Abstract
Obesity is common in heart failure with preserved ejection fraction (HFpEF). Whether obesity modifies the response to spironolactone in patients with HFpEF remains unclear. We aimed to investigate the effect of obesity, defined by body mass index (BMI) and waist circumference (WC), on response to spironolactone in patients with HFpEF enrolled in TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist) trial. This was a post-hoc, exploratory analysis of the Americas cohort of TOPCAT. BMI≥30 kg/m2 was used to define the obese group and WC≥102cm in men and ≥88cm in women were defined as high WC (HWC). In separate analyses, BMI and WC were treated as continuous variables. The effect of spironolactone vs. placebo on outcomes was calculated by BMI and WC using Cox proportional hazard models. Obese patients were younger and had more comorbidities. In multivariate analysis, spironolactone use was associated with a significant reduction in the primary endpoint, compared to placebo in obese [hazard ratio (HR=0.618, 95% CI 0.460–0.831, p=0.001), but not in non-obese subjects (HR=0.946, 95% CI 0.623–1.437, p=0.796; p for interaction=0.056). There was a linear association between continuous BMI and the effect of spironolactone, with the effect becoming significant at 33kg/m2. Similar results were obtained for the WC-based analysis. In conclusion, use of spironolactone in obese patients with HFpEF was associated with a decreased risk of the primary endpoint, cardiovascular death and HF hospitalizations, compared to placebo. Further prospective randomized studies in obese subjects are required.
Keywords: heart failure with preserved ejection fraction, obesity, spironolactone
Heart failure (HF) with preserved ejection fraction (HFpEF) is one of the most prevalent cardiovascular conditions, is associated with significant morbidity and mortality, and unlike HF with reduced ejection fraction (HFrEF) there is no evidence-based treatment that improves clinical outcomes 1,2. Obesity is a well-established risk factor for HFpEF, and is associated with a systemic pro-inflammatory state and activation of the renin–angiotensin-aldosterone system with established deleterious cardiovascular effects 3,4. Drugs that antagonize aldosterone have been shown to decrease the systemic pro-inflammatory state and could be an attractive therapeutic option for patients with obesity-related HFpEF. Nonetheless, in the TOPCAT (Aldosterone Antagonist Therapy for Adults with Heart Failure and Preserved Systolic Function; NCT00094302) trial, spironolactone failed to show any beneficial effect compared to placebo on the primary composite endpoint of cardiovascular death, HF hospitalization, or aborted cardiac arrest patients with HFpEF 5. However, a post-hoc analysis, which included patients enrolled in the Americas, demonstrated a significant reduction in the primary and several secondary endpoints with spironolactone treatment 6. In light of the inflammatory phenotype associated with obesity and the anti-inflammatory effects of spironolactone, we hypothesized that spironolactone would result in a better outcome in obese compared with non-obese patients enrolled in TOPCAT.
Methods
TOPCAT was a multicenter, randomized, double-blind, placebo-controlled trial that evaluated the effects of spironolactone in patients with symptomatic HFpEF. The details of the study design and primary findings were previously reported 5. Briefly, the trial included patients older than 50 years with signs and symptoms of heart failure, left ventricular ejection fraction >45%, who fulfilled at least 1 of the following inclusion criteria: (1) history of hospitalization for HF within the past 12 months; or (2) brain natriuretic peptide (BNP)≥100 pg/mL or an N-terminal-pro-BNP (NT-pro-BNP)≥360 pg/mL within 60 days of randomization. The study included 3445 participants from 233 sites across the Americas (United States, Canada, South America) (n=1767 participants), and Europe (Russia and Republic of Georgia, n=1678 participants). The mean duration of follow-up was 3.4±1.7 years. The primary endpoint was time to cardiovascular death, HF hospitalization, or aborted cardiac arrest. All endpoints were adjudicated by a central adjudication committee blinded to treatment assignment. HF hospitalization was defined as an overnight stay for the acute management of HF with ≥1 symptom and ≥2 signs of HF with qualified treatment 5. The data and study materials were made available through the National Institutes of Health and the Institutional Review Board of the University of Oklahoma Health Sciences Center approved the present analysis.
The primary endpoint for the present study was the composite of cardiovascular death, HF hospitalization, or aborted cardiac arrest. Secondary endpoints analyzed were cardiovascular death, HF hospitalization and all-cause death. Due to the very small number of aborted cardiac arrest (n=6), we did not include this individual endpoint in the analysis.
Because of the previously reported significant regional differences between the Americas and Russia and Georgia, and with very few events in Russia and Georgia 6, the primary analysis was carried out on the 1751 patients from the Americas cohort (USA, Canada, Argentina, Brazil) with available data about waist circumference, weight and height.
Obesity was defined according to World Health Organization criteria: BMI≥30Kg/m2 for obese group and <30Kg/m2 for non-obese group. Subjects were divided into two groups according to waist circumference (WC) using the American Heart Association defined cut-offs 7. Men and women with WC values <102cm and <88cm, respectively, were considered to have a normal WC (NWC), whereas those with WC values ≥102cm and ≥88 cm, respectively, were considered to have high WC (HWC).
Actual plasma volume (aPV) was calculated for participants with available hematocrit and weight data (n=1734). These values were generated from equations previously validated against both measured plasma volume and clinical outcomes in patients with HF 8,9, as follows: aPV = (1–hematocrit) x [a + (b × weight in kg)], where hematocrit is a proportion. In this equation, a=1530 or 864; and b=41 or 47.9, for men and women, respectively.
Echocardiographic data were available for 642 patients in our analysis and were not used in the multivariate analysis as the analysis would have been underpowered.
Of the 1751 patients enrolled in the Americas cohort, 786 (44.9%) patients were enrolled in the natriuretic peptide (NP) stratum and 965 patients in the HF hospitalization stratum. The study-qualifying BNP or NT-pro BNP values were available in 1047 patients. According to NP values, we divided patients into tertiles: NP tertile I (BNP<177pg/ml, NT-Pro-BNP<684pg/ml), NP tertile II (BNP 177–366pg/ml, NT-Pro-BNP 684–1496pg/ml) and NP tertile III (BNP>366pg/ml, NT-Pro-BNP>1496pg/ml).
Baseline characteristics between WC and BMI groups were compared using the chi-square test and Student’s t-test test for categorical and continuous variables, respectively. Associations between BMI or WC (both as a continuous and categorical variable) and end points were determined using Cox proportional hazards models. The effect of spironolactone vs. placebo on end points was calculated for BMI and WC categories. Interactions between BMI or WC and spironolactone effect on end points were assessed by introducing an interaction term BMI or WC variable × spironolactone. Multivariate associations were adjusted for all patient characteristics that differed significantly between BMI and WC categories in frequency or magnitude with backwards elimination until a parsimonious model was achieved. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated. P values <0.05 were considered statistically significant for the main effect. Due to the low power of interaction tests, a p value <0.1 was considered statistically for the interaction effect, as previously described 10. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
The baseline characteristics of obese and non-obese patients are summarized in Table 1. Compared with non-obese patients, obese patients were younger and had a higher frequency of hypertension, diabetes, dyslipidemia, asthma and atrial fibrillation. Use of diuretics, angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARB), calcium channel blockers (CCB) and statins were more frequent in obese compared to non-obese patients. Furthermore, obese patients had higher blood pressure, aPV and higher incidence of edema, were enrolled more frequently through the HF hospitalization stratum and had lower NP values.
Table 1.
Baseline characteristics according to BMI group for TOPCAT Americas population
| BMI (Kg/m2) | |||
|---|---|---|---|
| Characteristic | < 30 (n=616) | ≥ 30 (n=1135) | P value |
| Age (years) | 75.3 ± 9 | 69.4±9.3 | <0.001 |
| Spironolactone | 303 (49%) | 580 (51%) | 0.453 |
| BMI (Kg/m2) | 25.9 ± 2.7 | 38 ± 6.7 | < 0.001 |
| Waist circumference (cm) | 95.9 ±11 | 118±15.9 | < 0.001 |
| Women | 297 (48%) | 578 (51%) | 0.293 |
| Men | 319 (52%) | 557 (49%) | |
| White | 508 (83%) | 865 (76%) | < 0.001 |
| Black | 70 (11%) | 229 (20%) | |
| Others | 38 (6%) | 41 (4%) | |
| Enrollment strata: | |||
| HF hospital admission | 274 (44%) | 691 (61%) | < 0.001 |
| Natriuretic Peptide | 342 (56%) | 444 (39%) | |
| BNP (pg/ml) | 458 ± 538 (n=244) | 339 ± 343 (n=447) | 0.002 |
| NT Pro-BNP (pg/ml) | 1983 ± 2141 (n= 156) | 1368 ± 1699 (n= 200) | 0.004 |
| Natriuretic Peptide tertiles: | < 0.001 | ||
| I (n= 348) | 106 (27%) | 242 (37%) | |
| II (n= 349) | 134 (33%) | 215 (33%) | |
| III (n= 350) | 160 (40%) | 190 (29%) | |
| Actual plasma volume (aPV) (ml) | 2702.4 ± 387.2 | 3603.4 ± 662.4 | <0.001 |
| Heart rate (beats/min) | 67 ± 10.4 | 69 ± 11.5 | < 0.001 |
| Systolic Blood Pressure (mm Hg) | 125.8 ± 15.4 | 128.7 ± 15.8 | < 0.001 |
| Diastolic Blood Pressure (mm Hg) | 70.1 ± 11 | 72 ± 11.6 | 0.001 |
| NYHA | < 0.001 | ||
| I or II | 446 (72%) | 687 (61%) | |
| III or IV | 170 (28%) | 445 (39%) | |
| Edema over the past year | 536 (90%) | 1069 (96%) | < 0.001 |
| Hypertension | 529 (87%) | 1047 (92%) | < 0.001 |
| Diabetes mellitus | 177 (29%) | 608 (54%) | < 0.001 |
| Dyslipidemia | 407 (66%) | 837 (74%) | 0.001 |
| Atrial fibrillation | 279 (45%) | 458 (40%) | 0.048 |
| Stroke | 49 (8%) | 109 (10%) | 0.294 |
| Myocardial Infarction | 126 (21%) | 231 (20%) | 0.951 |
| PCI | 115 (19%) | 229 (20%) | 0.488 |
| CABG | 118 (19%) | 216 (19%) | 0.949 |
| Angina | 178 (29%) | 306 (27%) | 0.401 |
| Peripheral arterial diseases | 61 (10%) | 142 (13%) | 0.118 |
| COPD | 91 (15%) | 197 (17%) | 0.177 |
| Asthma | 48 (8%) | 146 (13%) | 0.001 |
| Sodium | 139.38 ± 3.4 | 139.86 ± 2.9 | 0.004 |
| Potassium | 4.2 ± 0.42 | 4.1 ± 0.43 | 0.063 |
| Glomerular filtration rate (ml/min/1.73 m2) | 64.3 ± 22.2 | 64.5 ± 21 | 0.836 |
| Hemoglobin | 12.8 ± 1.6 | 12.8 ± 1.6 | 0.618 |
| Hematocrit | 38.6 ± 4.8 | 38.6 ± 4.7 | 0.889 |
| Albumin | 3.9 ± 0.47 | 3.9 ± 1.9 | 0.502 |
| Ejection fraction | 57 ± 7 | 58 ± 7 | 0.252 |
| Left ventricular end diastolic volume | 87.3 ± 31.3 | 100.4 ± 31.6 | < 0.001 |
| Left ventricular end systolic volume | 36.6 ± 19.4 | 40.4 ± 17.3 | 0.015 |
| Stroke volume | 50.6 ± 15.5 | 60 ± 18.1 | < 0.001 |
| Left ventricular mass | 199.2 ± 63.1 | 236.4 ± 71.9 | < 0.001 |
| Left atrial volume | 60 ± 23.4 | 63.1 ± 28.2 | 0.151 |
| Global longitudinal strain | −15.3 ± 3.4 | −15.5 ± 3.4 | 0.598 |
| E/e’ lateral | 11.2 ± 5.5 | 13.1 ± 6.2 | 0.003 |
| E/e’ medial | 15.8 ± 7.1 | 16.6 ± 7.2 | 0.308 |
| Diuretics | 513 (83%) | 1045 (92%) | < 0.001 |
| ACEIs/ARBs | 447 (73%) | 936 (83%) | < 0.001 |
| Beta blockers | 484 (79%) | 893 (79%) | 0.951 |
| Calcium channel blockers | 211 (34%) | 464 (41%) | 0.006 |
| Nitrates | 100 (16%) | 203 (18%) | 0.391 |
| Aspirin | 343 (56%) | 681 (60%) | 0.084 |
| Statin | 372 (60%) | 769 (68%) | 0.002 |
| Warfarin | 220 (36%) | 367 (32%) | 0.168 |
| Study drug discontinuation | 573 (93%) | 1066 (93%) | 0.475 |
| Discontinuation due to permanent | 18 (3%) | 33 (3%) | 1.00 |
| Discontinuation due to abnormal renal function | 33 (5%) | 70 (6%) | 0.594 |
A total of 1643 patients were included in the WC analysis (124 patients were excluded due to missing information about WC). Table 2 shows differences in baseline characteristics between NWC and HWC subgroups. Similar to patients with high BMI, those with HWC had more complications related to obesity, including diabetes, hypertension, dyslipidemia and asthma.
Table 2.
Baseline characteristics according to WC group for TOPCAT Americas population
| Characteristic | NWC (n=349) | HWC (n= 1294) | P value |
|---|---|---|---|
| Age (years) | 75.9 ± 9 | 71±9.4 | <0.001 |
| Spironolactone | 181 (52%) | 647 (50%) | 0.547 |
| BMI (kg/m2) | 26.11 ± 4.4 | 35.7 ± 7.4 | < 0.001 |
| Waist circumference (cm) | 89.7 ± 9.2 | 115 ± 15.6 | <0.001 |
| Women |
107 (31%) |
701 (54%) |
<0.001 |
| Men | 242 (69%) | 593 (46%) | |
| White | 282 (81%) | 1031 (80%) | < 0.001 |
| Black | 38 (11%) | 215 (17%) | |
| Others | 29 (8%) | 48 (3%) | |
| Enrollment strata: | |||
| HF hospital admission | 158 (45%) | 733 (57%) | < 0.001 |
| Natriuretic peptide | 191 (55%) | 561 (43%) | |
| BNP (pg/ml) | 481 ± 630 (n=127) | 367 ± 363 (n=484) | 0.052 |
| NT Pro-BNP (pg/ml) | 2158 ± 2276 (n= 106) | 1428 ± 1714 (n=253) | 0.003 |
| Natriuretic Peptide Tertiles: | < 0.006 | ||
| I (n= 320) | 65 (28%) | 255 (35%) | |
| II (n= 318) | 68 (29%) | 250 (34%) | |
| III (n= 332) | 100 (43%) | 232 (31%) | |
| Actual plasma volume (aPV) (ml) | 2736.7 ± 477.2 | 3405.9 ± 692.2 | <0.001 |
| Heart rate (beats/min) | 67.5 ± 10.7 | 69 ± 11.2 | 0.027 |
| Systolic Blood Pressure (mm Hg) | 124.2 ± 15.4 | 128.3 ± 15.8 | < 0.001 |
| Diastolic Blood Pressure (mm Hg) | 69.6 ± 11.1 | 71.9 ± 11.5 | 0.001 |
| NYHA | |||
| I or II | 262 (75%) | 827 (64%) | < 0.001 |
| III or IV | 87 (25%) | 467 (36%) | |
| Edema over the past year | 304 (90%) | 1202 (94%) | < 0.008 |
| Hypertension | 293 (84%) | 1188 (92%) | < 0.001 |
| Diabetes mellitus | 96 (28%) | 642 (50%) | < 0.001 |
| Dyslipidemia | 227 (65%) | 951 (74%) | 0.003 |
| Atrial fibrillation | 144 (41%) | 559 (43%) | 0.542 |
| Stroke | 25 (7%) | 121 (9%) | 0.243 |
| Myocardial Infarction | 78 (22%) | 267 (21%) | 0.505 |
| PCI | 73 (21%) | 252 (20%) | 0.545 |
| CABG | 76 (22%) | 245 (19%) | 0.254 |
| Angina | 107 (31%) | 355 (27%) | 0.254 |
| Peripheral arterial diseases | 41 (12%) | 151 (12%) | 1 |
| COPD | 49 (14%) | 214 (17%) | 0.285 |
| Asthma | 21 (6%) | 155 (12%) | 0.001 |
| Sodium | 139.1 ± 3.2 | 139.8 ± 3.1 | < 0.001 |
| Potassium | 4.2 ± 0.42 | 4.1 ± 0.43 | 0.195 |
| Glomerular filtration rate (ml/min/1.73 m2) | 67 ± 24 | 63.7 ± 20.6 | 0.012 |
| Hemoglobin | 12.9 ± 1.6 | 12.8 ± 1.6 | 0.288 |
| Hematocrit | 38.9 ± 4.9 | 38.7 ± 4.7 | 0.546 |
| Albumin | 3.9 ± 0.49 | 4 ± 1.8 | 0.618 |
| Ejection fraction | 57.1 ± 8 | 58.3 ± 7.6 | 0.015 |
| Left ventricular end diastolic volume | 93.7 ± 31.3 | 96.3 ± 32.5 | 0.425 |
| Left ventricular end systolic volume | 40.1 ± 19.9 | 39.3 ± 17.9 | 0.664 |
| Stroke volume | 53.5 ± 16 | 57 ± 18.1 | 0.056 |
| Left ventricular mass | 210.7 ± 65.1 | 227.14 ± 71.7 | 0.018 |
| Left atrial volume | 58.7 ± 23 | 62.9 ± 28.4 | 0.093 |
| Global longitudinal strain | −14.9 ± 3.5 | −15.5 ± 3.4 | 0.191 |
| E/e’ lateral | 11.2 ± 5.3 | 12.5 ± 6.1 | 0.09 |
| E/e’ medial | 16.8 ± 7.5 | 16.1 ± 7.1 | 0.502 |
| Diuretics | 284 (81%) | 1176 (91%) | < 0.001 |
| ACEIs/ARBs | 252 (72%) | 1050 (81.2%) | < 0.001 |
| Beta blockers | 279 (80%) | 1016 (79%) | 0.606 |
| Calcium channel blockers | 114 (33%) | 516 (40%) | 0.015 |
| Nitrates | 57 (16%) | 224 (17%) | 0.69 |
| Aspirin | 208 (60%) | 749 (58%) | 0.583 |
| Statin | 210 (60%) | 857 (66%) | 0.037 |
| Warfarin | 115 (33%) | 443 (34%) | 0.656 |
| Study drug discontinuation | 320 (92%) | 1216 (94%) | 0.142 |
| Discontinuation due to permanent hyperkalemia | 11 (3%) | 38 (3%) | 0.724 |
| Discontinuation due to abnormal renal function | 15 (5%) | 84 (7%) | 0.161 |
There was no significant difference in the incidence of permanent hyperkalemia and abnormal renal function adverse events that led to drug discontinuation across BMI or WC subgroups (Table 1 and 2, respectively).
After multivariate adjustment, there was no difference in the primary endpoint in the primary endpoint or any of the secondary endpoints between the two groups (Figure 1 and Table 3). Importantly, there was no interaction between NP tertiles and BMI (p for interaction=0.210). When treated as a continuous variable in multivariate analysis, higher BMI was not associated with the primary endpoint or any of the secondary endpoints. Likewise, there was no difference in the primary endpoint or any of the secondary endpoints between the two groups (Figure 2 and Table 4). In addition, there was no interaction between NP tertiles and WC (p for interaction=0.130). When treated as a continuous variable in multivariate analysis, higher WC was not associated with the primary endpoint or HF hospitalization, but was associated with increased risk of cardiovascular death (HR=1.019, 95% CI 1.001–1.037, p=0.036) and all-cause death (HR=1.013, 95% CI 1.000–1.026, p=0.045).
Figure 1.
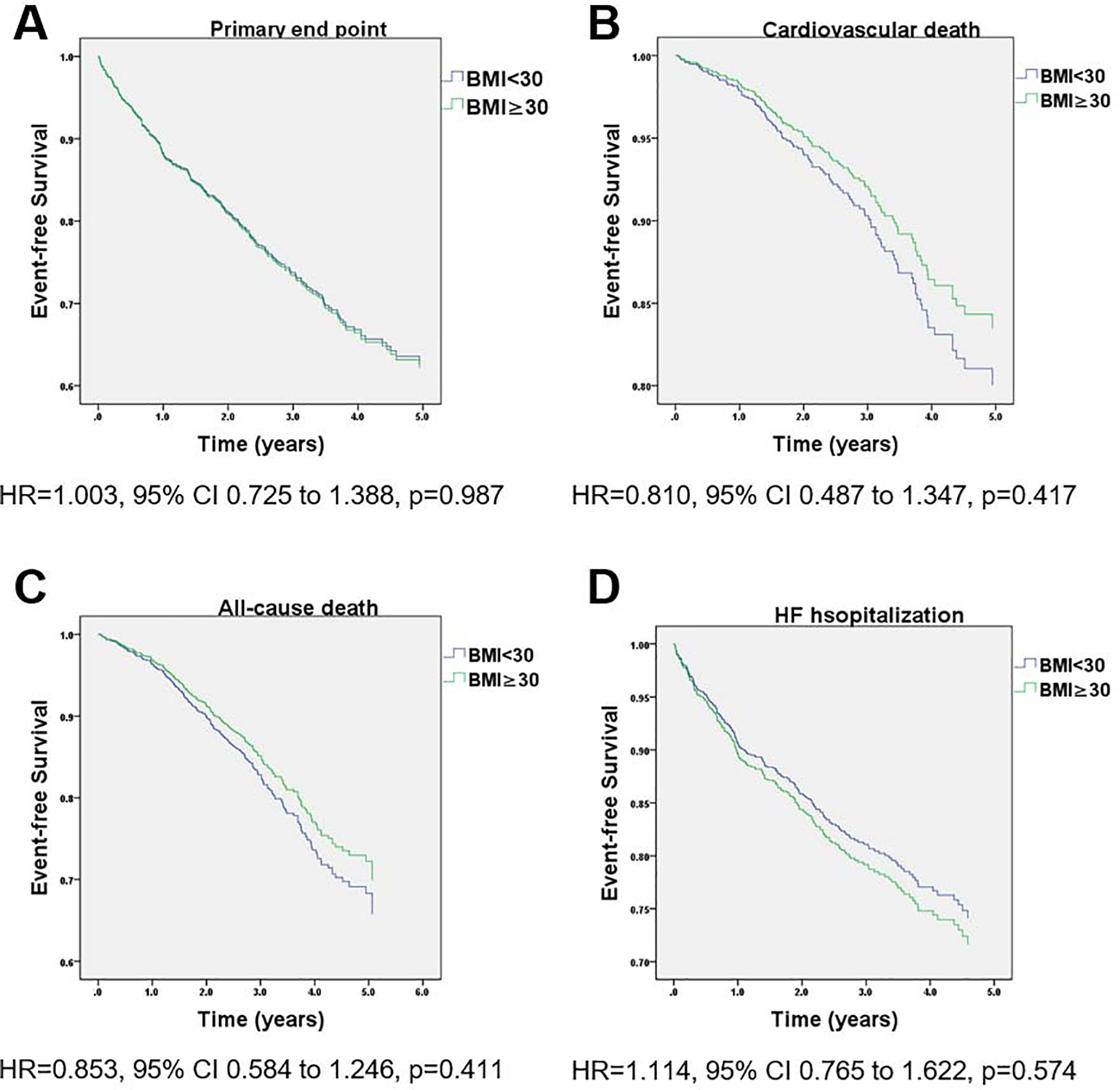
Kaplan-Meier survival curves for event-free survival stratified by BMI group in the Americas TOPCAT cohort. (A) primary outcome (cardiovascular death, aborted cardiac arrest, or hospitalization for heart failure), (B) cardiovascular death and (C) all-cause death. Adjustment was done for the following covariates: age, race, study enrollment status, actual plasma volume, NP tertiles, heat rate, systolic BP, edema over the past year, NYHA class, sodium, hypertension, diabetes, atrial fibrillation, dyslipidemia, asthma, diuretics, ACEIs/ARBS, CCB, statin.
Table 3.
Adjusted outcomes according to BMI group
| TOPCAT Americas cohort | ||||
|---|---|---|---|---|
| BMI (Kg/m2) | ||||
| < 30 (n=616) | ≥ 30 (n=1135) | Hazard ratio (95% CI) | P value | |
| Primary end point | 169 (27%) | 346 (31%) | 1.003 (0.981 – 1.441) | 0.987 |
| Cardiovascular death | 94 (15%) | 124 (11%) | 0.810 (0.582 – 1.020) | 0.417 |
| All-cause death | 159 (26%) | 219 (19%) | 0.853 (0.688 – 1.055) | 0.411 |
| HF hospitalizations | 118 (19%) | 277 (24%) | 1.114 (0.765 – 1.622) | 0.574 |
Figure 2.
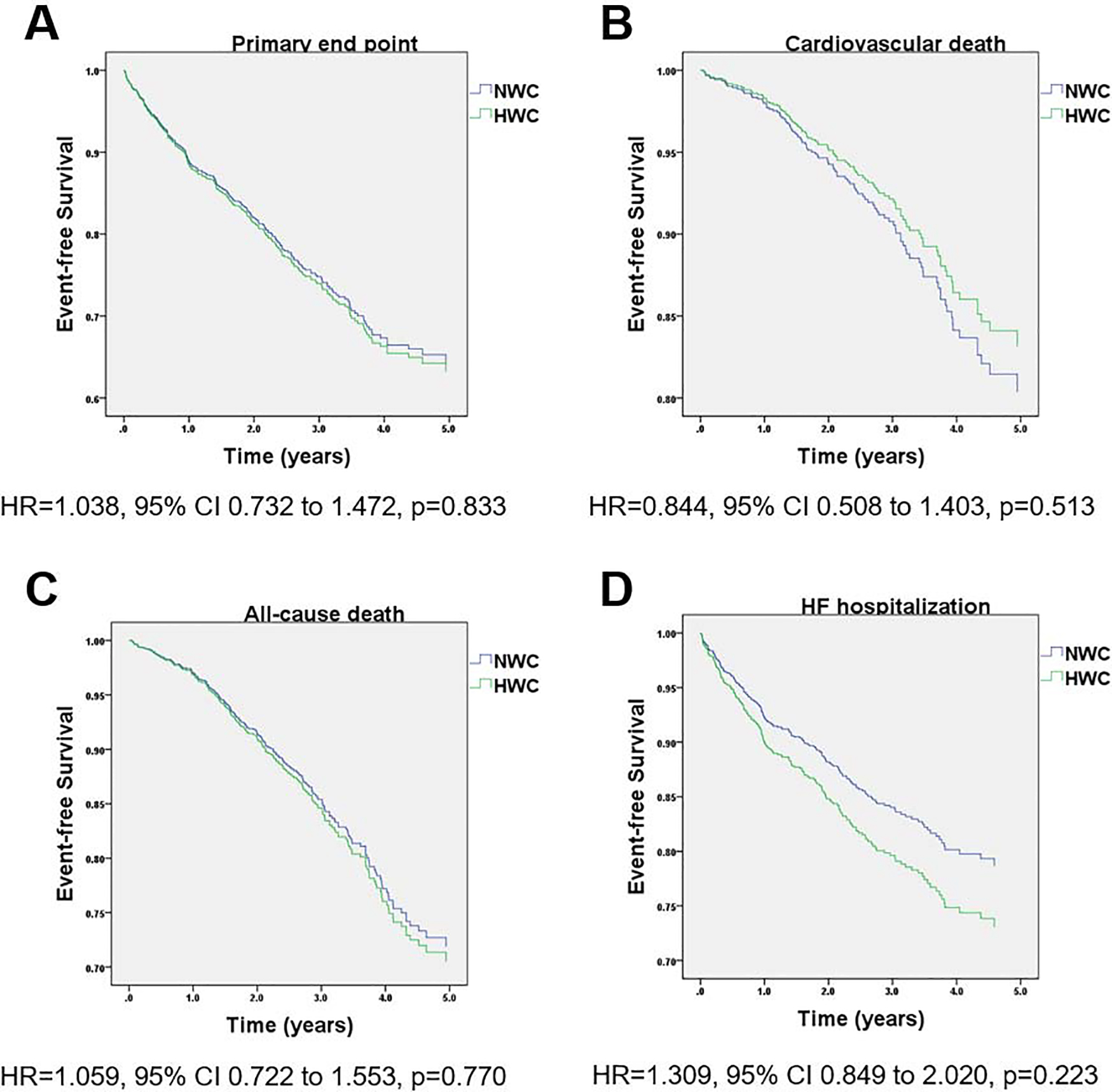
Kaplan-Meier survival curves for event-free survival stratified by WC group in the Americas TOPCAT cohort. (A) primary outcome (cardiovascular death, aborted cardiac arrest, or hospitalization for heart failure), (B) cardiovascular death and (C) all-cause death. Adjustment was done for the same covariates in Figure 1.
Table 4.
Adjusted outcomes according to WC group
| TOPCAT Americas cohort | ||||
|---|---|---|---|---|
| NWC (n=349) | HWC (n=1294) | Hazard ratio (95% CI) | P value | |
| Primary end point | 102 (29%) | 373 (29%) | 1.030 (0.731 – 1.472) | 0.834 |
| Cardiovascular death | 53 (15%) | 156 (12%) | 0.841 (0.501 – 1.403) | 0.513 |
| All-cause death | 86 (25%) | 274 (21%) | 1.052 (0.722 – 1.553) | 0.762 |
| HF hospitalizations | 70 (20%) | 288 (22%) | 1.301 (0.840 – 2.021) | 0.221 |
Kaplan Meier curves for multivariate adjusted outcomes in obese vs. non-obese subjects stratified by treatment arm are shown in Figure 3. In the obese group there was a 39% significant decrease in the primary endpoint rates in the spironolactone arm compared to placebo (HR=0.618, 95% CI 0.460–0.831, p=0.001), but not in the non-obese group (HR=0.946, 95% CI 0.623–1.437, p=0.796; p for BMI category by treatment arm interaction=0.056). Cardiovascular death was significantly decreased by 52% in the spironolactone arm compared to placebo in obese (HR=0.483, 95% CI 0.281–0.833, p=0.009), but not non-obese subjects (HR=0.742, 95% CI 0.415–1.326, p=0.313; p for interaction=0.412). All-cause death was not significantly different between spironolactone or placebo arms in obese (HR=0.759, 95% CI 0.518–1.112, p=0.157) and non-obese groups (HR=0.843, 95% CI 0.548–1.298, p=0.438; p for interaction=0.734). The rate of HF hospitalization was significantly lower in the spironolactone arm compared to placebo in obese (HR=0.641, 95% CI 0.465–0.883, p=0.007), but not in non-obese subjects (HR=1.029, 95% CI 0.613–1.728, p=0.913; p for interaction=0.130). When BMI was treated as a continuous variable, there was a linear association between BMI and the effect of spironolactone vs. placebo for the primary outcome and cardiovascular death, with the benefit becoming statistically significant at 33kg/m2 and 30kg/m2, respectively (Figure 4). A similar linear association between the effect of spironolactone and BMI as a continuous variable was observed for all cause death and HF hospitalizations, but none of them reached statistical significance (Figure 4).
Figure 3.
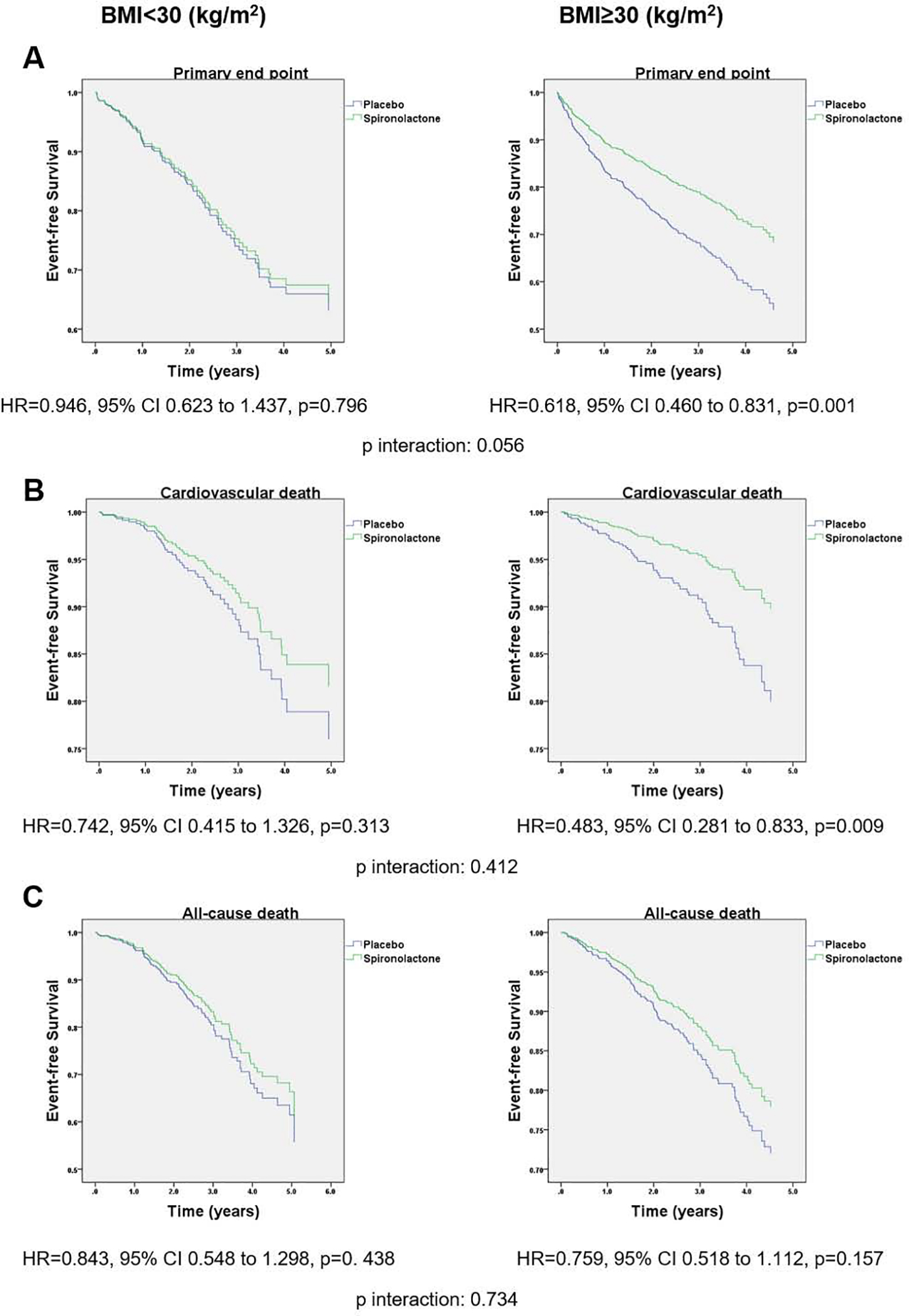
Kaplan-Meier survival curves for event-free survival stratified by BMI and treatment group in the Americas only cohort of TOPCAT. (A) primary outcome (cardiovascular death, aborted cardiac arrest, or hospitalization for heart failure), (B) cardiovascular death and (C) all-cause death. Adjustment was done for the same covariates in Figure 1.
Figure 4.
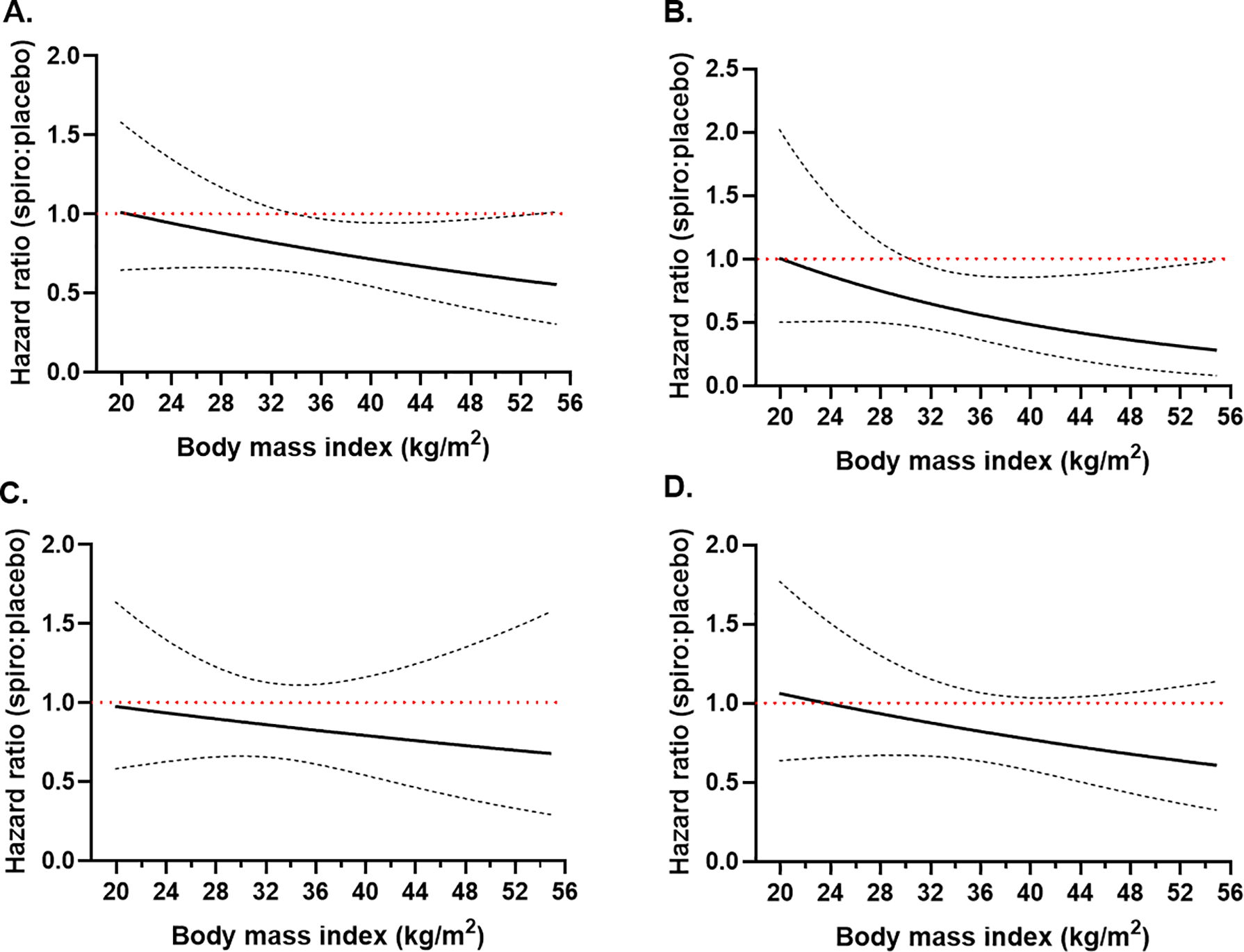
Plot of the spironolactone effect vs. placebo as a function of continuous BMI in the adjusted model for the primary outcome (A), cardiovascular death (B), all-cause death (C) and heart failure hospitalizations (D). Dashed lines represent 95% confidence intervals of the hazard ratio. The red horizontal line depicts where the upper limit of the confidence intervals crosses 1, indicating a statistically significant effect.
Kaplan Meier curves and HRs for multivariate adjusted outcomes in HWC vs. NWC subjects stratified by treatment arm are shown in Figure 5. In HWC group, use of spironolactone was associated with a 26% significant reduction of primary end point compared to placebo (HR=0.740, 95% CI 0.559–0.980, p=0.035) but not in the NWC group (adjusted HR=0.639, 95% CI 0.355–1.149, p=0.134; p for WC category by treatment arm interaction=0.930). Cardiovascular death was significantly decreased by 46% in the spironolactone arm compared to placebo in HWC group (HR= 0.541, 95% CI 0.335–0.873, p=0.012), but not the NWC group (HR 0.650, 95% CI 0.288–1.466, p=0.299; p for interaction=0.887). All-cause mortality did not different between the two arms in HWC and NWC groups. Likewise, there was no significant difference in rate of HF hospitalization between the two arms in HWC (HR= 0.777, 95% CI 0.570–1.061, p= 0.112) and NWC group (HR=0.607, 95% CI 0.278–1.328, p=0.211; p for interaction=0.990). When waist circumference was treated as a continuous variable, there was a linear association between WC and the effect of spironolactone vs. placebo for the primary outcome, cardiovascular death and HF hospitalizations, with the benefit becoming statistically significant at 109cm, 103cm and 123cm, respectively (Figure 6). The association between the effect of spironolactone and waist circumference as a continuous variable for all-cause death did not reach statistical significance (Figure 6).
Figure 5.
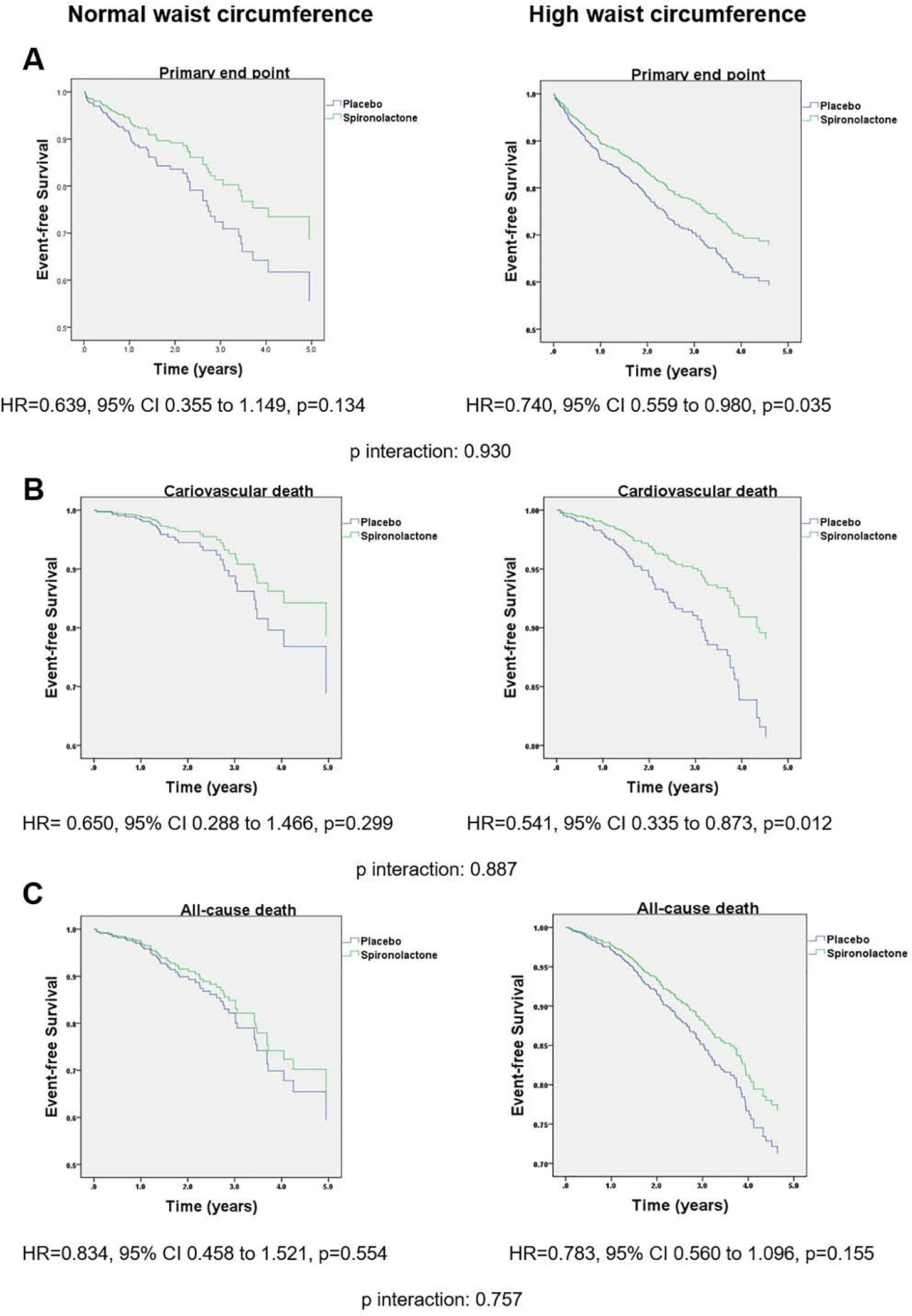
Kaplan-Meier survival curves for event-free survival stratified by WC and treatment group in the Americas only cohort of TOPCAT. (A) primary outcome (cardiovascular death, aborted cardiac arrest, or hospitalization for heart failure), (B) cardiovascular death and (C) all-cause death. Adjustment was done for the same covariates in Figure 1.
Figure 6.
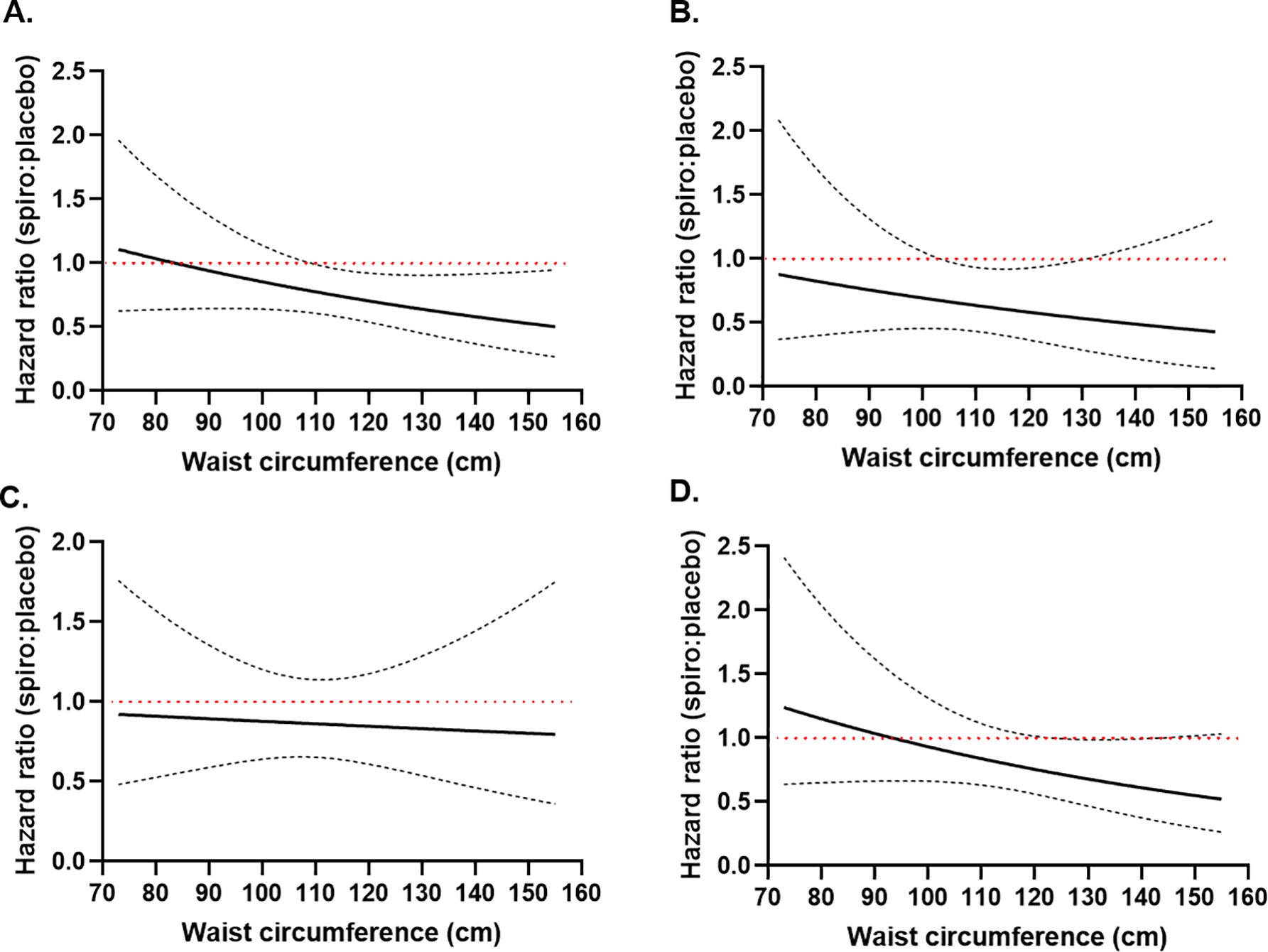
Plot of the spironolactone effect vs. placebo as a function of continuous waist circumference in the adjusted model for the primary outcome (A), cardiovascular death (B), all-cause death (C) and heart failure hospitalizations (D). Dashed lines represent 95% confidence intervals of the hazard ratio. The red horizontal line depicts where the upper limit of the confidence intervals crosses 1, indicating a statistically significant effect.
Discussion
The results of this post hoc analysis for the Americas cohort from the TOPCAT study demonstrated that there are significant differences between obese and non-obese groups in terms of both clinical characteristics and outcomes related to spironolactone use. Obese patients with HFpEF were younger, had lower NP values and had higher prevalence of comorbidities. After adjusting for these differences, use of spironolactone in obese patients with HFpEF was associated with a significantly decreased risk of the primary end point, cardiovascular death and HF hospitalizations. In addition, our analysis indicated that the benefit of spironolactone over placebo was more pronounced at higher BMI (and WC) values, suggesting a possible causal association between obesity and aldosterone blockade in this patient population. However, in light of the fact that formal interaction testing was significant only for the primary endpoint, but not the other end points analyzed, the results of our analyses would suggest that a larger dedicated trial may be able to detect a smaller treatment effect interaction by obesity for spironolactone. The significance of these results is based on the fact that evidence-based treatments that improve morbidity or mortality in HFpEF are lacking 1. Nonetheless, this analysis represents a post-hoc, secondary analysis and should only be regarded as hypothesis-generating. Further prospective randomized studies in obese subjects are required to confirm the validity of this finding prior to clinical application. It is also worth noting that some non-obese patients with high WC also benefitted from spironolactone. A future study may randomize obese and non-obese patients according to WC, which provides information on body fat distribution, in contrast to BMI, which does not distinguish between adipose mass and muscle mass 11.
Obesity has reached epidemic proportions worldwide and is a common comorbidity in patients with HFpEF 12. Obesity has many deleterious effects on the cardiovascular system, mediated by changes in volume status, cardiac loading, tissue metabolism, and systemic inflammation, which are believed to promote disease progression 13,14. Consequently, obesity-related HFpEF may represent a clinically relevant phenotype within the broader spectrum of HFpEF that may require specific treatments 15. Consistent with the notion that aldosterone blockade exerts anti-inflammatory and anti-fibrotic effects in experimental models of obesity 16,17, and based on our results, we propose that spironolactone may reverse the U-shaped relationship between BMI and the risk of adverse clinical outcomes at the higher end of the BMI spectrum 18, as suggested by the linear relationship between BMI and the effect of spironolactone, with the benefit for the primary outcome becoming statistically significant at 33kg/m2. In a recent post-hoc analysis of the TOPCAT trial, obese phenotype HFpEF was associated with increased levels of renin, impaired natriuresis and fluid retention 19. In our analysis, obese patients had higher aPV, end diastolic volume, stroke volume and LV mass index, which lends credence to this theory.
It has been previously shown that low NP levels possibly predicted response to therapy in TOPCAT 20 but also in I-PRESERVE 21 with spironolactone and irbesartan, respectively. Notably, obese patients with HF have lower NP levels compared to non-obese patients 22. Consistent with a secondary analysis of the same trial, which showed that there was a U-shaped association between BMI and NP levels, with elevated NP levels noted at the extremes of BMI distribution 18, in our analysis, more patients in the obese group were in the lower NP tertiles compared to the nonobese group (Table 1). Based on the finding that the interaction between BMI and NP levels was not significant, our analysis suggests that the lower NP levels in obese patients do not fully explain the beneficial effect of spironolactone in this group of patients. In addition, elevation of NP levels potentially reflects an advanced stage in the pathophysiological process of HFpEF in this patient population, when decompensation has occurred 23, rendering therapies targeting the renin angiotensin aldosterone system less effective. This notion is supported by a secondary analysis of TOPCAT, which showed that patients in the high BMI/high NP category had the worse outcome 18.
Several theories have been proposed to explain the “obesity paradox”, but the possibility that it may be due to residual confounding, unintentional weight loss, or selection bias, cannot be excluded 24,25. Our results suggested that there was no obesity paradox, after controlling for various comorbidities in multivariate analysis. These data are consistent with a recent analysis from the same trial, which showed that obese patients with high NP levels experienced the worst outcomes 18. Furthermore, detailed phenomapping of HFpEF patients in TOPCAT identified a group of obese, diabetic patients with higher inflammation and renal impairment, who had a higher risk of adverse outcomes, but also responded better to spironolactone 19.
This is a post-hoc exploratory analysis, that stratified patients according to BMI and WC, and should thus be regarded as hypothesis-generating only. In such an analysis, randomization is breached and even though we adjusted for differences between the 2 groups, there may be unknown confounders which may have biased the results. Because of the significantly smaller sample size of the analysis when the subjects enrolled from Russia and Georgia were excluded, the results of the analysis in the Americas only cohort may have been underpowered, illustrated by the absence of a statistically significant interaction, even though the spironolactone effect was numerically better in the obese group. These issues highlight the importance of conducting an appropriately powered, prospective trial examining the role of spironolactone in obese patients with HFpEF prior to making firm recommendations regarding the use of spironolactone in this patient population. In our primary analysis, we treated BMI as a dichotomous variable in order to have the maximum power to detect small differences. However, the association between BMI and spironolactone effect appears to be rather linear, with the benefit being more pronounced at higher BMI levels.
In conclusion, use of spironolactone in obese patients with HFpEF was associated with a decreased risk of the primary end point, cardiovascular death and HF hospitalizations, compared to placebo. Further prospective randomized studies in obese subjects are required to confirm the validity of this finding prior to clinical application.
Acknowledgments
Funding: This study was partially funded by NIH/NIA R21AG057879 to Stavros Stavrakis
Footnotes
Disclosures: The authors declare no conflicts of interest
Declaration of interests
☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Butler J, Fonarow GC, Zile MR, Lam CS, Roessig L, Schelbert EB, Shah SJ, Ahmed A, Bonow RO, Cleland JG, Cody RJ, Chioncel O, Collins SP, Dunnmon P, Filippatos G, Lefkowitz MP, Marti CN, McMurray JJ, Misselwitz F, Nodari S, O’Connor C, Pfeffer MA, Pieske B, Pitt B, Rosano G, Sabbah HN, Senni M, Solomon SD, Stockbridge N, Teerlink JR, Georgiopoulou VV and Gheorghiade M. Developing therapies for heart failure with preserved ejection fraction: current state and future directions. JACC Heart Fail 2014;2:97–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunlay SM, Roger VL and Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2017;14:591–602 [DOI] [PubMed] [Google Scholar]
- 3.Packer M. Leptin-Aldosterone-Neprilysin Axis: Identification of Its Distinctive Role in the Pathogenesis of the Three Phenotypes of Heart Failure in People With Obesity. Circulation 2018;137:1614–1631 [DOI] [PubMed] [Google Scholar]
- 4.Panagiotakos DB, Pitsavos C, Yannakoulia M, Chrysohoou C and Stefanadis C. The implication of obesity and central fat on markers of chronic inflammation: The ATTICA study. Atherosclerosis 2005;183:308–315 [DOI] [PubMed] [Google Scholar]
- 5.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O’Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM and Investigators T. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014;370:1383–1392 [DOI] [PubMed] [Google Scholar]
- 6.Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner JF, Lewis EF, O’Meara E, Rouleau JL, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, McKinlay SM and Pitt B. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015;131:34–42 [DOI] [PubMed] [Google Scholar]
- 7.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, Loria CM, Millen BE, Nonas CA, Pi-Sunyer FX, Stevens J, Stevens VJ, Wadden TA, Wolfe BM, Yanovski SZ, American College of Cardiology/American Heart Association Task Force on Practice G and Obesity S. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014;63:2985–3023 [DOI] [PubMed] [Google Scholar]
- 8.Fudim M and Miller WL. Calculated Estimates of Plasma Volume in Patients With Chronic Heart Failure-Comparison With Measured Volumes. J Card Fail 2018;24:553–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ling HZ, Flint J, Damgaard M, Bonfils PK, Cheng AS, Aggarwal S, Velmurugan S, Mendonca M, Rashid M, Kang S, Papalia F, Weissert S, Coats CJ, Thomas M, Kuskowski M, Cohn JN, Woldman S, Anand IS and Okonko DO. Calculated plasma volume status and prognosis in chronic heart failure. Eur J Heart Fail 2015;17:35–43 [DOI] [PubMed] [Google Scholar]
- 10.Olivier A, Pitt B, Girerd N, Lamiral Z, Machu JL, McMurray JJV, Swedberg K, van Veldhuisen DJ, Collier TJ, Pocock SJ, Rossignol P, Zannad F and Pizard A. Effect of eplerenone in patients with heart failure and reduced ejection fraction: potential effect modification by abdominal obesity. Insight from the EMPHASIS-HF trial. Eur J Heart Fail 2017;19:1186–1197 [DOI] [PubMed] [Google Scholar]
- 11.Iliodromiti S, Celis-Morales CA, Lyall DM, Anderson J, Gray SR, Mackay DF, Nelson SM, Welsh P, Pell JP, Gill JMR and Sattar N. The impact of confounding on the associations of different adiposity measures with the incidence of cardiovascular disease: a cohort study of 296 535 adults of white European descent. Eur Heart J 2018;39:1514–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy YN and Borlaug BA. Heart Failure With Preserved Ejection Fraction. Curr Probl Cardiol 2016;41:145–188 [DOI] [PubMed] [Google Scholar]
- 13.Lauer MS, Anderson KM, Kannel WB and Levy D. The impact of obesity on left ventricular mass and geometry. The Framingham Heart Study. JAMA 1991;266:231–236 [PubMed] [Google Scholar]
- 14.Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV and Ventura HO. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail 2013;1:93–102 [DOI] [PubMed] [Google Scholar]
- 15.Obokata M, Reddy YNV, Pislaru SV, Melenovsky V and Borlaug BA. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure With Preserved Ejection Fraction. Circulation 2017;136:6–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo C, Ricchiuti V, Lian BQ, Yao TM, Coutinho P, Romero JR, Li J, Williams GH and Adler GK. Mineralocorticoid receptor blockade reverses obesity-related changes in expression of adiponectin, peroxisome proliferator-activated receptor-gamma, and proinflammatory adipokines. Circulation 2008;117:2253–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bender SB, DeMarco VG, Padilla J, Jenkins NT, Habibi J, Garro M, Pulakat L, Aroor AR, Jaffe IZ and Sowers JR. Mineralocorticoid receptor antagonism treats obesity-associated cardiac diastolic dysfunction. Hypertension 2015;65:1082–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pandey A, Berry JD, Drazner MH, Fang JC, Tang WW and Grodin JL. Body mass index, natriuretic peptides, and risk of adverse outcomes in patients with heart failure and preserved ejection fraction: analysis from the TOPCAT trial. Journal of the American Heart Association 2018;7:e009664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen JB, Schrauben SJ, Zhao L, Basso MD, Cvijic ME, Li Z, Yarde M, Wang Z, Bhattacharya PT, Chirinos DA, Prenner S, Zamani P, Seiffert DA, Car BD, Gordon DA, Margulies K, Cappola T and Chirinos JA. Clinical Phenogroups in Heart Failure With Preserved Ejection Fraction: Detailed Phenotypes, Prognosis, and Response to Spironolactone. JACC Heart Fail 2020;8:172–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anand IS, Claggett B, Liu J, Shah AM, Rector TS, Shah SJ, Desai AS, O’Meara E, Fleg JL and Pfeffer MAJJHF. Interaction between spironolactone and natriuretic peptides in patients with heart failure and preserved ejection fraction: from the TOPCAT trial. JACC: Heart Failure 2017;5:241–252 [DOI] [PubMed] [Google Scholar]
- 21.Anand IS, Rector TS, Cleland JG, Kuskowski M, McKelvie RS, Persson H, McMurray JJ, Zile MR, Komajda M, Massie BM and Carson PE. Prognostic value of baseline plasma amino-terminal pro-brain natriuretic peptide and its interactions with irbesartan treatment effects in patients with heart failure and preserved ejection fraction: findings from the I-PRESERVE trial. Circ Heart Fail 2011;4:569–577 [DOI] [PubMed] [Google Scholar]
- 22.Ibrahim NE, Burnett JC Jr., Butler J, Camacho A, Felker GM, Fiuzat M, O’Connor C, Solomon SD, Vaduganathan M, Zile MR and Januzzi JL Jr. Natriuretic Peptides as Inclusion Criteria in Clinical Trials: A JACC: Heart Failure Position Paper. JACC Heart Fail 2020;8:347–358 [DOI] [PubMed] [Google Scholar]
- 23.Lewis GA, Schelbert EB, Williams SG, Cunnington C, Ahmed F, McDonagh TA and Miller CA. Biological Phenotypes of Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol 2017;70:2186–2200 [DOI] [PubMed] [Google Scholar]
- 24.Tadic M and Cuspidi C. Obesity and heart failure with preserved ejection fraction: a paradox or something else? Heart Fail Rev 2019;24:379–385 [DOI] [PubMed] [Google Scholar]
- 25.Simonenko M. Obesity paradox in heart failure: A matter of debate. Eur J Prev Cardiol 2019;26:1748–1750 [DOI] [PubMed] [Google Scholar]


