Abstract
Simple Summary
The tumor microenvironment plays an important role in tumor development and metastasis. Collagens are major components of the extracellular matrix and can influence tumor development and metastasis by activating discoidin domain receptors (DDRs). This work shows the different roles of DDRs in various cancers and highlights the complexity of anti-DDR therapies in cancer treatment.
Abstract
The tumor microenvironment is a complex structure composed of the extracellular matrix (ECM) and nontumoral cells (notably cancer-associated fibroblasts (CAFs) and immune cells). Collagens are the main components of the ECM and they are extensively remodeled during tumor progression. Some collagens are ligands for the discoidin domain receptor tyrosine kinases, DDR1 and DDR2. DDRs are involved in different stages of tumor development and metastasis formation. In this review, we present the different roles of DDRs in these processes and discuss controversial findings. We conclude by describing emerging DDR inhibitory strategies, which could be used as new alternatives for the treatment of patients.
Keywords: discoidin domain receptors, extracellular matrix, tumor growth, metastasis
1. Introduction
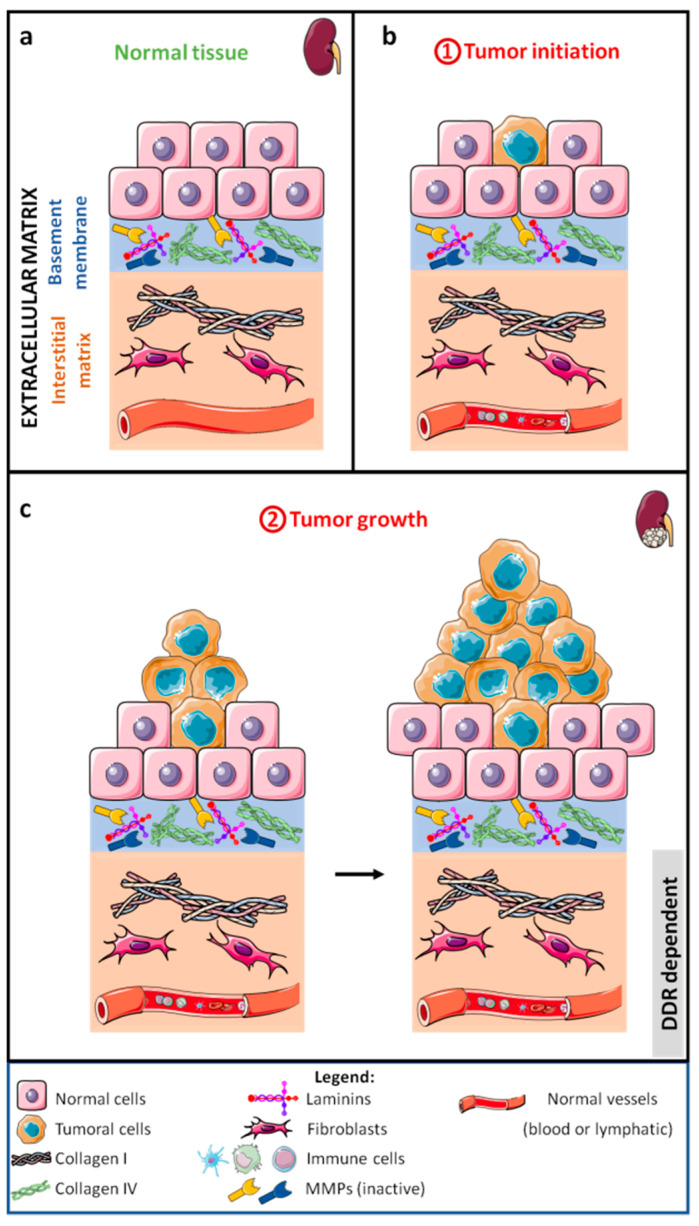
The development of tumors, particularly those of epithelial origin that lead to carcinoma (Figure 1a,b), begins with rapid and anarchic proliferation of cells, giving a tumor mass (Figure 1c). Soon, the tumor stops growing due to nutrient and oxygen deficiency.
Figure 1.
Schematic representation of normal tissue (a), carcinoma initiation (b), and initial stages of carcinoma development (c).
To grow further, the tumor cell mass attracts cells of different types such as endothelial, immune, and fibroblastic cells, together forming the tumor stroma or tumor microenvironment (TME, Figure 2a) [1]. At this stage, the primary tumor is well confined and surgical resection combined with adjuvant therapies can cure a vast majority of cancers. Nevertheless, tumor cells can escape from the primary tumor and colonize other organs in a process called metastasis.
Figure 2.
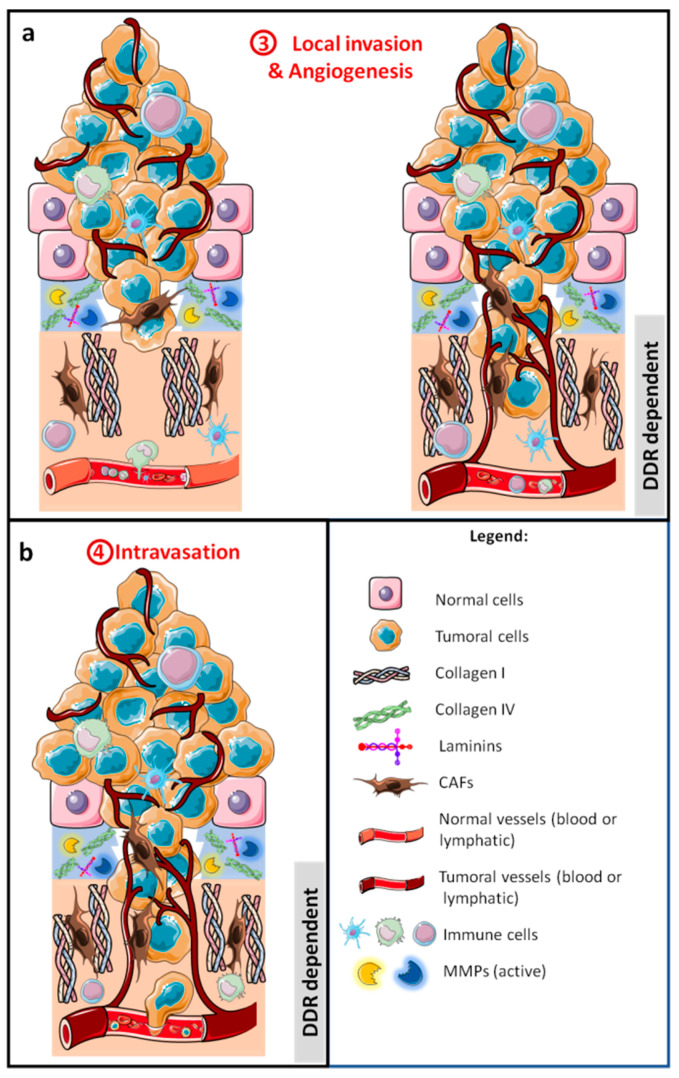
Schematic representation of the development of a carcinoma with the first metastatic stages, local invasion of tumor cells, and angiogenesis (a), allowing tumor cells to intravasate into lymphatic and/or blood vessels (b).
The multistep metastatic cascade was established in 2003 by Fidler [2] and refined several times since then [3,4]. The first step in the cascade is the invasion by tumor cells of the extracellular matrix (ECM) and the multilayer of stromal cells, forming the primary tumor (Figure 2a). Then, to disseminate, tumor cells intravasate into lymphatics or blood vessels (Figure 2b) where they survive anoikis, flow pressure, and the presence of immune cells (Figure 3a).
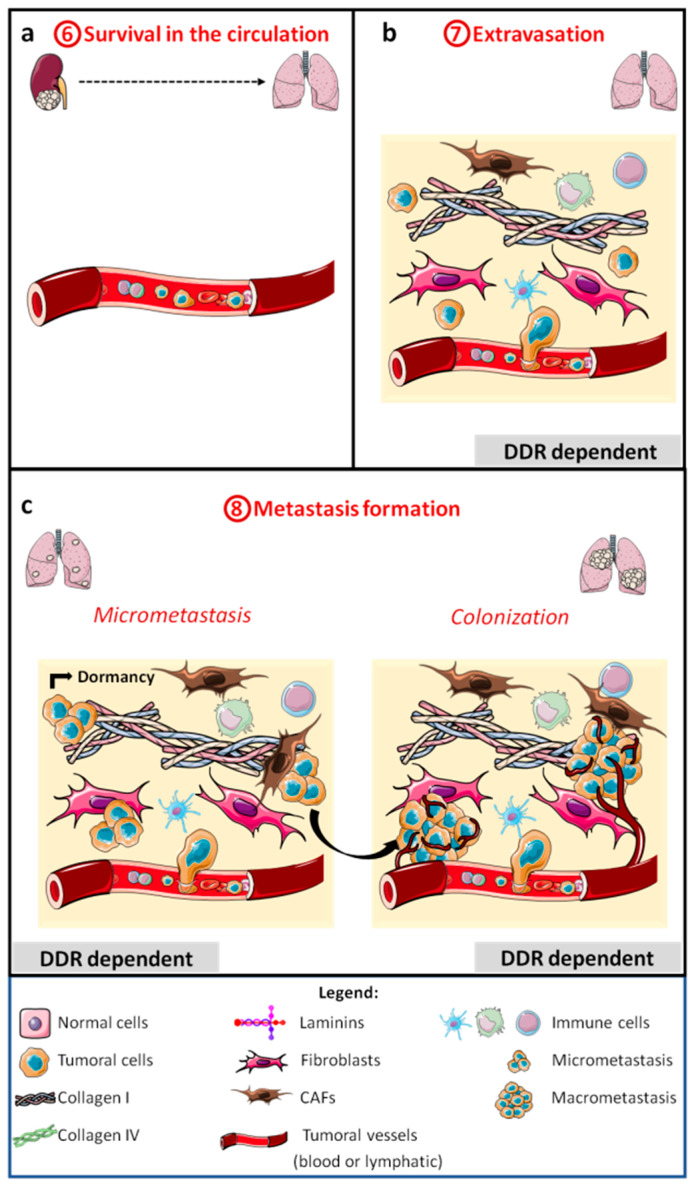
Figure 3.
Schematic representation of the late metastatic stages: survival in the circulation (a), extravasation (b), micrometastasis, and colonization or macrometastasis formation (c).
Subsequently, tumor cells arrest in the vasculature of distant organs and extravasate (Figure 3b). In the target organ tissues, tumor cells survive in a hostile microenvironment and form quiescent micrometastasis (tumor dormancy) (Figure 3c), before growing again in a step called macrometastasis or metastatic colonization (Figure 3c). During this process, tumor cells are exposed to different microenvironments, including different ECMs.
The ECM, one of the noncellular components of the TME, is composed of proteins, glycoproteins, proteoglycans, and polysaccharides, and it can be divided into the basement membrane and interstitial matrix. Epithelial cells are surrounded by the basement membrane, which is mainly composed of laminins, type IV collagen, nidogen, and heparan sulfate proteoglycans. The basement membrane separates the epithelial cells from the interstitial matrix. The latter is composed of collagens, elastin, proteoglycans, fibronectin, and other proteins. As a whole, the ECM forms an interconnected network. During tumor development, the ECM is continuously remodeled, with new synthesis and proteolytic degradation of the matrix components [5]. Collagens are the most abundant proteins in the ECM and, to date, 28 different collagens (I to XXVIII) have been found expressed in tissues and tumors. All collagens have in common the presence of three α chains forming a triple-helicoidal domain. Depending of the collagen, the triple-helix domain represents a mass ranging from more than 90% (collagen I) to less than 10% (collagen XII) of the protein. The diversity of the collagens is increased by the existence of molecules with more than one chain in some of them [6]. Collagens are classified into six different families, such as fibril-forming collagens (I–III, V, XI, XXIV, and XXVII) and network-forming collagens (IV, VIII, and X) [6].
In most cancers, e.g., colorectal or breast cancer, high collagen expression is associated with a poor prognosis. However, in some cancers, collagens may play an important role in limiting tumor cell activation or in constituting an obstacle to tumor growth [7]. In addition, proteolytic fragments of some collagens, such as endostatin, may inhibit tumor growth [8]. These antagonistic roles need to be further investigated in the perspective of anti-collagen therapies [7]. Collagens are primarily responsible for tumor rigidity and can affect tumor behavior through mechanical processes, e.g., by providing either a barrier for tumor development or a track for tumor cell migration [9,10]. On the other hand, collagens can influence tumor cell behavior by binding to extracellular receptors that induce intracellular signaling. The major collagen receptors present in carcinoma cells are integrins, α1β1, α2β1, α10β1, and α11β1. Intracellular integrin signaling is primarily mediated by activation of FAK (Focal Adhesion Kinase) and SFK (Src Family Kinase) proteins. In addition to these well-known receptors, collagens can bind cluster of differentiation 44 (CD44) and discoidin domain receptors (DDRs) [11].
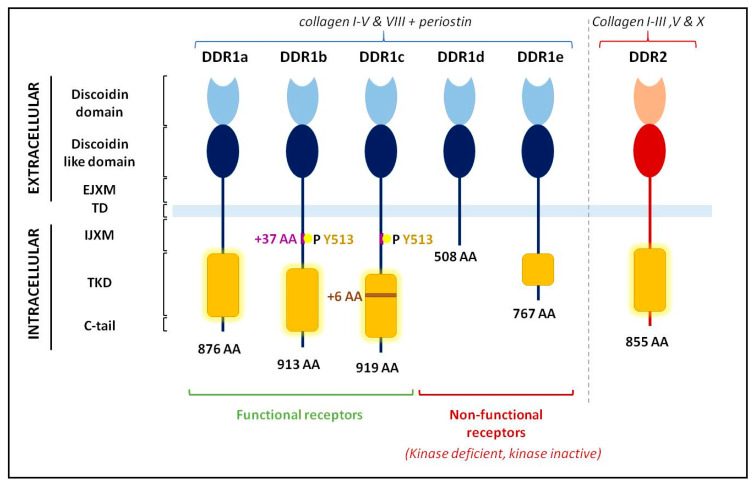
DDRs are a family of tyrosine kinase receptors (TKRs) with two members, DDR1 and DDR2. DDRs share an extracellular domain similar to the discoidin I lectin of the amoeba Dictyostelium discoideum, a discoidin-like domain, and an extracellular juxta-membrane domain (EJXM). The transmembrane domain is followed by an intracellular juxta-membrane domain (IJXM) and by the tyrosine kinase domain (TKD). DDR1 and DDR2 are encoded by single genes present in chromosome 6 (6p21.33) and 1 (1q23.3), respectively. Alternative splicing results in DDR1 isoforms, a, b, c, d, and e (Figure 4). DDR1b and c share a 37 amino-acid sequence inserted within the IJXM domain, compared to DDR1a. This stretch of 37 amino-acid insertion includes a tyrosine residue (Tyr 513) essential for DDR1 signaling. DDR1c has six extra amino acids in the kinase domain compared to DDR1b. DDR1a and b are the major isoforms expressed. Isoforms d and e are deleted or mutated in the TKD, resulting in inactive tyrosine kinase isoforms. In contrast, only one form of DDR2 is found (Figure 4) [12].
Figure 4.
Schematic representation of the five discoidin domain receptor 1 (DDR1) isoforms and of the only DDR2 form. EJXM: extracellular juxta-membrane domain, TD: transmembrane domain, IJXM: intracellular juxta-membrane domain, TKD: tyrosine kinase domain, C-tail: C-terminal part of the receptors. Adapted from [12,17].
Both receptors bind to fibrillar collagens with certain ligand specificities; DDR1 preferentially binds to collagen I–V and VIII while DDR2 binds to collagen I–III, V, and X [13]. In addition, DDR1 is able to bind periostin, another component of the ECM [14]. In the absence of any ligand and as for other TKRs, a mixed population of DDR monomers and homodimers is expressed at the cell surface. However, unlike other TKRs, fibrillar ligand-induced DDR activation is very slow, taking hours to set up and persisting for more than 24 h. In this step, the DDRs cluster, the tyrosine kinase activity is induced, and the dimers phosphorylate each other [15,16].
Several tyrosine residues are phosphorylated, notably Tyr513 and Tyr792, 796, and 797 within a clustering DDR1b, allowing the recruitment of various adaptor proteins involved in signal transduction [17]. Recently, a noncanonical activation mode was proposed for DDR1b, in which the binding of monomeric collagen to DDR1b induces a rapid (30 min) clustering, leading to internalization and re-expression at the cell surface as Tyr513-phosphorylated DDR1b. Then, if fibrillar collagens are present, linear clusters of DDR1b are formed, and canonical activation of the receptors occurs with the phosphorylation of Tyr740 and 792. Since Tyr513 is not present in DDR1a and has no equivalent in DDR2, this noncanonical pathway is only possible with DDR1b and c [18,19]. Activation of DDR2 appears to be more complex and requires phosphorylation by Src kinase. Binding of collagen to DDR2 induces phosphorylation of Tyr740 by Src, thereby releasing the autoinhibitory activity of the kinase, allowing phosphorylation of other tyrosine residues and recruitment of adaptor proteins [20]. Heterodimers or at least heteroclusters between DDR1 and DDR2 have been found in coimmunoprecipitation assays [21]. DDRs activate various intracellular signaling pathways such as extracellular signal-related kinase (ERK), signal transducer and activator of transcription (STAT), Src, protein kinase B (AKT), or Yap [17,21,22,23].
Nevertheless, DDRs can induce intracellular signaling independent of tyrosine kinase activity or of collagen binding. The formation of linear invadosomes is mediated by collagen binding to DDR1 and activation of the Cdc42-GEF (guanine nucleotide-exchange factor) Tuba, and it is independent of tyrosine kinase activity [24]. The involvement of DDR1 in the process of collective invasion is collagen-independent and only requires DDR1 interaction with E-cadherin to induce activation of Par3 and 6 [25]. DDR1 is also able to interact with other partners such as the tetraspanin-like protein TM4SF1 (Transmembrane 4 L Six Family Member 1) to mediate intracellular signaling [26]. Less is known about DDR2 interactions with its partners.
The regulation of DDR expression is not well understood. Hypoxia is a positive regulator of DDR1 and DDR2 expression in nontumor cells, such as mesenchymal stem cells and smooth muscle cells [27,28], and in pituitary adenoma and breast tumor cells [29,30]. The transcription factors Zeb1 and Twist1, involved in the epithelial-to-mesenchymal transition (EMT) process, increase the expression of DDR1 or DDR2, respectively [31,32]. E2F1 and P53, two cell-cycle transcription factors, increase DDR1 messenger RNA (mRNA) expression in different tumor cell lines [33,34]. In hepatocellular carcinoma, DDR1 activates STAT3 which, in turn, increases DDR1 mRNA expression [35]. ATF4 (Activating Transcription Factor 4) mediates osteoclast differentiation by increasing DDR2 gene transcription [36]. Angiotensin II and TNFα (Tumor Necrosis Factor alpha) increase DDR2 mRNA expression [37,38], whereas TGFβ1 increases DDR1 at both mRNA and protein level [39]. In lung fibroblasts, collagen I activates DDR2 and, in turn, JAK2 (Janus Kinase 2) and ERK1/2 signaling pathways leading to up-regulation of DDR1 [40]. MicroRNAs (miRNAs) are potent regulators of DDR expression, with miR-199-a/b 5p [41] and miR-486-3p [42] for DDR1 and miR-615-5p [43] for DDR2. Lastly, a growing body of evidence suggests epigenetic control of DDR1 mRNA expression. In patients with idiopathic nonobstructive azoospermia or ovarian or lung cancers, DDR1 expression was found to correlate with the methylation of the promoter [44,45,46].
DDR1 activity may be regulated by a shedding mechanism. Collagens are able to activate DDR1 and some batimastat-sensitive proteinases. In turn, active proteinases, such as MT1-MMP-1, MT2-MMP, MT3-MMP (Membrane Type Matrix Metalloproteinases), and a disintegrin and metalloproteinase (ADAM) 10 cleave DDR1 at EJXM, leaving a 62 kDa intracellular membrane anchored protein. This cleavage is responsible for inhibiting the tyrosine kinase activity of DDR1, but nothing is known about the function of the extracellular part of DDR1 [13,47,48].
In this review, we focus primarily on the involvement of DDRs in carcinoma development and metastasis. We discuss the potential roles of these receptors in certain stages of metastasis.
2. Roles of DDRs in Cancer Progression
2.1. Tumor Growth
2.1.1. DDRs and Tumor Cells
The transformation of a normal cell into a cancer cell involves different mechanisms such as gene activation/inactivation and chromosomal abnormalities. DDR1 is not important for tumor initiation, but it is critical in early tumor development and progression in KRAS (Ki-ras2 Kirsten rat sarcoma viral oncogene homolog)-driven lung adenocarcinoma [49] (Figure 1a,b). DDR1 is not expressed in normal gastric epithelial cells, but it is expressed in half of gastric carcinoma, showing that DDR1 is an important mediator of gastric cancer aggressiveness [50]. DDR2 has been shown to be a cancer-related gene associated with prostate cancer aggressiveness and progression, and its expression is upregulated in advanced benign prostate hyperplasia and prostate cancer compared to normal tissue [51]. This involvement of DDRs in the development and aggressiveness of tumors translates into an effect in several biological processes. Thus, numerous studies have shown that DDRs have an impact in vitro and in vivo on tumor growth by promoting or inhibiting proliferation and apoptosis in a cancer- and context-dependent manner, alone or in combination with other molecules (Figure 1c).
DDR1 has been shown to play a critical role in promoting the proliferation of several cancer cells, in vitro and in vivo. In different cancer cell lines, DDR1 promotes survival by regulating the Ras/Raf/MEK/MAPK (Rat sarcoma/Rapidly Accelerated Fibrosarcoma/Mitogen-activated protein kinase kinase/Mitogen-activated protein kinase) pathway, resulting in positive feedback on the expression of the tumor suppressor P53 and the activation of AKT [34]. In Hodgkin’s lymphoma, latent membrane protein 1 (LMP1) induces DDR1 expression in germinal center B cells and protects tumor cells from death [52]. In prostate cancer, prostate cancer antigen 1 (PCA-1) increases the level of DDR1 and Bcl-xl, an antiapoptotic molecule, resulting in the inhibition of apoptosis [53]. In non-small-cell lung carcinoma (NSCLC), DDR1 induces tumor cell proliferation in vivo. Inhibition of DDR1 or TMPRSS4 (Transmembrane protease serine 4), a membrane-bound serine protease, reduces proliferation to various extents with little alteration of cell-cycle progression or apoptosis. These effects are potentiated when DDR1 and TMPRSS4 are co-inhibited, resulting in complete inhibition of proliferation with disappearance of cyclin A and B1, increased P21 expression, and gap 0 (G0)/G1 cell-cycle arrest [54]. DDR1 can also promote proliferation in vivo. For example, inhibition of DDR1 in a subcutaneous gastric cancer xenograft slows tumor growth [50]. Furthermore, in pancreatic ductal adenocarcinoma, tumors from DDR1−/− mice have significantly fewer Ki-67+ proliferative cells [55]. This is consistent with an in vitro study of pancreatic cancer cells which showed that DDR1 inhibits TGFβ1 expression, thereby promoting tumor cell proliferation [56]. The role of DDR1 in breast cancer proliferation or survival appears to depend, at least in part, on tumor type or culture method. In vitro, targeting DDR1 expression inhibits proliferation of T47D and MCF-7 luminal cells or MDA-MB-435 triple-negative cells [57,58]. In contrast, in other breast cancer studies, DDR1 was found to be a proapoptotic receptor. Luminal breast cancer MCF-7 cells and basal-like breast cancer MDA-MD-231 cells express high and low levels of DDR1 and low and high levels of MT1-MMP, respectively. When cells are cultured in a three-dimensional (3D) collagen matrix, DDR1 in MCF-7 cells is involved in apoptosis and cell growth inhibition through upregulation of the proapoptotic mediator BIK (Bcl-2-interacting killer) [59]. Conversely, in MDA-MD-231 cells, MT1-MMP has a protective effect through degradation of collagen I and cleavage of DDR1, altering the collagen/DDR1/BIK pathway to induce apoptosis and suppress tumor growth [60]. In colon cancer, DDR1 interacts with LRP1, a lipoprotein receptor inducing DDR1 endocytosis, thereby controlling its expression at the plasma membrane. The DDR1/LRP1 interaction increases 3D cell proliferation and cell-cycle progression, as well as decreases apoptosis [61].
DDR2 has been shown to promote proliferation in human melanoma [62], oral squamous cell carcinoma [63], gastric cancer [64], hepatocellular carcinoma [65], lymphoma [43], and lung cancer [66] among others. Indeed, in vitro inhibition of DDR2 expression results in decreased proliferation in melanoma cell lines via JNK (c-Jun N-terminal kinase) phosphorylation [62] and induction of apoptosis in hepatocellular carcinoma cells [65]. In oral squamous cell carcinoma, downregulation of colon cancer-associated transcript 1 (CCAT1) prevents proliferation via inhibition of DDR2, resulting in inactivation of ERK/AKT pathways [63]. Lastly, Circ-LAMP1 (Lysosomal-associated membrane protein 1), the circular RNAs most expressed in T-cell lymphoblastic lymphoma, is capable of promoting proliferation by inhibiting apoptosis through modulation of miR-615-5P and its target DDR2 [43]. DDR2 has also been shown to enhance tumor growth in vivo. Indeed, in hepatocellular carcinoma xenograft, small interfering RNA (siRNA)-mediated inhibition of DDR2 expression in SNU182 cells led to decrease tumor growth [65]. In gastric cancer, overexpression of DDR2 promotes tumorigenesis in vivo by inducing tumor growth [67]. On the contrary, DDR2 may also inhibit cell proliferation in melanoma and fibrosarcoma cells in vitro. In these cells, fibrillar collagen activates DDR2 resulting in inhibition of proliferation characterized by growth arrest in the G0/G1 phase of the cell cycle [68].
Tumor cell proliferation can be modulated by DDR mutations. Indeed, some mutations have been identified in DDR1 and DDR2 throughout their structures, but the impact of mutations has been studied mainly for DDR2 and more particularly in lung cancer. DDR2 is mutated in 3–4% of cases. Some mutations such as E655K play a role in tumor progression by weakening the growth-inhibitory effect induced by collagen via DDR2, while V582E or L595F mutations also increase colony size in vitro [69,70].
As shown above, the impact of DDRs may be different in in vitro and in vivo situations, and it may depend on the structural organization of the TME. In the case of breast carcinoma [71], in vitro inhibition of DDR1 promotes tumor growth only in 3D. The rigidity of the microenvironment plays a critical role and can either activate or inhibit proliferation and invasion. The NSCLC cell line H1299 grown on a soft matrix expresses p300 at low levels, leading to reduced acetylation of c-myb and weak interaction between the DDR2 promoter and c-myb or LEF1 (Lymphoid enhancer-binding factor-1). Under these conditions, the DDR2 promoter is not very active, resulting in low proliferation and invasion. On the contrary, when the matrix stiffens, the DDR2 promoter is highly active, resulting in increased proliferation and invasion [66]. Table 1 summarizes the different roles of DDRs in cancer-cell survival or proliferation.
Table 1.
Roles of DDRs in tumor cell survival or proliferation. NSCLC, non-small-cell lung cancer; SCC, squamous cell carcinoma.
| Receptor | Cell Survival or Cell Proliferation | Cancers |
|---|---|---|
| DDR1 | increase | -Sarcoma [34] -Colon carcinoma [34] -Breast carcinoma [57,58] -Hodgkin’s lymphoma [52] -Prostate carcinoma [53] -Lung (NSCLC) [54], (adenocarcinoma) [49] -Gastric carcinoma [50] -Pancreatic carcinoma [55,56] |
| decrease | -Breast carcinoma [59,60,71] -Colon carcinoma [61] |
|
| DDR2 | increase | -Melanoma [62] -Oral SCC [63] -Gastric carcinoma [64,67] -Hepatocellular carcinoma [65] -Lymphoma [43] -Lung (NSCLC) [66] |
| decrease | -Melanoma [68] -Fibrosarcoma [68,73] |
The TME is not only composed of cancer cells but also of stromal cells such as CAFs. An interaction between DDRs and CAFs has been established in several cancers. In gastric carcinoma, CAFs induced the upregulation of DDR1 in tumor cells in vitro by potently activating STAT3, increasing its tumorigenic potential. In vivo, on gastric carcinoma, pharmacological inhibition of DDR1 in the mouse xenograft model impeded CAF-induced tumorigenesis with a reduction in the number of tumor nodules [72].
2.1.2. DDRs and Immune Cells
The immune system plays an essential role in the response against the tumor. In order to stop tumor progression, this response is mediated by T and B lymphocytes, natural killer cells, macrophages, and dendritic cells. The immune cells migrate through the matrix where collagen is very abundant and enter the bloodstream to reach the tumor site. In 2011, two new characteristics of cancer were revealed and, among them, is the escape from the immune system [74]. DDRs have been shown to control certain characteristics of immune cells.
DDR1 is able to enhance the migration of the T helper 17 (Th17) population of T lymphocytes in 3D collagen matrices through different pathways: RhoA/ROCK/MAPK/ERK (Ras homolog family member A/ Rho-associated protein kinase/ Mitogen-activated protein kinase/ Extracellular Signal-Regulated Kinase) in vitro and in vivo [75] or activation of p38 MAPK [76]. Recently, in breast cancer, an inverse correlation was made between DDR1 expression by tumor cells and the level of CD4+ and CD8+ T-lymphocyte infiltration. The molecular mechanism involved in these situations is not known [77].
DDR2 plays an important role in the collagen-mediated differentiation of dendritic cells to a mature phenotype producing a high amount of IL-12 (Interleukin-12). DDR2 enhances the functional abilities of these mature dendritic cells to activate T cells, thereby regulating immune responses [78]. While integrins are important mediators of neutrophil migration on a 2D collagen matrix, DDR2, but not DDR1, is involved in neutrophil migration in 3D collagen. DDR2 regulates neutrophil directionality through increased secretion of MMP8 [79]. Furthermore, in adipose tissue, T-cell activity is controlled by myeloid-derived CD45+/DDR2+ cells. These CD45+/DDR2+ cells express increased levels of MHC II (major histocompatibility complex II) and CD80, two markers of activation and antigen presentation, and lead to increased production of the proinflammatory cytokines, IFN-γ (Interferon gamma) and TNF-α, by CD4+ T cells [80]. The presence and the role of these myeloid-derived CD45+/DDR2+ cells in tumor progression are not known.
2.2. Local Invasion
2.2.1. Extracellular Matrix Breakdown
In order to spread into different tissues, tumor cells must first breach the surrounding basement membrane, which is mainly composed of collagen IV and laminins. The capacity of tumor cells to overcome the basement membrane is decisive for tumor progression (Figure 2a) [81]. DDRs are able to modulate the expression of MMPs, in particular MMP2 and MMP9, which are involved in the remodeling and degradation of the basement membrane.
In lung cancer, DDR1 inhibition decreases the activity of MMP2 and MMP9 [82]. Modulation and regulation of MMP2 and/or MMP9 activity by DDR1 have also been demonstrated in pituitary adenoma [83], colorectal cancer [84], and renal cancer (MMP2) [85]. In pancreatic cancer, a novel pathway including TM4SF1/DDR1/MMP2 and 9 enhances invadosome formation and activity, thereby enhancing cell migration and invasion capabilities [86]. In liver cancer, the interaction between C1q, a component of the complement system, and DDR1 induces the upregulation of MMP2 and MMP9 expression, leading to migration and invasion [87]. C1q is present in the microenvironment of many tumors such as colon, pancreatic, breast, lung adenocarcinoma, and melanoma, and it is expressed by stromal cells such as fibroblasts [88].
Like DDR1, DDR2 is involved in the modulation of MMP expression in ovarian cancer where it regulates the expression of MMP1–3, 7, and 13 mRNAs and the activity of MMP2 and MT1-MMP [32]. In human melanoma cells, downregulation of DDR2 is associated with decreased MMP2 and MMP9 activities [62]. Overexpression of DDR2, through ERK2/Snail signaling, increases MT1-MMP and MMP2 expression in hepatocellular carcinoma [89] and MT1-MMP expression in breast cancer [90]. Nevertheless, DDR2 was found to be required for collagen I-mediated activation of MT1-MMP in fibroblast but not in fibrosarcoma or in breast cancer [91].
Basement membrane degradation is mediated by the formation of specialized structures called invadosomes which degrade the basement membrane while maintaining cell-cell contacts and tissue integrity [92]. The involvement of DDRs in the formation of these structures has been demonstrated in several studies. In breast cancer cells, the binding of collagen I to DDR1 leads to the activation of the Cdc42-GEF Tuba, thereby inducing the formation of linear invadosomes, as well as promoting the activation of the proteolytic machinery and cell invasion. This process is independent of the tyrosine kinase activity of DDR1 [24]. In hepatocellular carcinoma, DDR1-induced linear invadosome formation is enhanced in the presence of TGFβ1 [39].
DDR2 is required to elaborate linear invadosomes in endothelial cells and to facilitate matrix degradation in association with VEGF (Vascular Endothelial Growth Factor), but it is not known if DDR2 plays the same role in cancer cells [93]. Table 2 summarizes the different roles of DDRs in MMP activation and invadosome formation.
Table 2.
Roles of DDRs in matrix metalloproteinase (MMP) activation and invadosome formation.
| Receptor | MMP Activation/Invadosome Formation | Cancers |
|---|---|---|
| DDR1 | Increase | -Lung carcinoma [82] -Pituitary adenoma [83] -Colorectal carcinoma [84] -Renal carcinoma [85] -Pancreatic [86] -Liver [87] -Breast carcinoma [24] |
| Decrease | ||
| DDR2 | Increase | -Ovarian [32] -Melanoma [62]-Hepatocellular carcinoma [89] -Breast [90] |
| Decrease |
After basement membrane degradation, cells encounter collagen I in the interstitial matrix (Figure 2a). Aligned collagen oriented perpendicular to the tumor boundary has been shown to promote invasion, and it is considered as a poor prognostic marker for survival or recurrence in patients with breast carcinoma [94]. Three tumor-associated collagen signatures (TACS) have been defined. TACS-1 is characterized by increased collagen deposition near the tumor that appears very early in tumor formation. As the tumor grows, TACS-2 and -3 have been defined. TACS-2 corresponds to collagen fibers stretched around the tumor, and TACS-3 is the situation in which collagen fibers are aligned perpendicular to the tumor, promoting local invasion [95]. DDR1 and DDR2 participate in collagen fiber alignment and have been associated with TACS-3 [90,96,97,98]. Accordingly, inhibition of DDR2 expression decreases collagen deposition and fiber alignment and thereby tumor growth, migration and invasion are reduced [90,99].
The structure of collagen I can be impacted by aging, affecting many biological processes. DDR1 is very sensitive to these changes and, consequently, the regulation of proliferation and apoptosis is altered. For instance, the proteolytic degradation of collagen I by MT1-MMP, which increases with aging, was shown to decrease DDR1 activation, tumor growth, and inhibition of apoptosis [71]. This effect is also observed with DDR2; collagen I aging reduces DDR2 activation, resulting in induction of fibrosarcoma cell proliferation. In the presence of old collagen I, DDR2 and the tyrosine phosphatase SHP-2 (Src homology region 2-containing protein tyrosine phosphatase 2) are activated to a lesser extent, JAK2 and ERK1/2 remain phosphorylated, and expression of the cell-cycle negative regulator p21CIP1 decreases [73].
DDR2 expressed by cells of the TME is also involved in collagen remodeling.Inhibition of DDR2 in CAFs or in mesenchymal stem cells impacts their mechanotransduction function, disturbs collagen fiber organization, and decreases matrix stiffness and TACS-3 signature, leading to inhibition of breast tumor cell invasion [97,99].
2.2.2. Epithelial–Mesenchymal Transition
EMT is a critical process involved in cancer development and progression. Depending on the context, this mechanism can lead epithelial cells to undergo multiple intermediate steps accompanied by physiological and morphological changes, transforming them in extreme cases into mesenchymal cells. EMT can be reversed by an event called mesenchymal-to-epithelial transition (MET). Both transitions are regulated by the induction or repression of transcription factors such as Zeb1/2, Snail1/2, and Twist among others, as well as the modulation of the expression of epithelial (E-cadherin) and mesenchymal (N-cadherin, vimentin, MMP9) markers [100]. DDRs have been shown to be associated with EMT in several cancers.
Some studies highlighted that DDR1 can induce EMT in vitro. For instance, in prostate cancer, DDR1 activates Pyk2 and MKK7 (Mitogen-activated protein kinase kinase 7), resulting in increased expression of the mesenchymal markers vimentin and N-cadherin and decreased expression of the epithelial marker E-cadherin [101]. In gastric cancer, a correlation among DDR1, E-cadherin, and vimentin has been demonstrated in cancer tissues. Overexpression of DDR1 increases the expression levels of vimentin and Snail1 and decreases that of E-cadherin [102]. Moreover, a negative correlation between miR-221-5p and DDR1 has been demonstrated. Overexpression of miR-221-5p in gastric cancer cells inhibits not only EMT, but also proliferation and invasion through modulation of DDR1 expression [103]. DDR1 induces EMT in hepatocellular carcinoma by regulating STAT3 in vitro and in vivo [35]. In colorectal cancer, downregulation of miR-199a-5p increases DDR1 expression, resulting in increased expression of mesenchymal markers [84]. In renal cancer OS-RC-2 and ACHN cell lines, collagen-mediated activation of DDR1 leads to increased expression of mesenchymal markers (vimentin/N-cadherin) and decreased expression of epithelial markers (E-cadherin) [85].
Conversely, several studies have shown that DDR1 expression is decreased during EMT. In breast cancer, a negative correlation between DDR1 and Zeb1 was found in carcinoma tissues. In the tumor, H-Ras is able to induce EMT by increasing Zeb1 and decreasing miR-200c expression. Zeb1 leads to suppression of E-cadherin expression and a reduction in DDR1 expression [31]. This negative correlation between Zeb1 and DDR1 is significantly associated in a female-specific manner in breast, ovarian, and liver cancers but not in lung or colon cancers [104]. Similarly, in epithelial ovarian cancer, a decrease in DDR1 expression is observed during the EMT process due to CpG hypermethylation of its promoter, and DDR1 inhibition did not affect E-cadherin expression at the protein level [45]. The effect of DDR1 on EMT inhibition has also been demonstrated in vivo in breast cancer. Crossing DDR1−/− and MMTV-PyMT mice revealed that a loss of DDR1 expression increases the level of vimentin in the primary tumor while E-cadherin expression decreases. In the absence of DDR1, tumor cells exhibit a basal phenotype leading to enhanced invasion and are associated with the development of breast cancers of poor prognosis [105]. In the Madin-Darby Canine Kidney (MDCK) cell line, Slug (Snail2) induces EMT with no detectable level of E-cadherin and increases the level of mesenchymal markers (MMP9 or vimentin). During this transition, a switch from DDR1 to DDR2 expression is observed. Indeed, MDCK cells expressing Slug show a decrease in DDR1 expression accompanied by an increase in DDR2 expression [106]. This notion of switching between DDR1 and DDR2 has been recently suggested in ovarian cancer, where DDR2 mRNA expression is low in epithelial-like cells and increases in mesenchymal-like cells. Nevertheless, DDR2 protein expression could not be found in these mesenchymal-like cells [45].
The role of DDR2 in EMT activation is less controversial. An association between DDR2 and Snail1 has been revealed in several studies on breast cancer [30,90,98], ovarian cancer [32], papillary thyroid carcinoma [107], and hepatocellular carcinoma [89] via different signaling pathways. Indeed, immunohistochemistry on breast cancer tissues revealed an association of DDR2 expression with nuclear localization of Snail1, as well as with a loss of E-cadherin expression. The tyrosine kinase activity of DDR2 induces stabilization of Snail1 protein and involves stimulation of ERK2 in a Src-dependent manner. Importantly, in this study, DDR2 was induced during EMT but was not required for EMT induction [90]. Similarly, in hepatocellular carcinoma, DDR2 stabilizes Snail1 through ERK2 activity [89]. In gastric cancer, DDR2 promotes EMT by modulating the mTORC2 (mechanistic target of rapamycin complex 2)/AKT pathway [67].
Although a link has been established between DDRs and some factors modulating the EMT process, especially in vitro where a few signaling pathways have started to emerge, the mechanisms involved need to be further elucidated in vivo. Table 3 summarizes the different roles of DDRs in EMT.
Table 3.
Roles of DDRs in the mechanism of EMT.
| Receptor | EMT | Cancers |
|---|---|---|
| DDR1 | Increase | -Prostate [101] -Gastric [102,103] -Hepatocellular carcinoma [35] -Colorectal [84] -Renal carcinoma [85] |
| Decrease | -Breast carcinoma [31,104,105] -Ovarian [45,104] -Liver (female, [104]) |
|
| DDR2 | Increase | -Breast [30,90,98] -Ovarian [32] -Papillary thyroid carcinoma [107] -Hepatocellular carcinoma [89] -Gastric [67] |
| Decrease |
2.2.3. Migration and Invasion
DDRs have been extensively studied for their impact on migration and invasion. There are two main mechanisms of migration: cells moving individually (amoeboid and mesenchymal migration) or collectively in a cohesive multicellular unit (collective migration). Migration can be influenced by cytoskeletal organization, remodeling of the surrounding matrix by migrating cells, and cell–matrix interaction and force generation [108]. Adhesion to the ECM is important for cancer cells to migrate and, subsequently, to disseminate to different tissues. Some studies have shown that adhesion can occur in a DDR-independent manner, e.g., in breast cancer, the adhesion of MCF-7 cells to collagen is predominantly driven by β1 integrin rather than DDR1 [109]. Recently, using different combinations of HEK293T cell populations expressing either DDR1 and/or DDR2, we showed that the extracellular part of DDR1 or DDR2 is sufficient to mediate cell adhesion to collagen I in absence of integrin activities [21]. In human A375 melanoma, HT29 colon carcinoma and SK-HEP hepatoma cells, inhibition of DDR1 reduces by 75% very early tumor cell adhesion to collagen I with significant impairment of the cell–cell adhesion molecules ICAM1 (InterCellular Adhesion Molecule 1) and VCAM1 (vascular cell adhesion molecule 1) [110]. In prostate cancer, overexpression of DDR2 improved adhesion to collagen I [111].
DDRs are involved in migration and invasion processes as shown in numerous in vitro studies.DDR1 modulates the organization of the cytoskeleton and, consequently, has an impact on cell migration. In squamous cell carcinoma, DDR1 is localized at cell–cell contacts in association with E-cadherin and is able to regulate cytoskeletal organization, particularly actomyosin organization, and collective migration. The DDR1/E-cadherin/Par3/Par6 complex controls the localization of RhoE which antagonizes Rho/ROCK, modulating actomyosin contractility at cell–cell contacts. At these sites, actomyosin forces are weaker, allowing coordinated movement of the clustered cells [25]. In oral squamous cell carcinoma, DDR1 is involved in collective migration and invasion into a collagen matrix, leading to an angiolymphatic invasion in vivo [112]. In hepatocellular carcinoma cells, a cross-talk between DDR1 and STAT3 enhances tumor progression by promoting proliferation, migration and invasion in vitro, and tumorigenesis in vivo [35]. In pancreatic cancer, TM4SF1-induced migration and invasion require DDR1. Overexpression of DDR1 increases cell ability to form invadosomes to degrade the matrix and increases MMP2 and MMP9 [86]. In breast cancer cells, in a pathway involving the CD9 tetraspanin, DDR1 induces cell migration [113]. Collagen causes shedding of the DDR1 ectodomain by the metalloproteinase ADAM10. This ADAM10 and collagen binding-dependent DDR1 shedding is important for more efficient migration of epithelial cells on the collagen I matrix in vitro [48]. Consequently, migration is influenced by the control of DDR1 expression. In gastric cancer, miR-221-5p modulates DDR1 expression. When miR-221-5p is downregulated, DDR1 expression increases and promotes migration and invasion [103]. On the contrary, in pancreatic carcinoma, the interplay of DDR1 with E-cadherin and the Par3 signaling pathway was shown to prevent 3D invasion by enhancing cell–cell adhesion [114].
DDR2 has also been shown to promote migration and invasion in many cancers. Inhibition of DDR2 expression in gastric cancer [64] or hepatocellular carcinoma [65] reduces migration and invasion. Inhibition of DDR2 expression in melanoma suppresses migration and invasion through decreased activation of the ERK1/2 and NF-κB (Nuclear Factor Kappa B) pathways and, consequently, reduced expression of MMP2 and 9 [115]. In oral squamous cell carcinoma, downregulation of CCAT1 prevents proliferation, migration, and invasion through inhibition of DDR2 leading to inactivation of ERK/AKT pathway [63]. In head and neck squamous cell carcinoma, overexpression of DDR2 enhances migration and invasion [116]. In breast cancer, DDR2 can induce tumor cell migration and invasion when expressed by tumor cells and/or by CAF or multipotent stromal cells (MSCs) [90,117].
Although, in most cases, DDR1 and DDR2 have been shown to promote migration and invasion, few studies have shown their involvement in inhibiting both processes. DARPP-32 (Dopamine- and cAMP-regulated phosphoprotein, Mr 32 kDa) binds to the IJXM domain of DDR1 and decreases collagen-stimulated invasion of breast cancer cells [118].
Very few studies have analyzed the impact of DDRs on migration and invasion in vivo; instead, they focused on the establishment of metastasis as in gastric cancer [119]. PCA-1 increased prostate cancer cell invasion through DDR1 and MMP9 expression in a chick chorioallantoic membrane (CAM) assay [53]. In colorectal cancer, NSD2 (Nuclear Receptor Binding SET Domain Protein 2) circular RNA inhibits miR-199b-5p expression, which leads to an upregulation of DDR1 expression and promotes migration and invasion in vitro and in vivo [120]. DDR2 increases metastasis in a peritoneal xenograft model of ovarian cancer [32]. In a mouse model of spontaneous breast tumor development, inhibition of DDR2 expression by gene ablation largely reduces metastasis without affecting lung tumor growth [99,117].
Table 4 summarizes the different roles of DDRs in EMT.
Table 4.
Roles of DDRs in tumor cell migration and/or invasion.
| Receptor | Migration/Invasion | Cancers |
|---|---|---|
| DDR1 | Increase | -Squamous cell carcinoma [25,112] -Hepatocellular carcinoma [35] -Pancreatic [86] -Breast [113] -Renal carcinoma [85] -Gastric [103] -Prostate [53] -Colorectal [120] -Epidermoid carcinoma [48] |
| Decrease | -Breast [118] -Pancreatic [114] |
|
| DDR2 | Increase | -Gastric [64] -Hepatocellular carcinoma [65] -Melanoma [115] -Squamous cell carcinoma [63,116] -Breast [99,117] -Ovarian [32] |
| Decrease |
2.3. Intravasation
There are two different routes for tumor dissemination: blood vessels or lymphatic vessels (Figure 2b). Angiogenesis is the primary vascularization process and is defined as the formation of new blood vessels from existing vasculature [1]. Likewise, lymphangiogenesis is the formation of new lymphatic vessels from pre-existent vessels [121]. During these processes, tumor blood and lymphatic vessels have the particularities of being disorganized and permeable to cancer cells, which facilitate their passage into the blood or lymphatic streams and, subsequently, the establishment of metastasis by their dispersion in different tissues. Tumor and endothelial DDRs are involved in angiogenesis.
DDR1, in renal cell carcinoma and in gastric cancer cells, induces the secretion of angiogenic factors involved in endothelial cell tube formation in vitro [85,119]. In gastric cancer cells, depletion of DDR1 suppresses the expression of key angiogenic factors such as VEGF-A, VEGF-C, and PDGF-B (Platelet-Derived Growth Factor B) [119] and leads to necrosis in vivo, as a consequence of impaired angiogenesis [102,119]. A direct role of DDR1 in angiogenesis was demonstrated by inhibiting DDR1 cell surface expression in microvascular endothelial cells (HMEC-1) [122].
DDR1 is also important for lymphangiogenesis. Gastric tumors develop fewer lymphatic vessels if DDR1 expression is inhibited [119] and lymphatic endothelial cells are unable to form tubes when DDR1 expression is suppressed by siRNA or with miR199a/b-5p [123].
In contrast to endothelial cells, in colon carcinoma, DDR2 is weakly expressed at the surface of normal endothelial cells; however, overexpression of the receptor enhances angiogenesis in vitro and in vivo. After injection of VEGF-containing Matrigel, angiogenesis is impacted in the slie mouse model carrying a deletion in the DDR2 gene. When melanoma cells are injected, tumor development is impacted with decreased tumor angiogenesis and increased tumor necrosis; nevertheless, the remaining vasculature is mature and functional. In these tumors, proangiogenic factors or receptors such as VEGFR2, Ang-2 (Angiopoietin-2) or MMP9 are decreased, and antiangiogenic factors such as Ang-1 are increased [124]. In hepatocellular carcinoma, an association between DDR2 and VEGF has been found. Indeed, during hypoxia, DDR2 can regulate the VEGF pathway [125].
Nothing is known about the role of DDRs in the mechanism of intravasation.
2.4. Survival in the Circulation, Extravasation, and Micrometastasis Formation
No evidence for a role of DDRs in tumor cell survival in the bloodstream or extravasation has been established (Figure 3a–c). Nevertheless, in a model of liver metastasis, Yuge and collaborators injected gastric cancer cells into the spleen of mice and found no difference in the number of liver micrometastases whether or not cells expressed DDR1. These data suggest that there is no difference for tumor cell survival in the bloodstream or tumor cell extravasation to the liver parenchyma [119]. On the contrary, DDR1 in tumor cells is important for the migration of lung cancer cells to the bone niche after intracardial injection. However, it is not clear which stage of metastasis (survival in blood, extravasation, and/or micrometastasis) is under the dependence of DDR1 [82].
Recently, DDR1 expressed by the liver metastatic niche was found to be important for micrometastasis implantation. DDR1 siRNA-injected mice have fewer hepatic stellate cells (HSCs), differentiated myofibroblasts, and angiogenesis (CD31-positive liver sinusoidal endothelial cell (LSECs)). Consequently, less collagen is secreted into the niche and the number of micrometastases is reduced [126].
The importance of DDR2 in the metastatic niche depends of the metastatic tissue. In a model of liver metastasis, intrasplenic injection of colon cancer cells into DDR2−/− mice showed an increase in micrometastasis foci compared to a DDR2+/+ mice. This result can be explained by increased HSC differentiation into myofibroblasts and increased LSEC activation and angiogenesis in DDR2−/− mice. Furthermore, tumor cell adhesion to LSECs from DDR2−/− mice is increased if these cells are exposed to tumor cell supernatants, suggesting that this may cause an increase in tumor cell extravasation in vivo. Lastly, the liver tumor niche in DDR2−/− mice promotes tumor cell colonization (see below) [127]. In contrast, in a model of lung metastasis, intravenous tail injection of melanoma cells in slie mice showed a reduction in the number of lung metastases [124].
However, different types of collagen are found at higher levels in the serum from patients with cancer, and serum collagen IV is a biomarker for peritoneal dissemination of gastric cancer [128]. In breast cancer, HSP47 induces collagen secretion in the bloodstream where it can bind to circulating tumor cells and platelets, thereby promoting metastases [129]. C1q, which is part of the complement activation complex, contains a collagen-like domain. It was recently shown that DDR1 is activated in the presence of C1q, with phosphorylation at Tyr513 of DDR1 in hepatocellular carcinoma [87]. Furthermore, DDRs are involved in the activation of the pro-survival AKT pathway [130,131]. It is tempting to speculate that serum collagens and C1q can activate DDRs in circulating tumor cells to induce their survival in the blood stream.
The involvement of DDRs in tumor cell extravasation is still poorly documented.
2.5. Macrometastasis (Colonization)
The first demonstration of DDR1 involvement in tumor colonization (Figure 3c) was elegantly established by the Giancotti group [26]. In lung micrometastases, breast tumor cells produce and deposit collagen I in the ECM. Collagen can bind to DDR1 inducing the recruitment of the tetraspanin TM4SF1. In turn, TM4SF1 induces a large clustering of DDR1 and brings PKCα bound to the adaptor protein syntenin-2 in close proximity to the intracellular part of DDR1. PKCα phosphorylates and activates JAK2 and, consequently, STAT3 is phosphorylated and can, in turn, activate the transcription of genes such as Sox2. This mechanism of DDR1-induced metastatic colonization is independent of its tyrosine kinase activity. The formation of large DDR1 clusters is crucial for this mechanism, because collagen IV, which induces only small-size clusters, is not involved in the colonization process. Inhibition of TM4SF1 alters not only the metastases of breast tumors to the lungs, but also to the brain and bone. However, the involvement of DDR1 in brain and bone metastasis has not been assessed [26]. Recently, it was shown that TM4SF1 can regulate AKT and ERK activation via DDR1 in lung carcinoma [130], but it is unclear whether or not these regulations require DDR1 tyrosine kinase activity and whether these pathways are involved in DDR1-induced colonization. DDR1 is involved in gastric liver metastasis colonization, and intrasplenic injection of DDR1-inactivated gastric tumor cells shows no difference in micrometastasis formation but an inhibition of metastasis colonization [119]. In contrast, DDR1b expression in fibrosarcoma cells completely inhibits the formation of lung macrometastases after intravenous tail injection of these cells, but the step in which the metastasis process is altered remains unknown [23].
DDR2 has also been found to play a role in metastasis formation; however, again, it is not clear which step(s) of the metastatic process is (are) involved. In addition, DDR2 expression in tumor cells and in the metastatic niche may influence tumor metastatic colonization. In lung metastases of breast tumor cells, DDR2 expression by tumor cells is important for metastasis development, but its expression in the metastatic niche is dispensable [117]. In contrast, melanoma cells require DDR2 expression in the lung metastatic niche and in tumor cells to develop metastatic colonization [62]. Intrasplenic colon carcinoma cells injected into DDR2−/− mice develop a liver niche favorable for micrometastasis development, as well as for tumor colonization. DDR2−/− HSCs exposed to colon cancer cell supernatants overexpress genes (IL-10, TGFβ, and VEGF-A) involved in immune suppression, angiogenesis, and cancer cell growth. Therefore, the number of proliferating tumor cells is increased in liver metastases if DDR2 is not expressed in the liver [127].
2.6. DDR Association and Crosstalk with Other Membrane Receptors
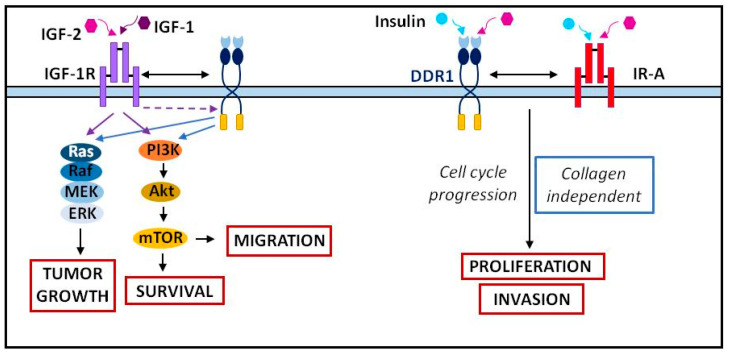
The association or crosstalk of DDR1 with other transmembrane proteins results in distinct outcomes. Through a collagen- and tyrosine kinase-independent mechanism, DDR1 binding to E-cadherin increases the strength of cell–cell interactions. In breast cancer, some studies showed crosstalk between DDR1 and the insulin and IGF receptors, IR-A and IGF-1R (Insulin like Growth Factor 1 Receptor) (Figure 5). IGF-1 and IGF-2 stimulation lead to the upregulation of DDR1 by activating the PI3K (Phosphoinositide 3-kinase)/AKT pathway, thereby inhibiting miR-199a-5p expression. In the absence of collagen and in the presence of IGFs, DDR1 enhances the IR-A and IGF-1R pathways, thereby increasing tumor cell migration and survival through the PI3K/AKT pathway and tumor growth through the Ras/Raf/MEK/ERK pathway [132]. In addition, insulin or IGF-1 and IGF-2 induce an association between IGF-1R or IR and DDR1. DDR1 can also modulate IR expression by stabilization of both the mRNA and the protein [133]. In thyroid cancer, crosstalk between DDR1 and IGF-2/IR-A is responsible for tumor cell proliferation and invasion in 2D and 3D assays, and it is involved in the maintenance of tumor cell stemness [134]. In bladder cancer, migration and anchorage-independent growth are dependent on DDR1/IGF-1R or DDR1/IR-A crosstalk [135]. In A549 lung cancer cells, as collagen I does, the binding of IGF-1 to its receptor IGF-1R mediates DDR1-induced cell migration [136].
Figure 5.
Crosstalk between DDR1 and the IGFs/insulin receptors. Adapted from [132,138].
Lastly, DDR1 can associate with TM4SF1 to induce metastatic tumor colonization as described above [26]. No crosstalk between DDR2 and other membrane receptors was found; however, in MCF-7 and NIH-3T3 cells, DDR2 is phosphorylated on tyrosine residues after IGF-2 or insulin stimulation, suggesting possible crosstalk between IGF-1R or/and IR and DDR2 [137].
3. Cancer Treatment
DDRs are upregulated in many cancers and appear to be promising biomarkers; thus, their inhibition an attractive strategy. DDR1 and DDR2 can be targeted by tyrosine kinase inhibitors such as dasatinib, nilotinib, and imatinib [139]. These inhibitors have been shown to be effective on multiple occasions. Dasatinib inhibited DDR1 in lung [54] and gastric cancers [140] and DDR2 in studies of head and neck cancers [141]. Similarly, nilotinib inhibited DDR1 in breast and colon cancer studies [60,61]. Although these compounds are effective in inhibiting DDRs, they have broad specificities and, thus, also inhibit other TKRs. Sitravatinib (MGCD-156) is a multitarget TKR inhibitor that can inhibit DDR2 [142].
Table 5 summarizes the imatinib, dasatinib, nilotinib, and sitravatinib specificities against different TKRs and their use in clinical trials targeting DDR1 or/and DDR2.
Table 5.
Nonspecific DDR inhibitors: specificities and clinical trials (adapted from ClinicalTrials.gov).
| Inhibitor | Targeted Kinases | In Vitro Assay (Cancer) | Preclinical (Cancer) |
Clinical (DDR Targeted) |
|---|---|---|---|---|
| Imatinib | BCR-ABL, DDR1, DDR2 | |||
| Nilotinib | ABL1, Kit, PDGFRA, PDGFRB, CSF1R, DDR1, DDR2 | DDR1: -Breast (decreased apoptosis and DDR1 phosphorylation) -Colon (increased proliferation, decreased DDR1 phosphorylation). |
-NCT02029001: Malignant solid neoplasms. Mutated DDR1, mutated DDR2. Phase 2 (recruiting). |
|
| Dasatinib | BCR-ABL, SRC kinase family, DDR1, DDR2 | DDR1: -Lung (decreased proliferation). -Gastric (decreased proliferation, migration, invasion). DDR2: -Head and neck (decreased proliferation, migration, invasion) |
DDR2: -Head and neck (zebrafish): decreased migration |
-NCT01491633: Squamous cell lung cancer. Mutated DDR2. Phase 2 (not evaluable, toxicity). -NCT04439305: lymphoma, myeloma, solid neoplasm. Mutated DDR2. Phase 2: withdrawn. -NCT01514864: NSCLC. Mutated DDR2. Phase 2: not completed, disease progression. |
| Sitravatinib (MGCD156) |
MET, AXL, VEGFR1, VEGFR2, VEGFR3, PDGFRA, PDGFRB, TRKA, TRKB, DDR2 | -NCT02219711: NSCLC, renal cell carcinoma. Mutated DDR2. Phase 1 (recruiting). |
In recent years, several DDR1-specific inhibitors have been developed. In 2013, the pyrazolo pyrimidin benzamide 7rh and 7rj selective DDR1 inhibitors were discovered. They can inhibit the enzyme activity with IC50 (Half-maximal inhibitory concentration) values of 6.8 and 7.0 nM, respectively [57]. In vitro and in vivo studies have shown 7rh effectiveness for pancreatic [143], gastric [72], and lung [144] cancers. KST9046, with a quinazoline urea scaffold, and 8v, a 3′-(imidazo[1,2-a]pyrazin-3-yl)-[1,1′-biphenyl]-3-carboxamide compound, were designed and optimized to inhibit DDR1 [145,146]. DDR1-IN-1 and DDR1-IN-2, two derivatives of the DDR1-interacting structure of imatinib, are capable of inhibiting DDR1 phosphorylation with EC50 (Half maximal effective concentration) values of 86 nM and 9 nM, respectively [147]. DDR1-IN-1 inhibits proliferation, migration, and invasion, and it increases apoptosis of prostate cancer cells [101]. Recently, a selective DDR1/DDR2 inhibitor called 2.45 was identified using a parallel DNA encoded library. The 2.45 compound preserves renal function and reduces tissue damage in mice with Alport syndrome, a syndrome in which DDR1 is involved [148]. DDR1 inhibition may be a promising solution in some cancers; however, in pancreatic cancer, long-term inhibition of DDR1 appears to lead to organ atrophy, as DDR1 is crucial for tissue homeostasis [55]. Furthermore, in some cancers such as renal cancer, DDR1 is downregulated in cancerous tissues and, therefore, its inhibition does not seem appropriate in this case [41].
Fewer compounds are known that can specifically and selectively inhibit DDR2. Actinomycin D, the potent mammalian transcription inhibitor, can impair the interaction between DDR2 and collagen, preventing DDR2 activation [149]. Compound 1 can inhibit DDR2 phosphorylation but without affecting NSCLC proliferation [150]. WRG-28 inhibits the interaction between DDR2 and collagen through allosteric modulation. WRG-28 impedes breast cancer cell migration and invasion in vitro and inhibits lung metastasis formation after injection of tumor cells in the mouse tail vein [117]. Other ways to inhibit DDR2 activity include the use of an anti-DDR2 antibody or a recombinant soluble DDR2 form, which prevents collagen binding to DDR2 [79].
Today, the difficulty with patient management lies in the tumor heterogeneity that does not allow a universal treatment, as well as in the lack of effective treatment, which leads to the appearance of resistance and cancer relapse. Some researchers have highlighted the role of DDR1 in treatment resistance in lung [144], breast [151], pancreas [143], glioma [152], ovarian [153], and head and neck cancers [154]. For example, in glioblastoma, DDR1 drives chemoresistance by collaborating with the 14-3-3/BECN1 (Beclin 1)/AKT1 multiprotein complex to enhance cell survival, anti-autophagy, and resistance by AKT/mTOR signaling [152]. However, DDR1 can improve the chemosensitivity of prostate cancer cells, with DDR1b increasing the sensitivity of cells to doxorubicin and paclitaxel [155].
DDR2 is also involved in treatment resistance. DDR2 has been identified as a critical target for improved response to anti-PD1 (Programmed death-ligand 1) immunotherapy. The combination of anti-PD1 therapy and DDR2 inhibition with dasatinib shows a reduction in tumor burden and results in an increase in CD8+ T-cell counts in bladder, breast, colon, and sarcoma cancers. Melanoma metastasis in mouse lungs is largely inhibited if mice receive both treatments in combination [156]. Despite the demonstrated efficacy in inhibiting DDR2, acquired resistance to dasatinib can occur through acquisition of the DDR2 gatekeeper mutation T654I in NSCLC [157]. On the other hand, other mutations such as L595P lead to increased sensitivity to dasatinib in squamous cell lung cancer [70]. No specific DDR inhibitors are in clinical trials. Table 6 summarizes the specificities of the different DDR inhibitors and their use in preclinical assays.
Table 6.
Specific DDR inhibitors and some examples of their used in preclinical assays. Specificity describes the strength of binding between a receptor and an inhibitor and it is measured by the dissociation constant (KD).
| Inhibitor | Specificity | In Vitro Assay (Cancer) | Preclinical (Cancer) |
|---|---|---|---|
| 7rh | DDR1 > DDR2 | -Pancreatic (decreased DDR1 phosphorylation, signaling, colony formation, migration) -Gastric (decreased proliferation) -Lung (increased apoptosis) |
-Pancreatic (mice): decreased tumor development and proliferation, increased apoptosis and survival (in association with chemotherapy). -Gastric (mice): decreased peritoneal tumor nodules. -Lung (mice): In association with chemotherapy, decreased tumor development and increased apoptosis |
| 7rj | DDR1 > DDR2 | ||
| KST9046 | DDR1 > ARAF, FLT3, LIMK1, LMK2 | -Decreased proliferation (leukemia, lung, colon, brain, melanoma, renal, ovarian, prostate and breast) and DDR1 phosphorylation | |
| 8v | DDR1 > DDR2, BCL-ABL, c-KIT | -Lung: decreased colony formation, proliferation, migration, invasion and DDR1 phosphorylation | |
| DDR1-IN1 | DDR1 > DDR2 | -Decreased DDR1 phosphorylation, cell proliferation (colon, breast, lung, uterus, liver). -Prostate: decreased proliferation, cell viability, colony formation, EMT and migration. Increased apoptosis. |
|
| DDR1-IN2 | DDR1 > DDR2 | Decreased DDR1 phosphorylation, cell proliferation (colon, breast, lung, uterus, liver) | |
| 2.45 | DDR1 > DDR2 | Decreased DDR1 phosphorylation | |
| Compound 1 | DDR2 | Decreased DDR2 phosphorylation and lung cell proliferation. | |
| WRG-28 | DDR2 | Breast: decreased migration and invasion. | Decreased breast metastasis to lung |
4. Discussion
DDRs are involved in different stages of tumor development and metastasis. No evidence has been observed for their involvement in tumor initiation, but DDR1 and DDR2 play a role in tumor cell proliferation (Figure 1c) and more particularly in TME remodeling (Figure 2a). Both receptors are important for MMP expression, collagen fiber alignment, and immune cell migration and activation, whilst DDR2 is important for CAF activity. They are also associated with tumor angiogenesis and lymphangiogenesis, both processes enabling tumor cell intravasation (Figure 2b). In addition, DDRs are also important in tumor cell migration and invasion. DDR1 is involved in collective migration and, certainly, in individual migration via EMT induction (Figure 2a). After intravasation, no evidence for the involvement of DDRs in the survival of circulating tumor cells was found (Figure 3a). Nevertheless, some collagens and other noncanonical ligands (e.g., C1q) are found in the serum of cancer patients. Furthermore, DDR1 increases cell–cell adhesion; hence, we can hypothesize a role of DDR1 in the adhesion of tumor cells to protective cells such as platelets, CAFs, or immune cells. No direct evidence for the involvement of DDRs in extravasation has been found, although there are clues for roles of DDR1 and DDR2 in extravasation in bone and liver, respectively. The metastatic niche plays a crucial role in the formation of micrometastases (Figure 3c). The role of DDRs in the metastatic niche depends on the metastatic tissue and on the DDR isoform. DDR1, but not DDR2, is important for the formation of the liver niche, whereas DDR2 is necessary for the formation of the lung niche. Only DDR1, in association with TM4SF1, is essential for the development of micrometastases in macrometastases (Figure 3c).
In almost all stages of metastasis, apparently contradictory results have been obtained. Different hypotheses may explain the contrasted effects of DDR1 and DDR2 manipulation on proliferation, EMT, MMP induction, migration, invasion, and metastasis. First, DDR1 activity may depend on the type of cell and of the presence or absence of an interacting protein (such as DARPP-32) [118]. Second, DDR1 and DDR2 activity may be assay-dependent. DDRs induce cell proliferation in a 2D assay; however, in a 3D assay, DDRs inhibit cell proliferation [60]. Third, the activity of DDR1 activities may be isoform-dependent. As mentioned above, DDR1 has five different isoforms, the most commonly studied being DDR1a and DDR1b, although many studies do not mention or distinguish them. In pancreatic cancer, DDR1b is able to upregulate N-cadherin in a collagen-dependent manner. This regulation is due to the interaction between the phosphotyrosine domain of the phosphatase Shc1 (SHC Adaptor Protein 1) and Tyr513, absent in DDR1a or DDR1b [158]. On the other hand, in glioma, DDR1a is able to induce invasion, adhesion, and MMP2 activation [159] and, in NSCLC, DDR1a has a stronger effect in cell migration and invasion than DDR1b [160]. A switch between DDR1 and DDR2 during tumor progression has been demonstrated, but it is not impossible that a switch between the different DDR1 isoforms during tumor progression may exist. These important aspects remain to be elucidated, and this will be important for a better adaptation of treatments in different cancers.
Most of the late metastasis cascade (i.e., blood survival, extravasation, micrometastasis, and macrometastasis) has not been studied individually but as a whole after injection of tumor cells into the bloodstream. It will be interesting to clarify in which step(s) DDRs are involved.
There are not many studies that focused on the analysis of the impact of DDR1 and DDR2 within the same cancer, although, very often, both receptors are expressed. Further studies analyzing the relationship between the two receptors in tumor development are needed.
DDR inhibitors used in cancer treatment, such as dasatinib or nilotinib, are not specific DDR inhibitors. Further in vivo analysis of cancer development during treatment using specific DDR1 or DDR2 inhibitors, such as 7rh, 2.45, or WRG-28, in different cancers are needed.
5. Conclusions
DDRs are receptors for ECM collagens. They are expressed at the extracellular membrane of tumor cells and of many cells of the TME such as CAFs and immune cells. In many cancers, DDR1 or DDR2 are overexpressed, suggesting a role for DDRs in tumor development and metastasis. They are involved in the proliferation and migration of tumor cells and in the formation of micro- and macrometastases. However, the impact of DDRs in tumor development or metastasis seems to be cancer-dependent and may be explained by the DDR1 isoform expressed or by interaction with other receptors. Further experiments are needed to clarify the activities of DDRs in different cancers. These studies will be facilitated by the development of specific DDR inhibitors that are being characterized in vitro and in vivo.
Acknowledgments
We are grateful to Malak Alannan and Elisabeth Genot for reading the manuscript. We apologize to the authors whose papers were not cited because of space limitations. Figures were generated with images from SMART-Servier medical art (https://smart.servier.com/).
Author Contributions
S.M. and P.A. wrote, reviewed, and edited the manuscript. Both authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from Inserm and University of Bordeaux and from “La Ligue Contre Le Cancer, Region Aquitaine et Charentes” to Patrick Auguste. Sandra Majo was supported by funds from “Region Nouvelle Aquitaine” (2018-1R30223) and by “Fondation Groupama pour la Sante, Groupama Centre Atlantique”. This study was supported by donations from the charity associations Aidons Marina, E.S.CA.P.E., Eva pour la Vie, Les Récoltes de l’Espoir, and Sphères to the MIRCADE team.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
No conflicts of interest, financial or otherwise, are declared by the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Auguste P., Lemiere S., Larrieu-Lahargue F., Bikfalvi A. Molecular Mechanisms of Tumor Vascularization. Crit. Rev. Oncol. Hematol. 2005;54:53–61. doi: 10.1016/j.critrevonc.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Fidler I.J. Timeline—The Pathogenesis of Cancer Metastasis: The “seed and Soil” Hypothesis Revisited. Nat. Rev. Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 3.Valastyan S., Weinberg R.A. Tumor Metastasis: Molecular Insights and Evolving Paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinbichler T.B., Savic D., Dudás J., Kvitsaridze I., Skvortsov S., Riechelmann H., Skvortsova I.-I. Cancer Stem Cells and Their Unique Role in Metastatic Spread. Semin. Cancer Biol. 2020;60:148–156. doi: 10.1016/j.semcancer.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Niland S., Eble J.A. Hold on or Cut? Integrin- and MMP-Mediated Cell-Matrix Interactions in the Tumor Microenvironment. Int. J. Mol. Sci. 2021;22:238. doi: 10.3390/ijms22010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ricard-Blum S. The Collagen Family. Cold Spring Harbor Perspect. Biol. 2011;3:a004978. doi: 10.1101/cshperspect.a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu S., Xu H., Wang W., Li S., Li H., Li T., Zhang W., Yu X., Liu L. The Role of Collagen in Cancer: From Bench to Bedside. J. Transl. Med. 2019;17:309. doi: 10.1186/s12967-019-2058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Reilly M.S., Boehm T., Shing Y., Fukai N., Vasios G., Lane W.S., Flynn E., Birkhead J.R., Olsen B.R., Folkman J. Endostatin: An Endogenous Inhibitor of Angiogenesis and Tumor Growth. Cell. 1997;88:277–285. doi: 10.1016/S0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 9.Ilina O., Gritsenko P.G., Syga S., Lippoldt J., La Porta C.A.M., Chepizhko O., Grosser S., Vullings M., Bakker G.-J., Starruß J., et al. Cell–Cell Adhesion and 3D Matrix Confinement Determine Jamming Transitions in Breast Cancer Invasion. Nat. Cell Biol. 2020;22:1103–1115. doi: 10.1038/s41556-020-0552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weigelin B., Bakker G.-J., Friedl P. Intravital Third Harmonic Generation Microscopy of Collective Melanoma Cell Invasion: Principles of Interface Guidance and Microvesicle Dynamics. IntraVital. 2012;1:32–43. doi: 10.4161/intv.21223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorusso G., Rueegg C., Kuonen F. Targeting the Extra-Cellular Matrix-Tumor Cell Crosstalk for Anti-Cancer Therapy: Emerging Alternatives to Integrin Inhibitors. Front. Oncol. 2020;10:1231. doi: 10.3389/fonc.2020.01231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rammal H., Saby C., Magnien K., Van-Gulick L., Garnotel R., Buache E., El Btaouri H., Jeannesson P., Morjani H. Corrigendum: Discoidin Domain Receptors: Potential Actors and Targets in Cancer. Front. Pharmacol. 2016;7:346. doi: 10.3389/fphar.2016.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu H.-L., Valiathan R.R., Arkwright R., Sohail A., Mihai C., Kumarasiri M., Mahasenan K.V., Mobashery S., Huang P., Agarwal G., et al. Discoidin Domain Receptors: Unique Receptor Tyrosine Kinases in Collagen-Mediated Signaling. J. Biol. Chem. 2013;288:7430–7437. doi: 10.1074/jbc.R112.444158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han T., Mignatti P., Abramson S.B., Attur M. Periostin Interaction with Discoidin Domain Receptor-1 (DDR1) Promotes Cartilage Degeneration. PLoS ONE. 2020;15:e0231501. doi: 10.1371/journal.pone.0231501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corcoran D.S., Juskaite V., Xu Y., Gorlitz F., Alexandrov Y., Dunsby C., French P.M.W., Leitinger B. DDR1 Autophosphorylation Is a Result of Aggregation into Dense Clusters. Sci. Rep. 2019;9:17104. doi: 10.1038/s41598-019-53176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Juskaite V., Corcoran D.S., Leitinger B. Collagen Induces Activation of DDR1 through Lateral Dimer Association and Phosphorylation between Dimers. eLife. 2017;6:e25716. doi: 10.7554/eLife.25716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leitinger B. Discoidin Domain Receptor Functions in Physiological and Pathological Conditions. In: Jeon K.W., editor. International Review of Cell and Molecular Biology. Volume 310. Elsevier Academic Press Inc.; San Diego, CA, USA: 2014. pp. 39–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeung D.A., Shanker N., Sohail A., Weiss B.A., Wang C., Wellmerling J., Das S., Ganju R.K., Miller J.L.C., Herr A.B., et al. Clustering, Spatial Distribution, and Phosphorylation of Discoidin Domain Receptors 1 and 2 in Response to Soluble Collagen I. J. Mol. Biol. 2019;431:368–390. doi: 10.1016/j.jmb.2018.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agarwal G., Smith A.W., Jones B. Discoidin Domain Receptors: Micro Insights into Macro Assemblies. Biochim. Biophys. Acta-Mol. Cell Res. 2019;1866:118496. doi: 10.1016/j.bbamcr.2019.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang K., Kim J.H., Kim H.J., Park I.S., Kim I.Y., Yang B.S. Tyrosine 740 Phosphorylation of Discoidin Domain Receptor 2 by Src Stimulates Intramolecular Autophosphorylation and Shc Signaling Complex Formation. J. Biol. Chem. 2005;280:39058–39066. doi: 10.1074/jbc.M506921200. [DOI] [PubMed] [Google Scholar]
- 21.Croissant C., Tuariihionoa A., Bacou M., Souleyreau W., Sala M., Henriet E., Bikfalvi A., Saltel F., Auguste P. DDR1 and DDR2 Physical Interaction Leads to Signaling Interconnection but with Possible Distinct Functions. Cell Adhes. Migr. 2018;12:324–334. doi: 10.1080/19336918.2018.1460012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hilton H.N., Stanford P.M., Harris J., Oakes S.R., Kaplan W., Daly R.J., Ormandy C.J. KIBRA Interacts with Discoidin Domain Receptor 1 to Modulate Collagen-Induced Signalling. Biochim. Biophys. Acta Mol. Cell Res. 2008;1783:383–393. doi: 10.1016/j.bbamcr.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Wasinski B., Sohail A., Bonfil R.D., Kim S., Saliganan A., Polin L., Bouhamdan M., Kim H.-R.C., Prunotto M., Fridman R. Discoidin Domain Receptors, DDR1b and DDR2, Promote Tumour Growth within Collagen but DDR1b Suppresses Experimental Lung Metastasis in HT1080 Xenografts. Sci. Rep. 2020;10:2309. doi: 10.1038/s41598-020-59028-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juin A., Di Martino J., Leitinger B., Henriet E., Gary A.-S., Paysan L., Bomo J., Baffet G., Gauthier-Rouvière C., Rosenbaum J., et al. Discoidin Domain Receptor 1 Controls Linear Invadosome Formation via a Cdc42-Tuba Pathway. J. Cell Biol. 2014;207:517–533. doi: 10.1083/jcb.201404079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hidalgo-Carcedo C., Hooper S., Chaudhry S.I., Williamson P., Harrington K., Leitinger B., Sahai E. Collective Cell Migration Requires Suppression of Actomyosin at Cell–Cell Contacts Mediated by DDR1 and the Cell Polarity Regulators Par3 and Par6. Nat. Cell Biol. 2011;13:49–59. doi: 10.1038/ncb2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao H., Chakraborty G., Zhang Z., Akalay I., Gadiya M., Gao Y., Sinha S., Hu J., Jiang C., Akram M., et al. Multi-Organ Site Metastatic Reactivation Mediated by Non-Canonical Discoidin Domain Receptor 1 Signaling. Cell. 2016;166:47–62. doi: 10.1016/j.cell.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu E.-H., Li H.-S., Zhao T., Fan J.-D., Ma X., Xiong L., Li W.-J., Zhu L.-L., Fan M. Effect of Hypoxia on the Gene Profile of Human Bone Marrow-Derived Mesenchymal Stem Cells. Sheng Li Xue Bao Acta Physiol. Sin. 2007;59:227–232. [PubMed] [Google Scholar]
- 28.Chen S.-C., Wang B.-W., Wang D.L., Shyu K.-G. Hypoxia Induces Discoidin Domain Receptor-2 Expression via the P38 Pathway in Vascular Smooth Muscle Cells to Increase Their Migration. Biochem. Biophys. Res. Commun. 2008;374:662–667. doi: 10.1016/j.bbrc.2008.07.092. [DOI] [PubMed] [Google Scholar]
- 29.Li S., Zhang Z., Xue J., Guo X., Liang S., Liu A. Effect of Hypoxia on DDR1 Expression in Pituitary Adenomas. Med. Sci. Monit. 2015;21:2433–2438. doi: 10.12659/MSM.894205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren T., Zhang W., Liu X., Zhao H., Zhang J., Zhang J., Li X., Zhang Y., Bu X., Shi M., et al. Discoidin Domain Receptor 2 (DDR2) Promotes Breast Cancer Cell Metastasis and the Mechanism Implicates Epithelial-Mesenchymal Transition Programme under Hypoxia. J. Pathol. 2014;234:526–537. doi: 10.1002/path.4415. [DOI] [PubMed] [Google Scholar]
- 31.Koh M., Woo Y., Valiathan R.R., Jung H.Y., Park S.Y., Kim Y.N., Kim H.-R.C., Fridman R., Moon A. Discoidin Domain Receptor 1 Is a Novel Transcriptional Target of ZEB1 in Breast Epithelial Cells Undergoing H-Ras-Induced Epithelial to Mesenchymal Transition. Int. J. Cancer. 2015;136:E508–E520. doi: 10.1002/ijc.29154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grither W.R., Divine L.M., Meller E.H., Wilke D.J., Desai R.A., Loza A.J., Zhao P., Lohrey A., Longmore G.D., Fuh K.C. TWIST1 Induces Expression of Discoidin Domain Receptor 2 to Promote Ovarian Cancer Metastasis. Oncogene. 2018;37:1714–1729. doi: 10.1038/s41388-017-0043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Z., Sun X., Bao Y., Mo J., Du H., Hu J., Zhang X. E2F1 Silencing Inhibits Migration and Invasion of Osteosarcoma Cells via Regulating DDR1 Expression. Int. J. Oncol. 2017;51:1639–1650. doi: 10.3892/ijo.2017.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ongusaha P.P., Kim J., Fang L., Wong T.W., Yancopoulos G.D., Aaronson S.A., Lee S.W. P53 Induction and Activation of DDR1 Kinase Counteract P53-Mediated Apoptosis and Influence P53 Regulation through a Positive Feedback Loop. EMBO J. 2003;22:1289–1301. doi: 10.1093/emboj/cdg129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin Y., Jin H., Wu X., Jian Z., Zou X., Huang J., Guan R., Wei X. The Cross-Talk between DDR1 and STAT3 Promotes the Development of Hepatocellular Carcinoma. Aging. 2020;12:14391–14405. doi: 10.18632/aging.103482. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Lin K.-L., Chou C.-H., Hsieh S.-C., Hwa S.-Y., Lee M.-T., Wang F.-F. Transcriptional Upregulation of DDR2 by ATF4 Facilitates Osteoblastic Differentiation through P38 MAPK-Mediated Runx2 Activation. J. Bone Miner. Res. 2010;25:2489–2503. doi: 10.1002/jbmr.159. [DOI] [PubMed] [Google Scholar]
- 37.George M., Vijayakumar A., Dhanesh S.B., James J., Shivakumar K. Molecular Basis and Functional Significance of Angiotensin II-Induced Increase in Discoidin Domain Receptor 2 Gene Expression in Cardiac Fibroblasts. J. Mol. Cell. Cardiol. 2016;90:59–69. doi: 10.1016/j.yjmcc.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Lim J., Ehsanipour A., Hsu J.J., Lu J., Pedego T., Wu A., Walthers C.M., Demer L.L., Seidlits S.K., Tintut Y. Inflammation Drives Retraction, Stiffening, and Nodule Formation via Cytoskeletal Machinery in a Three-Dimensional Culture Model of Aortic Stenosis. Am. J. Pathol. 2016;186:2378–2389. doi: 10.1016/j.ajpath.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ezzoukhry Z., Henriet E., Piquet L., Boyé K., Bioulac-Sage P., Balabaud C., Couchy G., Zucman-Rossi J., Moreau V., Saltel F. TGF-Β1 Promotes Linear Invadosome Formation in Hepatocellular Carcinoma Cells, through DDR1 up-Regulation and Collagen I Cross-Linking. Eur. J. Cell Biol. 2016;95:503–512. doi: 10.1016/j.ejcb.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Ruiz P.A., Jarai G. Collagen I Induces Discoidin Domain Receptor (DDR) 1 Expression through DDR2 and a JAK2-ERK1/2-Mediated Mechanism in Primary Human Lung Fibroblasts. J. Biol. Chem. 2011;286:12912–12923. doi: 10.1074/jbc.M110.143693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krazinski B.E., Kiewisz J., Sliwinska-Jewsiewicka A., Kowalczyk A.E., Grzegrzolka J., Godlewski J., Kwiatkowski P., Dziegiel P., Kmiec Z. Altered Expression of DDR1 in Clear Cell Renal Cell Carcinoma Correlates With MiR-199a/b-5p and Patients’ Outcome. Cancer Genom. Proteom. 2019;16:179–193. doi: 10.21873/cgp.20124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chou S.-T., Peng H.-Y., Mo K.-C., Hsu Y.-M., Wu G.-H., Hsiao J.-R., Lin S.-F., Wang H.-D., Shiah S.-G. MicroRNA-486-3p Functions as a Tumor Suppressor in Oral Cancer by Targeting DDR1. J. Exp. Clin. Cancer Res. 2019;38:281. doi: 10.1186/s13046-019-1283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deng L., Liu G., Zheng C., Zhang L., Kang Y., Yang F. Circ-LAMP1 Promotes T-Cell Lymphoblastic Lymphoma Progression via Acting as a CeRNA for MiR-615-5p to Regulate DDR2 Expression. Gene. 2019;701:146–151. doi: 10.1016/j.gene.2019.03.052. [DOI] [PubMed] [Google Scholar]
- 44.Ramasamy R., Ridgeway A., Lipshultz L.I., Lamb D.J. Integrative DNA Methylation and Gene Expression Analysis Identifies Discoidin Domain Receptor 1 Association with Idiopathic Nonobstructive Azoospermia. Fertil. Steril. 2014;102:968–973.e3. doi: 10.1016/j.fertnstert.2014.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chung V.Y., Tan T.Z., Huang R.-L., Lai H.-C., Huang R.Y.-J. Loss of Discoidin Domain Receptor 1 (DDR1) via CpG Methylation during EMT in Epithelial Ovarian Cancer. Gene. 2017;635:9–15. doi: 10.1016/j.gene.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 46.Nelson H.H., Marsit C.J., Christensen B.C., Houseman E.A., Kontic M., Wiemels J.L., Karagas M.R., Wrensch M.R., Zheng S., Wiencke J.K., et al. Key Epigenetic Changes Associated with Lung Cancer Development. Epigenetics. 2012;7:559–566. doi: 10.4161/epi.20219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vogel W.F. Ligand-Induced Shedding of Discoidin Domain Receptor 1. FEBS Lett. 2002;514:175–180. doi: 10.1016/S0014-5793(02)02360-8. [DOI] [PubMed] [Google Scholar]
- 48.Shitomi Y., Thogersen I.B., Ito N., Leitinger B., Enghild J.J., Itoh Y. ADAM10 Controls Collagen Signaling and Cell Migration on Collagen by Shedding the Ectodomain of Discoidin Domain Receptor 1 (DDR1) Mol. Biol. Cell. 2015;26:659–673. doi: 10.1091/mbc.E14-10-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ambrogio C., Gómez-López G., Falcone M., Vidal A., Nadal E., Crosetto N., Blasco R.B., Fernández-Marcos P.J., Sánchez-Céspedes M., Ren X., et al. Combined Inhibition of DDR1 and Notch Signaling Is a Therapeutic Strategy for KRAS-Driven Lung Adenocarcinoma. Nat. Med. 2016;22:270–277. doi: 10.1038/nm.4041. [DOI] [PubMed] [Google Scholar]
- 50.Hur H., Ham I.-H., Lee D., Jin H., Aguilera K.Y., Oh H.J., Han S.-U., Kwon J.E., Kim Y.-B., Ding K., et al. Discoidin Domain Receptor 1 Activity Drives an Aggressive Phenotype in Gastric Carcinoma. BMC Cancer. 2017;17:87. doi: 10.1186/s12885-017-3051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Azemikhah M., Ashtiani H.A., Aghaei M., Rastegar H. Evaluation of Discoidin Domain Receptor-2 (DDR2) Expression Level in Normal, Benign, and Malignant Human Prostate Tissues. Res. Pharm. Sci. 2015;10:356–363. [PMC free article] [PubMed] [Google Scholar]
- 52.Cader F.Z., Vockerodt M., Bose S., Nagy E., Brundler M.-A., Kearns P., Murray P.G. The EBV Oncogene LMP1 Protects Lymphoma Cells from Cell Death through the Collagen-Mediated Activation of DDR. Blood. 2013;122:10. doi: 10.1182/blood-2013-04-499004. [DOI] [PubMed] [Google Scholar]
- 53.Shimada K., Nakamura M., Ishida E., Higuchi T., Yamamoto H., Tsujikawa K., Konishi N. Prostate Cancer Antigen-1 Contributes to Cell Survival and Invasion Though Discoidin Receptor 1 in Human Prostate Cancer. Cancer Sci. 2008;99:39–45. doi: 10.1111/j.1349-7006.2007.00655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Villalba M., Redin E., Exposito F., Pajares M.J., Sainz C., Hervas D., Guruceaga E., Diaz-Lagares A., Cirauqui C., Redrado M., et al. Identification of a Novel Synthetic Lethal Vulnerability in Non-Small Cell Lung Cancer by Co-Targeting TMPRSS4 and DDR1. Sci. Rep. 2019;9:15400. doi: 10.1038/s41598-019-51066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruggeri J.M., Franco-Barraza J., Sohail A., Zhang Y., Long D., Pasca di Magliano M., Cukierman E., Fridman R., Crawford H.C. Discoidin Domain Receptor 1 (DDR1) Is Necessary for Tissue Homeostasis in Pancreatic Injury and Pathogenesis of Pancreatic Ductal Adenocarcinoma. Am. J. Pathol. 2020;190:1735–1751. doi: 10.1016/j.ajpath.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rudra-Ganguly N., Lowe C., Mattie M., Chang M.S., Satpayev D., Verlinsky A., An Z., Hu L., Yang P., Challita-Eid P., et al. Discoidin Domain Receptor 1 Contributes to Tumorigenesis through Modulation of TGFBI Expression. PLoS ONE. 2014;9:e111515. doi: 10.1371/journal.pone.0111515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao M., Duan L., Luo J., Zhang L., Lu X., Zhang Y., Zhang Z., Tu Z., Xu Y., Ren X., et al. Discovery and Optimization of 3-(2-(Pyrazolo[1,5- a ]Pyrimidin-6-Yl)Ethynyl)Benzamides as Novel Selective and Orally Bioavailable Discoidin Domain Receptor 1 (DDR1) Inhibitors. J. Med. Chem. 2013;56:3281–3295. doi: 10.1021/jm301824k. [DOI] [PubMed] [Google Scholar]
- 58.Wu A., Chen Y., Liu Y., Lai Y., Liu D. MiR-199b-5p Inhibits Triple Negative Breast Cancer Cell Proliferation, Migration and Invasion by Targeting DDR1. Oncol. Lett. 2018;16:4889–4896. doi: 10.3892/ol.2018.9255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Assent D., Bourgot I., Hennuy B., Geurts P., Noel A., Foidart J.-M., Maquoi E. A Membrane-Type-1 Matrix Metalloproteinase (MT1-MMP)—Discoidin Domain Receptor 1 Axis Regulates Collagen-Induced Apoptosis in Breast Cancer Cells. PLoS ONE. 2015;10:e0116006. doi: 10.1371/journal.pone.0116006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saby C., Collin G., Sinane M., Buache E., Van Gulick L., Saltel F., Maquoi E., Morjani H. DDR1 and MT1-MMP Expression Levels Are Determinant for Triggering BIK-Mediated Apoptosis by 3D Type I Collagen Matrix in Invasive Basal-Like Breast Carcinoma Cells. Front. Pharmacol. 2019;10:462. doi: 10.3389/fphar.2019.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Appert-Collin A. LRP-1 Promotes Colon Cancer Cell Proliferation in 3D Collagen Matrices by Mediating DDR1 Endocytosis. Front. Cell Dev. Biol. 2020;8:15. doi: 10.3389/fcell.2020.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Badiola I., Villacé P., Basaldua I., Olaso E. Downregulation of Discoidin Domain Receptor 2 in A375 Human Melanoma Cells Reduces Its Experimental Liver Metastasis Ability. Oncol. Rep. 2011;26:971–978. doi: 10.3892/or.2011.1356. [DOI] [PubMed] [Google Scholar]
- 63.Sun M., Shen Z. Knockdown of Long Non-Coding RNA (LncRNA) Colon Cancer-Associated Transcript-1 (CCAT1) Suppresses Oral Squamous Cell Carcinoma Proliferation, Invasion, and Migration by Inhibiting the Discoidin Domain Receptor 2 (DDR2)/ERK/AKT Axis. Med. Sci. Monit. 2020;26:e920020. doi: 10.12659/MSM.920020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kurashige J., Hasegawa T., Niida A., Sugimachi K., Deng N., Mima K., Uchi R., Sawada G., Takahashi Y., Eguchi H., et al. Integrated Molecular Profiling of Human Gastric Cancer Identifies DDR2 as a Potential Regulator of Peritoneal Dissemination. Sci. Rep. 2016;6:22371. doi: 10.1038/srep22371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park J.-W., Lee Y.-S., Kim J.S., Lee S.-K., Kim B.H., Lee J.A., Lee N.O., Kim S.H., Hong E.K. Downregulation of Discoidin Domain Receptor 2 Decreases Tumor Growth of Hepatocellular Carcinoma. J. Cancer Res. Clin. Oncol. 2015;141:1973–1983. doi: 10.1007/s00432-015-1967-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim D., You E., Jeong J., Ko P., Kim J.-W., Rhee S. DDR2 Controls the Epithelial-Mesenchymal-Transition-Related Gene Expression via c-Myb Acetylation upon Matrix Stiffening. Sci. Rep. 2017;7:6847. doi: 10.1038/s41598-017-07126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Y.-G., Xu L., Jia R.-R., Wu Q., Wang T., Wei J., Ma J.-L., Shi M., Li Z.-S. DDR2 Induces Gastric Cancer Cell Activities via Activating MTORC2 Signaling and Is Associated with Clinicopathological Characteristics of Gastric Cancer. Dig. Dis. Sci. 2016;61:2272–2283. doi: 10.1007/s10620-016-4116-3. [DOI] [PubMed] [Google Scholar]
- 68.Wall S.J., Werner E., Werb Z., DeClerck Y.A. Discoidin Domain Receptor 2 Mediates Tumor Cell Cycle Arrest Induced by Fibrillar Collagen. J. Biol. Chem. 2005;280:40187–40194. doi: 10.1074/jbc.M508226200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Terashima M., Togashi Y., Sato K., Mizuuchi H., Sakai K., Suda K., Nakamura Y., Banno E., Hayashi H., De Velasco M.A., et al. Functional Analyses of Mutations in Receptor Tyrosine Kinase Genes in Non–Small Cell Lung Cancer: Double-Edged Sword of DDR2. Clin. Cancer Res. 2016;22:3663–3671. doi: 10.1158/1078-0432.CCR-15-2093. [DOI] [PubMed] [Google Scholar]
- 70.Lee M.-S., Jung E.A., An S.B., Kim Y.J., Oh D.-Y., Song J.-Y., Um S.-W., Han J., Choi Y.-L. Prevalence of Mutations in Discoidin Domain-Containing Receptor Tyrosine Kinase 2 (DDR2) in Squamous Cell Lung Cancers in Korean Patients. Cancer Res. Treat. 2017;49:1065–1076. doi: 10.4143/crt.2016.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saby C., Rammal H., Magnien K., Buache E., Brassart-Pasco S., Van-Gulick L., Jeannesson P., Maquoi E., Morjani H. Age-Related Modifications of Type I Collagen Impair DDR1-Induced Apoptosis in Non-Invasive Breast Carcinoma Cells. Cell Adhes. Migr. 2018;12:335–347. doi: 10.1080/19336918.2018.1472182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jin H., Ham I.-H., Oh H.J., Bae C.A., Lee D., Kim Y.-B., Son S.-Y., Chwae Y.-J., Han S.-U., Brekken R.A., et al. Inhibition of Discoidin Domain Receptor 1 Prevents Stroma-Induced Peritoneal Metastasis in Gastric Carcinoma. Mol. Cancer Res. 2018;16:1590–1600. doi: 10.1158/1541-7786.MCR-17-0710. [DOI] [PubMed] [Google Scholar]
- 73.Saby C., Buache E., Brassart-Pasco S., El Btaouri H., Courageot M.-P., Van Gulick L., Garnotel R., Jeannesson P., Morjani H. Type I Collagen Aging Impairs Discoidin Domain Receptor 2-Mediated Tumor Cell Growth Suppression. Oncotarget. 2016;7:24908–24927. doi: 10.18632/oncotarget.8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hanahan D. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 75.Azreq M.-A.E., Kadiri M., Boisvert M., Pagé N., Tessier P.A., Aoudjit F. Discoidin Domain Receptor 1 Promotes Th17 Cell Migration by Activating the RhoA/ROCK/MAPK/ERK Signaling Pathway. Oncotarget. 2016;7:44975–44990. doi: 10.18632/oncotarget.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kadiri M., El Azreq M.-A., Berrazouane S., Boisvert M., Aoudjit F. Human Th17 Migration in Three-Dimensional Collagen Involves P38 MAPK: Th17 M IGRATION R EQUIRES P38. J. Cell. Biochem. 2017;118:2819–2827. doi: 10.1002/jcb.25932. [DOI] [PubMed] [Google Scholar]
- 77.Zhong X., Zhang W., Sun T. DDR1 Promotes Breast Tumor Growth by Suppressing Antitumor Immunity. Oncol. Rep. 2019;42:2844–2854. doi: 10.3892/or.2019.7338. [DOI] [PubMed] [Google Scholar]
- 78.Lee J.-E., Kang C.-S., Guan X.-Y., Kim B.-T., Kim S.-H., Lee Y.-M., Moon W.-S., Kim D.-K. Discoidin Domain Receptor 2 Is Involved in the Activation of Bone Marrow-Derived Dendritic Cells Caused by Type I Collagen. Biochem. Biophys. Res. Commun. 2007;352:244–250. doi: 10.1016/j.bbrc.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 79.Afonso P.V., McCann C.P., Kapnick S.M., Parent C.A. Discoidin Domain Receptor 2 Regulates Neutrophil Chemotaxis in 3D Collagen Matrices. Blood. 2013;121:1644–1650. doi: 10.1182/blood-2012-08-451575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sidles S.J., Xiong Y., Young M.R.I., LaRue A.C. High-Fat Diet Alters Immunogenic Properties of Circulating and Adipose Tissue-Associated Myeloid-Derived CD45 + DDR2 + Cells. Mediat. Inflamm. 2019;2019:1–15. doi: 10.1155/2019/1648614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chang J., Chaudhuri O. Beyond Proteases: Basement Membrane Mechanics and Cancer Invasion. J. Cell Biol. 2019;218:2456–2469. doi: 10.1083/jcb.201903066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Valencia K., Ormazabal C., Zandueta C., Luis-Ravelo D., Anton I., Pajares M.J., Agorreta J., Montuenga L.M., Martinez-Canarias S., Leitinger B., et al. Inhibition of Collagen Receptor Discoidin Domain Receptor-1 (DDR1) Reduces Cell Survival, Homing, and Colonization in Lung Cancer Bone Metastasis. Clin. Cancer Res. 2012;18:969–980. doi: 10.1158/1078-0432.CCR-11-1686. [DOI] [PubMed] [Google Scholar]
- 83.Yoshida D., Teramoto A. Enhancement of Pituitary Adenoma Cell Invasion and Adhesion Is Mediated by Discoidin Domain Receptor-1. J. Neurooncol. 2007;82:29–40. doi: 10.1007/s11060-006-9246-6. [DOI] [PubMed] [Google Scholar]
- 84.Hu Y., Liu J., Jiang B., Chen J., Fu Z., Bai F., Jiang J., Tang Z. MiR-199a-5p Loss Up-Regulated DDR1 Aggravated Colorectal Cancer by Activating Epithelial-to-Mesenchymal Transition Related Signaling. Dig. Dis. Sci. 2014;59:2163–2172. doi: 10.1007/s10620-014-3136-0. [DOI] [PubMed] [Google Scholar]
- 85.Song J., Chen X., Bai J., Liu Q., Li H., Xie J., Jing H., Zheng J. Discoidin Domain Receptor 1 (DDR1), a Promising Biomarker, Induces Epithelial to Mesenchymal Transition in Renal Cancer Cells. Tumor Biol. 2016;37:11509–11521. doi: 10.1007/s13277-016-5021-2. [DOI] [PubMed] [Google Scholar]
- 86.Yang J., Zhang Y., He S., Li M., Cai X., Wang H., Xu L., Cao J. TM4SF1 Promotes Metastasis of Pancreatic Cancer via Regulating the Expression of DDR1. Sci. Rep. 2017;7:45895. doi: 10.1038/srep45895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee J.-H., Poudel B., Ki H.-H., Nepali S., Lee Y.-M., Shin J.-S., Kim D.-K. Complement C1q Stimulates the Progression of Hepatocellular Tumor through the Activation of Discoidin Domain Receptor 1. Sci. Rep. 2018;8:4908. doi: 10.1038/s41598-018-23240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bulla R., Tripodo C., Rami D., Ling G.S., Agostinis C., Guarnotta C., Zorzet S., Durigutto P., Botto M., Tedesco F. C1q Acts in the Tumour Microenvironment as a Cancer-Promoting Factor Independently of Complement Activation. Nat. Commun. 2016;7:10346. doi: 10.1038/ncomms10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xie B., Lin W., Ye J., Wang X., Zhang B., Xiong S., Li H., Tan G. DDR2 Facilitates Hepatocellular Carcinoma Invasion and Metastasis via Activating ERK Signaling and Stabilizing SNAIL1. J. Exp. Clin. Cancer Res. 2015;34:101. doi: 10.1186/s13046-015-0218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang K., Corsa C.A., Ponik S.M., Prior J.L., Piwnica-Worms D., Eliceiri K.W., Keely P.J., Longmore G.D. The Collagen Receptor Discoidin Domain Receptor 2 Stabilizes SNAIL1 to Facilitate Breast Cancer Metastasis. Nat. Cell Biol. 2013;15:677–687. doi: 10.1038/ncb2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Majkowska I., Shitomi Y., Ito N., Gray N.S., Itoh Y. Discoidin Domain Receptor 2 Mediates Collagen-Induced Activation of Membrane-Type 1 Matrix Metalloproteinase in Human Fibroblasts. J. Biol. Chem. 2017;292:6633–6643. doi: 10.1074/jbc.M116.770057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Genot E., Gligorijevic B. Invadosomes in Their Natural Habitat. Eur. J. Cell Biol. 2014;93:367–379. doi: 10.1016/j.ejcb.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hammoud A.A., Marais S., Allain N., Ezzoukhry Z., Moreau V., Saltel F. DDR2 Induces Linear Invadosome to Promote Angiogenesis in a Fibrillar Type I Collagen Context. Cell Biol. 2020 doi: 10.1101/2020.04.11.036764. [DOI] [Google Scholar]
- 94.Conklin M.W., Eickhoff J.C., Riching K.M., Pehlke C.A., Eliceiri K.W., Provenzano P.P., Friedl A., Keely P.J. Aligned Collagen Is a Prognostic Signature for Survival in Human Breast Carcinoma. Am. J. Pathol. 2011;178:1221–1232. doi: 10.1016/j.ajpath.2010.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Provenzano P.P., Eliceiri K.W., Campbell J.M., Inman D.R., White J.G., Keely P.J. Collagen Reorganization at the Tumor-Stromal Interface Facilitates Local Invasion. BMC Med. 2006;4:38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Coelho N.M., Arora P.D., van Putten S., Boo S., Petrovic P., Lin A.X., Hinz B., McCulloch C.A. Discoidin Domain Receptor 1 Mediates Myosin-Dependent Collagen Contraction. Cell Rep. 2017;18:1774–1790. doi: 10.1016/j.celrep.2017.01.061. [DOI] [PubMed] [Google Scholar]
- 97.Bayer S.V., Grither W.R., Brenot A., Hwang P.Y., Barcus C.E., Ernst M., Pence P., Walter C., Pathak A., Longmore G.D. DDR2 Controls Breast Tumor Stiffness and Metastasis by Regulating Integrin Mediated Mechanotransduction in CAFs. eLife. 2019;8:e45508. doi: 10.7554/eLife.45508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Slocum E., Craig A., Villanueva A., Germain D. Parity Predisposes Breasts to the Oncogenic Action of PAPP-A and Activation of the Collagen Receptor DDR2. Breast Cancer Res. 2019;21:56. doi: 10.1186/s13058-019-1142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gonzalez M.E., Martin E.E., Anwar T., Arellano-Garcia C., Medhora N., Lama A., Chen Y.-C., Tanager K.S., Yoon E., Kidwell K.M., et al. Mesenchymal Stem Cell-Induced DDR2 Mediates Stromal-Breast Cancer Interactions and Metastasis Growth. Cell Rep. 2017;18:1215–1228. doi: 10.1016/j.celrep.2016.12.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang Y., Weinberg R.A. Epithelial-to-Mesenchymal Transition in Cancer: Complexity and Opportunities. Front. Med. 2018;12:361–373. doi: 10.1007/s11684-018-0656-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Azizi R., Salemi Z., Fallahian F., Aghaei M. Inhibition of Didscoidin Domain Receptor 1 Reduces Epithelial–Mesenchymal Transition and Induce Cell-cycle Arrest and Apoptosis in Prostate Cancer Cell Lines. J. Cell. Physiol. 2019;234:19539–19552. doi: 10.1002/jcp.28552. [DOI] [PubMed] [Google Scholar]
- 102.Xie R., Wang X., Qi G., Wu Z., Wei R., Li P., Zhang D. DDR1 Enhances Invasion and Metastasis of Gastric Cancer via Epithelial-Mesenchymal Transition. Tumor Biol. 2016;37:12049–12059. doi: 10.1007/s13277-016-5070-6. [DOI] [PubMed] [Google Scholar]
- 103.Jiang X., Jiang M., Guo S., Cai P., Wang W., Li Y. Promotion of MiR-221-5p on the Sensitivity of Gastric Cancer Cells to Cisplatin and Its Effects on Cell Proliferation and Apoptosis by Regulating DDR1. OTT. 2020;13:2333–2345. doi: 10.2147/OTT.S232953. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 104.Kim S., Lee S., Lee E., Lim H., Shin J., Jung J., Kim S., Moon A. Sex-biased Differences in the Correlation between Epithelial-to-mesenchymal Transition-associated Genes in Cancer Cell Lines. Oncol. Lett. 2019 doi: 10.3892/ol.2019.11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Takai K., Drain A.P., Lawson D.A., Littlepage L.E., Karpuj M., Kessenbrock K., Le A., Inoue K., Weaver V.M., Werb Z. Discoidin Domain Receptor 1 (DDR1) Ablation Promotes Tissue Fibrosis and Hypoxia to Induce Aggressive Basal-like Breast Cancers. Genes Dev. 2018;32:244–257. doi: 10.1101/gad.301366.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Maeyama M., Koga H., Selvendiran K., Yanagimoto C., Hanada S., Taniguchi E., Kawaguchi T., Harada M., Ueno T., Sata M. Switching in Discoid Domain Receptor Expressions in SLUG-Induced Epithelial-Mesenchymal Transition. Cancer. 2008;113:2823–2831. doi: 10.1002/cncr.23900. [DOI] [PubMed] [Google Scholar]
- 107.Liang Z., Xie W., Zhao M., Cheng G., Wu M. DDR2 Facilitates Papillary Thyroid Carcinoma Epithelial Mesenchymal Transition by Activating ERK2/Snail1 Pathway. Oncol. Lett. 2017;14:8114–8121. doi: 10.3892/ol.2017.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Friedl P., Wolf K. Plasticity of Cell Migration: A Multiscale Tuning Model. J. Cell. Biol. 2010;188:11–19. doi: 10.1083/jcb.200909003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dejmek J., Dib K., Jonsson M., Andersson T. Wnt-5a and G-Protein Signaling Are Required for Collagen-Induced DDR1 Receptor Activation and Normal Mammary Cell Adhesion. Int. J. Cancer. 2003;103:344–351. doi: 10.1002/ijc.10752. [DOI] [PubMed] [Google Scholar]
- 110.Romayor I., Badiola I., Olaso E. Inhibition of DDR1 Reduces Invasive Features of Human A375 Melanoma, HT29 Colon Carcinoma and SK-HEP Hepatoma Cells. Cell Adhes. Migr. 2020;14:69–81. doi: 10.1080/19336918.2020.1733892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yan Z., Jin S., Wei Z., Huilian H., Zhanhai Y., Yue T., Juan L., Jing L., Libo Y., Xu L. Discoidin Domain Receptor 2 Facilitates Prostate Cancer Bone Metastasis via Regulating Parathyroid Hormone-Related Protein. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2014;1842:1350–1363. doi: 10.1016/j.bbadis.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 112.Chen Y.-L., Tsai W.-H., Ko Y.-C., Lai T.-Y., Cheng A.-J., Shiah S.-G., Hsiao J.-R., Chang J.-Y., Lin S.-F. Discoidin Domain Receptor-1 (DDR1) Is Involved in Angiolymphatic Invasion in Oral Cancer. Cancers. 2020;12:841. doi: 10.3390/cancers12040841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Castro-Sanchez L., Soto-Guzman A., Navarro-Tito N., Martinez-Orozco R., Salazar E.P. Native Type IV Collagen Induces Cell Migration through a CD9 and DDR1-Dependent Pathway in MDA-MB-231 Breast Cancer Cells. Eur. J. Cell Biol. 2010;89:843–852. doi: 10.1016/j.ejcb.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 114.Chow C.R., Ebine K., Knab L.M., Bentrem D.J., Kumar K., Munshi H.G. Cancer Cell Invasion in Three-Dimensional Collagen Is Regulated Differentially by Gα 13 Protein and Discoidin Domain Receptor 1-Par3 Protein Signaling. J. Biol. Chem. 2016;291:1605–1618. doi: 10.1074/jbc.M115.669606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Poudel B., Lee Y.-M., Kim D.-K. DDR2 Inhibition Reduces Migration and Invasion of Murine Metastatic Melanoma Cells by Suppressing MMP2/9 Expression through ERK/NF- B Pathway. Acta Biochim. Biophys. Sin. 2015;47:292–298. doi: 10.1093/abbs/gmv005. [DOI] [PubMed] [Google Scholar]
- 116.Xu J., Lu W., Zhang S., Zhu C., Ren T., Zhu T., Zhao H., Liu Y., Su J. Overexpression of DDR2 Contributes to Cell Invasion and Migration in Head and Neck Squamous Cell Carcinoma. Cancer Biol. Ther. 2014;15:612–622. doi: 10.4161/cbt.28181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Grither W.R., Longmore G.D. Inhibition of Tumor-Microenvironment Interaction and Tumor Invasion by Small-Molecule Allosteric Inhibitor of DDR2 Extracellular Domain. Proc. Natl. Acad. Sci. USA. 2018;115:E7786–E7794. doi: 10.1073/pnas.1805020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hansen C., Greengard P., Nairn A.C., Andersson T., Vogel W.F. Phosphorylation of DARPP-32 Regulates Breast Cancer Cell Migration Downstream of the Receptor Tyrosine Kinase DDR1. Exp. Cell Res. 2006;312:4011–4018. doi: 10.1016/j.yexcr.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 119.Yuge R., Kitadai Y., Takigawa H., Naito T., Oue N., Yasui W., Tanaka S., Chayama K. Silencing of Discoidin Domain Receptor-1 (DDR1) Concurrently Inhibits Multiple Steps of Metastasis Cascade in Gastric Cancer. Transl. Oncol. 2018;11:575–584. doi: 10.1016/j.tranon.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen L., Zhi Z., Wang L., Zhao Y., Deng M., Liu Y., Qin Y., Tian M., Liu Y., Shen T., et al. NSD2 Circular RNA Promotes Metastasis of Colorectal Cancer by Targeting MiR-199b-5p-mediated DDR1 and JAG1 Signalling. J. Pathol. 2019;248:103–115. doi: 10.1002/path.5238. [DOI] [PubMed] [Google Scholar]
- 121.Larrieu-Lahargue F., Welm A.L., Bouchecareilh M., Alitalo K., Li D.Y., Bikfalvi A., Auguste P. Blocking Fibroblast Growth Factor Receptor Signaling Inhibits Tumor Growth, Lymphangiogenesis, and Metastasis. PLoS ONE. 2012;7:e39540. doi: 10.1371/journal.pone.0039540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xiao Q., Jiang Y., Liu Q., Yue J., Liu C., Zhao X., Qiao Y., Ji H., Chen J., Ge G. Minor Type IV Collagen A5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1. PLoS Genet. 2015;11:e1005249. doi: 10.1371/journal.pgen.1005249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Oh S., Seo M., Choi J.-S., Joo C.-K., Lee S.K. MiR-199a/b-5p Inhibits Lymphangiogenesis by Targeting Discoidin Domain Receptor 1 in Corneal Injury. Mol. Cells. 2018;41:93–102. doi: 10.14348/molcells.2018.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang S., Bu X., Zhao H., Yu J., Wang Y., Li D., Zhu C., Zhu T., Ren T., Liu X., et al. A Host Deficiency of Discoidin Domain Receptor 2 (DDR2) Inhibits Both Tumour Angiogenesis and Metastasis: DDR2 Regulates Endothelial Function and Tumour Angiogenesis. J. Pathol. 2014;232:436–448. doi: 10.1002/path.4311. [DOI] [PubMed] [Google Scholar]
- 125.Lee N.O., Park J.-W., Lee J.A., Shim J.H., Kong S.-Y., Kim K.T., Lee Y.-S. Dual Action of a Selective Cyclooxygenase-2 Inhibitor on Vascular Endothelial Growth Factor Expression in Human Hepatocellular Carcinoma Cells: Novel Involvement of Discoidin Domain Receptor 2. J. Cancer Res. Clin. Oncol. 2012;138:73–84. doi: 10.1007/s00432-011-1075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Romayor I., Badiola I., Benedicto A., Marquez J., Herrero A., Arteta B., Olaso E. Silencing of Sinusoidal DDR1 Reduces Murine Liver Metastasis by Colon Carcinoma. Sci. Rep. 2020;10:18398. doi: 10.1038/s41598-020-75395-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Badiola I., Olaso E., Crende O., Friedman S.L., Vidal-Vanaclocha F. Discoidin Domain Receptor 2 Deficiency Predisposes Hepatic Tissue to Colon Carcinoma Metastasis. Gut. 2012;61:1465–1472. doi: 10.1136/gutjnl-2011-300810. [DOI] [PubMed] [Google Scholar]
- 128.Kinoshita J., Fushida S., Harada S., Makino I., Nakamura K., Oyama K., Fujita H., Ninomiya I., Fujimura T., Kayahara M., et al. Type IV Collagen Levels Are Elevated in the Serum of Patients with Peritoneal Dissemination of Gastric Cancer. Oncol. Lett. 2010;1:989–994. doi: 10.3892/ol.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xiong G., Chen J., Zhang G., Wang S., Kawasaki K., Zhu J., Zhang Y., Nagata K., Li Z., Zhou B.P., et al. Hsp47 Promotes Cancer Metastasis by Enhancing Collagen-Dependent Cancer Cell-Platelet Interaction. Proc. Natl. Acad. Sci. USA. 2020;117:3748–3758. doi: 10.1073/pnas.1911951117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ye L., Pu C., Tang J., Wang Y., Wang C., Qiu Z., Xiang T., Zhang Y., Peng W. Transmembrane-4 L-Six Family Member-1 (TM4SF1) Promotes Non-Small Cell Lung Cancer Proliferation, Invasion and Chemo-Resistance through Regulating the DDR1/Akt/ERK-MTOR Axis. Respir. Res. 2019;20:106. doi: 10.1186/s12931-019-1071-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jia S., Agarwal M., Yang J., Horowitz J.C., White E.S., Kim K.K. Discoidin Domain Receptor 2 Signaling Regulates Fibroblast Apoptosis through PDK1/Akt. Am. J. Respir. Cell Mol. Biol. 2018;59:295–305. doi: 10.1165/rcmb.2017-0419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Matà R., Palladino C., Nicolosi M.L., Presti A.R.L., Malaguarnera R., Ragusa M., Sciortino D., Morrione A., Maggiolini M., Vella V., et al. IGF-I Induces Upregulation of DDR1 Collagen Receptor in Breast Cancer Cells by Suppressing MIR-199a-5p through the PI3K/AKT Pathway. Oncotarget. 2016;7:7683–7700. doi: 10.18632/oncotarget.6524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vella V., Malaguarnera R., Nicolosi M.L., Palladino C., Spoleti C., Massimino M., Vigneri P., Purrello M., Ragusa M., Morrione A., et al. Discoidin Domain Receptor 1 Modulates Insulin Receptor Signaling and Biological Responses in Breast Cancer Cells. Oncotarget. 2017;8:43248–43270. doi: 10.18632/oncotarget.18020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vella V., Nicolosi M.L., Cantafio P., Massimino M., Lappano R., Vigneri P., Ciuni R., Gangemi P., Morrione A., Malaguarnera R., et al. DDR1 Regulates Thyroid Cancer Cell Differentiation via IGF-2/IR-A Autocrine Signaling Loop. Endocr.-Relat. Cancer. 2019;26:197–214. doi: 10.1530/ERC-18-0310. [DOI] [PubMed] [Google Scholar]
- 135.Buraschi S., Morcavallo A., Neill T., Stefanello M., Palladino C., Xu S.-Q., Belfiore A., Iozzo R.V., Morrione A. Discoidin Domain Receptor 1 Functionally Interacts with the IGF-I System in Bladder Cancer. Matrix Biol. Plus. 2020;6–7:100022. doi: 10.1016/j.mbplus.2020.100022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Avino S., Marco P.D., Cirillo F., Santolla M.F., Francesco E.M.D., Perri M.G., Rigiracciolo D., Dolce V., Belfiore A., Maggiolini M., et al. Stimulatory Actions of IGF-I Are Mediated by IGF-IR Cross-Talk with GPER and DDR1 in Mesothelioma and Lung Cancer Cells. Oncotarget. 2016;7:52710–52728. doi: 10.18632/oncotarget.10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Morcavallo A., Gaspari M., Pandini G., Palummo A., Cuda G., Larsen M.R., Vigneri R., Belfiore A. Research Resource: New and Diverse Substrates for the Insulin Receptor Isoform A Revealed by Quantitative Proteomics After Stimulation With IGF-II or Insulin. Mol. Endocrinol. 2011;25:1456–1468. doi: 10.1210/me.2010-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vella V., Malaguarnera R., Nicolosi M.L., Morrione A., Belfiore A. Insulin/IGF Signaling and Discoidin Domain Receptors: An Emerging Functional Connection. Biochim. Biophys. Acta Mol. Cell Res. 2019;1866:118522. doi: 10.1016/j.bbamcr.2019.118522. [DOI] [PubMed] [Google Scholar]
- 139.Day E., Waters B., Spiegel K., Alnadaf T., Manley P.W., Buchdunger E., Walker C., Jarai G. Inhibition of Collagen-Induced Discoidin Domain Receptor 1 and 2 Activation by Imatinib, Nilotinib and Dasatinib. Eur. J. Pharmacol. 2008;599:44–53. doi: 10.1016/j.ejphar.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 140.Montenegro R.C., Howarth A., Ceroni A., Fedele V., Farran B., Mesquita F.P., Frejno M., Berger B.-T., Heinzlmeir S., Sailem H.Z., et al. Identification of Molecular Targets for the Targeted Treatment of Gastric Cancer Using Dasatinib. Oncotarget. 2020;11:535–549. doi: 10.18632/oncotarget.27462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.von Maessenhausen A., Sanders C., Braegelmann J., Konantz M., Queisser A., Vogel W., Kristiansen G., Duensing S., Schroeck A., Bootz F., et al. Targeting DDR2 in Head and Neck Squamous Cell Carcinoma with Dasatinib. Int. J. Cancer. 2016;139:2359–2369. doi: 10.1002/ijc.30279. [DOI] [PubMed] [Google Scholar]
- 142.Gajdosik Z. Sitravatinib Multitargeted Tyrosine Kinase Inhibitor Treatment of Solid Tumors. Drug Future. 2018;43:181–187. doi: 10.1358/dof.2018.043.03.2776627. [DOI] [Google Scholar]
- 143.Aguilera K.Y., Huang H., Du W., Hagopian M.M., Wang Z., Hinz S., Hwang T.H., Wang H., Fleming J.B., Castrillon D.H., et al. Inhibition of Discoidin Domain Receptor 1 Reduces Collagen-Mediated Tumorigenicity in Pancreatic Ductal Adenocarcinoma. Mol. Cancer Ther. 2017;16:2473–2485. doi: 10.1158/1535-7163.MCT-16-0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nokin M.-J., Darbo E., Travert C., Drogat B., Lacouture A., San José S., Cabrera N., Turcq B., Prouzet-Mauleon V., Falcone M., et al. Inhibition of DDR1 Enhances in Vivo Chemosensitivity in KRAS-Mutant Lung Adenocarcinoma. JCI Insight. 2020;5:e137869. doi: 10.1172/jci.insight.137869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Elkamhawy A., Park J.-E., Cho N.-C., Sim T., Pae A.N., Roh E.J. Discovery of a Broad Spectrum Antiproliferative Agent with Selectivity for DDR1 Kinase: Cell Line-Based Assay, Kinase Panel, Molecular Docking, and Toxicity Studies. J. Enzyme. Inhib. Med. Chem. 2016;31:158–166. doi: 10.3109/14756366.2015.1004057. [DOI] [PubMed] [Google Scholar]
- 146.Mo C., Zhang Z., Li Y., Huang M., Zou J., Luo J., Tu Z.-C., Xu Y., Ren X., Ding K., et al. Design and Optimization of 3′-(Imidazo[1,2-a]Pyrazin-3-Yl)-[1,1′-Biphenyl]-3-Carboxamides as Selective DDR1 Inhibitors. ACS Med. Chem. Lett. 2020;11:379–384. doi: 10.1021/acsmedchemlett.9b00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kim H.-G., Tan L., Weisberg E.L., Liu F., Canning P., Choi H.G., Ezell S.A., Wu H., Zhao Z., Wang J., et al. Discovery of a Potent and Selective DDR1 Receptor Tyrosine Kinase Inhibitor. ACS Chem. Biol. 2013;8:2145–2150. doi: 10.1021/cb400430t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Richter H., Satz A.L., Bedoucha M., Buettelmann B., Petersen A.C., Harmeier A., Hermosilla R., Hochstrasser R., Burger D., Gsell B., et al. DNA-Encoded Library-Derived DDR1 Inhibitor Prevents Fibrosis and Renal Function Loss in a Genetic Mouse Model of Alport Syndrome. ACS Chem. Biol. 2019;14:37–49. doi: 10.1021/acschembio.8b00866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Siddiqui K., Kim G.W., Lee D.H., Shin H.R., Yang E.G., Lee N.T., Yang B.-S. Actinomycin D Identified as an Inhibitor of Discoidin Domain Receptor 2 Interaction with Collagen through an Insect Cell Based Screening of a Drug Compound Library. Biol. Pharm. Bull. 2009;32:136–141. doi: 10.1248/bpb.32.136. [DOI] [PubMed] [Google Scholar]
- 150.Terai H., Tan L., Beauchamp E.M., Hatcher J.M., Liu Q., Meyerson M., Gray N.S., Hammerman P.S. Characterization of DDR2 Inhibitors for the Treatment of DDR2 Mutated Nonsmall Cell Lung Cancer. ACS Chem. Biol. 2015;10 doi: 10.1021/acschembio.5b00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Baltes F., Caspers J., Henze S., Schlesinger M., Bendas G. Targeting Discoidin Domain Receptor 1 (DDR1) Signaling and Its Crosstalk with Β1-Integrin Emerges as a Key Factor for Breast Cancer Chemosensitization upon Collagen Type 1 Binding. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21144956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vehlow A., Cordes N. DDR1 (Discoidin Domain Receptor Tyrosine Kinase 1) Drives Glioblastoma Therapy Resistance by Modulating Autophagy. Autophagy. 2019;15:1487–1488. doi: 10.1080/15548627.2019.1618540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Deng Y., Zhao F., Hui L., Li X., Zhang D., Lin W., Chen Z., Ning Y. Suppressing MiR-199a-3p by Promoter Methylation Contributes to Tumor Aggressiveness and Cisplatin Resistance of Ovarian Cancer through Promoting DDR1 Expression. J. Ovarian Res. 2017;10:50. doi: 10.1186/s13048-017-0333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lai S.L., Tan M.L., Hollows R.J., Robinson M., Ibrahim M., Margielewska S., Parkinson E.K., Ramanathan A., Zain R.B., Mehanna H., et al. Collagen Induces a More Proliferative, Migratory and Chemoresistant Phenotype in Head and Neck Cancer via DDR1. Cancers. 2019;11:1766. doi: 10.3390/cancers11111766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chappell W.H., Candido S., Abrams S.L., Akula S.M., Steelman L.S., Martelli A.M., Ratti S., Cocco L., Cervello M., Montalto G., et al. Influences of TP53 and the Anti-Aging DDR1 Receptor in Controlling Raf/MEK/ERK and PI3K/Akt Expression and Chemotherapeutic Drug Sensitivity in Prostate Cancer Cell Lines. Aging. 2020;12:10194–10210. doi: 10.18632/aging.103377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tu M.M., Lee F.Y.F., Jones R.T., Kimball A.K., Saravia E., Graziano R.F., Coleman B., Menard K., Yan J., Michaud E., et al. Targeting DDR2 Enhances Tumor Response to Anti–PD-1 Immunotherapy. Sci. Adv. 2019;5:eaav2437. doi: 10.1126/sciadv.aav2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Beauchamp E.M., Woods B.A., Dulak A.M., Tan L., Xu C., Gray N.S., Bass A.J., Wong K.-K., Meyerson M., Hammerman P.S. Acquired Resistance to Dasatinib in Lung Cancer Cell Lines Conferred by DDR2 Gatekeeper Mutation and NF1 Loss. Mol. Cancer Ther. 2014;13:475–482. doi: 10.1158/1535-7163.MCT-13-0817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Huang H., Svoboda R.A., Lazenby A.J., Saowapa J., Chaika N., Ding K., Wheelock M.J., Johnson K.R. Up-Regulation of N-Cadherin by Collagen I-Activated Discoidin Domain Receptor 1 in Pancreatic Cancer Requires the Adaptor Molecule Shc1. J. Biol. Chem. 2016;291:23208–23223. doi: 10.1074/jbc.M116.740605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ram R., Lorente G., Nikolich K., Urfer R., Foehr E., Nagavarapu U. Discoidin Domain Receptor-1a (DDR1a) Promotes Glioma Cell Invasion and Adhesion in Association with Matrix Metalloproteinase-2. J. Neurooncol. 2006;76:239–248. doi: 10.1007/s11060-005-6874-1. [DOI] [PubMed] [Google Scholar]
- 160.Yang S.H., Baek H.A., Lee H.J., Park H.S., Jang K.Y., Kang M.J., Lee D.G., Lee Y.C., Moon W.S., Chung M.J. Discoidin Domain Receptor 1 Is Associated with Poor Prognosis of Non-Small Cell Lung Carcinomas. Oncol. Rep. 2010;24:311–319. doi: 10.3892/or_00000861. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.