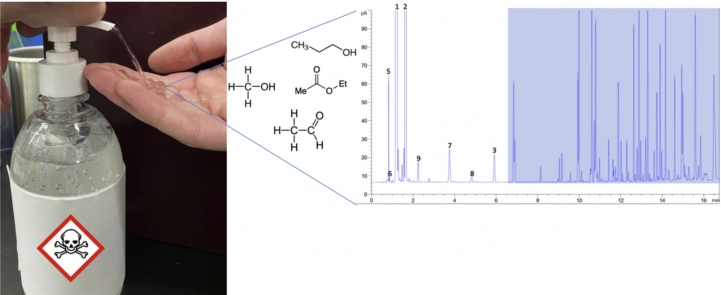
Graphical abstract
Abbreviations: COVID-19, Coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ABHR, Alcohol-based hand rubs; FCC, Food Chemical Codex; USP, United States Pharmacopeia; HC, Health Canada; FDA, Food and Drug Administration; NIOSH, National Institute for Occupational Safety and Health; DEP, Diethyl phthalate
Keywords: COVID-19, Technical-Grade, Hand sanitizer, Ethanol, Acetaldehyde, Contaminants
Highlights
-
•
Alcohol-based hand rubs formulated with technical-grade ethanol can increase exposure to alcoholic impurities.
-
•
Inexperienced manufacturers may introduce additional contaminants into the product.
-
•
More vigilant policing should be employed to ensure compliancy, safety and efficacy.
Abstract
Alcohol-based hand rubs (ABHRs) formulated with technical-grade ethanol were temporarily permitted in Canada and the U.S beginning April 2020 to meet the current demand due to COVID-19. ABHRs formulated with technical-grade ethanol are low risk for general use. In this review, we discuss the toxicity of common contaminants found in technical-grade ethanol, as well as contaminants that may have been introduced into the products during formulation and packaging of ABHRs. Although primary route of exposure is via dermal absorption and inhalation, there have been reported elevated concerns regarding to ingestion of ABHRs. Overall, the highest risks were associated with methanol (for its toxicity), ethyl acetate (skin defattening), and acetaldehyde (carcinogenic and teratogenic). For these reasons Health Canada and the United States Food and Drug Administration have issued recalls on products containing some of these contaminants. More vigilant policing by regulatory agencies and general product users are required to ensure compliance, safety, and efficacy of these new products, as demand continue to rise during this unprecedented pandemic.
1. Introduction
Community transmission of infectious diseases remains a significant concern, especially during the current severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; aka COVID-19) pandemic. Hand hygiene is the most important intervention to prevent the transmission of infectious disease and pathogenic microorganisms in shared facilities [1]. Alcohol-based hand rubs (ABHRs) are routinely used in healthcare settings, as they demonstrate convenience and effective antimicrobial activity [2,3]. The efficacy of ABHRs is determined by hand hygiene procedures,1 degree of hand soilage [4,5], as well as concentration, volume, formulation, and contact time of the ABHR [6,7].
Typically, raw materials (e.g. ethanol) used for hand sanitizer must adhere to specific monographs (e.g. Food Chemicals Codex; FCC [8], and the United States Pharmacopeia; USP [9]), and are regulated by governmental agencies (e.g. Health Canada; HC, and the United States Food and Drug Administration; US-FDA). Compliance with these monographs ensure the raw material is high-quality (e.g. pharmaceutical-grade or food-grade) and undesirable contaminants are minimized. In this paper, “food” and “pharmaceutical” grade ethanol are compliant with FCC and USP monographs, respectively. Recently, the COVID-19 pandemic has led to stockpiling of essential items (e.g. ABHRs) [10]. This increase in demand, has led to supply chain disruptions, and resulted in a global shortage of both products and raw materials. In response, governmental agencies including but not limited to Canada and the US have introduced relaxed quality guidelines regarding the production of ABHRs using ethanol [[11], [12], [13], [14]] (ABHRs using isopropanol have also been relaxed but use existing quality standards) [15]. This has enabled non-traditional manufacturers, including industrial ethanol plants, to produce “technical-grade” alcohol to help alleviate the growing demand, resulting in an 6-fold in the number of licenses and 7-fold increase in number of sales of ABHRs in Canada, and a spike in ethanol production worldwide in 2020 [[16], [17], [18]]. These ethanol manufacturers possess different infrastructure and feedstocks which influence the quality of the ethanol (e.g. presence of contaminants) [19]. Therefore, extra precautions must be taken to ensure the efficacy and safety of the product for public use.
Technical-grade ethanol can contain contaminants at concentrations that are orders of magnitude higher than USP or FCC compliant ethanol. For example, 1000 ppm acetaldehyde was initially permitted in technical-grade ethanol during the beginning of the COVID-19 pandemic, whereas USP permits only 10 ppm of acetaldehyde. The main exposure route for ABHRs is dermal adsorption followed by inhalation. Oral exposure is also a hazard due to accidental ingestion, with the highest risk to young children (via exploratory behaviour) or recreational ingestion [[20], [21], [22]]. Exposure to unregulated ethanol can potentially result in secondary toxic effects due to the additive effects of contaminants. This review will focus primarily on the formulations of ABHRs utilizing technical-grade ethanol and the common contaminants present therein.
1.1. Formulations
There are a wide range of active chemicals routinely employed in the production of hand sanitizers and disinfectants (e.g. alcohol, chlorine compounds, quaternary ammonium compounds, etc.) [23]. Each of these ingredients possess different mechanisms of action which determine their efficacy and applicability [24].
Alcohol demonstrates broad-spectrum applicability by denaturing the proteins in cell plasma membranes, making it an effective disinfectant against bacteria, viruses, and fungi [23]. Optimum germicidal activity occurs at concentrations between 60–95 % of either ethanol, isopropanol, or n-propanol [2], with higher alcohol concentrations demonstrating greater activity.2 Concentrations greater than 95 % are less effective as water is required for protein denaturation, and quicker evaporation reduces contact time. Humectants are typically added to ABHRs to improve contact time. Potential spores in the ABHR can be inactivated through the addition of hydrogen peroxide (< 0.5 % v/v) [2]. The World Health Organization (WHO) has released two formulations of ABHRs consisting of ethanol or isopropyl alcohol (isopropanol), with hydrogen peroxide and glycerol [25].
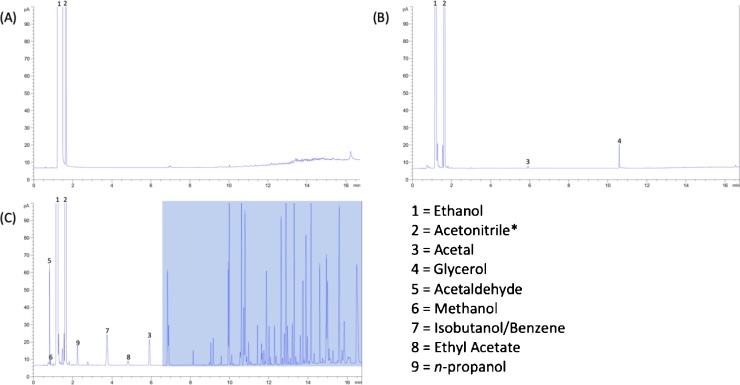
To ensure that process contaminants are minimized, ABHRs are normally formulated using raw materials conforming with USP or FCC guidelines. The FCC monograph previously set criteria using colorimetric tests and odor tests, however with an increase in ethanol demand, the FCC has released a “Notice of Intent to Revise” the current ethanol monograph. These revisions including implementing limitations on individual impurities [26] (Table 1). Meanwhile, the USP monograph combines colorimetric tests and analytical chromatography to determine purity of the ethanol. Common contaminants naturally coproduced during grain fermentation include, methanol, acetates, aldehydes, butanols, amyl alcohols, propanols, and pentanols [19]. These compounds can form azeotropes that are co-distilled with the ethanol fraction, making it difficult to purify ethanol. Impurities can be influenced by a variety of factors including yeast amount, the source and concentration of nitrogen (e.g. amino acids and urea supplement), process conditions (e.g. pH) [27], biological processes (e.g. Ehrlich pathway) [28], and distillation processes. Certain contaminants can exhibit some level of toxicity and contribute to undesirable odors (e.g. sulfur-containing compounds). However, HC has conducted risk assessments and calculated the tolerable lifetime daily exposure of acetaldehyde for an individual assuming a body weight of 50 kg and a daily exposure of 100 applications of technical-grade ethanol with a 2% absorption rate at 20 °C [11]. Nonetheless, a thorough examination of the quality of raw materials used for interim ABHR products has not been conducted, although these contaminants have been observed during convenience sampling of ABHRs (Fig. 1) [29].
Table 1.
Quality criteria and outcomes by recall for the ethyl alcohol.
| Compound | Acceptance criteria (USP [9]) | Current criteria (HC [11])* | Current criteria (US-FDA [39]) | Revised criteria (FCC [73])** | Recall incidents in Canada (7/20 to 1/21 [80]) | Recall incidents in US (7/20 to 1/21 [81]) |
|---|---|---|---|---|---|---|
| Methanol | ≤200 μL/L | ≤200 μL/L | ≤630 μL/L | ≤200 μL/L | 19 | 165 |
| Acetaldehyde and acetal | ≤10 μL/L | ≤400 μL/L | ≤50 μL/L | ≤1000 μL/L | 0 | 0 |
| Benzene | ≤2 μL/L | ≤2 μL/L | ≤2 μL/L | ** | 0 | 0 |
| All other impurities (summed) | ≤300 μL/L | ≤300 μL/L | ≤300 μL/L | ≤5000 μL/L | 62 (Ethyl acetate, 1-propanol) | 3 (1-propanol) |
The criteria are based off the range of acetaldehyde that is currently found in samples from ethanol producers that were submitted to HC for risk assessment. These criteria have decreased over time as distillation methods improve.
The revised FCC criteria now includes limitations of individual volatile organic impurities at 1000 μL/L and a limit of 5000 μL/L of the sum of all impurities. However, benzene is not specifically mentioned.
Fig. 1.
Gas chromatography flame ionization detector spectrum of (A) USP ethanol, (B) ABHR formulated with USP-compliant ethanol and, (C) ABHR formulated with technical-grade ethanol [22]. The shaded area represents other possible alcohol contaminants or additives (e.g. essential oils, fragrances, etc.) that were blended during production.
*Acetonitrile was used as the syringe rinsate.
1.2. Contaminants
Product recalls have been issued by HC and the US-FDA due to unauthorized sale, improper labeling, and contamination. Here we discuss the toxicity and risk associated with contaminants that may be observed in ABHRs formulated with technical-grade ethanol. These contaminants require further treatments (e.g. multi-stage distillation) to comply with FCC or USP monographs. During the fermentation, heating of the grain mash can result in reactions initiating the Maillard process (e.g. amino acid utilization) producing undesirable compounds which affect the taste, smell, and color of the mash and end-product [30]. Yeast can then transform these free amino acids to higher alcohols (e.g. fusel alcohols) via the Ehrlich pathway [28].
1.3. Methanol
Methanol is produced during fermentation by the hydrolysis of naturally occurring pectin in the wort, from yeast, fungi or bacteria [31]]. Improper distillation can result in methanol blended into ABHRs. Acute poisonings and deaths caused by methanol in ABHRs have been reported in Canada, USA, Hong Kong, and China [[32], [33], [34]]. Recently, the US-FDA and HC have issued advisories to consumers not to use certain ABHR products due to significant levels of methanol (ranging from 1% to 80 %) [35,36]].
Methanol toxicity is dose and exposure dependent [37]. Persons acutely exposed to high levels of methanol via ingestion, inhalation, or extensive skin contact (Table 2) can develop abraded skin, severe metabolic, ocular, and neurologic toxicity (e.g. Parkinsonism) [37]. Chronic short-term exposure can result in skin irritation and defatting, neurological impairment, and visual impairment [37,38]. Metabolism of methanol occurs in the liver and is mediated by alcohol and aldehyde dehydrogenases, where methanol is oxidized to formaldehyde then to formic acid. Formic acid is then detoxified into carbon dioxide and water 38]. Formic acid is the primary toxic metabolite of methanol and can result in organ damage [37].
Table 2.
Toxicity data and occupational exposure limits for common contaminants in technical-grade ethanol. Reconstructed from the National Center for Biotechnology Information - Pubchem and citations therein [34].
| Chemical | Exposure Route | LD50/LC50 | Reproductive effects | Skin and eye irritation | Genotoxicity | Carcinogenicity | NIOSH Exposure Limit | OSHA Exposure Limit |
|---|---|---|---|---|---|---|---|---|
| Methanol | Oral | 5600 mg/kg (rats) | 42 m L/kg (rats) | N/A | Mutagenic in mouse lymphoma assay, in a Basc test, in Drosophila sex-linked recessive lethal mutation assay | No evidence of carcinogenicity | 10-hr TWA =200 ppm | 8-hr TWA =200 ppm |
| Inhalation | 64,000 ppm/4 h (rats) | 1000 ppm (rats) | N/A | 15-min STEL =250 ppm | ||||
| Dermal | 15,800 mg/kg (rabbits) | ND | Skin: 20 mg (rabbits) | |||||
| Eyes: 100 mg (rabbits) | ||||||||
| Acetaldehyde | Oral | >661 mg/kg (rats) | >4800 mg/kg (21d pregnant rats) | N/A | Mutagenicity was measured in a variety of animal models at varying concentrations | Is carcinogenic in animal models, highly likely carcinogen in humans | N/A; carcinogenic, NIOSH recommends lowest feasible concentration | 8-hr TWA =200 ppm |
| Inhalation | 13,300 ppm/4 h (rats) | 5 mg/m3 (rats) | N/A | |||||
| Dermal | 3540 mg/kg (rabbits) | ND | Skin: 500 mg (rabbits) | |||||
| Eyes: 40 mg (rabbits) | ||||||||
| Isopropyl alcohol | Oral | >5000 mg/kg (rats) | >5040 mg/kg (1−20d pregnant rats) | N/A | Mutagenicity has been studied in mouse and drosophila models | No evidence of carcinogenicity. | 10-hr TWA =400 ppm | 8-hr TWA =400 ppm |
| Inhalation | >16,000 ppm/8 h (rats) | >3500 ppm/7 h (1−19d pregnant rats) | N/A | |||||
| Dermal | 12,800 mg/kg | ND | Skin: 500 mg (rabbits) | Isopropyl alcohol manufacturing via strong acid processes is carcinogenic | 15-min STEL =500 ppm | |||
| Eyes: >10 mg (rabbits) | ||||||||
| n-propanol | Oral | >1870 mg/kg (rats) | ND | N/A | No evidence of mutagenicity | No evidence of carcinogenicity | 10-hr TWA =200 ppm | 8-hr TWA =200 ppm |
| Inhalation | 48 g/m3 (mice) | >7000 ppm/7 h (6 w males; 1−19d pregnant rats) | N/A | |||||
| Dermal | 5040 mg/kg (rabbits) | ND | Skin: 500 mg (rabbits) | 15-min STEL =250 ppm | ||||
| Eyes: 20 mg (rabbits) | ||||||||
| Ethyl acetate | Oral | 5620 mg/kg (rats) | ND | N/A | No evidence of mutagenicity | No evidence of carcinogenicity | 10-hr TWA =400 ppm | 8-hr TWA =400 ppm |
| Inhalation | 1600 ppm/8 h (rats) | ND | N/A | |||||
| Dermal | >20 mL/kg | ND | Eyes: 400 ppm (humans) | |||||
| Isobutyl alcohol | Oral | 2460 mg/kg (rats) | ND | N/A | No evidence of mutagenicity | Possible carcinogen in rats | 10-hr TWA =50 ppm | 8-hr TWA =100 ppm |
| Inhalation | 19,200 ppm/4 h (rats) | 10,000 ppm (6−15d pregnant rats) | N/A | |||||
| Dermal | 3400 mg/kg (rabbits) | ND | N/A | |||||
| Tert-butyl alcohol | Oral | 2743 mg/kg (rats) | >103 g/kg (6−20d pregnant mice) | N/A | No evidence of mutagenicity | Tumorigenic in rat and mouse models; suggestive evidence of kidney and thyroid tumors as a potential human hazard. | 10-hr TWA =100 ppm | 8-hr TWA =100 ppm |
| Inhalation | >10,000 ppm/4 h (rats) | 2000 ppm (1−19d pregnant rats) | N/A | 15-min STEL =150 ppm | ||||
| Dermal | >2000 mg/kg (rabbits) | ND | Skin: 500 μL (rabbits) | |||||
| Eyes: 100 μL (rabbits) | ||||||||
| Benzene | Oral | 1 mL/kg (rats) | 9 g/kg (6−15d pregnant mice) | N/A | Mutagenicity was measured in a variety of animal models at varying concentrations. | Carcinogenic | 10-hr TWA =0.1 ppm | 8-hr TWA =1 ppm |
| Inhalation | 10,000 ppm/7 h (rats) | 50 ppm (7−14d pregnant rats) | N/A | |||||
| Dermal | >9400 μL/kg (rabbits) | ND | Skin: 20 mg (rabbits) | 15-min STEL =1 ppm | 15-min STEL =5 ppm | |||
| Eyes: 2 mg (rabbits) | ||||||||
| Ethanol | Oral | >7060 mg/kg (rats) | >4 g/kg (13d pregnant rats) | N/A | Mutagenicity has been observed in a variety of animal models at varying concentrations | Carcinogenic; enhances carcinogenesis. | 10-hr TWA =1000 ppm | 8-hr TWA =1000 ppm |
| Inhalation | >5900 ppm/6 h (rats) | >5000 ppm (9−20d pregnant rats) | N/A | |||||
| Dermal | ND | ND | Skin: 400 mg open irritation (rabbits) | |||||
| Eyes: 500 mg (rabbits) | ||||||||
| Diethyl Phthalates | Oral | 8600 mg/kg (rats) | >25 g/kg (6−15d pregnant rats) | N/A | Evidence of mutagenicity on bacteria, more studies needed to investigate mutagenicity on animal models | No evidence of carcinogenicity | 10-hr TWA =5 mg/m3 | N/A |
| Inhalation | >4640 mg/m3/6 h (rats) | ND | N/A | |||||
| Dermal | >20 mL/kg (guinea pigs) | 26 m L/kg (6−18d pregnant rabbits) | Eyes: 112 mg (rabbits) |
STEL = Short-term exposure limit.
TWA = Time-weighted average.
NIOSH = The National Institute for Occupational Safety and Health.
OSHA = Occupational Safety and Health Administration.
ND = No data available.
N/A = Not Applicable.
1.4. Acetaldehyde
Acetaldehyde is produced through the oxidation or metabolism of ethanol (via alcohol dehydrogenase) and other phenolic compounds by yeasts and bacteria [39]. Conversion between acetal and acetaldehyde can occur in the presence of methanol, ethanol, or an acidic catalyst during the fermentation process [27,40,41]. Acetaldehyde toxicity has been characterized in animal models [42] (Table 2), and has been demonstrated to elicit teratogenic, mutagenic, and carcinogenic effects. Application of ABHRs can cause a potential inhalation or dermal absorption hazards dependent on concentration and frequency of use. The presence of water or humectant can prolong contact time and increase exposure risk.
Recent studies suggest acetaldehyde to be a likely human carcinogen [43] (i.e. Group 2B according to the International Agency for Research on Cancer classification) and the main chemical involved in fetal alcohol spectrum disorder [44]. Dermal absorption and defatting of the skin is possible [45], although inhalation and ingestion are the primary routes of acetaldehyde exposure. Short-term inhalation studies of animals have demonstrated histopathological changes in nasal olfactory epithelia and functional changes in the lungs (Table 2). After chronic inhalation exposure, nasal carcinomas can also develop and can lead to esophageal cancer [43].
Currently, the limits of acetaldehyde in USP-compliant ethanol is 10 ppm, whereas interim licenced technical-grade ethanol in March 2020 could contain up to 1000 ppm, however that number has since decreased to < 400 ppm as of June 2020 in Canada, and further decreased to < 75 ppm as of November 2020 [11]. ABHRs exceeding 75 ppm of acetaldehyde are subject to additional labelling requirements [11]. The US-FDA requires < 50 ppm [46] (Table 1). Producers of ABHRs are required to provide warning labels for acetaldehyde concentration and are encouraged to reduce the levels of this contaminant. These criteria are temporary, and concentrations are expected to decrease further as ethanol producers improve distillation and other refinement methods. However, at 400 ppm, inhalation exposure in rats resulted in the degeneration of the nasal olfactory epithelium leading to loss of microvilli, thinning and disarrangement of epithelial cells, and loss of sensory cells [47]. For human health risk, the National Institute for Occupational Safety and Health (NIOSH), does not recommend occupational exposure limits for this chemical, but instead recommends working at the lowest feasible levels. Due to the potential exposure to at-risk population (i.e. breastfeeding or pregnant people, and children) [44], some healthcare services have opted to discontinue the use of ABHR produced using technical-grade ethanol.
1.5. Propanols
Isopropanol is routinely used as an active ingredient in hand sanitizers and surface disinfectants and is regulated as a separate product [48,49] with its own risks. It has demonstrated enhanced disinfection effectiveness of medical equipment used in healthcare settings [50,51]. This useful ingredient may be included unintentionally in technical-grade ethanol, and if further isopropanol is added in the ABHR formula, the resulting mixture will have a higher than intended isopropanol content in disagreement with the product formulation. In the current context of technical-grade ethanol as an ingredient, Health Canada identifies and controls isopropanol as an “impurity” and places a limit of 1000 ppm. With or without the use of technical-grade ethanol, recalls have been issued for ethanol-based ABHRs with elevated presence of combined denaturants, isopropanol (7.8 L in 100 L of alcohol) and ethyl acetate (3.3 L in 100 L of alcohol) [35]. These recalls may have been primarily attributed to the high ethyl acetate content rather than the presence of isopropanol, or the prevention of arbitrary mixtures of isopropanol and ethanol.
Isopropanol and n-propanol are naturally synthesized from amino acids and simple sugars during fermentation processes (e.g. Maillard reactions) [42,52,53]. Isopropanol can also be produced through the reduction of acetone by lactic acid bacteria [54] (i.e. bacterial contamination in the fermentation mash) [55]. Of these, n-propanol is nontoxic for animals and humans via the dermal, inhalation, and oral routes of exposure. However, development and reproductive effects has been observed at concentrations far exceeding occupational exposure limits [56,57] (Table 2).
Isopropanol is an isomer of n-propanol and is approximately two-fold more toxic than ethanol due to its higher molecular weight [58]. It is metabolized in the liver to produce acetone by the enzyme alcohol dehydrogenase [37], whereas, n-propanol is metabolized to propionaldehyde [59]. Isopropanol acts similarly to ethanol in regard to adsorption, distribution, metabolism, and excretion, but displays a stronger narcotic/intoxicating effect [60], due to depression of the central nervous system (CNS). Metabolism of isopropanol to acetone was previously thought to be the culprit leading to CNS depression; however, subsequent studies identified isopropanol itself as the major contributor. Clinical improvement was observed while acetone concentrations were increasing, due to the metabolism of isopropanol exposure [61]. Currently, isopropanol is not classifiable as carcinogenic to humans (Group 3), although the manufacture of isopropanol by strong-acid processes has been deemed carcinogenic to humans (Group 1) [62]. Fatalities due to isopropanol exposure are considered rare, although increased exposure can result in altered sensorium, hypotension, hypothermia, and cardiopulmonary collapse [37]. Repeated use can result in mild erythema and dermal absorption into the circulatory system [63]. A study on isopropanol exposure in rabbit showed toxicity with dermal absorption. Furthermore, during exposure isopropanol was converted to acetone and with long term exposure acetone accumulation might contribute to prolonged activity and toxicity [64]. Prolonged dermal adsorption could be greater than lung adsorption if isopropanol was applied topically [64]. In spite of the demonstrated toxicity of propanols the concentration of these molecules in known technical and USP alcohols is much lower than used in any known study.
1.6. Ethyl acetate
Ethyl acetate and other esters are produced by alcohol acyltransferases and can be hydrolyzed by esterases [65]. This can occur through the esterification of acetic acid by ethanol, enzyme-catalyzed esterification, or ester formation within the cell prior to diffusion into the solution [66]. It is one of the most abundant esters produced by yeasts and is difficult to separate by simple distillation processes [67]. Ethyl acetate is an ester of ethanol and acetic acid and is used as an industrial solvent (e.g. paints, plasticizers, denaturant, etc.) [66]. Acute toxicity for this chemical is unlikely [68], although moderate toxicity has been observed via intraperitoneal, subcutaneous, and oral routes [62] (Table 2). Exposure to ethyl acetate vapours (∼ 400 ppm) can result in irritations to the eyes, nose and throat, and headaches, nausea, vomiting, sleepiness, and unconsciousness [68]. Meanwhile, dermal absorption of ethyl acetate can cause reduced fertility in men, defatted or erupted skin, and adverse effects to the liver and kidneys [68]. Recently, due to the use of technical-grade ethanol in ABHR formulation, HC has issued recalls for 21 products due to the elevated presence of ethyl acetate (July 2020) [35].
1.7. Butanols
Butanols, like propanols, are produced from the Maillard reactions that occur during Saccharomyces cerevisiae fermentations and the reduction of amino acids [69]. Several animal studies have demonstrated that acute toxicity is unlikely at environmental levels [42] (Table 2). Minimal studies have been conducted to measure mutagenic or carcinogenic effects of isobutanol [42]; however, tumorigenic and teratogenic effects have been observed in animal models for tert-butanol [42]. Reproductive effects in animal models have also been observed for these chemicals (Table 2) [70]. This could create a risk for those who are breastfeeding or children due to the bioaccumulation of these compounds in the breastmilk.
1.8. Benzene
Benzene is an organic used in the production of gasoline. Benzene, as an entraining agent, can also be used to produce absolute ethanol by breaking the ethanol-water azeotrope and producing a ternary azeotrope [71]. Absolute ethanol can then be fractionally distilled to remove the water. Alternatively, ethanol produced from grain fermentations is typically benzene-free unless cross contamination occurs (e.g. mixing with gasoline products). Therefore, depending on the ethanol production processes, the risk of benzene contamination can be substantial.
Benzene toxicity and human health risk assessments have been well-characterized (Table 2). It is a known human carcinogen and mutagen that can be absorbed into the body via inhalation, dermal and oral routes [72]. Common symptoms include drowsiness, tremors, headaches, vomiting, irritation, convulsions, irregular heartbeat, and death. In fact, due to the inherent risks associated with benzene, occupational exposure limits, are set at 0.1 ppm (8 h time-weighted average), and 1 ppm (15-minute time-weighted average) [73]. The USP monograph for ethanol limits the amount of benzene to < 2 ppm [9]. Benzene is readily absorbed through the skin, although the amount absorbed is dose-dependent and varies upon the delivery vehicle [74]. The potential for benzene contaminant in ABHR formulations can be detrimental to the health and safety of those who use these products, as repeated use and the inclusion of water or gelation agent (e.g. carbomer) can greatly prolong exposure time and dermal absorption [74]. Epidemiological studies on inhalation of benzene have identified that low exposure to benzene significantly reduced total white blood cells and red blood cells [75]. The major effect of benzene from long-term exposure is on the blood and bone marrow, leading to anemia [75]. Therefore, ABHRs utilizing technical-grade ethanol should be monitored to minimize potential and repeated exposure to this toxic compound.
1.9. Other additives
Many manufacturers have resorted to using non-compliant containers for ABHRs, this can increase child exploratory behavior risks and accidental poisonings [20,21]. In Canada, we have observed some manufacturers resorting to canned beverage containers to ship their products as single-use, “refill” ABHRs. However, these manufacturers may be unaware of the chemical changes due to carbonation, via the injection of carbon dioxide during the canning process. Carbon dioxide is acidic in nature and can therefore alter the pH of ABHRs, chemically modifying the composition of the ABHR by altering the equilibrium between acetal and acetaldehyde compounds [27,41]. Consequently, this can influence the toxicity of the impurities in the product.
Some manufacturers have also opted to mix non-approved dyes and fragrances into their products, potentially to mask undesirable odors and colors associated with technical-grade ethanol. This can present a risk for consumers, as fragrance ingredients are one of the most frequent causes of type IV contact allergies [76]. The use of fragrances in ABHRs could act as a vehicle for phthalate exposure [77]. Exposure to phthalates can also occur from leeching from polyethylene terephthalate and high-density polyethylene containers [78] that are currently permitted for ABHRs during this interim period. The amount of leeching is influenced by the acidity of the solution, packaging material, period of storage, and storage temperature [78]. We have observed one ABHR product that contained diethyl phthalate (DEP), a commonly used phthalate in the manufacturing of personal care products. Although this compound exhibits a low order of acute toxicity [42,77] (Table 2), it is readily adsorbed via percutaneous or dermal routes, which can result in dermal irritation and sensitization [77]. Once absorbed, DEP will primarily localize to the liver and kidneys [77]. Despite the identification of DEP in one ABHR product, the presence of other phthalates is probable (e.g. fragrances and other raw ingredients), and should be monitored. As different phthalates can evoke different toxicities [79], it is of concern to public health that regulatory compliance is ensured in the formulation of ABHRs to minimize exposures to these potentially toxic contaminants.
2. Conclusion
The COVID-19 pandemic has resulted in increased demand for essential hygiene items and interim changes to government regulations. To combat this virus, the use of technical-grade ethanol ABHR formulations has been permitted. Health agencies in Canada and the US have deemed the risk of technical-grade ethanol as acceptable to help contain the spread of disease. With hundreds of new products becoming available on the marketplace daily, it is incredibly difficult for government agencies to investigate each individual product for compliance. In a sampling of newly licensed ABHRs, we have found some producers that are aware of the contaminants and are taking steps to reduce them. However, other producers seem unfamiliar with the risks of contamination and incorrect formulation. The implementation of technical-grade ethanol could have consequences in a healthcare setting, especially if the ingredients are non-compliant to current regulations.
Due to the current pandemic, ABHR usage has significantly increased in the public, private and healthcare sectors, it is of utmost importance to provide compliant products to ensure consumer safety and efficacy of the product. For consumers who use ABHRs less frequently, the presence of technical-grade ethanol with higher levels of impurities still results in elevated risk compared to standard ABHRs. It is important for both doctors and the public to be aware that the interim ABHRs are higher in contaminants, even if they come from food sources (e.g. local distilleries). Although the elevated concentrations for most of the contaminants found may not elicit detrimental health effects, the combined effects for these contaminants have not been studied. Defatting of the skin can create further human health risk for elevated dermal absorption of these contaminants. There is the potential for additive adverse effects, or effects because of underlying health conditions. The main contaminants for health concern that may be present in ABHRs appear to be methanol due to risk of acute toxicity when ingested, ethyl acetate due to risk of dermatitis and added exposure routes related to this, acetaldehyde due to teratogenicity, and benzene due to mutagenicity and carcinogenicity. Although, most products are deemed safe-to-use in the interim, there have been cases where dangerous levels of contaminants have been reported. Therefore, more vigilant policing is recommended to ensure the safety, efficacy and compliance of ABHRs with regulatory authorities. Health practitioners and the public must also be aware of the role they play in enforcing product compliance with the surge of new products from such a wide variety of sources.
Funding
This work was supported by the Saskatchewan Agricultural Development Fund (grant numbers 20190155, 20190154, 20180281, 20180248, 20180255, 20170133); National Sciences and Engineering Research Council of Canada Discovery Grant (grant number RGPIN-2018-06631); and Mitacs (grant numbers IT19122, IT16156).
Conflict of Interest
Dr. Martin J. T. Reaney is the founder of, and has an equity interest in, Prairie Tide Diversified Inc. (PTD, Saskatoon, SK, Canada: previous company name is Prairie Tide Chemicals Inc.).
Declaration of Competing Interest
The authors report no declarations of interest.
Edited by Dr. A.M Tsatsaka
Contributor Information
Timothy J. Tse, Email: timothy.tse@usask.ca.
Martin J.T. Reaney, Email: martin.reaney@usask.ca.
References
- 1.Bloomfield S.F., Aielloo A.E., Cookson B., O’Boyle C., Larson E.L. The effectiveness of hand hygiene procedures in reducing the risks of infections in home and community settings including handwashing and alcohol-based hand sanitizers. Am. J. Infect. Control. 2007;35:S27–S64. [Google Scholar]
- 2.Trampuz A., Widmer A.F. Hand hygiene: a frequently missed lifesaving opportunity during patient care. Mayo Clin. Proc. 2004;79:109–116. doi: 10.4065/79.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Picheansathian W. Effectiveness of alcohol-based solutions for hand hygiene: a systematic review. JBI Libr. Sys. Rev. 2004;2:1–27. doi: 10.11124/01938924-200402090-00001. [DOI] [PubMed] [Google Scholar]
- 4.Pickering A.J., Davis J., Boehm A.B. Efficacy of alcohol-based hand sanitizer on hands soiled with dirt and cooking oil. J. Water Health. 2011;9:429–433. doi: 10.2166/wh.2011.138. [DOI] [PubMed] [Google Scholar]
- 5.Fabiszewski de Aceituno A., Bartz F.E., Hodge D.W., Shumaker D.J., Grubb J.E., Arbogast J.W., Dávila-Aviña J., Venegas F., Heredia N., Barcía S., Leon J.S. Ability of hand hygiene interventions using alcohol-based hand sanitizers and soap to reduce microbial load on farmworker hands soiled during harvest. J. Food Prot. 2015;78:2024–2032. doi: 10.4315/0362-028X.JFP-15-102. [DOI] [PubMed] [Google Scholar]
- 6.Macinga D.R., Schumaker D.J., Werner H.-P., Edmonds S.L., Leslie R.A., Parker A.E., Arbogast J.W. The relative influences of product volume, delivery, format, and alcohol concentration on dry-time and efficacy of alcohol-based hand rubs. BMC Infect. Dis. 2014;14:511. doi: 10.1186/1471-2334-14-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suchomel M., Kundim M., Pittet D., Weinlich M., Rotter M.L. Testing the World Health Organization recommended formulations in their application as hygienic hand rubs and proposals for increased efficacy. Am. J. Infect. Control. 2012;40:328–331. doi: 10.1016/j.ajic.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Institute of Medicine . fifth edition. The National Academies Press; Washington, DC: 2003. Food Chemicals Codex. [DOI] [Google Scholar]
- 9.United States Pharmacopeia and National Formulary (USP 41-NF 36). Rockville, MD: United States Pharmacopeial Convention; 2016. https://online.uspnf.com/uspnf/document/GUID-AC788D41-90A2-4F36-A6E7-769954A9ED09_1_en-US. Accessed January 18, 2019.
- 10.Kelly S., Weinraub M. 2020. Ethanol Makers See Demand Surge on Hand Sanitizer Stockpiling. Reuters. March 9. Available online: https://www.reuters.com/article/us-health-coronavirus-cargill-idUSKBN20W1WW. (accessed on February 2, 2021). [Google Scholar]
- 11.Government of Canada . 2020. Health Canada’s Decision on Technical-grade Ethanol for the Manufacture of Hand Sanitizers: Notice to Industry. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/natural-non-prescription/legislation-guidelines/covid19-technical-grade-ethanol-hand-sanitizer.html. (Accessed on June 17, 2020) [Google Scholar]
- 12.Australian Government Department of Health. Hand sanitisers: Information for manufacturers, suppliers and advertisers. Available online: https://www.tga.gov.au/hand-sanitisers-information-manufacturers-suppliers-and-advertisers. (Accessed on February 2, 2021).
- 13.Government of Brazil Ministry of Health. Resolution – RDC No. 350, of 19 March 2020. Available online: https://www.in.gov.br/en/web/dou/-/resolucao-rdc-n-350-de-19-de-marco-de-2020-249028045. (Accessed on February 2, 2021).
- 14.United States Food and Drug Administration. March 2020 – Updated February 10, 2021. Temporary policy for preparation of certain alcohol-based hand sanitizer products during the public health emergency (COVID-19) Guidance for Industry.
- 15.Government of Canada. Production of isopropyl alcohol for use in alcohol-based hand sanitizers: Interim guide. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/natural-non-prescription/legislation-guidelines/guidance-documents/covid-19-production-isopropyl-alcohol-hand-sanitizers-interim-guide.html#_Technical_grade. (Accessed on February 2, 2021).
- 16.Government of Canada . 2020. Hard-surface Disinfectants and Hand Sanitizers (COVID-19): List of Hand Sanitizers Authorized by Health Canada. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/disinfectants/covid-19/hand-sanitizer.html. (accessed on August 12, 2020) [Google Scholar]
- 17.Health Canada . 2020. Health Canada is Temporarily Authorizing the Use of Technical-grade Ethanol in Hand Sanitizer Products: Always Follow the Label Directions when Using Alcohol-based Hand Sanitizers. Available online: https://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2020/72739a-eng.php. (accessed on May 18, 2020) [Google Scholar]
- 18.Statista. Hand Sanitizer. Available online: https://www.statista.com/outlook/18060000/100/hand-sanitizer/worldwide. (Accessed on February 2, 2021).
- 19.Onuki S., Koziel J.A., Jenks W.S., Cai L., Grewell D., van Leeuwen J.H. Taking ethanol quality beyond fuel grade: a review. J. Inst. Brew. 2016;122:588–598. [Google Scholar]
- 20.Joseph M.M., Zeretzke C., Reader S., Sollee D.R. Acute ethanol poisoning in a 6-year-old girl following ingestion of alcohol-based hand sanitizer at school. World J. Emerg. Med. 2011;2:232–233. doi: 10.5847/wjem.j.1920-8642.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engel J.S., Spiller H.A. Acute ethanol poisoning in a 4-year-old as a result of ethanol-based hand-sanitizer ingestion. Pediatr. Emerg. Care. 2010;26:508–509. doi: 10.1097/PEC.0b013e3181e5bfc9. [DOI] [PubMed] [Google Scholar]
- 22.British Columbia Centre for Disease Control . BC CDC; 2020. Poison Control Records Spike in Calls about Children and Adults Accidentally Ingesting Hand Sanitizer. May 25. http://www.bccdc.ca/about/news-stories/stories/2020/poison-control-records-spike-in-calls-about-children-and-adults-accidentally-ingesting-hand-sanitizer. (Accessed February 26, 2021) [Google Scholar]
- 23.Jing J.L.J., Yi T.P., Bose R.J.C., McCarthy J.R., Tharmalingam N., Madheswaran T. Hand sanitizers: a review on formulation aspects, adverse effects, and regulations. Int. J. Environ. Res. Public Health. 2020;17:3326. doi: 10.3390/ijerph17093326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golin A.P., Choi D., Ghahary A. Hand sanitizers: a review of ingredients, mechanisms of action, modes of delivery, and efficacy against coronaviruses. Am. J. Infect. Control. 2020 doi: 10.1016/j.ajic.2020.06.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization . 2009. Guide to Local Production: WHO-recommended Handrub Formulations. Retrieve from: https://www.who.int/gpsc/information_centre/handrub-formulations/en/. (Accessed on May 15, 2020) [Google Scholar]
- 26.Food Chemical Codex . FCC; 2020. Ethyl Alcohol. Available online: https://www.foodchemicalscodex.org/notices/ethyl-alcohol-nitr-20200818. (Accessed on January 28, 2021) [Google Scholar]
- 27.Capeletti M.R., Balzano L., de la Puente G., Laborde M., Sedran U. Synthesis of acetal (1,1-diethoxyethane) from ethanol and acetaldehyde over acidic catalysts. Apple Catal. A-Gen. 2000;198:L1–L4. [Google Scholar]
- 28.Hazelwood L.A., Daran J.-M., van Maris A.J.A., Pronk J.T., Dickinson R.J. The Ehrlich Pathway for fusel alcohol production: a century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008;74:2259–2266. doi: 10.1128/AEM.02625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tse T.J., Nelson F.B., Reaney M.J.T. Analyses of commercially available alcohol-based hand rubs formulated with compliant and non-compliant ethanol. Int. J. Environ. Res. Public Health. 2021;18:3766. doi: 10.3390/ijerph18073766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pielech-Przybylska K., Balcerek M., Dziekońska-Kubczak U., Pacholczyk-Sienicka B., Ciepielowski G., Albrecht Ł., Patelski P. The role of Saccharomyces cerevisiae yeast and lactic acid bacteria in the formation of 2-propanol from acetone during fermentation of rye mashes obtained using thermal-pressure method of starch liberation. Molecules. 2019;24:610. doi: 10.3390/molecules24030610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohimain E.I. Springerplus; 2016. Methanol Contamination in Traditionally Fermented Alcoholic Beverages: the Microbial Dimension. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Health Canada . 2013. Two Deaths Linked to Ingestion of Hand Sanitizer Containing Methanol. Available online: http://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2013/36469-eng.php (accessed on May 18, 2020) [Google Scholar]
- 33.Mowry J.B., Spyker D.A., Brooks D.E., McMillan N., Schauben J.L. 2014 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 32nd annual report. Clin. Toxicol. 2015;50:823–831. doi: 10.3109/15563650.2015.1102927. [DOI] [PubMed] [Google Scholar]
- 34.Chan G.C.K., Chan J.C.M., Szeto C.C., Chow K.M. Mixed isopropanol-methanol intoxication following ingestion of alcohol-based hand rub solution. Clin. Nephrol. 2017;88:218–220. doi: 10.5414/CN109103. [DOI] [PubMed] [Google Scholar]
- 35.Government of Canada . 2020. Recall of Certain Hand Sanitizers That May Pose Health Risks. Available online: https://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2020/73385a-eng.php. (Accessed on July 4, 2020) [Google Scholar]
- 36.United States Food and Drug Administration . 2020. FDA Advises Consumers Not to Use Hand Sanitizer Manufactured by Eskbiochem. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-advises-consumers-not-use-hand-sanitizer-products-manufactured-eskbiochem. (accessed on June 22, 2020) [Google Scholar]
- 37.Ashurst J.V., Nappe T.M. StatPearls Publishing; Treasure Island (FL): 2020. StatPearls [Internet] Jan-. Available from: https//www.ncbi.nlm.nih.gov/books/NBK493181/ [Google Scholar]
- 38.Institute of Medicine . The National Academies Press; Washington, DC: 1995. Environmental Medicine: Integrating a Missing Element into Medical Evaluation. [DOI] [PubMed] [Google Scholar]
- 39.Liu S.Q., Pilone G.J. An overview of formation and roles of acetaldehyde in winemaking with emphasis on microbiological implications. Int. J. Food Sci. Technol. 2001;35:49–61. [Google Scholar]
- 40.Kłosowski G., Czupryński B. Kinetics of acetals and esters formation during alcoholic fermentation of various starchy raw materials with application of yeasts Saccharomyces cerevisiae. J. Food Eng. 2004;72:242–246. [Google Scholar]
- 41.Meadows G.W., Darwent B.D. The reactions of acetaldehyde with methanol. Can. J. Chem. 1952;30:501–506. [Google Scholar]
- 42.National Center for Biotechnology Information PubChem Database. https://pubchem.ncbi.nlm.nih.gov/ (accessed on July 20, 2020)
- 43.Seitz H.K., Mueller S. Molecular Aspects of Alcohol and Nutrition. 2016. Molecular mechanisms of alcohol-associated carcinogenesis; pp. 305–314. [DOI] [Google Scholar]
- 44.Shabtai Y., Bendelac L., Jubran H., Hirschberg J., Fainsod A. Acetaldehyde inhibits retinoic acid biosynthesis to mediate alcohol teratogenicity. Sci. Rep. 2018;8:347. doi: 10.1038/s441598-017-18719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thredgold L., Gaskin S., Heath L., Pisaniello D., Logan M., Baxter C. Understanding skin absorption of common aldehyde vapours from exposure during hazardous material incidents. J. Expo. Sci. Env. Epid. 2020;30:537–546. doi: 10.1038/s41370-019-0127-4. [DOI] [PubMed] [Google Scholar]
- 46.United States Food and Drug Administration. Preparation of certain alcohol-based hand sanitizer products during the public health emergency (COVID-19) guidance for industry. March 2020. Available online: https://www.fda.gov/media/136289. (Accessed on June 17, 2020).
- 47.Appelman L.M., Woutersen R.A., Feron V.J. Inhalation of acetaldehyde in rats. I. Acute and subacute studies. Toxicology. 1982;23:293–307. doi: 10.1016/0300-483x(82)90068-3. [DOI] [PubMed] [Google Scholar]
- 48.Health Canada . 2020. Production of Isopropyl Alcohol for Use in Alcohol-based Hand Sanitizers: Interim Guide. June 4. https://www.canada.ca/en/health-canada/services/drugs-health-products/natural-non-prescription/legislation-guidelines/guidance-documents/covid-19-production-isopropyl-alcohol-hand-sanitizers-interim-guide.html#_Recommended_formulations, 2020. (Accessed March 18, 2021) [Google Scholar]
- 49.Mahmood A., Eqan M., Pervez S., Alghamdi H.A., Tabinda A.B., Yasar A., Brindhadevi K., Pugazhendhi A. COVID-19 and frequent use of hand sanitizers; human health and environmental hazards by exposure pathways. Sci. Total Environ. 2020;742 doi: 10.1016/j.scitotenv.2020.140561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gouda S., Mattoo J., Kotian S., Kukanu S.F., Naveen G. Comparison of effectiveness of 70%-isopropanol, 65%-ethanol and 1%-chlorhexidine for stethoscope decontamination. J. Pure Appl. Microbiol. 2020;14:2053–2062. [Google Scholar]
- 51.Lecat P., Cropp E., McCord G., Haller N.A. Ethanol-based cleanser versus isopropyl alcohol to decontaminate stethoscopes. Am. J. Infect. Control. 2009;37:241–243. doi: 10.1016/j.ajic.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 52.Sentheshanmuganathan S. The mechanism of the formation of higher alcohols from amino acids by Saccharomyces cerevisiae. Biochem. J. 1960;74:568–576. doi: 10.1042/bj0740568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Janssen P.H. Propanol as an end product of threonine fermentation. Arch. Microbiol. 2004;182:482–486. doi: 10.1007/s00203-004-0732-y. [DOI] [PubMed] [Google Scholar]
- 54.Pielech-Przybylska K., Balcerek M., Dziekońska-Kubczak U., Pacholczyk-Sienicka B., Ciepielowski G., Albrecht Ł., Patelski P. The role of Saccharomyces cerevisiae yeast and lactic acid bacteria in the formation of 2-propanol from acetone during fermentation of rye mashes obtained using thermal-pressure method of starch liberation. Molecules. 2019;24 doi: 10.3390/molecules24030610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Narendranath N.V., Hynes S.H., Thomas K.C., Ingledew W.M. Effects of lactobacilli on yeast-catalyzed ethanol fermentations. Appl. Environ. Microbiol. 1997;63:4158–4163. doi: 10.1128/aem.63.11.4158-4163.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nelson B.K., Brightwell W.S., Burg J.R. Comparison of behavioural teratogenic effects of ethanol and n-propanol administered by inhalation to rats. Neurotoxicol. Teratol. 1985;7:779–783. [PubMed] [Google Scholar]
- 57.Nelson B.K., Brightwell W.S., MacKenzie-Taylor D.R., Khan A., Burg J.R., Weigel W.W., Goad P.T. Teratogenicity of n-propanol and isopropanol administered at high inhalation concentrations to rats. Food Chem. Toxicol. 1988;26:247–254. doi: 10.1016/0278-6915(88)90126-3. [DOI] [PubMed] [Google Scholar]
- 58.Thrall M.A., Hamar D.W. Alcohols and glycols. Veterinary Toxicology. 2012:735–744. doi: 10.1016/b978-0-12-385926-6.00071-5. [DOI] [Google Scholar]
- 59.Bessonneau V., Clément M., Thomas O. Can intensive use of alcohol-based hand rubs lead to passive alcoholization? Int. J. Environ. Res. Public Health. 2010;7:3038–3050. doi: 10.3390/ijerph7083038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Slaughter R.J., Mason R.W., Beasley D.M.G., Vale J.A., Schep L.J. Isopropanol poisoning. Clin. Toxicol. 2014;52:470–478. doi: 10.3109/15563650.2014.914527. [DOI] [PubMed] [Google Scholar]
- 61.Blanchet B., Charachon A., Lukat S., Huet E., Hulin A., Asiter A. A case of mixed intoxication with isopropyl alcohol and propanol-1 after ingestion of a topical antiseptic solution. Clin. Toxicol. Phila. (Phila) 2007;45:701–704. doi: 10.1080/15563650701517285. [DOI] [PubMed] [Google Scholar]
- 62.Lewis R.J. Sr., editor. Sax’s Dangerous Properties of Industrial Materials. 11th edition. Wiley-Interscience, Wiley & Sons, Inc.; Hoboken, NJ: 2004. p. 1625. [Google Scholar]
- 63.Turner P., Saeed B., Kelsey M.C. Dermal absorption of ispropyl alcohol from a commercial hand rub: implications for its use in hand decontamination. J. Hosp. Infect. 2004;56:287–290. doi: 10.1016/j.jhin.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 64.Martinez T.T., Jaeger R.W., deCastro F.J., Thompson M.W., Hamilton M.F. A comparison of the absorption and metabolism of isopropyl alcohol by oral, dermal and inhalation routes. Vet. Hum. Toxicol. 1986;28:233–236. [PubMed] [Google Scholar]
- 65.Yoshioka K., Hasimoto N. Ester formation by alcohol acetyltransferase from brewers’ yeast. Agric. Biol. Chem. 1981;45:2183–2190. [Google Scholar]
- 66.Nordström K. Formation of ethyl acetate in fermentation with brewer’s yeast. J. Inst. Brew. 1961;68:188–196. [Google Scholar]
- 67.Furzer I.A. Critical distillation experiments in a region near the homoegenous ternary azeotrope in the system ethyl acetate-ethanol-water. Ind. Eng. Chem. Res. 2001;40:990–992. [Google Scholar]
- 68.DECOS (Dutch Expert Committee for Occupational Standards) Sdu Uitgeverij; Den Haag: 1991. Ethyl Acetate. Health-based Recommended Occupational Exposure Limit, RA 10/91. [Google Scholar]
- 69.He Y., Dong J., Yin H., Zhao Y., Chen R., Wan X., Chen P., Hou X., Liu J., Chen L. Wort composition and its impact on the flavour-active higher alcohol and ester formation of beer. J. Inst. Brew. 2014;120:157–163. [Google Scholar]
- 70.Pellizzari E.D., Hartwell T.D., Harris B.S.H., Waddell R.D., Whitaker D.A., Erickson M.D. Purgeable organic compounds in mother’s milk. Bull. Environ. Contam. Toxicol. 1982;28(3):322–328. doi: 10.1007/bf01608515. [DOI] [PubMed] [Google Scholar]
- 71.Mattson G., hertel G.R. Drying ethanol by azeotropic distillation. J. Chem. Educ. 1990;67:46–47. [Google Scholar]
- 72.IARC . World Health Organization, International Agency for Research on Cancer, 1972 to present; Geneva: 1987. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans; pp. S7–120. (multivolume work). Available at: http://monographs.iarc.fr/ENG/Classification/index.php, (Accessed July 19, 2020) [Google Scholar]
- 73.Yardley-Jones A., Anderson D., Parke D.V. The toxicity of benzene and its metabolism and molecular pathology in human risk assessment. Br. J. Ind. 1991;48:437–444. doi: 10.1136/oem.48.7.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wester R.C., Maibach H.I. Benzene percutaneous absorption: dermal exposure relative to other benzene sources. Int. J. Occup. Environ. Health. 2000;6:122–126. doi: 10.1179/oeh.2000.6.2.122. [DOI] [PubMed] [Google Scholar]
- 75.Qu Q., Shore R., Li G., Jin X., Chen L.C., Cohen B., Melikian A.A., Eastmond D., Rappaport S.M., Yin S., Li H., Waidyanatha S., Li Y., Mu R., Zhang X., Li K. Hematological changes among Chinese workers with a broad range of benzene exposures. Am J Ind. 2002;42:275–285. doi: 10.1002/ajim.10121. [DOI] [PubMed] [Google Scholar]
- 76.Johansen J.D. Fragrance contact allergy. Am. J. Clin. Dermatol. 2003;4:789–798. doi: 10.2165/00128071-200304110-00006. [DOI] [PubMed] [Google Scholar]
- 77.Api A.M. Toxicological profile of diethyl phthalate: a vehicle for fragrance and cosmetic ingredients. Food Chem. Toxicol. 2001;39:97–108. doi: 10.1016/s0278-6915(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 78.Rastkari N., Jeddi M.Z., Yunesian M., Ahmadkhaniha R. The effects of storage time, temperature and type of packaging on the release of phthalate esters into packed acidic liquids. Food Technol Biotech. 2017;55:562–569. doi: 10.17113/ftb.55.04.17.5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.National Research Council (US) National Academies Press (US); Washington (DC): 2008. Committee on the Health Risks of Phthalates. Phthalates and Cumulative Risk Assessment: The Tasks Ahead.https://www.ncbi.nlm.nih.gov/books/NBK215030/ 3, Toxicity Assessment. Available from: [PubMed] [Google Scholar]
- 80.Government of Canada. Recall of certain hand sanitizers that may pose health risks. Available online: https://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2020/73385a-eng.php. (Accessed on February 2, 2021).
- 81.United States Food and Drug Administration. FDA updates on hand sanitizers consumers should not use. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-hand-sanitizers-consumers-should-not-use#products. (Accessed on February 2, 2021).