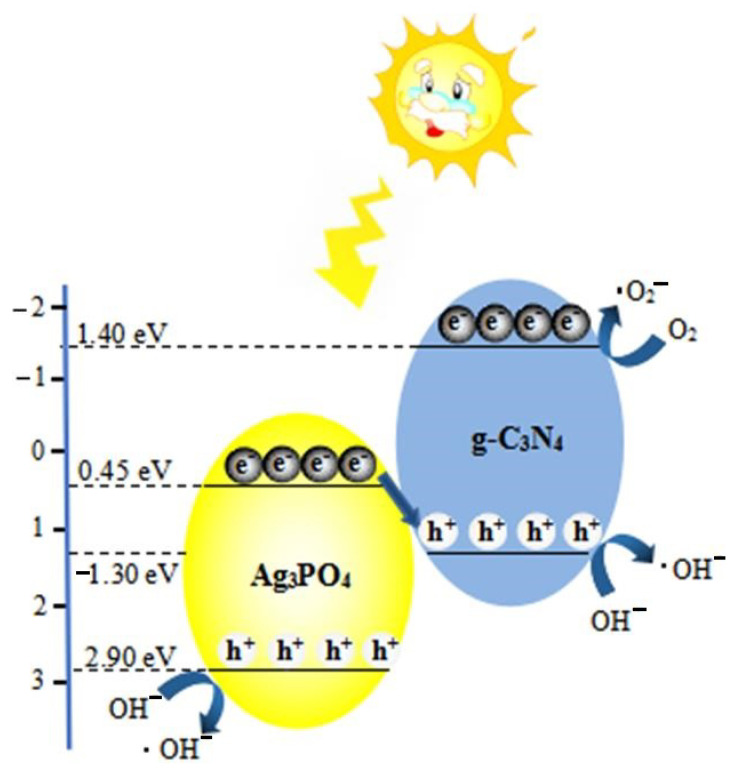
Figure 8 presents the Z-scheme charge transfer pathway of the Ag3PO4/g-C3N4 composite photocatalyst for the degradation of organic pollutants. The bandgap of g-C3N4 was 2.7 eV with the VB potential of ~ 1.4 eV and CB potential of ~ −1.3 eV [27,28]. The potential of e− on the CB of g-C3N4 was −1.3 eV, which can reduce the molecular oxygen O2 to·O2 because the potential of O2/·O2– was −0.44 eV vs. NHE. Therefore, O2− was the main active substance during the photocatalytic process by g-C3N4. The bandgap of Ag3PO4 was 2.45 eV with a VB potential of ~2.9 eV and CB potential of ~0.45 eV [29]. The generated electrons (e−) in the CB of Ag3PO4 are insufficient to reduce O2 into O2−. Therefore, holes (h+) play a major role during the photocatalytic degradation of organic matter by Ag3PO4.
Figure 8.
Energy band structure and Z-scheme photocatalytic mechanism of Ag3PO4/g-C3N4.
Based on the energy band analysis, it can be inferred that the photogenerated e− in the CB of Ag3PO4 can combine with h+ in the VB of g-C3N4 due to the formation of a heterojunction interface between Ag3PO4 particles and g-C3N4 nanosheets, resulting in the accumulation of e− in the CB of g-C3N4 and h+ in VB of Ag3PO4. The h+ in the VB of Ag3PO4 can directly react with pollutants, whereas the electrons in CB of g-C3N4 can reduce O2 into O2−, which reacts with pollutants. The Z-scheme charge transfer mechanism promotes the separation of electron-hole pairs, slows down the photocorrosion of Ag+, and improves photocatalyst activity and stability.
4. Conclusions
In summary, the Z-scheme heterojunction Ag3PO4/g-C3N4 photocatalyst was synthesized using an in situ deposition method and exhibited excellent photocatalytic degradation activity for Rh B and phenol under Xenon lamp irradiation. The observed rate constant (k) for the degradation of Rh B by Ag3PO4/g-C3N4 was found to be 0.4227 min−1, which was 4.09 and 20.24 times higher than pure Ag3PO4 and g-C3N4, respectively. Moreover, the k value for the degradation of phenol by Ag3PO4/g-C3N4 was 0.0540 min−1, which was 5.35 and 20.00 times higher than pure Ag3PO4 and g-C3N4, respectively. Overall, the formation of the Z-scheme heterojunction hindered the recombination of photogenerated electrons and holes, and accelerated the electron transfer, thus improving the activity and stability of photocatalysts.
Acknowledgments
The authors would like to thank Xiangguang Meng of North China University of Science and Technology for helpful discussions on topics related to this work.This work was supported by the National Natural Science Fund of China (Grant No. 51772099, 51872091), it is also supported by the Scientific and Technological Research Projects of Colleges and Universities in Hebei Province (QN2019049), the Doctoral Initiation Fund (BS2017025), and Innovation and Entrepreneurship Training Program for College Students of North China University of Science and Technology.
Supplementary Materials
Figure S1: The spectra of xenon lamp, Figure S2: The picture of experimental setup.
Author Contributions
Validation, M.Z., J.J., C.Q.; investigation, Z.Z.; resources, Y.S.; data curation, H.D.; writing—original draft preparation, M.Z.; writing—review and editing, F.L.; supervision, Y.C.L.; project administration, Y.C.L.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Fund of China, grant number 51772099 and 51872091; the Scientific and Technological Research Projects of Colleges and Universities in Hebei Province, grant number QN2019049; the Postdoctoral Program of Hebei Province, grant number B2020003015; the Doctoral Initiation Fund, grant number BS2017025.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the ongoing follow-up studies.
Conflicts of Interest
The authors declare no conflict of interest. The authors had no any personal circumstances or interest that may be perceived as inappropriately influencing the representation or interpretation of reported research results. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Sample Availability
Samples of the Ag3PO4/g-C3N4.photocatalyst are available from the authors. However, it may be necessary to pay properly for the synthesis and mailing of samples.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Choi J.H., Hong J., Son Y.R., Wang J., Kim H.S., Lee H., Lee H. Comparison of Enhanced Photocatalytic Degradation Efficiency and Toxicity Evaluations of CeO2 Nanoparticles Synthesized Through Double- Modulation. Nanomaterials. 2020;10:1543. doi: 10.3390/nano10081543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoo H., Lee M., Lee S., Lee J., Cho S., Lee H., Cha H.G., Kim H.S. Enhancing Photocatalytic β-O-4 Bond Cleavage in Lignin Model Compounds by Silver-Exchanged Cadmium Sulfide. ACS Catal. 2020;10:8465–8475. doi: 10.1021/acscatal.0c01915. [DOI] [Google Scholar]
- 3.Byrne J.A., Dunlop P.S.M., Hamilton J.W.J., Fernandez-Ibanez P., Polo-Lopez I., Sharma P.K., Vennard A.S.M. A review of heterogeneous photocatalysis for water and surface disinfection. Molecules. 2015;20:5574–5615. doi: 10.3390/molecules20045574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H.L., Zhang L.S., Chen Z.G., Hu J.Q., Li S.J., Wang Z.H., Liu J.S., Wang X.C. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014;43:5234–5244. doi: 10.1039/C4CS00126E. [DOI] [PubMed] [Google Scholar]
- 5.Rong X., Chen H., Rong J., Zhang X., Wei J., Liu S., Zhou X., Xu J., Qiu F., Wu Z. An all-solid-state Z-scheme TiO2/ZnFe2O4 photocatalytic system for the N2 photofixation enhancement. Chem. Eng. J. 2019;371:286–293. doi: 10.1016/j.cej.2019.04.052. [DOI] [Google Scholar]
- 6.Qi K., Cheng B., Yu J., Ho W. A review on TiO2-based Z-scheme photocatalysts. Chin. J. Catal. 2017;38:1936–1955. doi: 10.1016/S1872-2067(17)62962-0. [DOI] [Google Scholar]
- 7.Kumar A., Raizada P., Singh P., Saini R.V., Saini A.K., Hosseini-Bandegharaei A. Perspective and status of polymeric graphitic carbon nitride based Z-scheme photocatalytic systems for sustainable photocatalytic water purification. Chem. Eng. J. 2020;391:123496. doi: 10.1016/j.cej.2019.123496. [DOI] [Google Scholar]
- 8.Xu Q., Zhang L., Yu J., Wageh S., Al-Ghamdi A.A., Jaroniec M. Direct Z-scheme photocatalysts: Principles, synthesis, and applications. Mater. Today. 2018;21:1042–1063. doi: 10.1016/j.mattod.2018.04.008. [DOI] [Google Scholar]
- 9.Shi Y., Chen J., Mao Z., Bradley D., Fahlman C., Wang D. Construction of Z-scheme heterostructure with enhanced photocatalytic Hoevolution for g-C3N4 nanosheets via loading porous silicon. J. Catal. 2017;356:22–31. doi: 10.1016/j.jcat.2017.10.007. [DOI] [Google Scholar]
- 10.Chen X., Dai Y., Wang X. Methods and mechanism for improvement of photocatalytic activity and stability of Ag3PO4: A review. J. Alloys Compd. 2015;649:910–932. doi: 10.1016/j.jallcom.2015.07.174. [DOI] [Google Scholar]
- 11.Ge M., Li Z. Recent progress in Ag3PO4-based all-solid-state Z-scheme photocatalytic systems. Chin. J. Catal. 2017;38:1794–1803. doi: 10.1016/S1872-2067(17)62905-X. [DOI] [Google Scholar]
- 12.Martin D.J., Liu G., Moniz S.J.A., Bi Y., Beale A.M., Ye J., Tang J. Efficient visible driven photocatalyst, silver phosphate: Performance, understanding and perspective. J. Alloys Compd. 2015;649:910–932. doi: 10.1039/C5CS00380F. [DOI] [PubMed] [Google Scholar]
- 13.Lang X., Chen X., Zhao J. Heterogeneous visible light photocatalysis for selective organic transformations. Chem. Soc. Rev. 2015;44:7808–7828. doi: 10.1039/C3CS60188A. [DOI] [PubMed] [Google Scholar]
- 14.Zhu C., Zhang L., Jiang B., Zheng J., Hu P., Li S., Wu M., Wu W. Fabrication of Z-scheme Ag3PO4/MoS2 composites with enhanced photocatalytic activity and stability for organic pollutant degradation. Appl. Surf. Sci. 2016;377:99–108. doi: 10.1016/j.apsusc.2016.03.143. [DOI] [Google Scholar]
- 15.Wang Z., Lv J., Dai K., Lu L., Liang C., Geng L. Large scale and facile synthesis of novel Z-scheme Bi2MoO6/Ag3PO4 composite for enhanced visible light photocatalyst. Mater. Lett. 2016;169:250–253. doi: 10.1016/j.matlet.2016.01.147. [DOI] [Google Scholar]
- 16.Wang Z., Hu T., Dai K., Zhang J., Liang C. Construction of Z-scheme Ag3PO4/Bi2WO6 composite with excellent visible-light photodegradation activity for removal of organic contaminants. Chin. J. Catal. 2017;38:2021–2029. doi: 10.1016/S1872-2067(17)62942-5. [DOI] [Google Scholar]
- 17.Zhu P., Chen Y., Duan M., Ren Z., Hu M. Construction and mechanism of a highly efficient and stable Z-scheme Ag3PO4/reduced graphene oxide/BiMoO4 visible-light photocatalyst. Catal. Sci. Technol. 2018;8:3818–3832. doi: 10.1039/C8CY01087K. [DOI] [Google Scholar]
- 18.Bu Y., Chen Z., Sun C. Highly efficient Z-Scheme AgPO4/Ag/WO3-x photocatalyst for its enhanced photocatalytic performance. Appl. Catal. B Environ. 2015;179:363–371. doi: 10.1016/j.apcatb.2015.05.045. [DOI] [Google Scholar]
- 19.Chen X., Zhang W., Zhang L., Feng L., Wen J., Yang J., Zhang C., Jiang J., Wang H. An urchin-like Ag3PO4/Pd/LaPO4 photocatalyst with Z-scheme heterojunction for enhanced hydrogen evolution. Appl. Surf. Sci. 2019;497:143771. doi: 10.1016/j.apsusc.2019.143771. [DOI] [Google Scholar]
- 20.Ren Y., Zeng D., Ong W. Interfacial engineering of graphitic carbon nitride g-C3N4-based metal sulfide heterojunction photocatalysts for energy conversion: A review. Chin. J. Catal. 2019;40:289–319. doi: 10.1016/S1872-2067(19)63293-6. [DOI] [Google Scholar]
- 21.Ong W., Tan L., Ng Y.H., Yong S., Chai S. Graphitic Carbon Nitride (g-C3N4)—Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability? Chem. Rev. 2016;116:7159–7329. doi: 10.1021/acs.chemrev.6b00075. [DOI] [PubMed] [Google Scholar]
- 22.Li Y., Yang L., Don G., Ho W. Mechanism of NO Photocatalytic Oxidationon g-C3N4 Was Changed by Pd-QDs Modification. Molecules. 2015;21:36–45. doi: 10.3390/molecules21010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groenewolt M., Antonietti M. Synthesis of g-C3N4 Nanoparticles in Mesoporous Silica Host Matrices. Adv. Mater. 2010;17:1789–1792. doi: 10.1002/adma.200401756. [DOI] [Google Scholar]
- 24.Xu L., Shen X., Wu J., Ji Z., Wang J., Kong L., Liu M., Song C. Fabrication of an all solid Z-scheme photocatalyst g-C3N4/GO/AgBr with enhanced visible light photocatalytic activity. Appl. Catal. A Gen. 2017;5:104–113. [Google Scholar]
- 25.Chen X., Li R., Pan X., Huang X., Yi Z. Fabrication of In2O3-Ag-Ag3PO4 composites with Z-scheme configuration for photocatalytic ethylene degradation under visible light irradiation. Chem. Eng. J. 2017;320:644–652. doi: 10.1016/j.cej.2017.03.072. [DOI] [Google Scholar]
- 26.Liu L., Ding L., Liu Y., An W., Lin S., Liang Y., Cui W. A stable Ag3PO4@PANI core@shell hybrid: Enrichment photocatalytic degradation with r-r conjugation. Appl. Catal. B Environ. 2017;201:92–104. doi: 10.1016/j.apcatb.2016.08.005. [DOI] [Google Scholar]
- 27.Rawool S.A., Samanta A., Ajithkumar T.G., Kar Y., Polshettiwar V. Photocatalytic Hydrogen Generation and CO2 Conversion Using g-C3N4 Decorated Dendritic Fibrous Nanosilica: Role of Interfaces between Silica and g-C3N4. ACS Appl. Energy Mater. 2020;3:8150–8158. doi: 10.1021/acsaem.0c01265. [DOI] [Google Scholar]
- 28.Wei Z., Liang F., Liu Y., Luo W., Wang J., Yao W., Zhu Y. Photoelectrocatalytic degradation of phenol-containing wastewater by TiO2/g-C3N4 hybrid heterostructure thin film. Appl. Catal. B Environ. 2017;201:600–606. doi: 10.1016/j.apcatb.2016.09.003. [DOI] [Google Scholar]
- 29.Yi Z., Ye J., Kikugawa N., Kako T., Ouyang S., Stuart-Williams H., Yang H., Cao J., Luo W., Li Z., et al. An orthophosphate semiconductor with photooxidation properties under visible-light irradiation. Nat. Mater. 2010;9:559–564. doi: 10.1038/nmat2780. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the ongoing follow-up studies.