Abstract
Background: This systematic review aimed at comparing performances of ultrasonography (US), magnetic resonance imaging (MRI), and fluorodeoxyglucose positron emission tomography (PET) for axillary staging, with a focus on micro- or micrometastases. Methods: A search for relevant studies published between January 2002 and March 2018 was conducted in MEDLINE database. Study quality was assessed using the QUality Assessment of Diagnostic Accuracy Studies checklist. Sensitivity and specificity were meta-analyzed using a bivariate random effects approach; Results: Across 62 studies (n = 10,374 patients), sensitivity and specificity to detect metastatic ALN were, respectively, 51% (95% CI: 43–59%) and 100% (95% CI: 99–100%) for US, 83% (95% CI: 72–91%) and 85% (95% CI: 72–92%) for MRI, and 49% (95% CI: 39–59%) and 94% (95% CI: 91–96%) for PET. Interestingly, US detects a significant proportion of macrometastases (false negative rate was 0.28 (0.22, 0.34) for more than 2 metastatic ALN and 0.96 (0.86, 0.99) for micrometastases). In contrast, PET tends to detect a significant proportion of micrometastases (true positive rate = 0.41 (0.29, 0.54)). Data are not available for MRI. Conclusions: In comparison with MRI and PET Fluorodeoxyglucose (FDG), US is an effective technique for axillary triage, especially to detect high metastatic burden without upstaging majority of micrometastases.
Keywords: meta-analysis, ultrasound, magnetic resonance imaging, positron emission tomography, breast cancer, lymph node, micrometastasis
1. Introduction
Breast cancer is the most commonly diagnosed cancer among women worldwide [1], accounting for 25% of cancer cases and 15% of cancer-related deaths [2]. Axillary lymph node (ALN) metastases are detected in 30 to 40% of women with breast cancer and are associated with a less favorable prognostic [3,4]. Sentinel lymph node biopsy (SLNB) is the classical staging procedure for breast cancer patients with clinically and radiologically negative axilla [5,6,7,8]. Preoperative detection of ALN involvement by imaging may change management in several ways, from first-line ALN dissection to neoadjuvant chemotherapy [9]. However, it is now well established that axillary micro- and macrometastases do not have the same prognostic and therapeutic impact, and the detection of micrometastasis should not lead to an ALN dissection or an inappropriate chemotherapy. Consequently, the axillary staging by imaging should help selecting patients with macrometastatic ALN and patients with negative or micrometastatic ALN.
To our knowledge, no study has systematically evaluated the performance of each of the 3 main imaging techniques as a triage test for axilla staging for breast cancer patients, especially without palpable ALN, with a focus on the type of nodal involvement (micro-or macrometastases). Many of the previous analyses concerning axillary staging did not include nodal ultrastadification and were performed in a population in which a significant proportion of patients had palpable ALN. Palpable ALN constitute a contraindication for SLNB as grossly involved nodes may not retain the dye or the radio-colloid agent due to the replacement of macrophages by cancer cells [10,11,12,13]. Moreover, inclusion of a significant proportion of patients undergoing neoadjuvant chemotherapy may not allow an accurate evaluation, as node staging may change during neoadjuvant chemotherapy (false negative).
Hence, the role and performance of imaging (including ultrastadification) remains to be clarified for breast cancer patients without palpable ALN, as well as the choice of the adequate imaging modality.
In clinical routine, axillary ultrasound (US) is widely performed, followed by fine-needle aspiration or core needle biopsy of abnormal ALN [3]. In some patients, magnetic resonance imaging (MRI) and 2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography (PET) are performed, for local or distant staging, and are potential techniques to improve axillary staging [9,14,15,16].
This systematic review aimed at systematically evaluating the performances of US (with or without fine-needle aspiration or core needle biopsy), MRI, and fluorodeoxyglucose PET for axillary staging, with a focus on micro- or micrometastases in breast cancer patients without palpable axillary nodes, and to discuss their use in different clinical settings.
2. Materials and Methods
2.1. Search Strategy
This systematic review followed the recommendations in the PRISMA statement [17,18]. Two reviewers independently searched the relevant studies that assessed the accuracy and the utility of US, MRI, and PET in staging the axilla in patients with breast cancer. The MEDLINE database was used for all in vivo human studies. The discrepancies were resolved by consensus.
2.2. Inclusion and Exclusion Criteria
Studies with the following inclusion criteria were reviewed: (1) Published in English, (2) cohort studies (prospective or retrospective); (3) published between 1 January 2002 and 15 March 2018; (4) imaging was done to detect ALN involvement in patients with breast cancer, (5) imaging procedures were US, MRI, PET; (6) histopathological analysis of ALN obtained by SLNB or ALN dissection procedure were used as the reference standard test, and (7) true positive (TP), false positive (FP), true negative (TN), and false negative (FN) values were reported or, if there was sufficient data for them, were calculated.
We excluded studies with the following criteria: (1) Neoadjuvant chemotherapy was administered between imaging and axillary surgery; (2) patients with palpable ALN ipsilateral to the breast cancer; (3) no histopathological reference standard; (4) patients without breast cancer; (5) insufficient data available to calculate the TP, FP, TN, and FN values; (6) imaging was performed for the sole purpose of detecting sentinel ALN; (7) patients were shared with another study previously included; (8) experimental subject was an animal and ex vivo; (9) under 18 analyzable patients in the study, (10) the type of study was a case control study, review, case report, letter to the editor, and (11) we were unable to get the full text.
Some studies were also included if we could manually exclude patients with exclusion criteria—such as patients treated with neoadjuvant chemotherapy or with palpable node, or patients without breast cancer and if we could calculate VP, FP, VN, and FN in the new population.
2.3. Data Extraction and Quality Assessment
Data were extracted by one reviewer, checked by a second, and discrepancies resolved by discussion. Study quality was assessed using the QUality Assessment of Diagnostic Accuracy Studies (QUADAS) checklist [19]. All the 14 items in the checklist were used.
2.4. Data Synthesis and Statistical Analysis
Patients were classified as TP when both imaging techniques and the reference standard (e.g., ALN dissection or SLNB) detected axillary metastases; TN when neither imaging techniques nor reference standard detected metastasis; FN when the imaging technique failed to detect metastasis identified by the reference standard; and FP when the imaging technique incorrectly suggested metastasis not detected by the reference standard. Sensitivity was defined as TP/(TP + FN) and SP as TN/(TN + FP). The diagnostic odd ratio (DOR) values was obtained with different combinations of SE and SP and could be used as a single summary measure. It was defined as the ratio of odds of positivity in disease relative to non-diseased. The DOR value ranges from 0 to infinity and a higher value means better diagnostic performance. A value of 1 indicates that a test cannot distinguish between patients with or without the disease and values of <1 introduce more FN results among the diseased [20].
| (1) |
Considering the correlation between sensitivity and specificity, a bivariate random effects model was used to summarize performance estimates and their 95% confidence intervals (CI) [21]. Heterogeneity was assessed using the quantity I2 that lies between 0 and 100% (a value of 0% indicates no observed heterogeneity, values lower than 50% were considered as an acceptable level of heterogeneity) [22]. When no significant heterogeneity was observed between studies or when the number of considered studies was too small, a pooled analysis was undertaken. For all statistical tests, differences were considered significant at the 0.05 level. All statistical analyses were conducted using STATA 13.0® software (copyright College Station, TX: StataCorp LP).
Forest plots were generated within Review Manager 5® (copyright The Cochrane Collaboration, Copenhagen: The Nordic Cochrane Centre).
2.5. Subgroup Analyses
Subgroup analyses were undertaken according to US technique; US grayscale, US + fine needle aspiration/core needle biopsy, fine needle aspiration, and elastosonography. Subgroup analyses were conducted according to which MRI technique was used; MRI without diffusion weighted imaging (DWI), MRI with DWI, and DWI alone. Subgroup analyses were conducted according to which PET technique was used; PET without computed tomography (CT), and PET with CT.
In some studies, several results for one imaging technique, like MRI, were available, for example, for each MRI subgroup (e.g., MRI without DWI, MRI with DWI, DWI alone). As these results came from the same population, only one result could be considered for the pool estimates. Additionally, the subgroup with the best accuracy result ((TP + TN)/(TP + FP + FN + TN)) was considered.
For US studies, the US + fine needle aspiration/core needle biopsy criterion was preferred over US grayscale, because in routine clinical practice, any suspicious ALN in breast cancer undergoes ultrasound guided fine needle aspiration ore core needle biopsy. In studies evaluating elastosonography, nodes were considered abnormal if either US grayscale, elastosonography, or both were abnormal (disjunctive method).
Subgroups analysis were undertaken according to ALN involvement (micrometastases versus macrometastases and less than 3 ALN metastases versus 3 or more ALN metastases) in patient with T1–T2 breast cancer.
3. Results
3.1. Number and Characteristics of Included Studies
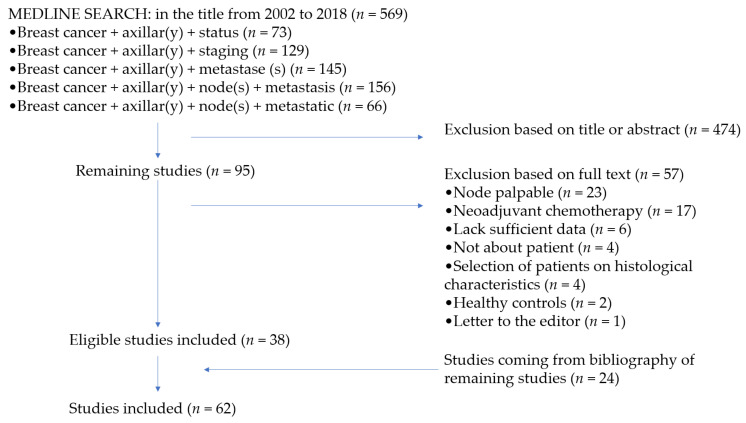
The search identified 569 citations from the MEDLINE data base, 95 were examined for full text review analysis after primary screening of titles and abstracts. Study characteristics of each subgroup are described in Table 1A–D.
Table 1.
Study characteristics.
| (A) Characteristics of ultrasound included studies | |||||||||||||
| Author | Year | Country | Index Test | Second Test | Reference Standard | Prospective/Retrospective | N Analysed | N with Axillary Metastases | Prevalence of Axillary Metastases | Mean Age | Years of Study | Other Criteria | |
| Chang W. [23] | 2018 | China | US | US + Elastosonography | Histology (SLNB/ALND) | Retrospective | 140 | 78 | 55.7% | 55.3 | 2013–2014 | Disjunctive method | |
| Wallis M.G. [24] | 2017 | UK | US | US + CNB | Histology (SLNB/ALND) | Retrospective | 769 | 134 | 17.4% | ND | 2008–2015 | ||
| Zhao Q.L. [25] | 2017 | China | US | US + Elastosonography | Histology (SLNB/ALND) | Prospective | 78 | 44 | 56.4% | 52.5 | 2012–2013 | Disjunctive method | |
| Akinci M. [26] | 2016 | Turkey | US | US + FNA | Histology (SLNB/ALND) | Prospective | 46 | 30 | 65.2% | ND | 2011–2013 | ||
| Gipponi M. [27] | 2016 | Italy | US | US + FNA | Histology (SLNB/ALND) | Prospective | 400 | 127 | 31.8% | 64.6 | 2013–2015 | Only T1-T2-T3 tumors | |
| Zhu Y. [28] | 2016 | China | US | US + FNA | Histology (SLNB/ALND) | Retrospective | 445 | 169 | 38.0% | 55.6 | 2013–2014 | Only T1-T2 tumors | |
| Hyun S.J. [29] | 2015 | South Korea | US | US + FNA | Histology (SLNB/ALND) | Retrospective | 497 | 159 | 32.0% | 52 | 2012–2013 | ||
| Zhang Y.N. [30] | 2015 | China | US | US grayscale | Histology (SLNB/ALND) | Retrospective | 1049 | 402 | 38.3% | 50.3 | 2010–2011 | ||
| Sohn Y.M.b[31] | 2014 | South Korea | US | US grayscale | Histology (SLNB/ALND) | Retrospective | 107 | 45 | 42.1% | 53.9 | 2009–2012 | ||
| Cools Lartique J. [32] | 2013 | Canada | US | US + FNA | Histology (SLNB/ALND) | Prospective | 234 | 90 | 38.5% | 57.8 | 2005–2007 | ||
| Stachs A. [33] | 2013 | Germany | US | US grayscale | Histology (SLNB/ALND) | Retrospective | 470 | 166 | 35.3% | ND | 2008–2010 | ||
| Riegger C. [34] | 2012 | Germany | US | US grayscale | Histology (SLNB/ALND) | Retrospective | 91 | 37 | 40.7% | 55.5 | 2007–2010 | ||
| Davey P. [35] | 2011 | Northern Ireland | US | US + FNA | Histology (SLNB/ALND) | Retrospective | 119 | 40 | 33.6% | ND | 2009 | ||
| Schiettecatte A. [36] | 2011 | Belgium | US | US + FNA | Histology (SLNB/ALND) | Retrospective | 147 | 67 | 45.6% | 56 | ND | Breast tumors < 3cm | |
| Baruah B.P. [37] | 2010 | UK | US | US + FNA | Histology (SLNB/ALND) | Retrospective | 502 | 137 | 27.3% | 61 | 2006–2009 | ||
| Jung J. [38] | 2010 | South Korea | US | US + FNA | Histology (SLNB/ALND) | Retrospective | 189 | 61 | 32.3% | ND | 2005–2006 | ||
| Luparia A. [39] | 2010 | Italy | US | US grayscale | Histology (SLNB/ALND) | Retrospective | 427 | 170 | 39.8% | 60.9 | 2005–2008 | ||
| Monzawa S. [40] | 2009 | Japan | US | US grayscale | Histology (SLNB/ALND) | Retrospective | 50 | 15 | 30.0% | 59 | 2005–2006 | ||
| Cowher M.S. [41] | 2008 | USA | US | US + FNA | Histology (SLNB/ALND) | Retrospective | 125 | 57 | 45.6% | 61.3 | 2004–2005 | ||
| Moore A. [42] | 2008 | USA | US | US + FNA | Histology (SLNB/ALND) | Retrospective | 112 | 58 | 51.8% | ND | ND | High risk of metastases | |
| Ueda S. [43] | 2008 | Japan | US | US grayscale | Histology (SLNB/ALND) | Prospective | 183 | 59 | 32.2% | 57 | 2005–2007 | ||
| Altomare V. [44] | 2007 | Italy | US | US + FNA | Histology (SLNB/ALND) | Retrospective | 100 | 30 | 30.0% | 53 | 2004–2005 | Only T1-T2-T3 tumors. FNA performed for all patients | |
| Davis J.T. [45] | 2006 | USA | US | US + FNA | Histology (SLNB/ALND) | Prospective | 37 | 22 | 59.5% | ND | 2004–2005 | High risk of metastases | |
| Lumachi F. [46] | 2006 | Italy | US | US grayscale | Histology (SLNB/ALND) | Prospective | 77 | 37 | 48.1% | 54 | ND | Only T1-T2 tumors. | |
| Popli M.B. [47] | 2006 | India | US | US + FNA | Histology (SLNB/ALND) | Prospective | 30 | 22 | 73.3% | ND | ND | ||
| Podkrajsek M. [48] | 2005 | Slovenia | US | US + FNA | Histology (SLNB/ALND) | Retrospective | 165 | 65 | 39.4% | 56 | 2001–2003 | ||
| Bedrosian I. [49] | 2003 | USA | US | US + FNA | Histology (SLNB/ALND) | Prospective | 208 | 53 | 25.5% | 55.4 | 1994–2000 | ||
| Deurloo E.E. [50] | 2003 | The Netherlands | US | US + FNA | Histology (SLNB/ALND) | Prospective | 268 | 121 | 45.1% | 56 | 1999–2001 | Only patients eligible for SLNB | |
| Kuenen-Boumeester V. [51] | 2003 | The Netherlands | US | US + FNA | Histology (SLNB/ALND) | Retrospective | 183 | 85 | 46.4% | ND | 1998–2003 | ||
| Sapino A. [52] | 2003 | Italy | US | US + FNA | Histology (SLNB/ALND) | Prospective | 298 | 88 | 29.5% | ND | 2000 | 31 in situ breast cancer | |
| TOTAL | 7546 | 2668 | 35.4% | 56 | |||||||||
| (B) Characteristics of Magnetic Resonance Imaging included studies | |||||||||||||
| Author | Year | Country | Index Test | Second Test | Number of Testla | Reference Standard | Prospective/Retrospective | N Analysed | N with Axillary Metastases | Prevalence of Axillary Metastases | MEAN AGE | Period of Study | Other Criteria |
| Kim S.H. [53] | 2017 | South Korea | MRI | With and without DWI + Gadolinium IV | 3T | Histology | Retrospective | 149 | 50 | 33.6% | 49.2 | 2014–2015 | |
| (SLNB/ALND) | |||||||||||||
| Yun S.J. [54] | 2016 | South Korea | MRI | With DWI + Gadolinium IV | 3T | Histology | Retrospective | 124 | 34 | 27.4% | 59.8 | 2011–2014 | |
| (SLNB/ALND) | |||||||||||||
| Schipper R.J. [55] | 2015 | The Netherlands | MRI | With and without DWI | 3T | Histology | Retrospective | 50 | 12 | 24.0% | 60 | 2012–2013 | Only T1-T2-T3 |
| (SLNB/ALND) | tumors | ||||||||||||
| Ergul N. [56] | 2015 | Turkey | MRI | With and without DWI | 1.5T | Histology | Prospective | 24 | 15 | 62.5% | 47 | 2012–2013 | Only T1-T2 |
| (SLNB/ALND) | tumors | ||||||||||||
| Kamitani T. [57] | 2013 | Japan | MRI | DWI alone | 1.5T | Histology | Retrospective | 110 | 26 | 23.6% | 54.9 | 2006–2007 | |
| (SLNB/ALND) | |||||||||||||
| Fornasa F. [58] | 2012 | Italy | MRI | With DWI + Gadolinium IV | 1.5T | Histology | Prospective | 43 | 19 | 44.2% | 58 | 2008–2010 | |
| (SLNB/ALND) | |||||||||||||
| Scaranelo M. [59] | 2012 | Canada | MRI | With and without DWI | 1.5T | Histology | Prospective | 65 | 28 | 43.1% | 53 | 2008–2009 | |
| (SLNB/ALND) | |||||||||||||
| Memarsadeghi M. [60] | 2006 | Austria | MRI | Without DWI + USPIO IV | 1T | Histology | Prospective | 22 | 6 | 27.3% | 62 | 5 months | |
| (SLNB/ALND) | |||||||||||||
| Michel S.C. [61] | 2002 | Switzerland | MRI | Without DWI + USPIO IV | 1.5T | Histology | Prospective | 18 | 11 | 61.1% | 53 | 2000–2001 | |
| (SLNB/ALND) | |||||||||||||
| Murray A.D. [62] | 2002 | UK | MRI | Without DWI + Gadolinium IV | 0.95T | Histology | ND | 47 | 10 | 21.3% | 63 | ND | |
| (SLNB/ALND) | |||||||||||||
| TOTAL | 652 | 211 | 32.4% | 55.4 | |||||||||
| (C) Characteristics of FDG Positron Emission Tomography included studies | |||||||||||||
| Author | Year | Country | Index Test | Second Test | Evaluation | Reference Standard | Prospective/Retrospective | N Analysed | N with Axillary Metastases | Prevalence of Axillary Metastases | Mean Age | Years of Study | Other Criteria |
| Ergul N. [56] | 2015 | Turkey | FDG PET | With CT | Visual and semi-quantitative | Histology | Prospective | 24 | 15 | 62.5% | 47 | 2012–2013 | Only T1-T2 tumors |
| (SLNB/ALND) | |||||||||||||
| Jeong Y.J. [63] | 2014 | South Korea | FDG PET | With CT | Visual and semi-quantitative | Histology | Retrospective | 178 | 48 | 27.0% | 54.9 | 2010–2013 | |
| (SLNB/ALND) | |||||||||||||
| Park J. [64] | 2014 | South Korea | FDG PET | With CT | Visual and semi-quantitative | Histology | Retrospective | 136 | 70 | 51.5% | 49.7 | 2009–2012 | 3 patients without FDG-avid breast tumors excluded |
| (SLNB/ALND) | |||||||||||||
| Sohn Y.M. [31] | 2014 | South Korea | FDG PET | With CT | Visual | Histology | Retrospective | 107 | 45 | 42.1% | 53.9 | 2009–2012 | |
| (SLNB/ALND) | |||||||||||||
| Machida Y. [65] | 2013 | Japan | FDG PET | With CT | Visual and semi-quantitative | Histology | Retrospective | 227 | 54 | 23.8% | ND | 2005–2009 | |
| (SLNB/ALND) | |||||||||||||
| Seok J.W. [66] | 2013 | South Korea | FDG PET | With CT | Visual and semi-quantitative | Histology | Retrospective | 104 | 21 | 20.2% | 49.4 | 2010–2012 | Only T1-T2 tumors |
| (SLNB/ALND) | |||||||||||||
| Hahn S. [67] | 2012 | Germany | FDG PET | With CT | Visual and semi-quantitative | Histology | Retrospective | 38 | 16 | 26.9% | 52 | 2008 | Only T1-T2 tumors |
| (SLNB/ALND) | |||||||||||||
| Riegger C. [34] | 2012 | Germany | FDG PET | With CT | Visual | Histology | Retrospective | 91 | 37 | 40.7% | 55.5 | 2007–2010 | |
| (SLNB/ALND) | |||||||||||||
| Choi W.H. [68] | 2011 | South Korea | FDG PET | With CT | Visual and semi-quantitative | Histology | Retrospective | 171 | 73 | 42.7% | 50.1 | 2003–2006 | |
| (SLNB/ALND) | |||||||||||||
| Heusner T.A. [69] | 2009 | Germany | FDG PET | With CT | Visual | Histology | Retrospective | 61 | 24 | 39.3% | 56 | 2007–2008 | |
| (SLNB/ALND) | |||||||||||||
| Kim J [70] | 2009 | South Korea | FDG PET | With CT | Visual | Histology | Prospective | 137 | 35 | 25.5% | 50.5 | 2007–2008 | Only T1-T2 tumors |
| (SLNB/ALND) | |||||||||||||
| Monzawa S. [40] | 2009 | Japan | FDG PET | With CT | Visual | Histology | Retrospective | 50 | 15 | 30.0% | 59 | 2005–2006 | |
| (SLNB/ALND) | |||||||||||||
| Taira N. [71] | 2008 | Japan | FDG PET | With CT | Visual and semi-quantitative | Histology | Retrospective | 92 | 27 | 29.3% | 54.6 | 2006–2007 | |
| (SLNB/ALND) | |||||||||||||
| Ueda S. [43] | 2008 | Japan | FDG PET | With CT | Visual | Histology | Prospective | 183 | 59 | 32.2% | 57 | 2005–2007 | |
| (SLNB/ALND) | |||||||||||||
| Veronesi U. [72] | 2007 | Italy | FDG PET | With CT | Visual and semi-quantitative | Histology | Retrospective | 236 | 103 | 43.6% | 49 | 2003–2005 | Only T1-T2-T3 tumors |
| (SLNB/ALND) | |||||||||||||
| Kumar R. [73] | 2006 | USA | FDG PET | Without CT | ND | Histology | Prospective | 80 | 36 | 45.0% | 52 | ND | |
| (SLNB/ALND) | |||||||||||||
| Weir L. [74] | 2005 | Canada | FDG PET | Without CT | Visual | Histology | Retrospective | 40 | 18 | 45.0% | 52 | 2000–2003 | |
| (SLNB/ALND) | |||||||||||||
| Fehr M.K. [75] | 2004 | Switzerland | FDG PET | Without CT | Visual | Histology | Prospective | 24 | 10 | 41.7% | 56 | ND | Tumors |
| (SLNB/ALND) | < 3 cm (clinical) | ||||||||||||
| Zornoza M.J. [76] | 2004 | Spain | FDG PET | Without CT | Visual | Histology | Prospective | 200 | 107 | 53.5% | 52.2 | ND | Tumors < 3.5 cm (ND) |
| (SLNB/ALND) | |||||||||||||
| Barranger E. [77] | 2003 | France | FDG PET | Without CT | Visual | Histology | Prospective | 32 | 15 | 46.9% | 58 | 2001 | Only T1-T2 tumors |
| (SLNB/ALND) | |||||||||||||
| Guller U. [78] | 2002 | Switzerland | FDG PET | Without CT | ND | Histology | Prospective | 31 | 14 | 45.2% | 64.8 | ND | |
| (SLNB/ALND) | |||||||||||||
| Nakamoto Y. [79] | 2002 | USA | FDG PET | Without CT | Visual | Histology | Prospective | 36 | 15 | 41.7% | 50.6 | ND | |
| (SLNB/ALND) | |||||||||||||
| Rieber A. [80] | 2002 | Germany | FDG PET | Without CT | ND | Histology | Retrospective | 40 | 20 | 50.0% | 52.9 | ND | |
| (SLNB/ALND) | |||||||||||||
| Van der Hoeven J.M. [81] | 2002 | The Netherlands | FDG PET | Without CT | Visual | Histology (SLNB/ALND) | Prospective | 70 | 32 | 45.7% | 58 | 1997–2000 | |
| TOTAL | 2388 | 909 | 38.1% | 52.9 | |||||||||
| (D) Characteristics of Fine Needle Aspiration included studies | |||||||||||||
| Author | Year | Country | Index Test | Evaluation | Prospective/Retrospective? | N Analysed | N with Axillary Metastases | Prevalence of Axillary Metastases | Mean Age | Years of Studies | Other Criteria | ||
| Zhu Y. [28] | 2016 | China | FNA | Histology | Retrospective | 445 | 169 | 38.0% | 55.6 | 2013–2014 | Only T1-T2 tumors | ||
| (SLNB/ALND) | |||||||||||||
| Sohn Y.M. [31] | 2014 | South Korea | FNA | Histology | Retrospective | 107 | 45 | 42.1% | 53.9 | 2009–2012 | |||
| (SLNB/ALND) | |||||||||||||
| Ganott M.A. [82] | 2014 | USA | FNA | Histology | Prospective | 44 | 26 | 59.1% | ND | 2008–2010 | |||
| (SLNB/ALND) | |||||||||||||
| Hayes B.D. [83] | 2011 | Ireland | FNA | Histology | Retrospective | 161 | 86 | 53.4% | ND | 2006–2009 | |||
| (SLNB/ALND) | |||||||||||||
| Schiettecatte A. [36] | 2011 | Belgium | FNA | Histology | Retrospective | 147 | 67 | 45.6% | 56 | ND | |||
| (SLNB/ALND) | |||||||||||||
| Luparia A. [39] | 2010 | Italy | FNA | Histology | Retrospective | 427 | 170 | 39.8% | 60.9 | 2005–2008 | FNA was not performed for all suspicious axillary US | ||
| (SLNB/ALND) | |||||||||||||
| Tahir M. [84] | 2008 | UK | FNA | Histology | Prospective | 38 | 17 | 44.7% | 56.7 | 2005–2006 | |||
| (SLNB/ALND) | |||||||||||||
| Cowher M.S. [41] | 2008 | USA | FNA | Histology | Retrospective | 125 | 57 | 45.6% | 61.3 | 2004–2005 | |||
| (SLNB/ALND) | |||||||||||||
| Moore A. [42] | 2008 | USA | FNA | Histology | Retrospective | 112 | 58 | 51.8% | ND | ND | Only high risk of metastases | ||
| (SLNB/ALND) | |||||||||||||
| Davis J.T. [45] | 2006 | USA | FNA | Histology | Prospective | 37 | 22 | 59.5% | ND | 2004–2005 | Only high risk of metastases | ||
| (SLNB/ALND) | |||||||||||||
| Popli M.B. [47] | 2006 | India | FNA | Histology | Prospective | 30 | 22 | 73.3% | ND | ND | |||
| (SLNB/ALND) | |||||||||||||
| Podkrajsek M. [48] | 2005 | Slovenia | FNA | Histology | Retrospective | 165 | 65 | 39.4% | 56 | 2001–2003 | |||
| (SLNB/ALND) | |||||||||||||
| Deurloo E.E. [50] | 2003 | The Netherlands | FNA | Histology | Prospective | 268 | 121 | 45.1% | 56 | 1999–2001 | |||
| (SLNB/ALND) | |||||||||||||
| Sapino A. [52] | 2003 | Italy | FNA | Histology | Prospective | 298 | 88 | 29.5% | ND | 2000 | |||
| (SLNB/ALND) | |||||||||||||
| TOTAL | 2404 | 1013 | 42.1% | 49.9 | |||||||||
ALND: Axillary Lymph Nodes Dissection; SLNB: Sentinel Lymph Node Biopsy; CNB: Core Needle Biopsy; FNA: Fine Needle Aspiration; DWI: Diffusion Weighted Imaging; IV: Intravenous injection; MRI: Magnetic Resonance Imaging; CT: Computed Tomography; FDG: Fluorodeoxyglucose; PET: Positron Emission Tomography; USPIO: Ultrasmall Superparamagnetic Iron Oxide; US: Ultrasonography; N: Number of patients; ND: Not Determined; UK: United Kingdom; USA: United States of America.
In total, 62 studies were suitable for inclusion (Figure 1). There were 30 studies assessing US with or without fine needle aspiration/core needle biopsy, including 7546 patients of which 2668 had ALN metastases (prevalence = 35.4%), 10 studies assessing MRI, including 652 patients of which 211 had ALN metastases (prevalence = 32.4%), and 24 studies assessing PET, including 2388 patients of which 909 had ALN metastases (prevalence = 38.1%).
Figure 1.
Flowchart depicting the inclusion and exclusion of the identified studies.
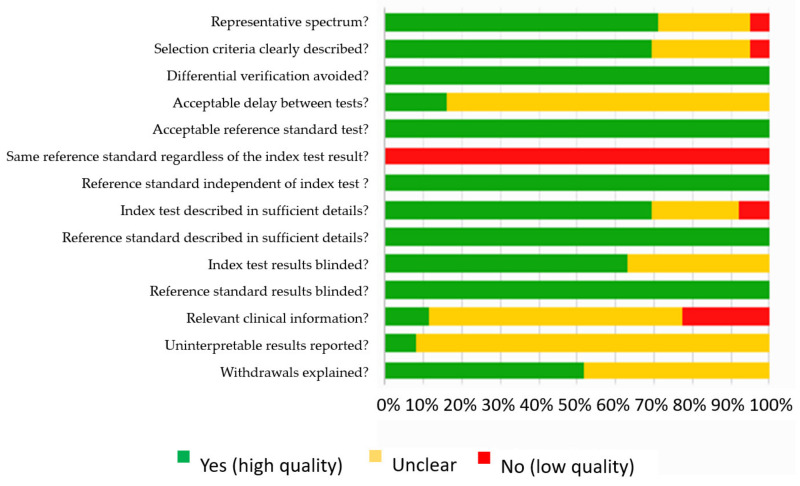
3.2. Quality of Included Studies
Figure 2 summarizes the methodological quality of the 62 included studies.
Figure 2.
Quality analysis of the included studies based on QUality Assessment of Diagnostic Accuracy Studies (QUADAS).
In general, the reference standard was adequate, but was not the same for all patients (either SLNB or ALN dissection), and the choice of the reference standard depended on the index test results (for instance, ALN dissection was performed for biopsy-proven metastatic nodes). The reference standard and the index test were well described in every study.
The index test was interpreted by reviewers blinded to reference standard results in all studies. The index test was often interpreted by reviewers blinded to other clinical data, most of the cases for MRI and PET studies, but rarely in US studies. Uninterpretable results were discussed in only 5 studies.
3.3. Sensitivity and Specificity of US, MRI and PET
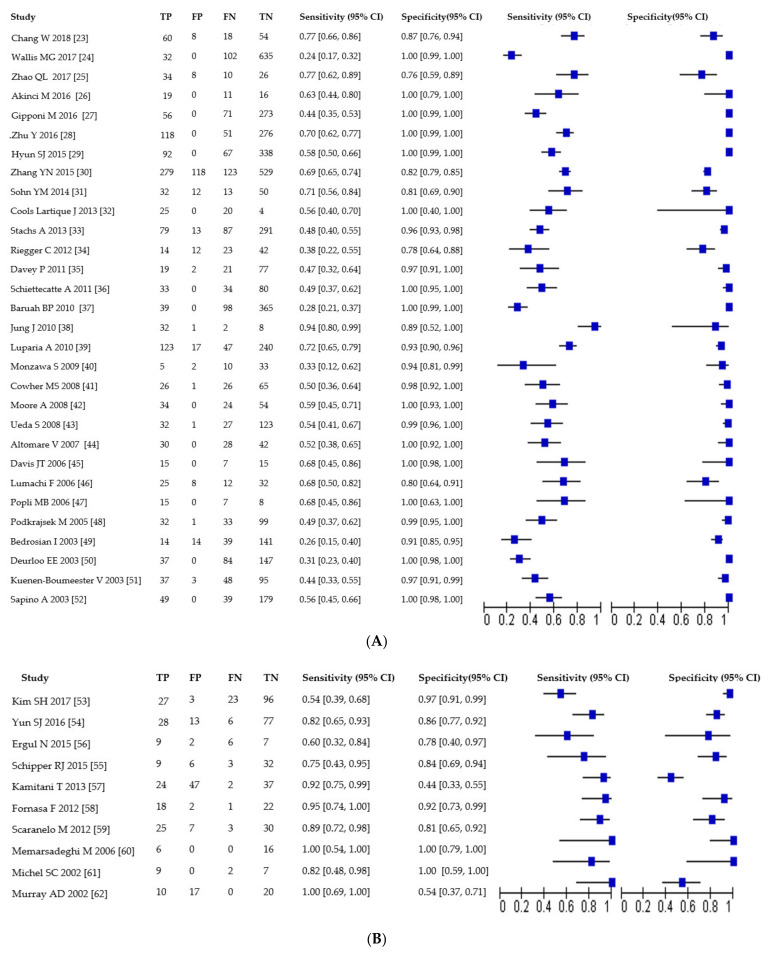
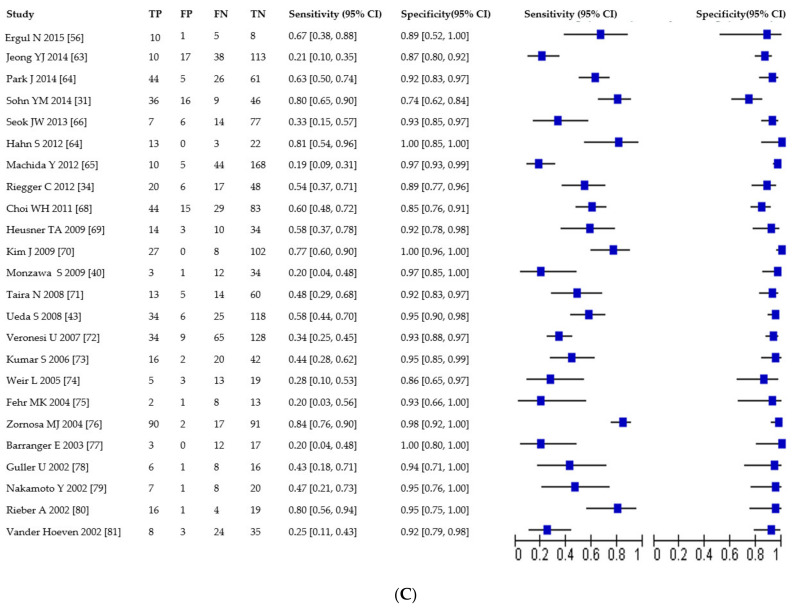
Of the 30 studies evaluating US, sensitivity was 55% (95% CI: 49–62%; range 24–94%) and specificity was 99% (95% CI: 97–100%; range 76–100%). Of the 10 studies evaluating MRI, sensitivity was 83% (95% CI: 72–91%; range 50–100%) and specificity was 85% (95% CI: 72–92%; range 44–100%). Of the 24 studies evaluating PET, sensitivity was 49% (95% CI: 39–59%; range 19–84%) and specificity was 94% (95% CI: 91–96%; range 74–100%).
Results are presented in Table 2 and Figure 3A–C.
Table 2.
Summary estimates of sensitivity, specificity, diagnostic odds ratio, and their 95% confidence intervals of US, MRI, and FDG PET.
| Imaging Technique | N Studies | Sensitivity | I2 | Specificity | I2 | DOR |
|---|---|---|---|---|---|---|
| US | 30 | 0.55 (0.49, 0.62) | 90.01 | 0.99 (0.97, 1.00) | 95.06 | 112 (39, 320) |
| US grayscale | 24 | 0.63 (0.56, 0.69) | 88.86 | 0.88 (0.82, 0.92) | 93.91 | 12 (8, 18) |
| US + FNA|CNB | 20 | 0.51 (0.43, 0.59) | 88.44 | 1.00 (0.99, 1.00) | 94.19 | 752 (98, 5765) |
| FNA | 14 | 0.78 (0.73, 0.83) | 55.40 | 0.99 (0.96, 1.00) | 48.73 | 560 (91, 3451) |
| MRI | 10 | 0.83 (0.72, 0.91) | 75.81 | 0.85 (0.72, 0.92) | 93.00 | 28 (16, 51) |
| MRI without DWI | 7 | 0.81 (0.49, 0.95) | 89.17 | 0.84 (0.74, 0.91) | 89.04 | 22 (7, 72) |
| MRI with DWI | 4 | 0.78 (0.60, 0.89) | 79.35 | 0.90 (0.82, 0.95) | 67.07 | 33 (17, 65) |
| DWI alone | 5 | 0.74 (0.50, 0.89) | 83.54 | 0.78 (0.51, 0.92) | 93.63 | 10 (5, 19) |
| PET FDG | 24 | 0.49 (0.39, 0.59) | 87.03 | 0.94 (0.91, 0.96) | 73.98 | 15 (8, 26) |
| PET FDG without CT | 9 | 0.44 (0.28, 0.62) | 90.90 | 0.95 (0.91, 0.97) | 0 | 14 (5, 44) |
| PET FDG with CT | 15 | 0.51 (0.40, 0.63) | 86.04 | 0.93 (0.89, 0.96) | 79.51 | 14 (8, 27) |
CNB: Core Needle Biopsy; CT: Computed Tomography; DOR: Diagnostic Odds Ratio; DWI: Diffusion Weighed Imaging; FDG: Fluorodeoxyglucose; FNA: Fine Needle Aspiration; MRI: Magnetic Resonance Imaging; PET: Positron Emission Tomography; US: Ultrasonography. The diagnostic odd ratio (DOR) values obtained with different combinations of sensitivity and specificity could be used as a single summary measure. It was defined as the ratio of odds of positivity in disease relative to non-diseased. The DOR value ranges from 0 to infinity, and a higher value signifies better diagnostic performance. A value of 1 indicates that a test cannot distinguish between patients with or without the disease and values of <1 introduce more FN results among the diseased [22]. Confidence intervals consider the heterogeneity beyond chance between studies (random effects models). The impact of unobserved heterogeneity is traditionally assessed statistically using the quantity I2. It describes the percentage of total variation across studies that is attributable to the heterogeneity rather than chance [22]. Magnetic resonance imaging (MRI) had a significantly higher sensitivity than other imaging modalities, whereas Ultrasonography (US) had a significantly higher specificity than MRI and to a lesser extent than fluorodeoxyglucose positron emission tomography (PET). DOR estimated for US was significantly greater than those of MRI, which in turn was significantly greater than those of FDG PET. Further analysis revealed that for all imaging modality, US + fine needle aspiration (FNA) or core needle biopsy (CNB) had the highest DOR value. For MRI studies, MRI with diffusion weighted imaging (DWI) had the highest DOR value and for PET studies. PET with or without computed tomography (CT) had the same DOR value.
Figure 3.
(A) Forest plot of sensitivity and specificity for US studies. TP = true positive, FP = false positive, FN = false negative, TN = true negative. Brackets show 95% confidence intervals. The figure shows the sensitivity and specificity for each study (squares) and 95% confidence intervals (horizontal lines). (B) Forest plot of sensitivity and specificity for MRI studies. TP = true positive, FP = false positive, FN = false negative, TN = true negative. Brackets show 95% confidence intervals. The figure shows the sensitivity and specificity for each study (squares) and 95% confidence intervals (horizontal lines). (C) Forest plot of sensitivity and specificity for PET studies. TP = true positive, FP = false positive, FN = false negative, TN = true negative. Brackets show 95% confidence intervals. The figure shows the sensitivity and specificity for each study (squares) and 95% confidence intervals (horizontal lines).
3.4. US Subgroups Analysis
Of 24 studies evaluating US grayscale only (N = 5575, prevalence: 37.4%), sensitivity was 63% (95% CI: 56–69%; range 28–88%) and specificity was 88% (95% CI: 82–92%; range 38–100%). Of 20 studies evaluating US + fine needle aspiration/core needle biopsy (N = 4874, prevalence: 33.1%), sensitivity was 51% (95% CI: 43–59%; range 24–94%) and specificity was 100% (95% CI: 99–100%; range 89–100%). Across 14 studies evaluating fine needle aspiration (N = 2404 patients, prevalence: 42.1%), sensitivity was 78% (95% CI: 73–83%; range 47–90%) and specificity was 99% (95% CI: 96–100%; range 91–100%). Only 2 studies evaluated elastosonography, not allowing meta-analysis: They both demonstrated a better sensitivity for US + elastosonography (disjunctive method) than elastosonography alone, but a lesser specificity. Results are presented in Table 2.
3.5. MRI Subgroups Analysis
Of the 7 studies evaluating MRI without DWI (N = 375, prevalence: 35.2%), sensitivity was 81% (95% CI: 49–95%; range 24–82%) and specificity was 84% (95% CI: 74–91%; range 54–100%). Of the 4 studies evaluating MRI with DWI (N = 366, prevalence: 31.4%), sensitivity was 78% (95% CI: 60–89%; range 54–95%) and specificity was 90% (95% CI: 82–95%; range 84–97%). Of the 5 studies evaluating DWI only (N = 398, prevalence: 32.9%), sensitivity was 74% (95% CI: 50–89%; range 40–83%) and specificity was 78% (95% CI: 51–92%; range 44–100%). Results are presented in Table 2.
3.6. PET Subgroups Analysis
Of the 9 studies evaluating PET without CT (N = 553, prevalence: 48.3%), sensitivity was 44% (95% CI: 28–62%; range 20–84%) and specificity was 95% (95% CI: 91–97%; range 85–100%). Of the 15 studies evaluating PET with CT (N = 1835, prevalence: 35%), sensitivity was 51% (95% CI: 40–63%; range 19–81%) and specificity was 93% (95% CI: 89–96%; range 74–100%). Results are presented in Table 2.
3.7. Subgroup Analysis on Axillary Metastatic Burden
In 12 studies (1497 patients), data about axillary burden were presented, including the histological size of the largest ALN metastasis. The overall preoperative FN rate was 0.93 (0.87, 0.97) for micrometastasis and 0.56 (0.51, 0.61) for macrometastasis. For US (705 patients), the FN rate was 0.96 (0.86, 0.99) for micrometastasis, and 0.52 (0.45, 0.59) for macrometastasis. For PET (643 patients), the FN was 0.59 (0.46, 0.71) for micrometastasis, and 0.64 (0.56, 0.71) for macrometastasis. No subgroup analysis was possible for MRI due to the lack of data.
The number of involved ALN in early-stage breast cancer patients (T1 or T2) was given in 4 studies. For ultrasonography (632 patients), the FN rate was 0.63 (0.57, 0.68) for 1 or 2 involved node(s) and 0.28 (0.22, 0.34) when 3 or more nodes were involved.
4. Discussion
In this meta-analysis assessing the diagnostic performances of US, MRI, and PET for pretherapeutic ALN staging, we found that while MRI had a significant higher sensitivity than other imaging modalities, the performance of US significantly improved for macrometastases in more than 2 ALN. The association of US and fine needle aspiration had the highest diagnostic odd ratio, in part because of a specificity close to 100%.
Unlike other published meta-analysis, we chose to assess each of these 3 techniques to put in contrast their respective strengths and weaknesses and to offer an overview of the role of imaging for nodal staging and ultrastadification.
We did not include patients with clinically positive ALN, for which preoperative imaging is unlikely to change treatment plan [12]. We also chose not to include patients undergoing neoadjuvant chemotherapy, in order to have a gold-standard reference test available for every patient.
While previously published meta-analysis had a high prevalence of ALN metastasis [3,11], the metastasis rate in our study was in line with the commonly described rate of ALN metastasis in invasive breast cancer, between 30 and 40% [3,4].
Management of axilla has evolved with the increased use of neoadjuvant treatment. Furthermore, the ACOSOG Z0011 trial proved that women with micrometastases or less than 2 metastatic ALN and clinical T1-2 tumors undergoing lumpectomy and breast radiation therapy followed by systemic therapy, did not benefit from ALN dissection in terms of local control and 10-year overall survival [13]. An ideal preoperative axillary staging should therefore be able not only to detect macrometastasis with high accuracy, but also to evaluate the global axillary burden, in order to avoid unnecessary ALN dissection in low axillary burden.
We found that axillary US has a very high specificity (99%, 95% CI: 97–100%), in contrast with its much lower overall sensitivity [85,86], which indeed depends on the axillary burden: FN rate of US drops to 0.28 when more than 2 ALN are involved, while micrometastases are almost never detected. This data is fundamental to avoid over-treatment, as micrometastasis should not lead to an ALN dissection or the prescription of chemotherapy. A recent study on interobserver variability showed that the discrimination between low and high axillary burden on US is reliable and reproducible [87]. US should be used for first-line axillary triage, to detect high metastatic burden that could benefit from neoadjuvant chemotherapy, without diverting low-burden patients from SLNB procedure. Technical improvements, such as elastosonography [23,25] or the use of intradermal microbubbles to locate and biopsy the sentinel lymph node under ultrasound guidance [88] may further increase US sensitivity.
We found that MRI has a better sensitivity than US for detection of nodal metastasis. This is in line with the results of other meta-analysis, for example, Liang et al. [7] found a sensitivity of 82% (95%CI: 78–86). The main drawback of MRI is its relatively low specificity compared to other imaging modalities, which makes it unsuitable for surgical or oncological planning. The adjunction of diffusion-weighting imaging seems to significantly increase its specificity while only slightly decreasing its sensitivity. In one study by Hieken et al. [89], second-look US after abnormal axillary findings on MRI allowed detection of abnormal nodes not previously detected by US in only 10% of the cases. In the clinical situation of a positive MRI with negative US, there is a significant risk of axillary false positive.
In our study, PET shows a lower sensitivity than in Cooper’s less recent meta-analysis (49% vs. 63%) [4]. Indeed, performance of PET may vary depending on breast cancer histological subtypes, with higher performances in basal than luminal subtypes [90,91] and also depending on the histological gold standard (e.g., high rate of micrometastases in recent studies [15]). A functional, high-sensitivity imaging, PET has a much higher detection rate of micrometastases than US, which can theoretically lead to unnecessary ALN dissection or neoadjuvant chemotherapy. Yet, PET has the unique ability to detect extranodal distant metastasis and should be used preferentially in patients at high risk for extranodal disease. Further technical improvements, especially new markers for hormone-positive or HER2-positive breast tumors, may redefine the role of PET imaging in axillary staging.
Our study has some limits. A relatively low number of MRI studies were included in our metanalysis, as this imaging modality has only been studied more recently for axilla staging. Likewise, probably due to the lower availability of MRI and PET, these modalities are more widely used for T3-T4 than T1-T2 stages. It may explain why MRI and PET studies include fewer T1-T2 breast cancer than US studies. However, the prevalence of ALN metastases for each of 3 modalities was roughly the same, between 30 and 40%. High heterogeneity of MRI subgroup analysis was probably due to the lack of consensus on the criteria used to define a suspicious ALN on MRI, as well as difference in imaging protocol between centers (MRI field strength, imaging parameters). Finally, information about axillary burden was not widely available in MRI and PET studies.
Thus, future imaging studies should systematically include such parameters as the number of metastatic ALN, the presence of micrometastases versus macrometastases, and the presence of a capsular rupture to avoid over diagnosis and over treatment.
5. Conclusions
US is an effective technique for axillary triage, especially to detect high metastatic burden that could benefit from neoadjuvant chemotherapy or axillary clearance, without upstaging the majority of micrometastases.
Author Contributions
M.L.B. and J.G. wrote the manuscript, gathered, and analyzed the relevant studies that assessed the accuracy and utility of US, MRI, and PET in staging the axilla. J.G. did statistical analysis. Z.S., S.M., and C.M. reviewed the manuscript and suggested significant changes. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Torre L.A., Islami F., Siegel R.L., Ward E.M., Jemal A. Global cancer in women: Burden and trends. Cancer Epidemiol. Biomark. Prev. 2017;26:444–457. doi: 10.1158/1055-9965.EPI-16-0858. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D.M., Forman D., Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Houssami N., Ciatto S., Turner R.M., Cody H.S., MacAskill P. Preoperative ultrasound-guided needle biopsy of axillary nodes in invasive breast cancer: Meta-analysis of its accuracy and utility in staging the axilla. Ann. Surg. 2011;254:243–251. doi: 10.1097/SLA.0b013e31821f1564. [DOI] [PubMed] [Google Scholar]
- 4.Cooper K.L., Harnan S., Meng Y., Ward S.E., Fitzergerald P., Papioannou D., Wyld L., Ingram C., Wilkinson I.D., Lorenz E. Positron emission tomography (PET) for assessment of axillary lymph node status in early breast cancer: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2011;37:187–198. doi: 10.1016/j.ejso.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Crane-Okada R., Wascher R.A., Elashoff D., Giuliano A.E. Long-term morbidity of sentinel node biopsy versus complete axillary dissection for unilateral breast cancer. Ann. Surg. Oncol. 2008;15:1996–2005. doi: 10.1245/s10434-008-9909-y. [DOI] [PubMed] [Google Scholar]
- 6.McLaughlin S.A., Wright M.J., Morris K.T., Giron G.L., Sampson M.R., Brockway J.P., Hurley K.E., Riedel E.R., van Zee K.J. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: Objective measurements. J. Clin. Oncol. 2008;26:5213–5219. doi: 10.1200/JCO.2008.16.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu C., Guo Y., Shi J., Sheng Y. Late morbidity associated with a tumour-negative sentinel lymph node biopsy in primary breast cancer patients: A systematic review. Eur. J. Cancer. 2009;45:1560–1568. doi: 10.1016/j.ejca.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Kang B., Jun H., Lee K., Lee K., Kim S. Clinical application of sentinel lymph node biopsy based on axillary anatomy in breast cancer: A single institution experience. Breast. 2014;23:812–815. doi: 10.1016/j.breast.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Liang X., Yu J., Wen B., Xie J., Cai Q., Yang Q. MRI and FDG-PET/CT based assessment of axillary lymph node metastasis in early breast cancer: A meta-analysis. Clin. Radiol. 2017;72:295–301. doi: 10.1016/j.crad.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Sui W.F., Chen X., Peng Z.K., Ye J., Wu J.T. The diagnosis of metastatic axillary lymph nodes of breast cancer by diffusion weighted imaging: A meta-analysis and systematic review. World J. Surg. Oncol. 2016;14 doi: 10.1186/s12957-016-0906-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X.-W., Xiong Y.-H., Zen X.-Q., Lin H.-B., Liu Q.-Y. Diagnostic accuracy of ultrasonograph guided fine-needle aspiration cytologic in staging of axillary lymph node metastasis in breast cancer patients: A meta-analysis. Asian Pac. J. Cancer Prev. 2012;13:5517–5523. doi: 10.7314/APJCP.2012.13.11.5517. [DOI] [PubMed] [Google Scholar]
- 12.Lyman G.H., Giuliano A.E., Somerfield M.R., Benson A.B., Bodurka D.C., Burstein H.J., Cochran A.J., Cody H.S., Edge S.B., Galper S., et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J. Clin. Oncol. 2005;23:7703–7720. doi: 10.1200/JCO.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Giuliano A.E., Ballman K.V., McCall L., Beitsch P.D., Bennan M.B., Kelemen P.R., Ollila D.W., Hansen N.M., Whitworth P.W., Blumencranz P.W. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA. 2017;318:918–926. doi: 10.1001/jama.2017.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harnan S.E., Cooper K.L., Meng Y., Ward S.E., Fitzgerald P., Papaioannou D., Ingram C., Lorenz E., Wilkinson I.D., Wyld L. Magnetic resonance for assessment of axillary lymph node status in early breast cancer: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2011;37:928–936. doi: 10.1016/j.ejso.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Peare R., Staff R.T., Heys S.D. The use of FDG-PET in assessing axillary lymph node status in breast cancer: A systematic review and meta-analysis of the literature. Breast Cancer Res. Treat. 2010;123:281–290. doi: 10.1007/s10549-010-0771-9. [DOI] [PubMed] [Google Scholar]
- 16.Yang W.T., Le-Petross H.T., Macapinlac H., Carkaci S., Gonzalez-Angulo A.M., Dawood S., Resetkova E., Hortobagyi G.N., Cristofanilli M. Inflammatory breast cancer: PET/CT, MRI, mammography, and sonography findings. Breast Cancer Res. Treat. 2008;109:417–426. doi: 10.1007/s10549-007-9671-z. [DOI] [PubMed] [Google Scholar]
- 17.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 18.PRISMA Statement Website. [(accessed on 15 March 2018)];2015 Available online: www.prisma-statement.org.
- 19.Whiting P., Rutjes A.W.S., Reitsma J.B., Bossuyt P.M.M., Kleijnen J. The development of QUADAS: A tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BioMed Cent. Med. Res. Methodol. 2003;13:1–13. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glas A.S., Lijmer J.G., Prins M.H., Bonsel G.J., Bossuyt P.M.M. The diagnostic odds ratio: A single indicator of test performance. J. Clin. Epidemiol. 2003;56:1129–1135. doi: 10.1016/S0895-4356(03)00177-X. [DOI] [PubMed] [Google Scholar]
- 21.Reitsma J.B., Glas A.S., Rutjes A.W.S., Scholten R.J.P.M., Bossuyt P.M., Zwinderman A.H. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J. Clin. Epidemiol. 2005;58:982–990. doi: 10.1016/j.jclinepi.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 22.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 23.Chang W., Jia W., Shi J., Yuan C., Zhang Y., Chen M. Role of elastography in axillary examination of patients with breast cancer. J. Ultrasound Med. 2018;37:699–707. doi: 10.1002/jum.14538. [DOI] [PubMed] [Google Scholar]
- 24.Wallis M.G., Kilburn-Toppin F., Taylor-Phillips S. Does preoperative axillary staging lead to overtreatment of women with screen-detected breast cancer? Clin. Radiol. 2017:10–15. doi: 10.1016/j.crad.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Q.L., Xia X.N., He J.J., Sheng W., Ruan L.T., Yin Y.M., Lou H.L. Elastosonography and two-dimensional ultrasonography in diagnosis of axillary lymph node metastasis in breast cancer. Clin. Radiol. 2017:5–11. doi: 10.1016/j.crad.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Akinci M., Bulut S.P., Erozgen F., Gurbuzel M., Gulsen G., Kocakusak A., Gulen M., Kaplan R. Predictive value of fine needle aspiration biopsy of axillary lymph nodes in preoperative breast cancer staging. Turk. J. Surg. 2016;32:191–196. doi: 10.5152/UCD.2015.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gipponi M., Fregatti P., Garlaschi A., Murelli F., Margarino C., Depaoli F., Baccini P., Gallo M., Friedman D. Axillary ultrasound and Fine-Needle Aspiration Cytology in the preoperative staging of axillary node metastasis in breast cancer patients. Breast. 2016;30:146–150. doi: 10.1016/j.breast.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Y., Zhou W., Zhou J.Q., Fei X.C., Ye T.J., Huang O., Chen X.S., Zhan W.W. Axillary staging of early-stage invasive breast cancer by ultrasound-guided fine-needle aspiration cytology: Which ultrasound criteria for classifying abnormal lymph nodes should be adopted in the post-ACOSOG Z0011 trial era? J. Ultrasound Med. 2016;35:885–893. doi: 10.7863/ultra.15.06019. [DOI] [PubMed] [Google Scholar]
- 29.Hyun S.J., Kim E.K., Yoon J.H., Moon H.J., Kim M.J. Adding MRI to ultrasound and ultrasound-guided fine-needle aspiration reduces the false-negative rate of axillary lymph node metastasis diagnosis in breast cancer patients. Clin. Radiol. 2015;70:716–722. doi: 10.1016/j.crad.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y.N., Wang C.J., Xu Y., Zhu Q.L. Sensitivity, specificity and accuracy of ultrasound in diagnosis of breast cancer metastasis to the axillary lymph nodes in Chinese patients. Ultrasound Med. Biol. 2015;41:1835–1841. doi: 10.1016/j.ultrasmedbio.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 31.Sohn Y.-M., Hong I.K., Han K. Role of [18F] fluorodeoxyglucose positron emission tomography-computed tomography, sonography, and sonographically guided fine-needle aspiration biopsy in the diagnosis of axillary lymph nodes in patients with breast cancer. J. Ultrasound Med. 2014;33:1013–1021. doi: 10.7863/ultra.33.6.1013. [DOI] [PubMed] [Google Scholar]
- 32.Cools-Lartigue J., Sinclair A., TRabulsi N., Meguerditchian A., Mesurolle B., Fuhrer R., Meterissian S. Preoperative axillary ultrasound and fine-needle aspiration biopsy in the diagnosis of axillary metastases in patients with breast cancer: Predictors of accuracy and future implications. Ann. Surg. Oncol. 2013;20:819–827. doi: 10.1245/s10434-012-2609-7. [DOI] [PubMed] [Google Scholar]
- 33.Stachs A., Gode K., Hartmann S., Stengel B., Nierling U., Dieterich M., Reimer T., Gerber B. Accuracy of axillary ultrasound in preoperative nodal staging of breast cancer - size of metastases as limiting factor. Springerplus. 2013;2:1–9. doi: 10.1186/2193-1801-2-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riegger C., Koeninger A., Hartung V., Otterbach F., Kimmig R., Forsting M., Bockisch A., Antoch G., Heusner T.A. Comparison of the diagnostic value of FDG-PET / CT and axillary ultrasound for the detection of lymph node metastases in breast cancer patients. Acta Radiol. 2012;53:1092–1108. doi: 10.1258/ar.2012.110635. [DOI] [PubMed] [Google Scholar]
- 35.Davey P., Stokes M., Kennedy R., Kirk S., Newell J., Majury C., McKillen J. The value of axillary ultrasound with fine needle aspiration as a pre-operative staging procedure in breast cancer: Northern Irish experience. Ir. J. Med. Sci. 2011;180:509–511. doi: 10.1007/s11845-011-0684-6. [DOI] [PubMed] [Google Scholar]
- 36.Schiettecatte A., Bourgain C., Breucq C., Buls N., De Wilde V., De Mey J. Initial axillary staging of breast cancer using ultrasound-guided fine needle aspiration: A liquid-based cytology study. Cytopathology. 2011;22:30–35. doi: 10.1111/j.1365-2303.2010.00738.x. [DOI] [PubMed] [Google Scholar]
- 37.Baruah B.P., Goyal A., Young P., Douglas-Jones A.G., Mansel R.E. Axillary node staging by ultrasonography and fine-needle aspiration cytology in patients with breast cancer. Br. J. Surg. 2010;97:680–683. doi: 10.1002/bjs.6964. [DOI] [PubMed] [Google Scholar]
- 38.Jung J., Park H., Park J., Kim H. Accuracy of preoperative ultrasound and ultrasound-guided fine needle aspiration cytology for axillary staging in breast cancer. ANZ J. Surg. 2010;80:271–275. doi: 10.1111/j.1445-2197.2009.05090.x. [DOI] [PubMed] [Google Scholar]
- 39.Luparia A., Campanino P., Cotti R., Lucarelli D., Durando M., Mariscotti G., Gandini G. Role of axillary ultrasound in the preoperative diagnosis of lymph node metastases in patients affected by breast carcinoma. Radiol. Med. 2010;115:225–237. doi: 10.1007/s11547-009-0465-8. [DOI] [PubMed] [Google Scholar]
- 40.Monzawa S., Adachi S., Suzuki K., Hirokaga H., Takao S., Sakuma T., Hanioka H. Diagnostic performance of fluorodeoxyglucose-positron emission tomography/computed tomography of breast cancer in detecting axillary lymph node metastasis: Comparison with ultrasonography and contrast-enhanced CT. Ann. Nucl. Med. 2009;23:855–861. doi: 10.1007/s12149-009-0314-9. [DOI] [PubMed] [Google Scholar]
- 41.Cowher M.S., Erb K.M., Poller W., Julian T.B. Correlation of the use of axillary ultrasound and lymph node needle biopsy with surgical lymph node pathology in patients with invasive breast cancer. Am. J. Surg. 2008;196:756–759. doi: 10.1016/j.amjsurg.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 42.Moore A., Hester M., Nam M.W., Brill Y.M., McGrath P., Wright H., Weisinger K., Romond E., Samayoa L.M. Distinct lymph nodal sonographic characteristics in breast cancer patients at high risk for axillary metastases correlate with the final axillary stage. Br. J. Radiol. 2008;81:630–636. doi: 10.1259/bjr/21933846. [DOI] [PubMed] [Google Scholar]
- 43.Ueda S., Tsuda H., Asakawa H., Omata J., Fukatsu K., Kondo N., Kondo T., Hama Y., Tamura K., Ishida J., et al. Utility of 18F-fluoro-deoxyglucose emission tomography/ computed tomography fusion imaging (18F-FDG PET/CT) in combination with ultrasonography for axillary staging in primary breast cancer. BMC Cancer. 2008;8:1–10. doi: 10.1186/1471-2407-8-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Altomare V., Guerriero G., Carino R., Battista C., Primavera A., Altomare A., Vaccaro D., Esposito A., Ferri A.M., Rabitti C. Axillary lymph node echo-guided fine-needle aspiration cytology enables breast cancer patients to avoid a sentinel lymph node biopsy. Preliminary experience and a review of the literature. Surg. Today. 2007;37:735–739. doi: 10.1007/s00595-006-3366-7. [DOI] [PubMed] [Google Scholar]
- 45.Davis J.T., Brill Y.M., Simmons S., Sachleben B.C., Cibull M.L., McGrath P., Wright H., Romond E., Hester M., Moore A., et al. Ultrasound-guided fine-needle aspiration of clinically negative lymph nodes versus sentinel node mapping in patients at high risk for axillary metastasis. Ann. Surg. Oncol. 2006;13:1545–1552. doi: 10.1245/s10434-006-9095-8. [DOI] [PubMed] [Google Scholar]
- 46.Lumachi F., Tregnaghi A., Ferretti G., Povolato M., Marzola M.C., Zucchetta P., Checchin D., Bui F. Accuracy of ultrasonography and 99mTc-sestamibi scintimammography for assessing axillary lymph node status in breast cancer patients. A prospective study. Eur. J. Surg. Oncol. 2006;32:933–936. doi: 10.1016/j.ejso.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 47.Popli M.B., Sahoo M., Mehrota N., Choudhury M., Kumar A., Pathania O.P., Thomas S. Preoperative ultrasound-guided fine-needle aspiration cytology for axillary staging in breast carcinoma. Australas. Radiol. 2006;50:122–126. doi: 10.1111/j.1440-1673.2006.01545.x. [DOI] [PubMed] [Google Scholar]
- 48.Podkrajsek M., Music M.M., Kadivec M., Zgajnar J., Besic N., Pogacnik A., Hocevar M. Role of ultrasound in the preoperative staging of patients with breast cancer. Eur. Radiol. 2005;15:1044–1050. doi: 10.1007/s00330-004-2545-4. [DOI] [PubMed] [Google Scholar]
- 49.Bedrosian I., Bedi D., Kuerer H.M., Fornage B.D., Harker L., Ross M.I., Ames F.C., Krishnamurthy S., Edeiken-Monroe B.S., Meric F., et al. Impact of clinicopathological factors on sensitivity of axillary ultrasonography in the detection of axillary nodal metastases in patients with breast cancer. Ann. Surg. Oncol. 2003;10:1025–1030. doi: 10.1245/ASO.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 50.Deurloo E.E., Tanis P.J., Gilhuijs K.G., Muller S.H., Kroger R.P.J. Reduction in the number of sentinel lymph node procedures by preoperative ultrasonography of the axila in breast cancer. Eur. J. Cancer. 2003;39:1068–1073. doi: 10.1016/S0959-8049(02)00748-7. [DOI] [PubMed] [Google Scholar]
- 51.Kuenen-Boumeester V., Menke-Pluymers M., de Kanter A.Y., Obdeijn I.-M.A., Urich D., Van Der Kwast T.H. Ultrasound-guided fine needle aspiration cytology of axillary lymph nodes in breast cancer patients. A preoperative staging procedure. Eur. J. Cancer. 2003;39:170–174. doi: 10.1016/S0959-8049(02)00501-4. [DOI] [PubMed] [Google Scholar]
- 52.Sapino A., Cassoni P., Zanon E., Fraire F., Croce S., Coluccia C., Donadio M., Bussolati G. Ultrasonographically-guided fine-needle aspiration of axillary lymph nodes: Role in breast cancer management. Br. J. Cancer. 2003;88:702–706. doi: 10.1038/sj.bjc.6600744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim S.H., Shin H.J., Shin K.C., Chae E.Y., Choi W.J., Cha J.H., Kim H.H. Diagnostic performance of fused diffusion-weighted imaging using T1-weighted imaging for axillary nodal staging in patients with early breast cancer. Clin. Breast Cancer. 2017;17:154–163. doi: 10.1016/j.clbc.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 54.Yun S.J., Sohn Y.M., Seo M. Differentiation of benign and metastatic axillary lymph nodes in breast cancer: Additive value of MRI computer-aided evaluation. Clin. Radiol. 2016;71:403.e1–403.e7. doi: 10.1016/j.crad.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 55.Schipper R., Paiman M., Beets-Tan R., Nelemans P.J., de Vries B., Heuts E. Diagnostic performance of dedicated axillary T2-and diffusion-weighted MR imaging for nodal staging in breast cancer. Radiology. 2015;275:345–355. doi: 10.1148/radiol.14141167. [DOI] [PubMed] [Google Scholar]
- 56.Ergul N., Kadioglu H., Yildiz S., Yucel S.B., Gucin Z., Erdogan E.B., Aydin M., Muslumanoglu M. Assessment of multifocality and axillary nodal involvement in early-stage breast cancer patients using 18F-FDG PET/CT compared to contrast-enhanced and diffusion-weighted magnetic resonance imaging and sentinel node biopsy. Acta Radiol. 2015;56:917–923. doi: 10.1177/0284185114539786. [DOI] [PubMed] [Google Scholar]
- 57.Kamitani T., Hatakenaka M., Yabuuchi H., Matsuo Y., Fujita N., Jinnouchi M., Nagao M., Shirahane K., Tokunaga E., Honda H. Detection of axillary node metastasis using diffusion-weighted MRI in breast cancer. Clin. Imaging. 2013;37:56–61. doi: 10.1016/j.clinimag.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 58.Fornasa F., Nesoti M.V., Bovo C., Bonavina M.G. Diffusion-weighted magnetic resonance imaging in the characterization of axillary lymph nodes in patients with breast cancer. J. Magn. Reson. Imaging. 2012;36:858–864. doi: 10.1002/jmri.23706. [DOI] [PubMed] [Google Scholar]
- 59.Scaranelo A.M., Eiada R., Jacks L.M., Kulkarni S.R., Crystal P. Accuracy of unenhanced MR imaging in the detection of axillary lymph node metastasis: Study of reproducibility and reliability. Radiology. 2012;262:425–434. doi: 10.1148/radiol.11110639. [DOI] [PubMed] [Google Scholar]
- 60.Memarsadeghi M., Riedl C.C., Kaneider A., Galid A., Rudas M., Matzek W., Helbich T.H. Axillary lymph node metastases in patients with breast carcinomas: Assessment with nonenhanced versus USPIO-enhanced MR imaging. Radiology. 2006;241:367–377. doi: 10.1148/radiol.2412050693. [DOI] [PubMed] [Google Scholar]
- 61.Michel S.C., Keller T.M., Frohlich J.M., Fink D., Caduff R., Seifert B., Marincek B., Kubik-Huch R.A. Preoperative breast cancer staging: MR imaging of the axilla with ultrasmall superparamagnetic iron oxide enhancement. Radiology. 2002;225:527–536. doi: 10.1148/radiol.2252011605. [DOI] [PubMed] [Google Scholar]
- 62.Murray A.D., Staff R.T., Redpath T.W., Gilbert F.J., Ah-See A.K., Brookes J.A., Miller I.D., Payne S. Dynamic contrast enhanced MRI of the axilla in women with breast cancer: Comparison with pathology of excised nodes. Br. J. Radiol. 2002;75:220–228. doi: 10.1259/bjr.75.891.750220. [DOI] [PubMed] [Google Scholar]
- 63.Jeong Y.J., Kang D.Y., Yoon H.J., Son H.J. Additional value of F-18 FDG PET/CT for initial staging in breast cancer with clinically negative axillary nodes. Breast Cancer Res. Treat. 2014;145:137–142. doi: 10.1007/s10549-014-2924-8. [DOI] [PubMed] [Google Scholar]
- 64.Park J., Byun B.H., Noh W.C., Lee S.S., Kim H.-A., Kim E.-K., Choi C.W., Lim S.M. Lymph node to primary tumor SUV ratio by 18F-FDG PET/CT and the prediction of axillary lymph node metastases in breast cancer. Clin. Nucl. Med. 2014;39:e249–e253. doi: 10.1097/RLU.0b013e3182a75477. [DOI] [PubMed] [Google Scholar]
- 65.Machida Y., Kubota K., Katayama T., Toriihara A., Shibuya H. Diagnostic performance of fluorodeoxyglucose-positron emission tomography/computed tomography combined with ultrasonography-guided fine needle aspiration cytology for identifying axillary lymph node status in patients with breast cancer. Eur. J. Surg. Oncol. 2013;39:26–30. doi: 10.1016/j.ejso.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 66.Seok J.W., Kim Y., An Y.-S., Kim B.S. The clinical value of tumor FDG uptake for predicting axillary lymph node metastasis in breast cancer with clinically negative axillary lymph nodes. Ann. Nucl. Med. 2013;27:546–553. doi: 10.1007/s12149-013-0720-x. [DOI] [PubMed] [Google Scholar]
- 67.Hahn S., Hecktor J., Grabellus F., Hartung V., Poppel T., Kimmig R., Forsting M., Antoch G., Heusner T.A. Diagnostic accuracy of dual-time-point 18F-FDG PET/CT for the detection of axillary lymph node metastases in breast cancer patients. Acta Radiol. 2012;53:518–523. doi: 10.1258/ar.2012.110420. [DOI] [PubMed] [Google Scholar]
- 68.Choi W.H., Yoo I.R., O J.H., Kim S.H., Chung S.K. The value of dual-time-point 18 F-FDG PET/CT for identifying axillary lymph node metastasis in breast cancer patients. Br. J. Radiol. 2011;84:593–599. doi: 10.1259/bjr/56324742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heusner T.A., Kuemmel S., Hahn S., Koeninger A., Otterbach F., Hamami M.E., Kimmig K.R., Forsting M., Bockisch A., Antoch G., et al. Diagnostic value of full-dose FDG PET/CT for axillary lymph node staging in breast cancer patients. Eur. J. Nucl. Med. Mol. Imaging. 2009;36:1543–1550. doi: 10.1007/s00259-009-1145-6. [DOI] [PubMed] [Google Scholar]
- 70.Kim J., Lee J., Chang E., Kim S., Suh K., Sul J., Song I., Kim Y., Lee C. Selective sentinel node plus additional non-sentinel node biopsy based on an FDG-PET/CT scan in early breast cancer patients: Single institutional experience. World J. Surg. 2009;33:943–949. doi: 10.1007/s00268-009-9955-z. [DOI] [PubMed] [Google Scholar]
- 71.Taira N., Ohsumi S., Takabatake D., Hara F., Takashima S., Aogi K., Takashima S., Inoue T., Sugata S., Nishimura R. Determination of indication for sentinel lymph node biopsy in clinical node-negative breast cancer using preoperative 18F-fluorodeoxyglucose positron emission tomography/computed tomography fusion imaging. Jpn. J. Clin. Oncol. 2008;39:16–21. doi: 10.1093/jjco/hyn120. [DOI] [PubMed] [Google Scholar]
- 72.Veronesi U., De Cicco C., Galimberti V.E., Fernandez J.R., Rotmensz N., Viale G., Spano G., Luini A., Intra M., Veronesi P., et al. A comparative study on the value of FDG-PET and sentinel node biopsy to identify occult axillary metastases. Ann. Oncol. 2007;18:473–478. doi: 10.1093/annonc/mdl425. [DOI] [PubMed] [Google Scholar]
- 73.Kumar R., Zhuang H., Schnall M., Conant E., Damia S., Weinstein S., Chandra P., Czerniecki B., Alavi A. FDG PET positive lymph nodes are highly predictive of metastasis in breast cancer. Nucl. Med. Commun. 2006;27:231–236. doi: 10.1097/00006231-200603000-00005. [DOI] [PubMed] [Google Scholar]
- 74.Weir L., Worsley D., Bernstein V. The value of FDG positron emission tomography in the management of patients with breast cancer. Breast J. 2005;11:204–209. doi: 10.1111/j.1075-122X.2005.21625.x. [DOI] [PubMed] [Google Scholar]
- 75.Fehr M.K., Hornung R., Varga Z., Burger D., Hess T., Haller U., Fink D., von Schulthess G.K., Steinert H.C. Axillary staging using positron emission tomography in breast cancer patients qualifying for sentinel lymph node biopsy. Breast J. 2004;10:89–93. doi: 10.1111/j.1075-122X.2004.21455.x. [DOI] [PubMed] [Google Scholar]
- 76.Zornoza G., Gaŕcia-Velloso M.J., Sola J., Regueira F.M., Pina L., Beorlegui C. 18F-FDG PET complemented with sentinel lymph node biopsy in the detection of axillary involvement in breast cancer. Eur. J. Surg. Oncol. 2004;30:15–19. doi: 10.1016/j.ejso.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 77.Barranger E., Grahek D., Antoine M., Montravers F., Talbot J.N., Uzan S. Evaluation of fluorodeoxyglucose positron emission tomography in the detection of axillary lymph node metastases in patients with early-stage breast cancer. Ann. Surg. Oncol. 2003;10:622–627. doi: 10.1245/ASO.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 78.Guller U., Nitzsche E.U., Schirp U., Viehl C.T., Torhorst J., Moch H., Langer I., Marti W.R., Oertli D., Harder F., et al. Selective axillary surgery in breast cancer patients based on positron emission tomography with 18F-fluoro-2-deoxy-D-glucose: Not yet! Breast Cancer Res. Treat. 2002;71:171–173. doi: 10.1023/A:1013828710301. [DOI] [PubMed] [Google Scholar]
- 79.Nakamoto Y., Chang A.E., Zasadny K.R., Wahl R.L. Comparison of attenuation-corrected and non-corrected FDG-PET images for axillary nodal staging in newly diagnosed breast cancer. Mol. Imaging Biol. 2002;4:161–169. doi: 10.1016/S1536-1632(01)00005-1. [DOI] [PubMed] [Google Scholar]
- 80.Rieber A., Schirrmeister H., Gabelmann A., Nuessle K., Reske S., Kreienberg R., Brambs H.J., Kuehn T. Pre-operative staging of invasive breast CA with MR mammo and or PET: Boon or bunk? Br. J. Radiol. 2002;75:789–798. doi: 10.1259/bjr.75.898.750789. [DOI] [PubMed] [Google Scholar]
- 81.Van der Hoeven J.J.M., Hoekstra O.S., Comans E.F.I., Pijpers R. Determinants of diagnostic performance of [F-18] fluorodeoxyglucose positron emission tomography for axillary staging in breast cancer. Ann. Surg. 2002;236:619–624. doi: 10.1097/00000658-200211000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ganott M.A., Zuley M.L., Abrams G.S., Lu A.H., Kelly A.E., Sumkin J.H., Chivukula M., Carter G., Austin R.M., Bandos A.I. Ultrasound guided core biopsy versus fine needle aspiration for evaluation of axillary lymphadenopathy in patients with breast cancer. ISRN Oncol. 2014;2014:703160. doi: 10.1155/2014/703160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hayes B.D., Feeley L., Quinn C.M., Kennedy M.M., O’Doherty A., Flanagan F., O’Connell A.M. Axillary fine needle aspiration cytology for pre-operative staging of patients with screen-detected invasive breast carcinoma. J. Clin. Pathol. 2011;64:338–342. doi: 10.1136/jcp.2010.084772. [DOI] [PubMed] [Google Scholar]
- 84.Tahir M., Osman K.A., Shabbir J., Rogers C., Suarez R., Reynolds T., Bucknall T. Preoperative axillary staging in breast cancer–Saving time and resources. Breast J. 2008;14:369–371. doi: 10.1111/j.1524-4741.2008.00600.x. [DOI] [PubMed] [Google Scholar]
- 85.Kim T., Giuliano A.E., Lyman G.H. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma: A metaanalysis. Cancer. 2006;106:4–16. doi: 10.1002/cncr.21568. [DOI] [PubMed] [Google Scholar]
- 86.Tanaka K., Yamamoto D., Kanematsu S., Okugawa H., Kamiyama Y. A four node axillary sampling trial on breast cancer patients. Breast. 2006;15:203–209. doi: 10.1016/j.breast.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 87.Zhu Y., Zhou J.Q., Jia X.H., Zhou W., Zhan W.W. Interobserver variability between experienced radiologists in evaluating the number of abnormal lymph nodes seen on preoperative axillary ultrasound. Clin. Radiol. 2020;76:60–66. doi: 10.1016/j.crad.2020.03.041. [DOI] [PubMed] [Google Scholar]
- 88.Lowes S., Leaver A., Cox K., Satchithananda K., Cosgrove D., Lim A. Evolving imaging techniques for staging axillary lymph nodes in breast cancer. Clin. Radiol. 2018 doi: 10.1016/j.crad.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 89.Hieken T.J., Jones K.N., Boughey J.C., Shah S., Glazebrook K.N. Second-look axillary ultrasound after breast MRI for enhanced preoperative nodal staging in newly diagnosed breast cancer patients. J. Clin. Oncol. 2013;31:98. doi: 10.1200/jco.2013.31.26_suppl.98. [DOI] [Google Scholar]
- 90.Kajáry K., Lengyel Z., Tokes A.M., Kulka J., Dank M., Tímea T. Dynamic FDG-PET/CT in the initial staging of primary breast cancer: Clinicopathological correlations. Pathol. Oncol. Res. 2019;26:997–1006. doi: 10.1007/s12253-019-00641-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Acar E., Turgut B., Yiğit S., Kaya G. Comparison of the volumetric and radiomics findings of 18F-FDG PET/CT images with immunohistochemical prognostic factors in local/locally advanced breast cancer. Nucl. Med. Commun. 2019 doi: 10.1097/MNM.0000000000001019. [DOI] [PubMed] [Google Scholar]