Abstract
α-Methyl-L-tryptophan (α-MLT) is currently in use as a tracer in its 11C-labeled form to monitor the health of serotonergic neurons in humans. In the present study, we found this compound to function as an effective weight-loss agent at pharmacological doses in multiple models of obesity in mice. The drug was able to reduce the body weight when given orally in drinking water (1 mg/ml) in three different models of obesity: normal mice on high-fat diet, Slc6a14-null mice on high-fat diet, and ob/ob mice on normal diet. Only the l-enantiomer (α-MLT) was active while the d-enantiomer (α-MDT) had negligible activity. The weight-loss effect was freely reversible, with the weight gain resuming soon after the withdrawal of the drug. All three models of obesity were associated with hyperglycemia, insulin resistance, and hepatic steatosis; α-MLT reversed these features. There was a decrease in food intake in the treatment group. Mice on a high-fat diet showed decreased cholesterol and protein in the serum when treated with α-MLT; there was however no evidence of liver and kidney dysfunction. Plasma amino acid profile indicated a significant decrease in the levels of specific amino acids, including tryptophan; but the levels of arginine were increased. We conclude that α-MLT is an effective, reversible, and orally active drug for the treatment of obesity and metabolic syndrome.
Keywords: alpha-methyl-l-tryptophan, liver function, metabolic syndrome, obesity, weight loss
Introduction
Obesity, i.e. a body mass index ≥30 kg/m2, is a complex disorder arising from an imbalance between caloric intake and energy expenditure. Simply put, obesity is a ‘disease of the mouth'; i.e. when people eat more calories than they expend or ‘burn', they gain weight. What causes people to eat more and/or burn less calories might however vary from patient to patient. Whatever the cause, obesity has become a major health concern. The issue is not just the undesirable body mass index; obesity is the underlying cause for other health issues, including diabetes, metabolic syndrome, hypertension, myocardial infarction, stroke, and cancer. As such, we are in a dire need for effective treatment strategies to address this major health issue. Lifestyle changes such as eating fewer calories and/or expending more calories via exercise represent the most logical way to tackle this problem. But it has proven to be not that easy to practice this strategy. Pharmacotherapy seems the only option; what can't be achieved through willpower to reduce food intake and/or burn more calories should be possible with a drug.
Currently available FDA-approved anti-obesity drugs include a combination of phentermine and topiramate, a combination of naltrexone and bupropion, Liraglutide, and orlistat (https://www.niddk.nih.gov/health-information/weight-management/prescription-medications-treat-overweight-obesity) [1–3]. Amongst these, the first three are active in the brain; the first two are taken orally and Liraglutide by injection. Alli, a lipase inhibitor, acts in the intestine by inhibiting the digestion of dietary fat. Liraglutide, a GLP1 receptor agonist, shows robust efficacy for weight loss; but as an injectable drug, it is less than desirable for most patients. Furthermore, it is used for weight loss at a higher dose than for treatment of diabetes, thus increasing the risk for cholelithiasis, pancreatitis, and pancreatic cancer [4,5]. Several other drug candidates are at various stages of development. This intense interest in the development of anti-obesity drugs highlights the dire need for safe and effective pharmacotherapy to fight the obesity epidemic.
α-Methyl-l-tryptophan (α-MLT) is used in humans as a probe for serotonergic neurons by positron emission tomography [6,7]. Toxicology and pharmacokinetic studies have been done with α-MLT in rodents and non-human primates [8,9]. In mice and rats, there were no adverse effects with oral administration of the dl-enantiomeric mixture of α-methyltryptophan (150 mg/kg). Interestingly, it was observed 60 years ago that the enantiomeric mixture had a weight-loss effect in rats [10], but this outcome was not pursued further. Here we investigated the weight-reducing effect of α-methyltryptophan using multiple models of obesity in mice. The results of the study show that the l-enantiomer, not the d-enantiomer, is pharmacologically active in reducing body weight and obesity-associated metabolic syndrome and that the effect is freely reversible.
Materials and methods
Animals
C57BL/6 mice and ob/ob (leptin-deficient) mice were from Jackson Laboratories (Bar Harbor, ME). Slc6a14-null mice (Slc6a14y/− males and Slc6a14−/− females) on C57BL/6 background were generated in our lab and have been used to assess the role of this transporter in breast cancer [11] and colon cancer [12]. Mice had free access to water and diet ad libitum. Age- and gender-matched mice were used in control groups (no treatment). Mice were fed a normal diet (ND) (50 IF/6F 5V5R, Labdiet, St. Louis, MO) or a high-fat diet (HFD; 55% calorie from fat) (TD93075; Teklad diets, Madison, WI). The protocol was approved by the Institutional Animal Care and Use Committee of the Texas Tech University Health Sciences Center, Lubbock, TX, U.S.A. (IACUC approval number: 15002 for breeding protocol and 18005 for experimental protocol). All the experiments described in this study, including the animal experiments, were conducted at this institution. At the termination of the study, mice were sacrificed by cervical dislocation under CO2 anesthesia in accordance with the guidelines from the American Veterinary Medical Association.
Intraperitoneal glucose tolerance test (GTT)
Intraperitoneal GTT was done in mice fasted for 4 h. Blood glucose was determined using the TRUE track blood glucose monitor (Trividia Health Inc, Fort Lauderdale, FL) at 0, 15, 30, 60, 90 and 120 min after i.p. injection of glucose (2 g/kg body weight) as described previously [13,14].
Serum metabolic panel analysis
At the end of the experiment, mice were fasted for 4 h, blood was collected via orbital sinus using local anesthesia proparacaine. Serum was prepared by centrifugation of the clotted blood, and used for metabolic panel. This was done at our Laboratory Animal Resources Center.
Histology and assessment of steatosis in liver
Liver tissue was excised and weighed immediately, snap-frozen in liquid nitrogen, and stored at −80°C. For Oil-red-O (ORO) staining, tissues were OCT embedded, frozen at −20°C, and cut, followed by staining. Slides made from formalin-fixed, paraffin-embedded liver blocks were stained with hematoxylin and eosin (H & E).
Leptin assay
Animals were fasted for 4 h and blood was collected as described above. Blood samples were centrifuged at 1500×g for 20 min and the serum samples were collected and kept at −80°C until analyzed. Leptin was measured using the mouse/rat Quantikine ELISA kit (MOB00B, R & D Systems, MN).
Amino acid analysis
Plasma samples were used for the determination of amino acids at the Molecular Structural Facility (Genome Center, University of California, Davis, CA) with Hitachi-8900 ion-exchange chromatography amino acid analyzer.
Statistical analyses
Statistical analysis was performed with a two-tailed, paired Student's t-test for single comparison and a P value <0.05 was considered statistically significant. Data are given as means ± SEM.
Results
Weight-reducing effect of α-methyltryptophan
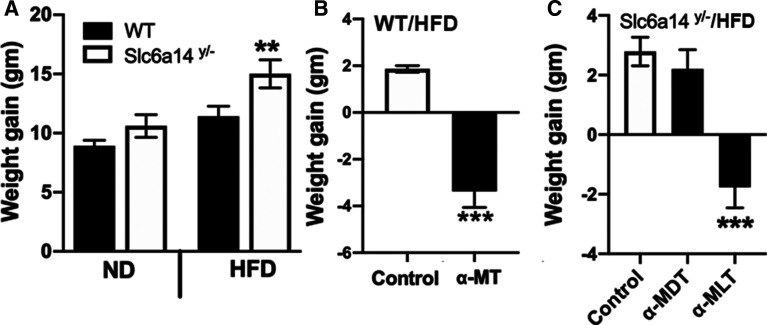
A while ago, we discovered that α-methyltryptophan is a blocker of the amino acid transporter SLC6A14 [15]. At that time, we were using the dl-enantiomeric mixture of the compound. We established the efficacy of blocking SLC6A14 with the dl-enantiomeric mixture in reducing the growth of cancers that exhibit overexpression of the transporter: estrogen receptor-positive breast cancer [15,16], pancreatic cancer [17], and colon cancer [12]. SNPs in SLC6A14 gene are associated with obesity [18–21]; however, the mechanism underlying this association was not known. Because of the connection between SLC6A14 and obesity in humans, we monitored the body weight in wild type and Slc6a14-null mice. We found that Slc6a14-null male mice became obese, but only when fed a HFD; there was a little, but not significant, change in body weight when fed a ND [22] (Figure 1A). As we were working on α-methyltryptophan as a blocker of this transporter, we thought that administration of this compound to wild type mice on a HFD would make the mice gain more weight as we found in Slc6a14-null male mice. The results turned out to be quite the opposite. When exposed to the drug for 1 week (2 mg/ml in drinking water; dl-enantiomeric mixture, α-MT), untreated wild type male mice on a HFD gained weight whereas mice exposed to the drug lost weight (Figure 1B). Since the deletion of Slc6a14 increased the weight gain whereas administration of α-MT to wild type mice decreased the weight gain, the effect of the drug on the body weight has little to do with its ability to block the transporter.
Figure 1. Effect of α-methyltryptophan (the dl-enantiomeric mixture and the individual d- and l-isomers) on body weight in wild type and Slc6a14-null male mice.
(A) Body weight gain in wild type (WT) male and Slc6a14y/− male mice on a normal diet (ND) and a high-fat diet (HFD) for 3 months. (B) Body weight gain measured in wild type male mice on HFD without treatment (Control) or exposed to α-methyl-dl-tryptophan (α-MT, 2 mg/ml in drinking water) ad libitum for a week. (C) HFD-fed male Slc6a14y/− mice were exposed either to drinking water (Control) or α-methyl-d-tryptophan (α-MDT; 1 mg/ml) or α-methyl-L-tryptophan (α-MLT; 1 mg/ml) ad libitum for a week. At the end of the experiment, gain/loss in body weight was monitored. Data are means ± S. E. M (N = 5 mice/group). ** P < 0.01; *** P < 0.001.
Identification of the l-isomer (α-methyl-l-tryptophan, α-MLT) as the active form for the weight-reducing effect
Upon review of the literature, we discovered that the weight-reducing effect of α-methyltryptophan has been reported ∼60 years ago; these studies were done in rats [10]. Surprisingly, this finding had not been pursued. But, similar to our current study, the earlier report on the weight-reducing effect of α-methyltryptophan was noticed with the dl-enantiomeric mixture. Therefore, we wanted to know which isomer in the enantiomeric mixture elicited the weight-reducing effect. For this, we used the Slc6a14-null male mice on a HFD as a model of obesity [22]. We selected this mouse model of obesity for initial experiments because the weight-reducing effect of α-MT is independent of what the drug does to the transport function of Slc6a14. We administered the d- and l-isomers separately to these mice on a HFD at a dose of 1 mg/ml in drinking water for 1 week. Only the l-isomer (α-MLT) was active as a weight-reducing agent (Figure 1C); the d-isomer (α-MDT) had negligible effect. This is the first time that the weight-reducing effect of α-methyltryptophan is being assigned specifically to the l-isomer.
Effect of α-MLT on body weight in other models of obesity in mice
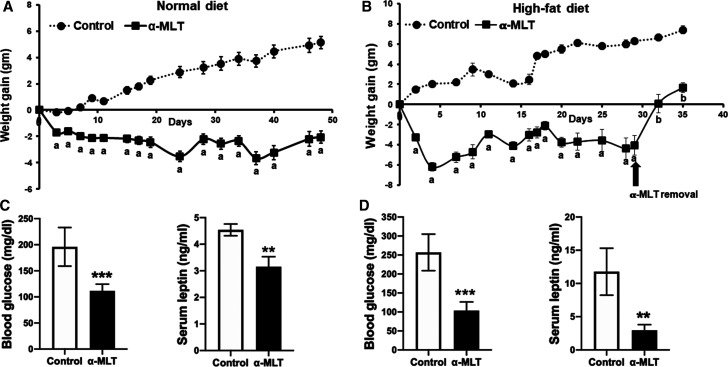
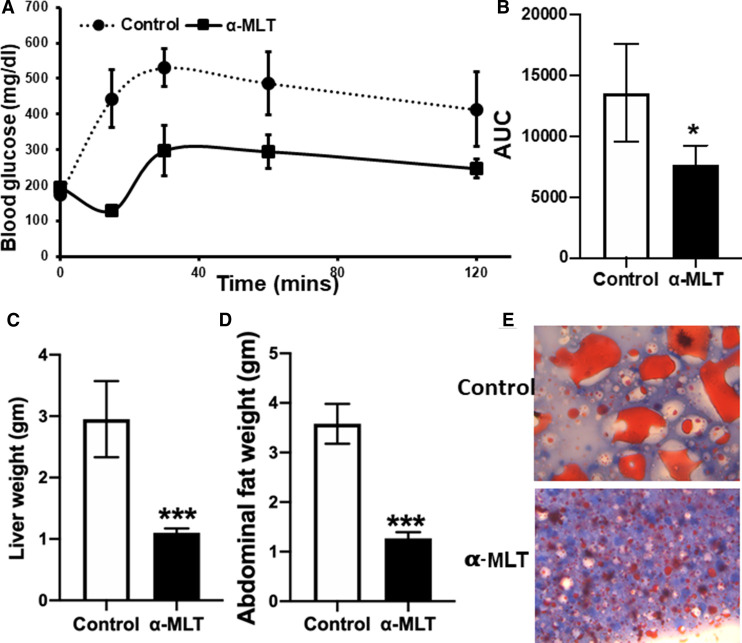
We examined the effect of α-MLT on the body weight in WT C57BL/6 male mice on a ND or a HFD (55% calories from fat). The drug was given in drinking water (1 mg/ml). Both with the ND and the HFD, mice gained weight when not treated with α-MLT (Figure 2A,B). In contrast, the mice treated with α-MLT showed a decrease in body weight (Figure 2A,B). This weight-reducing effect was evident with both diets. We were also able to show that the effect of the drug is reversible. When the drug was withdrawn, mice began to gain weight (Figure 2B). At 4-weeks of drug exposure, circulating levels of glucose and leptin were measured. Blood glucose was higher in control (i.e. untreated) mice on the HFD than in those on the ND (Figure 2C,D). The same was true with leptin levels, which correspond to fat content (Figure 2C,D). In mice treated with α-MLT, there was a significant decrease in circulating levels of glucose and leptin (Figure 2C,D). These changes were seen in response to the drug independent of the diet. We then performed glucose-tolerance test in control and α-MLT-treated (2-week treatment; 1 mg/ml in drinking water) mice which were fed the HFD for 12 weeks. The mice on α-MLT showed better glucose tolerance, indicative of improved insulin sensitivity (Figure 3A,B). At the end of the experiment, mice were sacrificed, and liver and abdominal fat pad were excised and weighed. Treatment with α-MLT reduced the weight of the liver and the abdominal fat (Figure 3C,D).
Figure 2. Weight-loss effect of α-MLT in wild type mice fed a normal diet or a high-fat diet and the consequences of the treatment on blood glucose and serum leptin levels.
Wild type mice (males) fed with ND (A,C) or HFD (B,D) were subjected to drinking water alone or to α-MLT (1 mg/ml) in drinking water. The body weight was measured twice a week (A,B). At end of the 4-week period, blood was collected after 4 h fasting to measure blood glucose and leptin levels (C,D). Data are means ± S. E. M (N = 5 mice/group). ** P < 0.01; *** P < 0.001; a, P < 0.001 compared with body weight in untreated control; b, P < 0.001 compared with body weight at the time of drug withdrawal.
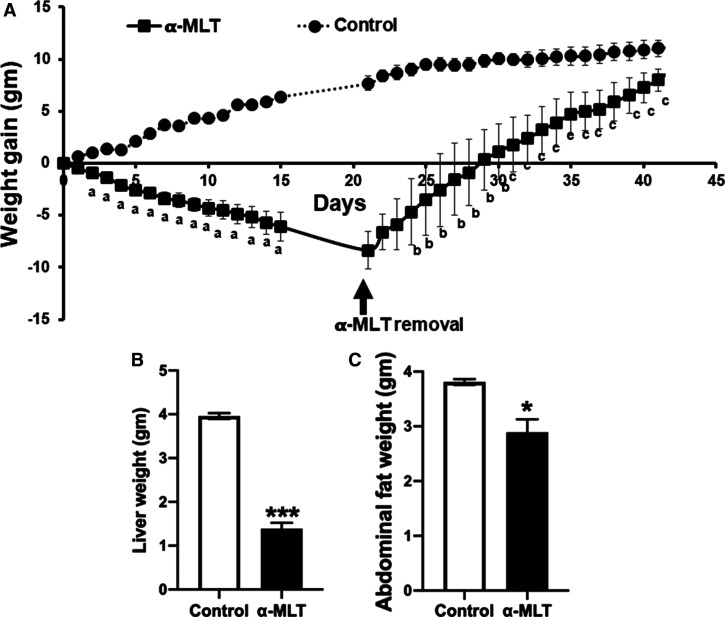
Figure 3. Impact of α-MLT on glucose tolerance test, liver and abdominal fat weight, and hepatic steatosis in wild type mice on a high-fat diet.
Wild type male mice were fed with HFD for 12 weeks and then the mice were divided into two groups. One group of mice received α-MLT (1 mg/ml in drinking water) ad libitum for 3 weeks and the other group received drinking water alone ad libitum. At the end of 2 weeks, blood glucose was measured following 4 h fasting and then the intraperitoneal glucose tolerance test was performed (A,B). The diet and α-MLT administration continued another week; then the mice were sacrificed, and the weights of liver and abdominal fat pad were determined (C,D). Liver tissue was fixed and stained with Oil-O-red for neutral lipids (63×) (E). Data means ± S. E. M (N = 5 mice/group). * P < 0.05; *** P < 0.001.
Prevention of hepatic steatosis by α-MLT in wild type mice on a HFD
Wild type mice developed fatty liver when fed a HFD for 14 weeks as evident from Oil-red-O staining of the liver sections for neutral lipids (Figure 3E). The hepatic steatosis was almost completely prevented by α-MLT treatment during the last 2 weeks of this 14-week period (Figure 3E). In this experiment, mice were fed the HFD for 12-weeks, after which the mice were divided into two groups, one with no α-MLT exposure and the other with α-MLT administration. The drug treatment was continued for two additional weeks, and all through the experiment, both groups were continuously fed the same HFD.
Effect of α-MLT on body weight in female mice on a ND
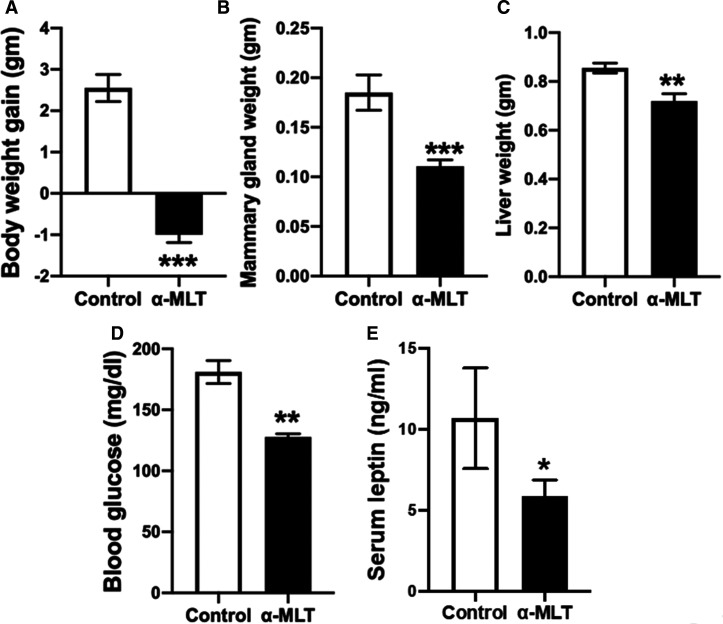
The experiments described above were done with male mice. To see if α-MLT elicits a similar weight-loss effect in female mice, wild type female mice were exposed to α-MLT (1 mg/ml in drinking water) at 3 months of age. The untreated mice gained weight and α-MLT-exposed mice lost weight (Figure 4A). At end of 2 weeks treatment, mice were sacrificed and mammary fat pad and liver were excised and weighed. The weights of both tissues decreased in the treated mice (Figure 4B,C). Blood glucose levels and serum leptin levels were also decreased in the treated mice (Figure 4D,E). These data confirm the weight-reducing effect of α-MLT in both males and females.
Figure 4. Effect of α-MLT on body weight and other biological parameters in wild type female mice fed with HFD.
Wild type female mice were maintained on the HFD for 2 months prior to use in the experiment. Mice were then divided into two groups: control and treatment (α-MLT; 1 mg/ml in drinking water). Gain/loss in body weight was monitored for this 2-week period (A). At the end of experiment, blood was collected and mice were sacrificed. Mammary gland and liver weights were measured (B,C). Blood glucose levels (D) and serum leptin levels (E) were measured. Data means ± S. E. M (N = 5 mice/group). * P < 0.05; ** P < 0.01; *** P < 0.001.
Effect of α-MLT in ob/ob mice on a ND
ob/ob mice are deficient in leptin and therefore obese even on a ND. These mice exhibit increased food intake, insulin resistance, and elevated blood glucose. ob/ob mice on ND are widely used as a model for diabetes and obesity. We used 12-week-old male ob/ob mice to evaluate the weight-reducing effect of α-MLT (1 mg/ml in drinking water). There was a gradual weight gain on a ND in control mice without the drug (Figure 5A). In contrast, mice exposed to α-MLT lost weight. At the end of 3-week drug exposure, the reversibility of α-MLT effect was evaluated by withdrawing the drug. The animals started gaining weight upon the drug withdrawal. At the end of the three-week treatment with α-MLT, mice were sacrificed and the weights of the liver and abdominal fat pad were determined. The treatment with the drug significantly reduced the liver weight (Figure 5B) and abdominal fat (Figure 5C). We also monitored food intake, blood glucose, and glucose tolerance in control mice and α-MLT-treated mice. We found that: (i) body weight increased in mice without α-MLT whereas body weight decreased in mice exposed to the drug (Figure 6A); (ii) α-MLT decreased food intake (Figure 6B); (iii) blood glucose was lower in mice exposed to α-MLT (Figure 6C); and (iv) α-MLT improved insulin sensitivity as seen with glucose tolerance test (Figure 6D,E).
Figure 5. Effect of α-MLT on body weight and its reversibility in ob/ob mice on a normal diet.
ob/ob male mice (3-month-old) were exposed to drinking water alone or to α-MLT (1 mg/ml) in drinking water. Body weight was measured throughout the experiment (A). After three weeks of treatment, mice were sacrificed, and the weights of the liver (B) and abdominal fat pad (C) were determined. Data are means ± S. E. M (N = 5 mice/group). * P < 0.05; *** P < 0.001. To assess the reversibility of α-MLT effect, the drug was withdrawn in the treatment group after 3-week treatment period, and the monitoring of the body weight continued for an additional 3 weeks (A). a, P < 0.001 compared with the control group; b, P < 0.01 and c, P < 0.001 compared with the body weight on the day of the drug withdrawal.
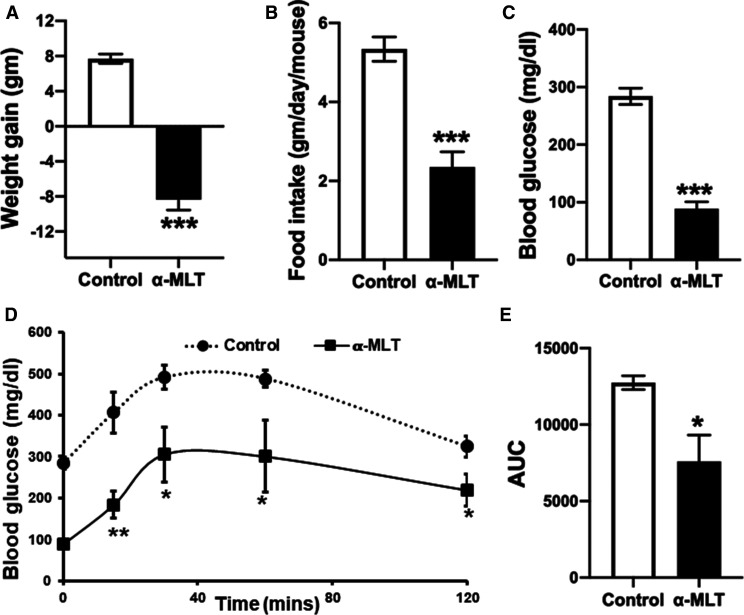
Figure 6. Effects of α-MLT on body weight and other biological parameters in ob/ob mice on a normal diet.
Twelve-week-old ob/ob male mice were administered either drinking water alone or α-MLT (1 mg/ml) in drinking water. Body weight (A) and food intake (B) were measured daily. After three weeks of this treatment regimen, blood glucose (C) was measured and the intraperitoneal glucose tolerance test (D,E) was performed. Data are means ± S. E. M (N = 5 mice/group). * P < 0.05; *** P < 0.001.
Serum biochemical profile in response to α-MLT treatment in wild type mice on a HFD
We evaluated the serum profile for metabolites and enzymes to assess the impact of α-MLT (4 weeks treatment; 1 mg/ml in drinking water) in normal mice which were on the HFD for three months prior to initiation of the drug treatment. These tests were designed to assess the function of the kidneys and liver. Plasma lipid profile indicated no significant change in triglycerides, but a significant decrease in cholesterol levels (Table 1). HFD caused a marked increase in liver weight, and the treatment with α-MLT reversed this effect; accordingly, there was a small, but significant, decrease in plasma albumin levels. There was however no compromise in liver function as evident from normal levels of bilirubin and the enzyme aspartate transaminase (glutamate-oxaloacetate transaminase) (AST). The same was true with the kidney function. There was no evidence of compromise in kidney function as seen with the data on blood urea nitrogen and blood creatinine.
Table 1. Effect of α-MLT treatment (1 mg/ml in drinking water for 4 weeks) on serum lipid profile, kidney function and liver function in wild type mice on the high-fat diet.
| Control | Treated | P value | |
|---|---|---|---|
| Lipid profile | |||
| Cholesterol (mg/dl) | 145 ± 4 | 106 ± 3 | 0.0001 |
| Triglycerides (mg/dl) | 73 ± 5 | 97 ± 17 | 0.1 |
| Kidney function | |||
| BUN (mg/dl) | 28.6 ± 2.9 | 34.2 ± 2.6 | 0.1 |
| Creatinine (mg/dl) | 0.25 ± 0.03 | 0.30 ± 0.04 | 0.2 |
| Liver function | |||
| Total protein (g/dl) | 4.78 ± 0.09 | 4.25 ± 0.06 | 0.001 |
| Albumin (g/dl) | 2.25 ± 0.05 | 1.90 ± 0.04 | 0.001 |
| Globulin (g/dl) | 2.53 ± 0.05 | 2.35 ± 0.09 | 0.06 |
| AST (U/I) | 70.5 ± 7.0 | 79.5 ± 16.3 | 0.32 |
| Total bilirubin (mg/dl) | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.56 |
Values are means ± S.E.M (N = 5 mice/group).
At the end of the treatment period, blood was collected from control (i.e. untreated) and treated mice to prepare serum, which was used for analysis.
Plasma amino acid profile in response to α-MLT treatment
For the determination of plasma amino acids, we had two mouse models of obesity: wild type mice fed the HFD for 12 weeks and 12-week-old ob/ob mice fed with the ND. In both models, the treatment with α-MLT was for 2 weeks. At the end of this period, mice were sacrificed and blood collected. Plasma levels of amino acids were then determined in all four groups: wild type mice with no α-MLT, wild type mice with α-MLT; ob/ob mice with no α-MLT; ob/ob mice with α-MLT. The data are given in Table 2. In wild type mice on the HFD, treatment with α-MLT reduced the plasma levels of serine, glutamine, glycine, tryptophan, and ornithine. The levels of other amino acids were not altered. In ob/ob mice with the ND, treatment with α-MLT caused a significant decrease in a broader spectrum of amino acids: taurine, aspartate, serine, asparagine, glutamine, glycine, alanine, phenylalanine, tryptophan, proline, and ornithine. This list includes all six amino acids whose levels were altered in wild type mice. The most robust decrease occurred with tryptophan in both models. Interestingly, the plasma levels of arginine increased in both models.
Table 2. Plasma concentration of amino acids in high-fat diet (HFD)-fed C57BL/6 mice and normal diet (ND)-fed ob/ob mice with and without α-MLT treatment.
| Amino acids | WT-HFD | WT-HFD-α-MLT | P value | ob/ob-ND | ob/ob-ND-α-MLT | P value |
|---|---|---|---|---|---|---|
| Taurine | 519.08 ± 32 | 506.98 ± 22 | 0.280 | 878.32 ± 118 | 610.13 ± 40 | 0.010 |
| Aspartic acid | 15.78 ± 2 | 15.06 ± 2 | 0.294 | 19.88 ± 4 | 8.13 ± 1 | 0.004 |
| Threonine | 302.73 ± 70 | 284.15 ± 10 | 0.309 | 185.88 ± 30 | 196.92 ± 11 | 0.292 |
| Serine | 182.91 ± 25 | 150.56 ± 8 | 0.025 | 186.45 ± 46 | 123.75 ± 6 | 0.041 |
| Asparagine | 68.54 ± 13 | 48.18 ± 9 | 0.214 | 87.02 ± 37 | 39.78 ± 4 | 0.018 |
| Glutamine | 767.74 ± 50 | 712.05 ± 32 | 0.063 | 853.17 ± 179 | 669.6 ± 32 | 0.013 |
| Glycine | 210.49 ± 35 | 144.63 ± 14 | 0.006 | 206.88 ± 54 | 140.7 ± 8 | 0.050 |
| Alanine | 502.99 ± 55 | 468.31 ± 67 | 0.227 | 721.18 ± 194 | 466.4 ± 15 | 0.043 |
| Citrulline | 60.63 ± 12 | 56.4 ± 6 | 0.272 | 73.37 ± 10 | 82.73 ± 9 | 0.151 |
| Valine | 318.23 ± 50 | 304.66 ± 27 | 0.323 | 249.57 ± 45 | 216.97 ± 28 | 0.174 |
| Methionine | 102.63 ± 30 | 98.7 ± 27 | 0.426 | 85.18 ± 10 | 87.06 ± 26 | 0.456 |
| Isoleucine | 115.98 ± 13 | 104.25 ± 8 | 0.092 | 104.38 ± 23 | 78.42 ± 13 | 0.085 |
| Leucine | 168.20 ± 23 | 163.15 ± 15 | 0.364 | 278.42 ± 45 | 224.92 ± 34 | 0.088 |
| Tyrosine | 103.3 ± 22 | 120.39 ± 17 | 0.132 | 121.65 ± 9 | 94.68 ± 25 | 0.074 |
| Phenylalanine | 88.35 ± 3 | 89.86 ± 15 | 0.426 | 132.85 ± 9 | 108.9 ± 15 | 0.041 |
| Ornithine | 115.44 ± 25 | 48.9 ± 2 | 0.001 | 172.83 ± 23 | 97.75 ± 22 | 0.007 |
| Lysine | 446.44 ± 66 | 402.98 ± 29 | 0.135 | 289.35 ± 62 | 306.73 ± 52 | 0.364 |
| 1-Methylhistidine | 4.65 ± 0.5 | 4.23 ± 0.5 | 0.099 | 6.77 ± 1.6 | 8.71 ± 1.4 | 0.096 |
| Histidine | 87.23 ± 4 | 88.04 ± 4 | 0.394 | 97.55 ± 10 | 90.4 ± 10 | 0.209 |
| Tryptophan | 112.60 ± 12 | 6.06 ± 12 | 0.000 | 121.72 ± 15 | 24.53 ± 29 | 0.003 |
| Arginine | 71.64 ± 38 | 124.36 ± 12 | 0.018 | 46.73 ± 7 | 94.42 ± 20 | 0.008 |
| Proline | 180.41 ± 57 | 173.99 ± 17 | 0.419 | 194.62 ± 33 | 137.93 ± 19 | 0.031 |
Values are given as nmoles/ml (μM) in plasma (means ± S.D; 3 mice/group).
Discussion
The present study has identified the tryptophan derivative α-methyl-l-tryptophan (α-MLT) as an effective and reversible weight-loss agent. The compound is orally active and efficacious in different mouse models of obesity, both dietary and genetic. α-MLT is already in use in humans as a tracer to evaluate the health of serotonergic neurons in the brain. The rationale for this use is that α-MLT crosses the blood-brain barrier, gets taken up into serotonergic neurons, and is converted into α-methylserotonin via the tryptophan hydroxylase pathway that is selective for serotonergic neurons in the brain. When used in the form of 11C-labeled α-MLT (a positron emitter) as the tracer, serotonergic neurons selectively light up in positron emission tomography. The present findings that α-MLT has additional pharmacological functions are not entirely new because studies performed almost six decades ago have shown that the dl-enantiomeric mixture of α-methyltryptophan reduced body weight in rats [10]. The weight-reducing effect of the drug was observed in that study in rats fed the ND, and most of the experiments were done at a drug dose of 1 mmol/kg body weight (i.e. 0.2 mg/g body weight) given once either intraperitoneally or intragastrically. In our study, the drug was given orally in drinking water and thus the animals were exposed to the drug continuously. With an estimate that mice drink ∼4 ml water per day, the drug dose used in the present study (1 mg/ml of the l-isomer) translates to ∼0.13 mg/day/g body weight). In our study as well as in the previous study, the weight-reducing effect of the drug was observed within a day of the drug administration. What is new in the present study is the observation that only the l-isomer is pharmacologically active and that the efficacy of the compound as a weight-loss agent is evident in multiple models of obesity in mice. Furthermore, the effect of α-MLT on body weight is reversible; mice resume weight gain immediately upon withdrawal of the drug. It is orally active and the effect is seen at a dose of 1 mg/ml in drinking water. The weight-loss effect is associated with decreased food intake as was observed in the previous study [10]. The actual molecular mechanism by which α-MLT reduces food intake and body weight is not known. It most likely involves the effect mediated by α-methylserotonin as a satiety signal in the brain. It is well known that α-MLT crosses the blood-brain barrier and gets converted into α-methylserotonin [23] as evident from its use in clinics as a probe to monitor the health of serotonergic neurons. It is also known that α-methylserotonin serves as an agonist for serotonin receptors, particularly for the 5HT2 receptor subtype [24] and that activation of 5HT2 receptor controls appetite [25]. Nonetheless, elucidation of the exact molecular mechanism underlying the weight-loss effect of α-MLT requires further investigation.
The changes in the plasma amino acid profile in response to α-MLT treatment are interesting. The primary change is a decrease in the concentrations of certain specific amino acids. This could be explained to a major extent because α-MLT is a blocker of the broad-specific amino acid transporter SLC6A14 [15], which is expressed highly in the ileum and colon [26]. The substrates for SLC6A14 include all of the neutral amino acids as well as the cationic amino acids [27]. It is possible that the transport function of SLC6A14 contributes to absorption of these amino acids, derived from either diet or colonic bacteria. Therefore, chronic exposure to α-MLT in drinking water is expected to interfere with this absorption process, thus leading to a decrease in the plasma levels of specific amino acids. It is important to note however that the decrease in tryptophan levels is much more marked than the decrease in other amino acids, suggesting additional mechanisms, at least for this particular amino acid. In the same 60-year-old study in which the dl-enantiomeric mixture of α-methyltryptophan was shown to elicit a weight-reducing effect, the drug treatment also induced the expression of the tryptophan-degrading enzyme tryptophan pyrrolase (also known as tryptophan-2,3-dioxygenase or TDO) in the liver [10]. Therefore, it is possible that chronic exposure to α-MLT activates tryptophan metabolism in the liver, thus contributing to the decrease in the circulating levels of this amino acid. The changes in the plasma levels of two other amino acids also need attention. In contrast with most amino acids, arginine levels increase in α-MLT-treated mice. This could be due to changes in adipose tissue content and the associated changes in inflammation. Obesity is associated with inflammation, and inflammation is associated with increased metabolism of arginine via arginase isoforms in activated macrophages [28]. Since α-MLT decreases body weight and reduces adipose tissue, these changes are most likely accompanied with reduced inflammation and consequently decreased metabolism of arginine in immune cells, thus explaining the increase in the circulating levels of this amino acid in α-MLT-treated animals. This idea is strongly supported by the marked decrease in the levels of ornithine, a product of arginases. Taurine is not a substrate for SLC6A14 [29], but its levels are decreased in plasma as a result of α-MLT treatment. This change is most likely due to obesity-associated changes in the expression of the taurine transporter (TauT or SLC6A6) in various tissues. Obesity is known to decrease the expression of this transporter in the intestinal tract [30] and placenta [31]. Taurine supplementation is also known to have an anti-obesity effect [32]. Taurine promotes the conversion of white adipose tissue into brown adipose tissue, thus enhancing energy expenditure [32]. Furthermore, ob/ob mice show increased expression of the taurine transporter in adipose tissue [33] and mice lacking the taurine transporter have reduced body weight [34]. The circulating levels of taurine are differentially affected by the taurine transporter in the intestinal tract versus the taurine transporter in other tissues. The intestinal transporter increases the absorption of exogenous taurine, thus increasing the plasma levels. In contrast, the transporter in other tissues mediate the clearance of taurine from blood by promoting cellular uptake. Thus, the relationship between plasma levels of taurine and obesity is complex, which needs to be investigated further to get a better understanding of this relationship.
α-MLT has been shown to be effective for the treatment of cancers that are associated with up-regulation of the amino acid transporter SLC6A14 [11,12,16,17]. But the weight-loss effect of the compound is independent of its ability to block this transporter as evident from its efficacy to reduce body weight even in Slc6a14-null mice. This suggests that α-MLT is ideal for treatment of obesity-associated cancers. This is particularly so in the case of the estrogen receptor-positive breast cancer, which is promoted by obesity and is also characterized by up-regulation of SLC6A14, thus providing two molecular targets for α-MLT (obesity and SLC6A14) to elicit a synergistic therapeutic effect. α-MLT would also be useful in the treatment of other obesity-related health problems such as diabetes, hypertension, cardiovascular diseases, and fatty liver.
Based on these pharmacologic features of α-MLT, there is potential for this compound as an anti-obesity drug in humans. In preclinical animal models, α-MLT given orally at a dose of 150 mg/kg per day showed no noticeable adverse effects in rats [8]. Our studies have shown that at a daily dose of 1 mg/ml in drinking water, which translates approximately to 115 mg/kg (the average daily water intake in male C57BL/6 mice, 4 ml/30 g body weight), there was no evidence of significant changes in liver function and kidney function. The dose of 115 mg/kg in mice approximates to 1 g of the drug in humans with body weight of 70 kg [35], a practically feasible dose for use in humans. As such, the findings of the present study provide a strong rationale and scientific basis for a more detailed evaluation of α-MLT as an anti-obesity drug in preclinical animal models and for subsequent clinical trials in humans.
Abbreviations
- AST
aspartate transaminase
- HFD
high-fat diet
- α-MLT
α-Methyl-L-tryptophan
- ND
normal diet
Data Availability
All supporting data in relation to the studies reported here are provided in this manuscript.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the Welch Endowed Chair in Biochemistry, Grant No. BI-0028, at Texas Tech University Health Sciences Center.
Open Access
Open access for this article was enabled by the participation of the Texas Tech University Health Sciences Center in an all-inclusive Read & Publish pilot with Portland Press and the Biochemical Society under a transformative agreement with EBSCO.
CRediT Contribution
Vadivel Ganapathy: Conceptualization, Formal analysis, Funding acquisition, Writing — original draft, Writing — review and editing. Sathish Sivaprakasam: Conceptualization, Data curation, Investigation, Writing — review and editing. Sabarish Ramachandran: Investigation, Methodology. Mohd Omar Faruk Sikder: Investigation, Methodology. Yangzom Doma Bhutia: Conceptualization, Formal analysis, Writing — review and editing. Mitchell Wachtel: Investigation, Methodology.
References
- 1.Rodgers, R.J., Tschop, M.H. and Wilding, J.P. (2012) Anti-obesity drugs: past, present and future. Dis. Model Mech. 5, 621–626 10.1242/dmm.009621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson, V.M., Breen, D.M., Fortin, J.P., Liou, A., Kuzmiski, J.B., Loomis, A.K.et al. (2015) Latest approaches for the treatment of obesity. Expert Opin. Drug Discov. 10, 825–839 10.1517/17460441.2015.1044966 [DOI] [PubMed] [Google Scholar]
- 3.Martinussen, C., Bojsen-Moller, K.N., Svane, M.S., Dejgaard, T.F. and Madsbad, S. (2017) Emerging drugs for the treatment of obesity. Expert Opin. Emerg. Drugs 22, 87–99 10.1080/14728214.2017.1269744 [DOI] [PubMed] [Google Scholar]
- 4.Consoli, A. and Formoso, G. (2015) Potential side effects to GLP-1 agonists: understanding their safety and tolerability. Expert Opin. Drug Saf. 14, 207–218 10.1517/14740338.2015.987122 [DOI] [PubMed] [Google Scholar]
- 5.Monami, M., Nreu, B., Scatena, A., Cresci, B., Andreozzi, F., Sesti, G.et al. (2017) Safety issues with glucagon-like peptide-1 receptor agonists (pancreatitis, pancreatic cancer and cholelithiasis): data from randomized controlled trials. Diabetes Obes. Metab. 19, 1233–1241 10.1111/dom.12926 [DOI] [PubMed] [Google Scholar]
- 6.Kumar, A., Asano, E. and Chugani, H.T. (2011) α-[11C]-Methyl-L-tryptophan PET for tracer localization of epileptogenic brain regions: clinical studies. Biomark. Med. 5, 577–584 10.2217/bmm.11.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diksic, M. and Young, S.N. (2001) Study of the brain serotonergic system with labeled α-methyl-L-tryptophan. J. Neurochem. 78, 1185–1200 10.1046/j.1471-4159.2001.00536.x [DOI] [PubMed] [Google Scholar]
- 8.Curzon, G. and Marsden, C.A. (1976) Effects of p-chlorophenylalanine and α-methyltryptophan on rat social behaviour. Br. J. Pharmacol. 58, 455–456 10.1111/j.1476-5381.1976.tb08621.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shoaf, S.E. and Schmall, B. (1996) Pharmacokinetics of α-methyl-L-tryptophan in Rhesus monkeys and calculation of the lumped constant for estimating the rate of serotonin synthesis. J. Pharmacol. Exp. Ther. 277, 219–224 PMID: [PubMed] [Google Scholar]
- 10.Sankoff, I. and Sourkes, T.L. (1962) The weight-depressing action of α-methyl-DL-tryptophan in the rat. Can. J. Biochem. Physiol. 40, 739–747 10.1139/y62-086 [DOI] [PubMed] [Google Scholar]
- 11.Babu, E., Bhutia, Y.D., Ramachandran, S., Gnanaprakasam, J.P., Prasad, P.D., Thangaraju, M.et al. (2015) Deletion of the amino acid transporter Slc6a14 suppresses tumour growth in spontaneous mouse models of breast cancer. Biochem. J. 469, 17–23 10.1042/BJ20150437 [DOI] [PubMed] [Google Scholar]
- 12.Sikder, M.O.F., Sivaprakasam, S., Brown, T.P., Thangaraju, M., Bhutia, Y.D. and Ganapathy, V. (2020) SLC6A14, a Na+/Cl− -coupled amino acid transporter, functions as a tumor promoter in colon and is a target for Wnt signaling. Biochem. J. 477, 1409–1425 10.1042/BCJ20200099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen, C.L., Kaur, G., Wanders, D., Sharma, S., Tomison, M.D., Ramalingam, L.et al. (2018) Annatto-extracted tocotrienols improve glucose tomeostasis and bone properties in high-fat diet-induced type 2 diabetic mice by decreasing the inflammatory response. Sci. Rep. 8, 11377 10.1038/s41598-018-29063-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung, E., Elmassry, M.M., Kottapalli, P., Kottapalli, K.R., Kaur, G., Dufour, J.M.et al. (2020) Metabolic benefits of annatto-extracted tocotrienol on glucose homeostasis, inflammation, and gut microbiome. Nutr. Res. 77, 97–107 10.1016/j.nutres.2020.04.001 [DOI] [PubMed] [Google Scholar]
- 15.Karunakaran, S., Umapathy, N.S., Thangaraju, M., Hatanaka, T., Itagaki, S., Munn, D.H.et al. (2008) Interaction of tryptophan derivatives with SLC6A14 (ATB0,+) reveals the potential of the transporter as a drug target for cancer chemotherapy. Biochem. J. 414, 343–355 10.1042/BJ20080622 [DOI] [PubMed] [Google Scholar]
- 16.Karunakaran, S., Ramachandran, S., Coothankandaswamy, V., Elangovan, S., Babu, E., Periyasamy-Thandavan, S.et al. (2011) SLC6A14 (ATB0,+) protein, a highly concentrative and broad specific amino acid transporter, is a novel and effective drug target for treatment of estrogen receptor-positive breast cancer. J. Biol. Chem. 286, 31830–31838 10.1074/jbc.M111.229518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coothankandaswamy, V., Cao, S., Xu, Y., Prasad, P.D., Singh, P.K., Reynolds, C.P.et al. (2016) Amino acid transporter SLC6A14 is a novel and effective drug target for pancreatic cancer. Br. J. Pharmacol. 173, 3292–3306 10.1111/bph.13616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suviolahti, E., Oksanen, L.J., Ohman, M., Cantor, R.M., Ridderstrale, M., Tuomi, T.et al. (2003) The SLC6A14 gene shows evidence of association with obesity. J. Clin. Invest. 112, 1762–1772 10.1172/JCI200317491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durand, E., Boutin, P., Meyre, D., Charles, M.A., Clement, K., Dina, C.et al. (2004) Polymorphisms in the amino acid transporter solute carrier family 6 (neurotransmitter transporter) member 14 gene contribute to polygenic obesity in French caucasians. Diabetes 53, 2483–2486 10.2337/diabetes.53.9.2483 [DOI] [PubMed] [Google Scholar]
- 20.Corpeleijn, E., Petersen, L., Holst, C., Saris, W.H., Astrup, A., Langin, D.et al. (2010) Obesity-related polymorphisms and their associations with the ability to regulate fat oxidation in obese europeans: the NUGENOB study. Obesity 18, 1369–1377 10.1038/oby.2009.377 [DOI] [PubMed] [Google Scholar]
- 21.Miranda, R.C., Vetter, S.B., Genro, J.P., Campagnolo, P.D., Mattevi, V.S., Vitolo, M.R.et al. (2015) SLC6A14 and 5-HTR2C polymorphisms are associated with food intake and nutritional status in children. Clin. Biochem. 48, 1277–1282 10.1016/j.clinbiochem.2015.07.003 [DOI] [PubMed] [Google Scholar]
- 22.Sivaprakasam, S., Sikder, M.O.F., Ramalingam, L., Kaur, G., Dufour, J.M., Moustaid-Moussa, N.et al. (2021) SLC6A14 deficiency is linked to obesity, fatty liver, and metabolic syndrome but only under conditions of a high-fat diet. Biochim. Biophys. Acta Mol. Basis Dis. 1867, 166087 10.1016/j.bbadis.2021.166087 [DOI] [PubMed] [Google Scholar]
- 23.Sourkes, T.L., Montine, T.J. and Missala, K. (1990) Alpha-methylserotonin, a substitute transmitter for serotonergic neurons. Prog. Neuropsychopharmacol. Biol. Psychiatry 14, 829–832 10.1016/0278-5846(90)90055-L [DOI] [PubMed] [Google Scholar]
- 24.Sourkes, T.L. (1991) Alpha-methyltryptophan as a therapeutic agent. Prog. Neuropsychopharmacol. Biol. Psychiatry 15, 935–938 10.1016/0278-5846(91)90020-2 [DOI] [PubMed] [Google Scholar]
- 25.Halford, J.C.G. and Harrold, J.A. (2012) 5-HT2C receptor agonists and the control of appetite. Handb. Exp. Pharmacol. 209, 349–356 10.1007/978-3-642-24716-3_16 [DOI] [PubMed] [Google Scholar]
- 26.Hatanaka, T., Huang, W., Nakanishi, T., Bridges, C.C., Smith, S.B., Prasad, P.D.et al. (2002) Transport of D-serine via the amino acid transporter ATB0,+ expressed in the colon. Biochem. Biophys. Res. Commun. 291, 291–295 10.1006/bbrc.2002.6441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhutia, Y.D. and Ganapathy, V. (2016) Glutamine transporters in mammalian cells and their functions in physiology and cancer. Biochim. Biophys. Acta 1863, 2531–2539 10.1016/j.bbamcr.2015.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang, Z. and Ming, X.F. (2014) Functions of arginase isoforms in macrophage inflammatory responses: impact on cardiovascular diseases and metabolic disorders. Front. Immunol. 5, 533 10.3389/fimmu.2014.00533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson, C.M.H., Howard, A., Walters, J.R.F., Ganapathy, V. and Thwaites, D.T. (2009) Taurine uptake across the human intestinal brush-border membrane is via two transporters: H+-coupled PAT1 (SLC36A1) and Na+- and Cl− -dependent tauT (SLC6A6). J. Physiol. 587, 731–744 10.1113/jphysiol.2008.164228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irving, B.A., Wood, G.C., Bennotti, P.N., Babu, E., Deshpande, A., Lent, M.R., et al. (2016) Nutrient transporter expression in the jejunum in relation to body mass index in patients undergoing bariatric surgery. Nutrients 8, 683 10.3390/nu8110683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ditchfield, A.M., Desforges, M., Mills, T.A., Glazier, J.D., Wareing, M., Mynett, K.et al. (2015) Maternal obesity is associated with a reduction in placental taurine transporter activity. Int. J. Obes. (Lond.) 39, 557–564 10.1038/ijo.2014.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo, Y.Y., Li, B.Y., Peng, W.Q., Guo, L. and Tang, Q.Q. (2019) Taurine-mediated browning of white adipose tissue is involved in its anti-obesity effect in mice. J. Biol. Chem. 294, 15014–15024 10.1074/jbc.RA119.009936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hou, X., Wang, Z., Ding, F., He, Y., Wang, P., Liu, X.et al. (2019) Taurine transporter regulates adipogenic differentiation of human adipose-derived stem cells through affecting Wnt/(-catenin signaling pathway. Int. J. Biol. Sci. 15, 1104–1112 10.7150/ijbs.31794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han, X., Patters, A.B., Ito, T., Azuma, J., Schaffer, S.W. and Chesney, R.W. (2015) Knockout of the tauT gene predisposes C57BL/6 mice to streptozotocin-induced diabetic nephropathy. PLoS ONE 10, e0117718 10.1371/journal.pone.0117718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guidance for Industry (2005) Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). (www.fda.gov/downloads/Drugs/Guidances/UCM078932.pdf) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All supporting data in relation to the studies reported here are provided in this manuscript.