Abstract
Membrane-bound angiotensin-converting enzyme 2 (ACE2) is important in regulation of the renin-angiotensin-aldosterone system, but the association of cleaved soluble ACE2 (sACE2) with cardiovascular disease (CVD) is unclear. We evaluated the association of sACE2 with cardiac biomarkers, structure, and function and cardiovascular events in the Atherosclerosis Risk in Communities Study. sACE2 was measured in a subset of 497 participants (mean age 78±5.4 years, 53% men, 27% black); Cox regression analyses assessed prospective associations of sACE2 with time to first CVD event at median 6.1-year follow-up. sACE2 was higher in men, blacks, and participants with prevalent CVD, diabetes, or hypertension. Higher sACE2 levels were associated with significantly higher biomarkers of cardiac injury (high-sensitivity cardiac troponin I and T, N-terminal pro–B-type natriuretic peptide), greater left ventricular mass index, and impaired diastolic function in linear regression analyses, and with increased risk for heart failure hospitalization (adjusted hazard ratio per natural log unit increase [HR] 1.32, 95% confidence interval [CI] 1.10–1.58), CVD events (HR 1.34, 95% CI 1.13–1.60), and all-cause death (HR 1.26, 95% CI 1.01–1.57). In an elderly biracial cohort, sACE2 was positively associated with biomarkers reflecting myocardial injury and neurohormonal activation, left ventricular mass index, impaired diastolic function, CVD, events and all-cause death.
Keywords: ACE2, biomarkers, cardiac structure, cardiovascular disease
Introduction
The renin–angiotensin–aldosterone system (RAAS) is an important regulator of blood pressure, but excess activation contributes to development of cardiovascular disease (CVD).1 In pathological RAAS activation, active tissue-bound, or membrane-bound, angiotensin-converting enzyme 2 (mbACE2) expression is increased, and increased shedding of plasma, or soluble, ACE2 (sACE2) into circulation results in increased sACE2 and relative deficiency of mbACE2.2 mbACE2 is cardioprotective,3–5 whereas increased sACE2 levels predict poor outcomes in patients with CHD, HF, or atrial fibrillation.6–8 Data on the relation of sACE2 levels and cardiovascular events are limited to relatively small cohorts of hospitalized patients with established CVD, with few data on the relation of sACE2 with cardiac biomarkers, structure, and function. We investigated the relations between levels of sACE2 and cardiac biomarkers, echocardiographic measurements of cardiac structure and function, and risk for CVD events in the Atherosclerosis Risk in Communities (ARIC) study, a biracial cohort of older adults with a high prevalence of CVD risk factors, to test the hypothesis that individuals with pathological RAAS activation have altered expression of sACE2, higher prevalence of CVD, elevated biomarkers of subclinical cardiac injury, abnormal cardiac structure and function, and increased risk for incident CVD.
Methods
Detailed methods are provided in the Supplementary Data. The Atherosclerosis Risk in Communities (ARIC) Study is a population-based study that recruited residents aged 45–65 years in 1987–1989 from 4 communities in North Carolina, Mississippi, Minnesota, and Maryland. A detailed description of the ARIC Study has been published.9 The study protocol was approved by each field center’s institutional review board and complies with the Declaration of Helsinki; all participants provided written informed consent. This analysis used data from participants in ARIC visit 5 (2011–2013; aged 66–90 years).
Of the 6538 participants in visit 5, we excluded those with self-reported race neither black nor white (N=14) and black participants at the Minnesota and Washington County field centers (N=17) because of small enrollment numbers and those with missing information on cardiac biomarkers (high-sensitivity cardiac troponin I [hs-cTnI] or T [hs-cTnT], N-terminal pro–B-type natriuretic peptide [NT-proBNP]; N=1227) to include 5280 for cardiac biomarker analysis. sACE2 measurements were available from a case-control study of incident HF for a subset of 497 individuals, 152 with prevalent CVD and 345 without (Supplementary Figure 1S).
The selection of the sACE2 subset from ARIC visit 5 was based on a case–control study design. Both cases and controls had to be free of HF at visit 5. For each case (incident HF between visit 5 and December 31, 2016), a matched control was selected, matched on age, sex, and being free of HF after the same follow-up time since visit 5 (incidence density sampling).
hs-cTnI was measured using a highly sensitive chemiluminescent immunoassay (Architect Stat Troponin-I; Abbott) on an automated chemistry analyzer (Architect i 2000sr; Abbott).10 hs-cTnT was measured using a highly sensitive assay (Elecsys Troponin T Gen 5 STAT; Roche).10 NT-proBNP was measured using a electrochemiluminescent immunoassay on an automated analyzer (Cobas e411; Roche).11 Participants with hs-cTnI, hs-cTnT, or NT-proBNP levels below the lower limits of detection were assigned values equal to half the lower limits of detection.
sACE2 protein levels were measured using the cardiovascular panel II of the Olink Multiplex platform (Olink Proteomics, Uppsala, Sweden) in a subset of 497 participants from ARIC visit 5. Cardiovascular panel II is validated with respect to sensitivity, dynamic range, specificity, precision (repeatability and reproducibility), and scalability.12
Echocardiography was performed according to a study-specific protocol and using uniform equipment by dedicated sonographers as described.13 Quantitative measures of cardiac structure and function were determined by a central reading center according to American Society of Echocardiography recommendations14; reproducibility metrics are published.13
Clinical endpoints assessed included first CHD, ischemic stroke, and HF hospitalization events and all-cause mortality as described.15–17 All outcomes were assessed after ARIC visit 5, with follow-up through December 31, 2018. Global CVD was a composite of CHD, stroke, and HF hospitalization events; ASCVD was a composite of CHD and stroke events. Median (25th, 75th percentile) follow-up periods were 6.1 (4.6, 6.8) years for global CVD, 6.2 (5.1, 6.8) years for ASCVD, and 6.2 (5.2, 6.8) years for HF hospitalizations.
sACE2 was modeled as a continuous variable or classified by tertiles of its distribution for categorical analysis. Baseline characteristics of participants were tabulated by sACE2 tertile. Categorical variables were expressed as count (percentage); continuous variables were reported as mean ± standard deviation or median (25th, 75th percentile) depending on normality of the data.
We modeled hs-cTnT, hs-cTnI, and NT-proBNP at visit 5 as both categorical and continuous variables. For the hs-cTnT categorical analysis, individuals were classified by prespecified cutpoints: <6 ng/L (“low”; the limit of quantification for the assay), 6 to <14 ng/L (“intermediate”), and ≥14 ng/L (“high”; 90th percentile in ARIC18 and 99th percentile upper reference in multisociety guidelines for defining myocardial infarction19). Respective categories for hs-cTnI were <2 ng/L (“low”; lowest integer above limit of detection), 2 to <10 ng/L (“intermediate”), and ≥10 ng/L (“high”; as published10). For NT-proBNP, individuals were classified by prespecified reference cutpoints of <100 pg/mL (low), ≥100 to <300 pg/mL (intermediate), and ≥300 pg/mL (high).11 For the continuous analyses, hs-cTnI, hs-cTnT, and NT-proBNP values were natural log (ln) transformed. We examined cross-sectional correlations among sACE2, hs-cTnI, hs-cTnT, and NT-proBNP levels using Spearman rank correlation. We further assessed cross-sectional association of sACE2 with hs-cTnI, hs-cTnT, and NT-proBNP as categorical and continuous variables using multivariable logistic or linear regression models, respectively. Model 1 was adjusted for age, sex, and race. Model 2 was adjusted for all variables in model 1 plus total cholesterol, high-density lipoprotein cholesterol, smoking status, systolic blood pressure, antihypertensive medication use, diabetes status, lipid-lowering medication use, prevalent CVD (composite of CHD, stroke, and HF hospitalization), and estimated glomerular filtration rate (eGFR).
Linear regression models were used to assess cross-sectional association of sACE2 with echocardiographic measures of systolic function (left ventricular [LV] ejection fraction, global longitudinal strain [GLS]), cardiac structure (LV mass index [LVMi]), and diastolic function (left atrial volume index [LAVi], tissue doppler imaging [TDI] septal e′ and septal E/e′ ratio). Adjustments were made with model 1 and model 2 as above; model 3 included all variables in model 2 plus log hs-cTnI, log hs-cTnT, and log NT-proBNP.
Finally, we used Cox proportional hazards models to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for the prospective associations of sACE2 at visit 5 with time to first ASCVD, HF hospitalization, or global CVD event, adjusted by models 1–3 as above. P-trend was calculated for linear increase in log relative hazard with increasing categories. Sensitivity analyses were performed by stratifying the cohort into those with or without prevalent CVD and into NT-proBNP categories (<100, 100 to <300, ≥300 pg/mL), to determine if findings were consistent among individuals with or without prevalent CVD and across low, intermediate, and high NT-proBNP categories.
Results
Individuals in the higher sACE2 tertiles were more likely to be black men and have prevalent CHD, hypertension, or diabetes mellitus and higher fasting glucose, high-sensitivity C-reactive protein (hs-CRP), hs-cTnI, hs-cTnT, and NT-proBNP (Table 1). sACE2 levels were positively correlated with hs-cTnI (R=0.27), hs-cTnT (R=0.27), and NT-proBNP (R=0.18) (Supplementary Table 1S).
Table 1.
Characteristics across sACE2 tertiles at ARIC visit 5, 2011–2013
| Variable | sACE2 tertile* | |||
|---|---|---|---|---|
| 18.54–55.01 (n=166) |
55.09–88.84 (n=166) |
90.62–1491.37 (n=165) |
||
| Age (years) | 77.1±5.4 | 78.4±4.9 | 77.9±5.8 | 0.13 |
| Male | 38.6% | 63.3% | 60.0% | <0.001 |
| Black | 19.9% | 31.3% | 30.3% | 0.03 |
| Prevalent CHD | 16.6% | 23.8% | 30.9% | 0.002 |
| Prevalent stroke | 3.0% | 4.2% | 6.1% | 0.17 |
| Prevalent HF | 7.3% | 9.8% | 9.3% | 0.54 |
| Hypertension | 79.1% | 74.6% | 87.7% | 0.05 |
| Diabetes mellitus | 32.9% | 29.6% | 45.3% | 0.02 |
| Obesity | 34.0% | 34.6% | 36.1% | 0.69 |
| Current smoker | 7.7% | 6.5% | 6.9% | 0.79 |
| SBP (mmHg) | 130.9±19.1 | 132.3±19.6 | 136.7±20.7 | 0.009 |
| DBP (mmHg) | 64.7±12.1 | 66.0±11.4 | 67.3±13.4 | 0.04 |
| Pulse pressure (mmHg) | 66.2±17.0 | 66.2±15.6 | 69.4±15.3 | 0.06 |
| BMI (kg/m2) | 28.9±6.0 | 28.6±5.6 | 29.0±5.6 | 0.79 |
| Use of antihypertension medications | 76.5% | 78.3% | 87.8% | 0.009 |
| Use of ACEi or ARB | 36.4% | 41.6% | 43.9% | 0.16 |
| Fasting glucose (mg/dL) | 112.4±31.3 | 110.8±24.7 | 121.4±43.2 | 0.05 |
| Total cholesterol (mg/dL) | 178.5±43.1 | 170.3±39.3 | 172.8±45.2 | 0.17 |
| Triglycerides (mg/dL) | 103 (76, 141) | 103 (80, 136) | 112 (84, 168) | 0.07 |
| HDL-C (mg/dL) | 52.4±13.7 | 49.5±12.0 | 50.5±15.4 | 0.06 |
| LDL-C (mg/dL) | 103.0±35.4 | 97.3±32.6 | 95.4±36.9 | 0.03 |
| Lipid-lowering medications | 60.0% | 56.6% | 64.6% | 0.39 |
| eGFR (mL/min/1.73m2) | 69.3±18.1 | 63.8±18.2 | 67.0±19.7 | 0.19 |
| hs-CRP (mg/L) | 1.8 (0.8, 4.3) | 2.2 (1.1, 4.6) | 3.0 (1.4, 7.4) | 0.001 |
| Cardiac biomarkers | ||||
| hs-cTnI (ng/L) | 3.7 (2.5, 5.4) | 4.5 (3.1, 8.7) | 6.9 (3.5, 12.8) | <0.001 |
| hs-cTnT (ng/L) | 12 (8, 16) | 15 (10, 24) | 18 (12, 27) | <0.001 |
| NT-proBNP (pg/mL) | 176.4 (86.2, 343.3) | 231.2 (108.1, 536.3) | 330.6 (121.4, 1002.0) | <0.001 |
| LVEF (%) | 65.3 (60.8, 69.3) | 63.6 (59.4, 67.0) | 64.3 (58.1, 68.8) | 0.08 |
| LVMi (g/m2) | 81.0 (68.8, 93.0) | 83.0 (71.0, 99.1) | 89.8 (74.2, 105.7) | 0.001 |
| LAVi (mL/m2) | 26.5 (21.5, 32.6) | 27.7 (22.5, 34.0) | 30.0 (23.9, 37.9) | <0.001 |
| Septal e′ (cm/sec) | 5.3 (4.5, 6.4) | 5.3 (4.5, 6.2) | 5.1 (4.3, 5.8) | 0.02 |
| Septal E/e′ | 12.4 (9.5, 15.5) | 11.9 (9.4, 14.9) | 13.5 (10.9, 17.8) | 0.004 |
| GLS (%) | −17.53 (−19.1, −16.0) |
−17.1 (−18.9, −15.2) |
−16.5 (−18.7, −14.8) |
0.001 |
sACE2 measured in normalized protein expression (NPX)
Significant values in bold.
Data presented as mean±SD, median (25th, 75th percentiles), or percentage. P-values for linear trend were calculated by using trend test across ordered groups.
Obesity defined as BMI ≥30 kg/m2
Abbreviations: ACEi: ACE inhibitors; ARB: angiotensin receptor blockers; BMI: body mass index; DBP: diastolic blood pressure; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; LVEF: LV ejection fraction; SBP: systolic blood pressure; TR: tricuspid regurgitation.
Use of ACE inhibitors/angiotensin receptor blockers or mineralocorticoid receptor antagonists was not associated with statistically significant differences in median sACE2 levels. On the other hand, individuals using loop diuretics or beta-blockers had significantly higher sACE2 levels compared with individuals not using these medications (Supplementary Table 2S).
sACE2 (ln transformed) was significantly and positively associated with (ln-transformed) hs-cTnI (β 0.25, 95% CI 0.13–0.36), hs-cTnT (β 0.15, 95% CI 0.08–0.22), and NT-proBNP (β 0.29, 0.14–0.44) per log unit of sACE2, after adjusting for clinical variables (traditional risk factors + prevalent CVD and eGFR; model 2) (Table 2). Individuals with higher sACE2 levels had significantly higher odds of having “elevated” hs-cTnI, hs-cTnT, and NT-proBNP levels after adjusting for variables in model 2 (Supplementary Table 3S).
Table 2.
Association of sACE2 with hs-cTnI, hs-cTnT, and NT-proBNP as continuous variables (ln transformed) at ARIC visit 5
| Biomarker | Model | Beta-coefficient* | 95% CI | p-value |
|---|---|---|---|---|
| ln–hs-cTnI | 1 | 0.27 | 0.16–0.39 | <0.001 |
| 2 | 0.25 | 0.13–0.36 | <0.001 | |
| ln–hs-cTnT | 1 | 0.15 | 0.08–0.23 | <0.001 |
| 2 | 0.15 | 0.08–0.22 | <0.001 | |
| ln–NT-proBNP | 1 | 0.33 | 0.18–0.48 | <0.001 |
| 2 | 0.29 | 0.14–0.44 | <0.001 |
Increment in hs-cTnI, hs-cTnT, or NT-proBNP (log unit) per log unit increase of sACE2, where sACE2 is independent and hs-cTnI, hs-cTnT, or NT-proBNP the dependent variables.
Model 1: adjusted by visit 5 age, sex, and race; model 2: model 1 plus visit 5 total cholesterol, HDL-C, SBP; use of antihypertension medication, current smoking, diabetes status, use of lipid-lowering medication, prevalent CVD, and eGFR.
After adjustment for demographic and clinical covariates (model 2), higher sACE2 levels were associated with significantly greater LVMi, larger LAVi, and higher TDI E/e′ and less-negative GLS. Although septal e′ was associated with sACE2 levels in model 1, the association was not significant after adjustment for model 2. Association of sACE2 with all echocardiographic measures of systolic and diastolic function and cardiac structure were attenuated and no longer significant after further adjusting for cardiac biomarkers in model 3 (Table 3). The results remained unchanged when individuals with prevalent CVD were excluded (Supplementary Table 4S).
Table 3:
Association of sACE2 as continuous variable (ln transformed) and echocardiographic parameters at ARIC visit 5
| Echocardiographic parameter | Model | Beta-coefficient | 95% CI | p-value |
|---|---|---|---|---|
| Ln-LVEF | 1 | −0.02 | −0.04, 0.002 | 0.08 |
| 2 | −0.02 | −0.04, 0.002 | 0.07 | |
| 3 | −0.004 | −0.03, 0.02 | 0.68 | |
| Ln-LVMi | 1 | 0.06 | 0.02, 0.09 | 0.001 |
| 2 | 0.05 | 0.01, 0.08 | 0.009 | |
| 3 | 0.002 | −0.03, 0.03 | 0.91 | |
| Ln-LAVi | 1 | 0.08 | 0.03, 0.13 | 0.001 |
| 2 | 0.06 | 0.01, 0.11 | 0.011 | |
| 3 | 0.01 | −0.03, 0.05 | 0.73 | |
| Ln-septal e′ | 1 | −0.04 | −0.08, −0.01 | 0.02 |
| 2 | −0.03 | −0.07, 0.01 | 0.10 | |
| 3 | −0.01 | −0.04, 0.03 | 0.76 | |
| Ln-septal E/e′ | 1 | 0.10 | 0.05, 0.15 | <0.001 |
| 2 | 0.07 | 0.02, 0.12 | 0.01 | |
| 3 | 0.02 | −0.03, 0.07 | 0.47 | |
| GLS | 1 | 0.61 | 0.21, 1.00 | 0.003 |
| 2 | 0.53 | 0.14, 0.93 | 0.009 | |
| 3 | 0.16 | −0.21, 0.54 | 0.39 |
Significant values in bold.
Increment in LVEF, LVMi, septal e′, septal E/e′ (log unit) and GLS (%) per log unit increase of sACE2, where sACE2 is the independent and hs-cTnI, hs-cTnT, or NT-proBNP dependent variable.
Model 1 is adjusted by age, sex, and race; model 2 is model 1 plus total cholesterol, HDL-C, current smoking, SBP, antihypertension medication use, diabetes status, lipid-lowering medication use, history of CVD (stroke, total CHD, and HF), and eGFR; model 3 is model 2 plus log hs-cTnI, log hs-cTnT, and log NT-proBNP.
sACE2 (ln transformed) was significantly associated with global CVD events (HR 1.34 per ln unit increase, 95% CI 1.13–1.60), HF hospitalization (HR 1.32 per ln unit increase, 95% CI 1.10–1.58), and all-cause death (HR 1.26, 95% CI 1.01–1.57) after adjusting for variables in model 2. Further adjusting with (ln-transformed) hs-cTnT, hs-cTnT, and NT-proBNP attenuated the associations, which were no longer significant. sACE2 was borderline-significantly associated with ASCVD events when adjusted for model 1, but not after adjusting for model 2 (Table 4). Sensitivity analyses indicated that associations of sACE2 with incident global CVD and HF hospitalization were unchanged and the association with all-cause death was attenuated when individuals with prevalent CVD were excluded (Supplementary Table 5S).
Table 4.
Risk for CVD events, HF hospitalization, ASCVD events, and all-cause death by sACE2 levels as a continuous variable (natural log)
| Outcome | n/N | Model | HR | 95% CI | P value |
|---|---|---|---|---|---|
| Global CVD | 282/497 (56.7%) | 1 | 1.41 | 1.20–1.65 | <0.001 |
| 2 | 1.34 | 1.13–1.60 | 0.001 | ||
| 3 | 1.11 | 0.93–1.34 | 0.25 | ||
| HF | 260/497 (52.3%) | 1 | 1.40 | 1.19–1.66 | <0.001 |
| 2 | 1.32 | 1.10–1.58 | 0.003 | ||
| 3 | 1.07 | 0.88–1.30 | 0.48 | ||
| ASCVD | 150/497 (30.2%) | 1 | 1.24 | 0.99–1.55 | 0.06 |
| 2 | 1.09 | 0.85–1.39 | 0.49 | ||
| 3 | 0.95 | 0.73–1.24 | 0.71 | ||
| All-cause death | 190/497 (38.2%) | 1 | 1.26 | 1.03–1.54 | 0.02 |
| 2 | 1.26 | 1.01–1.57 | 0.04 | ||
| 3 | 1.01 | 0.80–1.28 | 0.91 |
Data are presented as number of events [n] / number at risk [N] (percent) and HR per natural log unit increase for sACE2 with 95% CI.
Model 1 is adjusted by age, sex, and race; model 2 is model 1 plus total cholesterol, HDL-C, current smoking, SBP, antihypertension medication use, diabetes status, lipid-lowering medication use, history of CVD (stroke, total CHD, and HF), and eGFR; model 3 is model 2 plus log hs-cTnI, log hs-cTnT, and log NT-proBNP.
Discussion
Despite many animal studies demonstrating the protective role of ACE2 in cardiovascular physiology through ameliorating cardiac fibrosis, remodeling, and hypertrophy,3–5 relatively little information is available on the relation of sACE2 with CVD in humans. Our study has important implications in this regard. In a large elderly population-based cohort with a high prevalence of cardiovascular risk factors, increased sACE2 levels were associated with significantly higher cardiac biomarkers (hs-cTnI, hs-cTnT, and NT-proBNP), reflecting neurohormonal activation and cardiac inflammation and injury; echocardiographic measures of myocardial hypertrophy and impaired diastolic function; and increased risk for cardiovascular events, driven primarily by an increased risk for HF hospitalizations.
The specific relations between mbACE2 and sACE2 protein (or circulating ACE2 activity) as measured in other studies are not completely understood. Active mbACE2 is shed into circulation as sACE2 by enzymatic cleavage by tumor necrosis factor alpha–converting enzyme (TACE). In states of pathological RAAS activation, mbACE2 expression is increased (as counter-regulatory response), and shedding into circulation is increased through upregulation of TACE by angiotensin II2 resulting in increased sACE2 and relative deficiency of active mbACE2. Therefore, circulating sACE2 protein or activity, perhaps similarly to BNP, serve protective biological functions but increase in counter-regulatory response to disease stimuli.
To our knowledge, this is the first study to investigate relations between sACE2 and biomarkers of cardiac injury and the largest study evaluating echocardiographic parameters. A recent case–cohort study in 10,753 participants in 14 countries (Prospective Urban Rural Epidemiology study) showed similar results as our study; increased concentrations of plasma ACE2 were associated with increased risk for CVD and non-CVD death, HF, and myocardial infarction independent of age, sex, ancestry, and traditional cardiac risk factors.20 However, the association with echocardiographic parameters or biomarkers was not explored. 20
Plasma ACE2 activity is usually low in healthy individuals,21 higher in individuals with CVD22,23 or diabetes,24 and correlated with extent of tissue damage or CVD progression.21 Higher plasma ACE2 activity has been associated with adverse CVD outcomes in patients with CHD,6 HF,7 or atrial fibrillation,8 with higher activity seen with greater infarct size, ventricular systolic dysfunction,7 and adverse cardiac remodeling.8
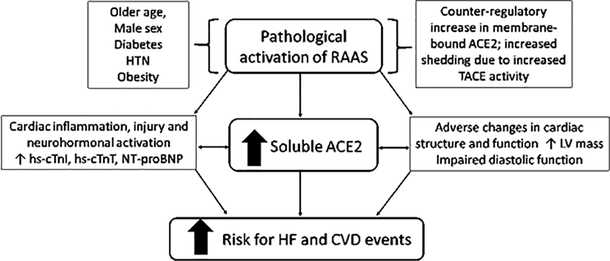
While these smaller studies measured ACE2 activity in patients with established CVD, we measured sACE2 protein levels in a substantially larger elderly population and observed that elevated plasma sACE2 levels were associated with higher odds of elevated hs-cTnI and hs-cTnT, greater LV mass, and worse LV diastolic function, mirroring the cardiac structural and functional correlates that we previously reported with higher hs-cTnT levels.25 Individuals with higher sACE2 had increased risk for global CVD events, driven by HF hospitalization, independent of traditional risk factors, kidney function, or prevalent CVD. Based on these data, we propose that elevated sACE2 may be a marker of pathological activation of RAAS (Figure 1), with concomitant elevation of biomarkers of cardiac injury and abnormalities of cardiac structure and function resulting in increased risk for HF.
Figure 1: sACE2 and cardiac biomarkers, structure, and function: potential implication for CVD.
Pathological activation of RAAS as evidenced by elevated sACE2 levels was associated with elevated cardiac biomarkers and echocardiographic measures of cardiac structural abnormalities and diastolic dysfunction, and independently predicted cardiovascular events including heart failure hospitalizations.
Although sACE2 levels were significantly associated with echocardiographic abnormalities and increased risk for HF events after adjusting for traditional risk factors, the associations were no longer significant after additional adjustment for cardiac biomarkers. This loss of significance may suggest that both sACE2 and cardiac biomarkers may be in the same biological pathway related to RAAS overactivation. The finding that sACE2 was associated with incident HF only in individuals who also had elevation of NT-proBNP may reflect increased cleavage present when there is increased pressure overload and needs confirmation in larger studies and ideally examination of both plasma and tissue levels of ACE2.
Limitations of our study include measurement of soluble plasma (not tissue) ACE2 in only a subset of participants at only one time-point; moreover, the number of black participants was small (n=134, 27%) and may provide insufficient power to study this subpopulation. The correlations of sACE2 with some biomarkers was weak, even though statistically significant because of the large number of samples. It is hypothesized that sACE 2 concentrations are associated with tissue concentration. ACE2 cleaves angiotensin-II to angiotensin 1–7; although measuring circulating levels of both angiotensins and evaluating their associations with sACE2 would increase our understanding of the RAAS axis, these measurements were not available in ARIC. Our study cohort included an elderly population with mean age 78 (SD 5); therefore, the results of our study may not be applicable to a younger age group. In addition, this study is hypothesis generating, and our proposal that increased levels of sACE2 and cardiac biomarkers may be useful to identify individuals with increased susceptibility to adverse cardiac outcomes is intended to encourage future investigation into this area.
In conclusion, elevated sACE2 levels were significantly and positively associated with increased levels of biomarkers of cardiac injury and neurohormonal activation, increased LV mass, impaired diastolic function, and increased risk for prospective CVD events in a large biracial elderly American cohort. sACE2 may therefore serve as an indicator of end-organ damage from pathological imbalance of the RAAS axis, which in turn increases risk for future CVD events.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions. Roche supplied reagents for hs-cTnT and NT-proBNP, Abbott supplied reagents for hs-cTnI, and SomaLogic provided assays without charge. The authors acknowledge Kerrie Jara, MLIS, Baylor College of Medicine, for editorial assistance.
Declaration of interests
☒ The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Olive Tang, Elizabeth Selvin, Vijay Nambi, Faiez Zannad, Salim S. Virani, Bing Yu, Jonathan W. Cunningham, Amil M. Shah, Christie M. Ballantyne reports financial support was provided by National Institutes of Health, US Department of Veterans Affairs, European Union 7th Framework Programme for Research and Technological Development, World Heart Federation, American Heart Association. Dr. Selvin reports a relationship with Novo Nordisk. that includes:. Dr. Nambi reports a relationship with Merck. that includes:. BALLANTYNE reports a relationship with Abbott Diagnostic, Denka Seiken, Roche Diagnostic. that includes:. Zannad reports a relationship with Janssen, Bayer, Boston Scientific, Amgen, CVRx, Boehringer, AstraZeneca, Vifor Fresenius, Cardior, Cereno Pharmacuetical, Applied Therapeutics, Merck, and Novartis, CVCT. that includes:. VIRANI reports a relationship with American College of Cardiology, PALM registry at Duke Clinical Research Institute that includes:. SHAH reports a relationship with Novartis, Philips Ultrasound that includes:. DE LEMOS reports a relationship with Roche Diagnostics, Abbott Diagnostics, Ortho Clinical Diagnostics, Quidel Cardiovascular, Inc. that includes:. Dr. Hoogeveen reports a relationship with Denka Seiken that includes:. Declaration of competing interests: Dr. Selvin: honoraria from Novo Nordisk. Dr. Nambi: site PI study sponsored by Merck. Dr. Zannad: personal fees from Janssen, Bayer, Boston Scientific, Amgen, CVRx, Boehringer, AstraZeneca, Vifor Fresenius, Cardior, Cereno Pharmacuetical, Applied Therapeutics, Merck, and Novartis; founder of CVCT. Dr. Virani: honorarium: American College of Cardiology (Associate editor for Innovations acc.org); Steering Committee member: PALM registry at Duke Clinical Research Institute (no financial remuneration). Dr Shah: research support (significant; paid to institution, not individual) from Novartis, and consultant (modest) for Philips Ultrasound. Dr. de Lemos: grant support and consulting income from Roche Diagnostics and Abbott Diagnostics, consulting income from Ortho Clinical Diagnostics and Quidel Cardiovascular, Inc. Dr. Hoogeveen: grant support and consulting fees from Denka Seiken outside the submitted work. Dr. Ballantyne: grants/research support (significant; paid to institution, not individual): Abbott Diagnostic, Roche Diagnostic; consultant (modest): Abbott Diagnostic, Denka Seiken, Roche Diagnostic. Dr. Hussain, Ms. Tang, Ms. Sun, Dr. Jia, Dr. Folsom, Dr. Heiss, Dr. Mosley, Dr. Coresh, Dr. Boerwinkle, Dr. Yu, Dr. Cunningham, Dr. Solomon: none. Grant support: The Atherosclerosis Risk in Communities study has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services (contract numbers HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). This work was supported by NIH grants F30-DK120160 (O.T.), K24-DK106414 (E.S.), R01-DK089174 (E.S.), R01-HL134320 (E.S. and C.M.B.), R01-HL141824 (B.Y.), R01-HL142003 (B.Y.), T32-HL094301 (J.W.C.), R01-HL135008 (A.M.S.), R01-HL143224 (A.M.S.), R01-HL150342 (A.M.S.), and K24-HL152008 (A.M.S.)]; US Department of Veterans Affairs grants 1I01CX001112-01 (V.N.), IIR 16-072 (S.V.), and IIR 19-069 (S.V.); European Union 7th Framework Programme for Research and Technological Development grant HEALTH-F7-305507 (F.Z., as part of Heart OMics in AGEing); World Heart Federation (S.V.); and American Heart Association grant 17SDG33661228 (B.Y.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, Raizada MK, Grant MB, Oudit GY. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res 2020;126:1456–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel VB, Clarke N, Wang Z, Fan D, Parajuli N, Basu R, Putko B, Kassiri Z, Turner AJ, Oudit GY. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: a positive feedback mechanism in the RAS. J Mol Cell Cardiol 2014;66:167–176. [DOI] [PubMed] [Google Scholar]
- 3.Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos AJ, da Costa J, Zhang L, Pei Y, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, Backx PH, Yagil Y, Penninger JM. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 2002;417:822–828. [DOI] [PubMed] [Google Scholar]
- 4.Kassiri Z, Zhong J, Guo D, Basu R, Wang X, Liu PP, Scholey JW, Penninger JM, Oudit GY. Loss of angiotensin-converting enzyme 2 accelerates maladaptive left ventricular remodeling in response to myocardial infarction. Circ Heart Fail 2009;2:446–455. [DOI] [PubMed] [Google Scholar]
- 5.Qi YF, Zhang J, Wang L, Shenoy V, Krause E, Oh SP, Pepine CJ, Katovich MJ, Raizada MK. Angiotensin-converting enzyme 2 inhibits high-mobility group box 1 and attenuates cardiac dysfunction post-myocardial ischemia. J Mol Med (Berl) 2016;94:37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramchand J, Patel SK, Srivastava PM, Farouque O, Burrell LM. Elevated plasma angiotensin converting enzyme 2 activity is an independent predictor of major adverse cardiac events in patients with obstructive coronary artery disease. PLoS One 2018;13:e0198144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epelman S, Shrestha K, Troughton RW, Francis GS, Sen S, Klein AL, Tang WH. Soluble angiotensin-converting enzyme 2 in human heart failure: relation with myocardial function and clinical outcomes. J Card Fail 2009;15:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walters TE, Kalman JM, Patel SK, Mearns M, Velkoska E, Burrell LM. Angiotensin converting enzyme 2 activity and human atrial fibrillation: increased plasma angiotensin converting enzyme 2 activity is associated with atrial fibrillation and more advanced left atrial structural remodelling. Europace 2017;19:1280–1287. [DOI] [PubMed] [Google Scholar]
- 9.ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 10.Jia X, Sun W, Hoogeveen RC, Nambi V, Matsushita K, Folsom AR, Heiss G, Couper DJ, Solomon SD, Boerwinkle E, Shah A, Selvin E, de Lemos JA, Ballantyne CM. High-sensitivity troponin I and incident coronary events, stroke, heart failure hospitalization, and mortality in the ARIC Study. Circulation 2019;139:2642–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ndumele CE, Matsushita K, Sang Y, Lazo M, Agarwal SK, Nambi V, Deswal A, Blumenthal RS, Ballantyne CM, Coresh J, Selvin E. N-terminal pro-brain natriuretic peptide and heart failure risk among individuals with and without obesity: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation 2016;133:631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olink Proteomics. Olink cardiovascular II validation data, article number 95500. https://www.olink.com/content/uploads/2019/12/Olink-CVD-II-Validation-Data-v2.1.pdf. Accessed 28 April 2020.
- 13.Shah AM, Cheng S, Skali H, Wu J, Mangion JR, Kitzman D, Matsushita K, Konety S, Butler KR, Fox ER, Cook N, Ni H, Coresh J, Mosley TH, Heiss G, Folsom AR, Solomon SD. Rationale and design of a multicenter echocardiographic study to assess the relationship between cardiac structure and function and heart failure risk in a biracial cohort of community-dwelling elderly persons: the Atherosclerosis Risk in Communities study. Circ Cardiovasc Imaging 2014;7:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39 e14. [DOI] [PubMed] [Google Scholar]
- 15.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol 1996;49:223–233. [DOI] [PubMed] [Google Scholar]
- 16.Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, Copper LS, Shahar E. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke 1999;30:736–743. [DOI] [PubMed] [Google Scholar]
- 17.Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the Atherosclerosis Risk in Communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail 2012;5:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, Hoogeveen RC, Liu X, Astor BC, Mosley TH, Folsom AR, Heiss G, Coresh J, Ballantyne CM. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation 2011;123:1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012;60:1581–1598. [DOI] [PubMed] [Google Scholar]
- 20.Narula S, Yusuf S, Chong M, Ramasundarahettige C, Rangarajan S, Bangdiwala SI, van Eikels M, Leineweber K, Wu A, Pigeyre M, Pare G. Plasma ACE2 and risk of death or cardiometabolic diseases: a case-cohort analysis. Lancet 2020;396:968–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Úri K, Fagyas M, Kertész A, Borbély A, Jenei C, Bene O, Csanádi Z, Paulus WJ, Édes I, Papp Z, Toth A, Lizanecz E. Circulating ACE2 activity correlates with cardiovascular disease development. J Renin Angiotensin Aldosterone Syst 2016;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou X, Zhang P, Liang T, Chen Y, Liu D, Yu H. Relationship between circulating levels of angiotensin-converting enzyme 2-angiotensin-(1–7)-MAS axis and coronary heart disease. Heart Vessels 2020;35:153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Epelman S, Tang WH, Chen SY, Van Lente F, Francis GS, Sen S. Detection of soluble angiotensin-converting enzyme 2 in heart failure: insights into the endogenous counter-regulatory pathway of the renin-angiotensin-aldosterone system. J Am Coll Cardiol 2008;52:750–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soro-Paavonen A, Gordin D, Forsblom C, Rosengard-Barlund M, Waden J, Thorn L, Sandholm N, Thomas MC, Groop PH, FinnDiane Study G. Circulating ACE2 activity is increased in patients with type 1 diabetes and vascular complications. J Hypertens 2012;30:375–383. [DOI] [PubMed] [Google Scholar]
- 25.Myhre PL, Claggett B, Ballantyne CM, Selvin E, Rosjo H, Omland T, Solomon SD, Skali H, Shah AM. Association between circulating troponin concentrations, left ventricular systolic and diastolic functions, and incident heart failure in older adults. JAMA Cardiol 2019;4:997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.