Abstract
Pancreatic cancer affects both male and female individuals with higher incidences and death rates among the male population. Detection of this malignancy is delayed due to the lack of symptoms in the early-stage cancer, which makes it extremely difficult to treat. Identifying effective strategies has been a challenge for improving the survival rates in pancreatic cancer patients. Resistance to chemotherapy is often developed in pancreatic cancer treatment. Although many strategies are under clinical trials to target certain markers associated with cancer, immunotherapeutic approaches are currently gaining importance. Immunotherapy for pancreatic cancer is in the limelight after preclinical research showed some promise. Immunotherapy approaches were tested along with other treatment options to enhance the treatment effect. Adoptive cell transfer and immune checkpoint inhibitors are currently in clinical trials. The Food and Drug Administration approved pembrolizumab in a fast-tracked review for advanced pancreatic cancer patients. Pembrolizumab blocks the checkpoint protein, programmed cell death protein 1 (PD-1), on T cells to boost the response of the immune system against cancer cells, thereby shrinking tumors. The recent developments in immunotherapy and the early success in other cancers are encouraging to further test immunotherapy in pancreatic cancer. The combination of pembrolizumab and pelareorep, an isolate of human reovirus, is in phase II clinical study in metastatic disease. Depending on the results of current clinical trials and testing, the strategies in the pipeline are expected to increase the use of immunotherapy in the clinical testing setting. Success in immunotherapy is urgently needed to address the side-effects, treating patients with advanced disease and reducing metastasis for increasing the survival rate in pancreatic cancer patients.
Keywords: pancreatic cancer, immunotherapy, clinical trials, programmed cell death protein 1
I. INTRODUCTION
Pancreatic cancer is one of the deadliest cancers for both men and women, with exocrine pancreatic ductal adenocarcinoma (PDAC) making up 85% of the cancers and endocrine pancreatic cancers making up less than 5%.1-6 Pancreatic cancer is the fourth leading cause of death in both genders, and survival for all stages combined is 9%.7 There were 56,770 new cases in the United States in 2018, with an estimated 45,750 deaths.8 The highest prevalence is in the male population of the developed world. Pancreatic cancer is considered one of the deadliest cancers because of its late detection. Currently, no tests are available for the early detection of pancreatic cancer, and thus, there have been minimal advances in treatment.
Many risk factors can contribute to the development of pancreatic cancer, some of which include tobacco use, obesity, exposure to certain chemicals, diabetes, and chronic pancreatitis. Genetic syndromes can also be risk factors that can contribute to the development of pancreatic cancer (Table 1).
TABLE 1:
The genetic syndromes and associated genes that are mutated
| Genetic Syndrome | Gene Mutation |
|---|---|
| Hereditary breast and ovarian cancer syndrome | Breast cancer genes (BRCA1/BRCA2) |
| Hereditary breast cancer | Partner and localizer of BRCA2 (PALB2) |
| Familial atypical multiple mole melanoma (FAMMM) syndrome | Cyclin-dependent kinase Inhibitor 2A (P16/CDKN2A) |
| Familial pancreatitis | Cationic trypsinogen (PRSS1) |
| Lynch syndrome | MutL homolog 1 and 2 (MLH1/MLH2) |
| Peutz-Jeghers syndrome | Serine/threonine kinase 11 (STK11) |
II. TYPES OF PANCREATIC CANCER
A. Exocrine Tumors
Pancreatic cancer is divided into two types: exocrine and endocrine. Exocrine cancers, such as PDAC, are formed in glands that secrete fluids and make up the majority of pancreatic cancer. The most common site for exocrine tumors of the pancreas is in the pancreatic duct. Patients with early stages of PDAC present with general symptoms such as fatigue, weakness, and loss of appetite. Later stages of the disease may present with more common symptoms such as jaundice and severe abdominal pain.9 However, these symptoms are very vague and as a result, pancreatic cancer is typically not diagnosed until it is already in the later stages.
B. Endocrine Tumors
Endocrine tumors are not as common as exocrine tumors and are usually benign. Because these tumors effect hormone production, they are called pancreatic neuroendocrine tumors (PNETs). PNETs develop from multipotent stem cells in the pancreatic epithelial lining.10 They are categorized as nonfunctional or functional. Nonfunctional pancreatic neuroendocrine tumors (NF-PNET) do not cause symptoms because they do not cause the production of hormones, or the hormones they secrete do not cause symptoms. On the other hand, functional pancreatic neuroendocrine tumors (F-PNET) produce hormones that cause symptoms.11
Because NF-PNETs do not cause the emergence of specific syndromes, they are diagnosed incidentally or because the tumor mass is causing symptoms. Common symptoms are weight loss, abdominal pain, a palpable mass, and jaundice.12 Hormones that can be secreted by NF-PNET include chromogranin A, ghrelin, HCG subunits, neurotensin. Levels of chromogranin A are the most widely used test for NF-PNET.13,14
Two of the most frequently occurring F-PNET tumors are insulinomas and gastrinomas. Insulinomas are the most common F-PNET and they secrete excess of insulin. Symptoms include hypoglycemia, visual disturbances, headaches, weakness, sweating, palpitations, and tremors.15 The next most common F-PNET is gastrinomas. Most of these are found in the duodenum, then the pancreas, and surrounding tissues.16 Gastrinomas can cause the Zollinger-Ellison syndrome due to the high production of gastrin. Also, because of this, patients can develop peptic ulcers and gastroesophageal reflux disease (GERD). Approximately 20%–30% of gastrinomas have been associated with the gene Multiple endocrine neoplasia type 1 (MEN1)17 In addition, several laboratory exams that can be performed to confirm the diagnosis of gastrinoma: FSG levels, basal acid output, stomach pH, and secretin levels.18
III. DIAGNOSIS
A. Imaging
Different imaging and screening modalities can be used as diagnostic tools for pancreatic cancer. Computed tomography (CT) scans are usually the first line of diagnostic imaging used when pancreatic cancer is suspected. Multidirectional CT scans can determine the size of the tumor and how far it has spread due to its ability to reconstruct 3D images. In addition, CT scans have advanced spatial resolution and sensitivity of up to 96% and accuracy of 86.8%. The lower cost and easy accessibility of CT scans make them the preferred choice over other diagnostic methods such as magnetic resonance imaging (MRIs). CT scans should be performed with IV contrast agents in both the pancreatic parenchymal phase and the portal venous phase.19
Endoscopic ultrasound (EUS) is one of the most sensitive techniques in pancreatic cancer detection; it has a higher sensitivity for detecting solid lesions that are smaller than 2 cm when compared to CT scans. EUS also has the option to be combined with fine-needle aspiration (FNA) to obtain tissue samples.20-25 With EUS, patients are able to avoid unnecessary exposure to ionizing radiation. However, advanced training is needed to operate the ultrasound, and there is significant variability among operators of the device.19
MRIs have a similar sensitivity for the detection of pancreatic cancer compared to CT scans. However, they have the advantage of being able to image larger areas of the abdomen at one time and not exposing the patient to ionizing radiation. Pancreatic cancer is shown on MRIs as a hypointense mass on T1-weighted MRIs and a slightly hyperintense mass on T2-weighted MRIs. When looking at the diffuse weighted images (DWIs), the apparent diffusion coefficient (ADC) is low in pancreatic cancer due to the increased cellularity and fibrotic changes that occur at sites of the cancer.20-25 These changes prevent the free movement of water, which results in a low ADC.
B. Biomarkers
The most common biomarker screened for in pancreatic cancer is the serum carbohydrate antigen 19-9 (CA 19-9). Although this is the only biomarker approved by the FDA, its specificity and sensitivity for pancreatic cancer are 77.6% and 75.4%, respectively. CA 19-9 can also be present in other gastrointestinal cancers. Carcinoembryonic acid (CEA) and CA 242 are two other biomarkers that can be tested for pancreatic cancer; however, they are not highly sensitive and specific for pancreatic cancer. CEA has a specificity of 81.3% and a sensitivity of 39.7%, whereas CA 242 has a specificity of 83% and a sensitivity of 67.8%. Therefore, CA 19-9 has the highest sensitivity and CA 242 has the highest specificity for pancreatic cancer.26
IV. PANCREATIC CANCER TREATMENT
A. Current Treatment Options
Surgical resection and adjuvant therapy involving chemotherapy alone or alongside radiation are the widely used options for the treatment of this malignancy. The chemotherapy regimen is planned typically using the chemotherapeutic agents, capecitabine, erlotinib, fluorouracil, gemcitabine, irinotecan, leucovorin and oxaliplatin.
V. IMMUNOTHERAPY
Immunotherapy is a heavily emerging science founded on the idea of manipulating the mechanisms of the body’s immune system to allow for recognition of antigens of our choosing. Currently, the three most significant approaches to immunotherapy are checkpoint inhibitors, vaccination, and adoptive T-cell transfer.
A. Checkpoint Inhibitors
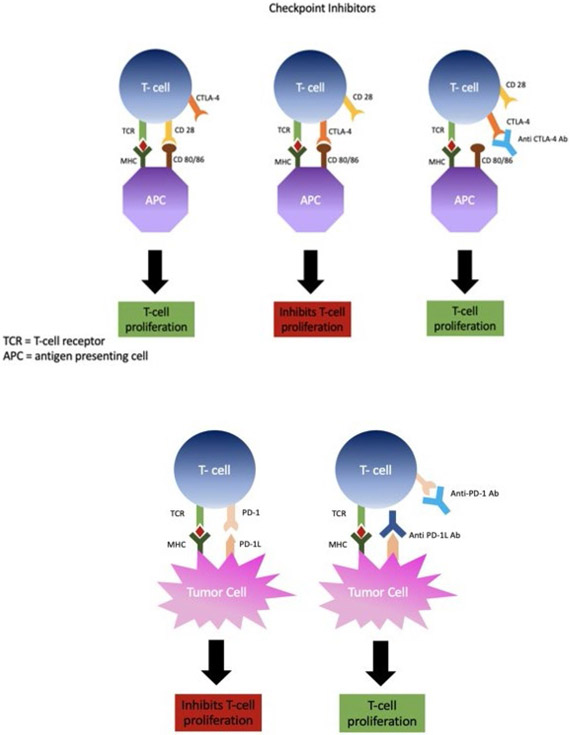
In an effort to avoid autoimmunity and immunopathologic conditions, the immune system contains various fail-safe type mechanisms to regulate the development and function of its effector cells.27-31 Exploiting such a mechanism has brought about the concept of immune checkpoint therapy, a treatment that has shown promise in clinical settings and has brought much attention to the field of cancer immunotherapy. The belief is that the ability of the tumor to gain control over these inhibitory pathways gives it the power to suppress the action and development of immune cells before they have the chance to carry out their anti-tumor functions. The process of T-cell suppression in regard to tumors is most notable via interactions between the activated T-cell cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and the dendritic cell CD 80/86. Another way is through T-cell programmed cell death protein 1 (PD-1) binding to PD-L1, which resides on tumor cells. In a healthy individual, both of these processes are normal physiologic mechanisms to avoid autoimmunity and immune system dysfunction. In cancer patients, the tumor has seized control of these T-cell suppressive mechanisms to promote its survival.32-37 The emergence of checkpoint inhibitors such as anti-CTLA-4, anti-PD-L1, and anti-PD-1 antibodies allows for the disinhibition of the tumor override mechanisms, reinstalling the antitumor T-cell effector functions (Fig 1).29,38-41
FIG. 1:
Checkpoint inhibition. Antibodies are created against specific receptors or ligands that prevent T-cell proliferation.
B. Preclinical Research
Several checkpoint inhibitors have been developed to target the previously described interactions. One of which is ipilimumab (YERVOY®), a humanized antibody that prevents the interaction between CTLA-4 and B7, and thereby enables increased T-cell activity. Preclinical evidence for CTLA-4 blockade has emerged primarily from prostate cancer murine models, which have demonstrated a five-fold reduction in tumor incidence when combined with an irradiated tumor vaccine.11,42-50
1. Clinical Trials
The success in preclinical studies led to the development of a human CTLA-4 antibody, which eventually underwent a double-blind, placebo-controlled phase III trial that compared the standard treatment of dacarbazine alone, to treatment in combination with ipilimumab in patients with metastatic melanoma. Ipilimumab gained FDA approval for use in metastatic melanoma in March 2011, after phase III studies showed a significant increase in survival with the addition of ipilimumab in patients.51-54
VI. VACCINATION
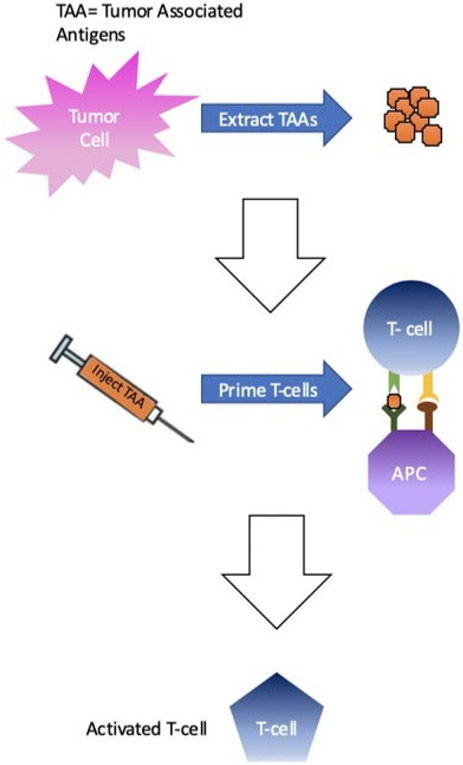
The idea behind vaccination in the setting of cancer involves tumor-associated antigens (TAAs), which are molecular components of the tumor cells. Although TAAs can vary in identity, the therapeutic benefit comes from using TAAs to incite the immune system against the tumor. TAA vaccines can be in the form of DNA, protein (antigen), DCs, or even whole cancer cells that are used to inoculate the immune system against the cancer. TAAs can even be derived from cancer-cell DNA mutations that differentiate the tumor cells from the normal cells of that tissue (Fig. 2). More specific and effective TAAs are constantly being researched. The more specific the TAA is to the cancer, and different it is from the normal tissue, the safer the treatment should be.55-59
FIG. 2:
Vaccines against tumor cells. Tumor associated antigens (TAAs) are extracted from tumor cells and used to create vaccines. Once injected, the TAAs in the vaccine activate T-cells specifically against the tumor cells.
A. Preclinical Trials
Preclinical trials have shown early success in the development of functional cancer vaccines. Gomez et al. showed this success in a B16 melanoma murine model.60-65 Melanoma cell lines were transduced with the gene coding for the MCPyV small T (ST) antigen, an antigen critical to the pathogenesis of Merkel cell carcinoma. From this antigen, they produced a DNA-coated particle vaccine (pcDNA3-MCC/ST). They administered the vaccine to ST-expressing cancerous mice and recorded significant levels of an ST-targeted T-cell immune response. Upon completion of a strict vaccination schedule, the tumor volumes of pcDNA3-MCC/ST vaccinated mice were significantly lower than that of the control.
1. Clinical Trials
Success in preclinical trials, such as those previously mentioned, have led to clinical trials and even to the development of Sipuleucel-T, the first ever FDA-approved cancer vaccine for the treatment of prostate cancer.66 Sipuleucel-T is a cellular vaccine consisting of serum mononuclear cells and antigen presenting cells (APC) activated against the prostate-specific PA2024 fusion protein. In a randomized double-blind placebo-controlled phase III trial concerning metastatic castration-resistant prostate cancer patients, 512 patients received either Sipuleucel-T or a placebo. The Sipuleucel-T group showed a significant immune response to the antigen of vaccination along with a 22% decrease in risk of death or 4.1-month increase in median survival time over the placebo.67 Receiving FDA approval for this new class of treatment shines a light on the enormous potential for cancer vaccination therapy and has broadened the field of immunotherapy in general.
VII. ADOPTIVE T-CELL TRANSFER (ACT)
ACT involves the identification and collection of T-cells based upon their specific antigen-recognition capabilities, or for the purpose of modification of receptor function. The two main methods for achieving these are the collection of tumor-infiltrating lymphocytes (TILs) and the engineering of chimeric antigen receptor (CAR) T cells.
Tumor-infiltrating lymphocytes are lymphocytes that have migrated out of the bloodstream and to the site of the tumor. These lymphocytes are obtained from the patient along with dendritic cells (DCs) and tumor DNA. The tumor DNA will be sequenced for the identification of mutations. Once identified the mutations, or neoepitopes, they are exposed to the DCs for uptake. These primed DCs are cultured together with the TILs and undergo expansion ex vivo. The TILs are then administered to the patient along with the cytokine interleukin-2 (IL-2) to enhance anticancer immunity.
CAR T-cells, are an emerging therapy with a similar mechanism. However, these T cells are extracted from the peripheral blood. The T cells undergo a modification process that yields an engineered T-cell receptor allowing the cells to bind a specific antigen residing on the surface of the tumor cells. These cells are expanded ex vivo and administered to the patient (Fig. 3).55-59,68
FIG. 3:
Adoptive T-cell transfer. T cells are removed from the peripheral blood and engineered to have tumor specific receptors.
A. Preclinical Research
Early in the CAR T-cell development, the first-generation cells resulted from the cloning of the intracellular CD3-zeta chain domain. Upon fusion with CD8, CD4, or CD25 extracellular domains, evidence of T-cell activation was apparent following antigen stimulation. Despite this early success, in murine models, the CD3-zeta fusion chain in CAR T-cells failed to significantly inhibit tumor growth due to suboptimal production of IFN-γ leading to eventual anergy. Although these CAR T-cells were equipped to initiate antigen-specific cytotoxicity, they failed to sustain significant T-cell expansion.
The second-generation CAR T cells combatted the issue of anergy or activation-induced cell death (AICD) with the addition of a CD28-based chimeric costimulatory receptor (CCR). This second-generation T-cell was able to mediate IL-2 synthesis, to support T-cell expansion following antigen interaction, and to improve tumor rejection function overall in murine models. These second-generation T-cells were then engineered to target CD19 surface antigens due to the presence of CD19 presence in the majority of B-cell malignancies. This first occurred nearly 20 years ago. Since then, clinical trials have shown significant results.69-74
1. Clinical Trials
In refractory B-cell lymphomas, CD19 targeting CAR T-cells (CTL019) showed promising results.15 In a study of 28 lymphoma patients administered CTL019 cells, a significant response was noted in 64% of the cohort. It was reported that 57% of patients underwent complete remission, and patients in remission at the 6-month mark remained so at 39.7 months. Due to these and other supporting results, CTL019-directed T cells received unanimous approval from the FDA advisory committee for the treatment of relapsed or refractory B-cell acute lymphoblastic leukemia (ALL).75-80
Currently, in pancreatic cancer research, no treatment regimens offer long-term benefits for late-stage patients. This may be due, in large part, to the uniquely suppressive tumor microenvironment (TME) of pancreatic cancer. Pancreatic tumors contain a dense desmoplastic stroma that limits blood and drug deliveries, enhancing immune escape of the tumor. Also, a severe combination of hypoxia, decreased pH, and significant interstitial fluid pressure contribute to tumor survival and downregulation of antitumor immune cells. This TME is thought to be a major limiter of pancreatic-based immunotherapy. Another major hindrance to the use of immunotherapy in pancreatic cancer is the uniquely low level of mutation and neoantigen formation in tumors. The mutation quantity may be correlated with increased potential for immunotherapy effectiveness. This also leads to lower levels of TILs, which combat the effectiveness of drugs, such as checkpoint inhibitors that exert their effects by promoting TIL activity.81-85
VIII. IMMUNOTHERAPY IN PANCREATIC CANCER
A. Checkpoint Inhibition
Although checkpoint inhibitors have shown efficacy in immunotherapy in some cancers, pancreatic cancer remains largely unsusceptible to their lone effects. This is likely due to the low levels of tumor-infiltrating lymphocytes and immunogenicity in pancreatic cancer as mentioned previously. A 0% overall response rate (ORR) was found in patients treated with an anti-PD-L1 monoclonal antibody, further demonstrating the ineffectiveness of checkpoint inhibition monotherapy.60-64 New PD-1 inhibitors pembrolizumab and nivolumab have received approval for therapy in melanoma, but they remain in the clinical trial phase of testing for pancreatic cancer. The most hopeful advances to checkpoint inhibition in pancreatic cancer seem to be combination therapy with chemotherapeutic agents. In a recent phase I study, the safety profile of the chemotherapeutic agent gemcitabine and CTLA-4 checkpoint inhibitor tremelimumab was examined. This combination showed success in the production of tolerable side effects, with 7 of 28 patients showing relatively stable disease for over a 10-week period. Despite these minor progressions, much work is yet to be done to find more efficacious treatments regarding checkpoint inhibition in PC.
B. Vaccines
Cancer vaccines have also been limited in their effectiveness due to the reasons previously mentioned. However, some progress has been seen in a preclinical murine model using the GVAX vaccine in combination with a checkpoint inhibitor. This vaccine is comprised of PC cells that have been irradiated and engineered on a genetic level to produce granulocyte macrophage colony-stimulating factor (GM-CSF). GM-CSF is a cellular signaling molecule that initiates the priming of T cells, presentation of antigens, and tumor-directed cytolytic action. In this study, GVAX was combined with an anti–PD-1 checkpoint inhibitor. The therapy promoted the secretion of IFN-γ and expansion of activated T-cells within the tumor microenvironment of mice receiving the combination therapy.81-85 Mice that were administered either treatment alone did not show these results, indicating that it was the synergistic effect of the combination therapy that was responsible. Combination therapy seems to be a growing idea at this point; however, more research needs to be done to develop vaccinations with increased specificity and potency.
C. Adoptive T-cell Transfer
Adoptive T-cell transfer (ACT) is made difficult in PC by the immunosuppressive TME along with previous lack of a suitable antigen for the CAR. The latter issue has recently been overcome by engineering the CAR T cell to recognize mesothelin, a protein with minimal expression in normal cells but with significant expression in pancreatic cancer cells. Mesothelin is thought to play a part in tumor aggressiveness, malignancy, and potentially metastasis. A concluded phase I clinical trial with mesothelin-targeted CAR T cells accomplished disease stability in two of the six patients who underwent therapy. The results showed that the treatment was well-tolerated and pointed toward evidence of antitumor effects in pancreatic cancer. A study of the usefulness and safety of using antimesothelin CAR T-cells in conjunction with chemotherapeutic drugs (e.g., cyclophosphamide) in metastatic pancreatic cancer patients is currently ongoing as a nonrandomized phase I/II clinical trial (Table 2).81-85
TABLE 2:
Adoptive T-cell transfer preclinical research and clinical trials
| Preclinical Trial Focus | Target Cancer | Results | Preclinical Trial Focus |
|---|---|---|---|
| Apoptosis and anergy of T cell induced by pancreatic stellate | Pancreatic Cancer | High expression of galectin-1 was associated with short survival as was low expression of CD3. | Apoptosis and anergy of T cell induced by pancreatic stellate |
| Virus-specific T cells engineered to coexpress tumor-specific receptors | Neuroblastoma | Infusion of these genetically modified cells was associated with tumor regression or necrosis in half of the subjects tested | Virus-specific T cells engineered to coexpress tumor-specific receptors |
| Tumor-infiltrating lymphocytes | Large pulmonary and hepatic metastatic tumors | 100% of mice (n = 12) bearing the MC-38 colon adenocarcinoma were cured of advanced hepatic metastases, and up to 50% of mice were cured of advanced pulmonary metastases. | Tumor-nfiltrating lymphocytes |
| Clinical Trial Focus | Target Cancer | Results | Trial Identifier |
| B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. | B-cell lymphoma | Of 15 patients, eight achieved complete remissions (CRs), four achieved partial remissions, one had stable lymphoma, and two were not evaluable for response. | NCT00924326 |
| Anti-tumor effect of B7-H3-blocking monoclonal antibody | Pancreatic Cancer | T-cell infiltration into the tumor and induced a substantial anti-tumor effect on murine pancreatic cancer | |
| T cells for the treatment of metastatic ovarian cancer | Ovarian Cancer | An inhibitory factor developed in the serum of three of six patients tested over the period of treatment, which significantly reduced the ability of gene-modified T cells to respond against FR+ tumor cells. |
IX. RECENT ADVANCEMENTS
Recent therapy options for pancreatic cancer are aimed at reducing the immunosuppressive TME. By targeting the immunosuppressive cells within the TME, immunotherapy options for treatment are more likely to be effective. The first of these targets is CSF1R. CSF1R is located on the tumor associated macrophages (TAM). The binding of CSF1 to CSF1-R allows for TAMs to proliferate and survive longer which then aids in tumor growth, resistance to treatments, and tumor metastasis. When CSF1-R is inhibited, fewer TAMs are present. This allows for a higher immune response, increases tumor regression, and increases survival.86
Another therapy option is targeted at the JAK/STAT pathway. Overactivation of this pathway by interferons upregulates the expression of PD-L1, as well as suppresses cytotoxic T-lymphocytes, in tumor cells. The use of JAK/STAT inhibitors can not only reduce the overexpression of PD-L1 but also reduce the growth of tumors and increase survival rates. This therapy can increase the response to anti–PD-L1 immunotherapies.82
X. FUTURE PERSPECTIVES
Pancreatic cancer is one of the most fatal cancers due to its late detection and aggressiveness. Therapeutic advancement in immunotherapy, such as vaccines and adoptive T-cell inhibitors, give hope to the future prognosis of pancreatic cancer. However, due to the highly immunosuppressive tumor microenvironment (TME) of the cancer, even these advances are proving to be of minimal help. Combination treatments of chemotherapy, immunotherapy, and radiation therapy work best to induce long-term antitumor activity and increase the body’s T-cell response. More research needs to be done into the optimal timing, order, and dosing of the different treatment options to best fight the disease.86
Research is being conducted on altering the TME to make the tumor more susceptible to treatment. The TME is a barrier to pharmacological intervention, increases the tumors progression, and increases tumor angiogenesis and stromal formation. Therapies targeted at inhibiting TGF-β, which aids in immunosuppression and stroma formation, are being further developed as a way to weaken the tumor defenses and allow drugs to enter the TME.87-92 Additionally, new technology is being developed that looks into T-cell receptor gene sequencing. This technology allows for a more detailed look into the number of T cells that are attacking the tumor as well as the specificity of those T cells.57,93-97 Much more research needs to be done to investigate whether this is an effective antitumor treatment method.
XI. CONCLUSION
Pancreatic cancer continues to be one of the leading causes of cancer-related deaths in both males and females. The development of new therapies has been slow due to the continual late diagnosis of pancreatic cancer. Immunotherapy has shed a light on a very dim future for many individuals. The use of checkpoint inhibitors diminishes the ability of cancer cells to downregulate T-cell proliferation. Vaccinations and adoptive T-cell transfer both increase the specificity of T cells to attack specific cancer cells. However, the use of these therapies alone is not enough. Although not completely treatable, combinations of immunotherapy, chemotherapy, and radiation therapy have proven to be the most effective method in the treatment of pancreatic cancer. In addition, altering the TME to be less immunosuppressive could lead to more successful treatments. Ultimately, immunotherapy has offered new and exciting opportunities for the treatment of pancreatic cancer, but much more research still needs to be done to ensure a higher success rate.
ACKNOWLEDGMENTS
Authors acknowledge the financial support from NIMHD (grant #: 2U54 MD006882-06), NCI (grant #: 1P20CA233355-01) and NHLBI (grant #: R25HL125447).
ABBREVIATIONS:
- ACT
adoptive T-cell transfer
- ADC
apparent diffusion coefficient
- AICD
activation-induced cell death
- ALL
acute lymphoblastic leukemia
- APC
antigen presenting cells
- CA
carbohydrate antigen
- CAR
chimeric antigen receptor
- CCR
chimeric co-stimulatory receptor
- CEA
carcinoembryonic acid
- CSF1R
colony-stimulating factor 1 receptor
- CT
computed tomography
- CTLA4
T-cell cytotoxic T-lymphocyte-associated protein 4
- DC
dendritic cells
- DWI
diffuse weighted images
- EUS
endoscopic ultrasound
- FDA
Federal Drug Administration
- ENA
fine-needle aspiration
- F-PNET
functional pancreatic neuroendocrine tumors
- GERD
gastroesophageal reflux disease
- GM-CSF
granulocyte macrophage colony-stimulating factor
- IFN-γ
interferon gamma
- MEN1
multiple endocrine neoplasia type 1
- NF-PNET
non-functional pancreatic neuroendocrine tumors
- ORR
overall response rate
- PD-1
programmed cell death protein 1
- PDAC
pancreatic ductal adenocarcinoma
- PNET
pancreatic neuroendocrine tumors
- TAA
tumor-associated antigen
- TAM
tumor-associated macrophage
- TGF
transforming growth factor
- TIL
tumor-infiltrating lymphocyte
- TME
tumor microenvironment
REFERENCES
- 1.Giardiello FM, Welsh SB, Hamilton SR, Offerhaus GJ, Gittelsohn AM, Booker SV, Krush AJ, Yardley JH, Luk GD. Increased risk of cancer in the Peutz-Jeghers syndrome. N Engl J Med. 1987; 316(24):1511–14. [DOI] [PubMed] [Google Scholar]
- 2.Grant RC, Selander I, Connor AA, Selvarajah S, Borgida A, Briollais L, Petersen GM, Lerner-Ellis J, Holter S, Gallinger S. Prevalence of germline mutations in cancer predisposition genes in patients with pancreatic cancer. Gastroenterology. 2015;148(3):556–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta R, Amanam I, Chung V. Current and future therapies for advanced pancreatic cancer. J Surg Oncol. 2017;116(1):25–34. [DOI] [PubMed] [Google Scholar]
- 4.Salo-Mullen EE, O’Reilly EM, Kelsen DP, Ashraf AM, Lowery MA, Yu KH, Reidy DL, Epstein AS, Lincoln A, Saldia A, Jacobs LM, Rau-Murthy R, Zhang L, Kurtz RC, Saltz L, Offit K, Robson ME, Stadler ZK. Identification of germline genetic mutations in patients with pancreatic cancer. Cancer. 2015;121(24):4382–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 6.Vasen HE, Grais NA, Frants RR, van Der Velden PA, Hille ET, Bergman W. Risk of developing pancreatic cancer in families with familial atypical multiple mole melanoma associated with a specific 19 deletion of p16 (p16-Leiden). Int J Cancer. 2000;87(6):809–11. [PubMed] [Google Scholar]
- 7.Siegel RL, Miller KD, Jemal A. Cancer statistics 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 8.Siegel RL, Miller KD, Jemal A. Cancer statistics 2018. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 9.Treadwell J, Mitchell M, Eatmon K, Jue J, Zafar H, Teitelbaum U, Schoelles K. In imaging tests for the diagnosis and staging of pancreatic adenocarcinoma. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014. September. Report No.: 14-EHC045-EF. [PubMed] [Google Scholar]
- 10.Anderson CW, Bennett JJ. Clinical presentation and diagnosis of pancreatic neuroendocrine tumors. Surg Oncol Clin. 2016. April 1;25(2):363–74. [DOI] [PubMed] [Google Scholar]
- 11.Thompson NW, Eckhauser FE. Malignant islet-cell tumors of the pancreas. World J Surg. 1984; 8 (6): 940–51. [DOI] [PubMed] [Google Scholar]
- 12.Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology. 2008; 135(5):1469–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Herder WW. Biochemistry of neuroendocrine tumours. Best Pract Res Clin Endocrinol Metab. 2007;21(1):33–41. [DOI] [PubMed] [Google Scholar]
- 14.Nobels FR, Kwekkeboom DJ, Coopmans W, Schoenmakers CH, Lindemans J, De Herder WW, Krenning EP, Bouillon R, Lamberts SW. Chromogranin A as serum marker for neuroendocrine neoplasia: comparison with neuron-specific enolase and the alpha-subunit of glycoprotein hormones. J Clin Endocrinol Metab. 1997;82(8):2622–28. [DOI] [PubMed] [Google Scholar]
- 15.De Herder WW, Niederle B, Scoazec JY, Pauwels S, Klöppel G, Falconi M, Kwekkeboom DJ, Öberg K, Eriksson B, Wiedenmann B, Rindi G. Well-differentiated pancreatic tumor/carcinoma: insulinoma. Neuroendocrinology. 2006;84(3):183–88. [DOI] [PubMed] [Google Scholar]
- 16.Zogakis TG, Gibril F, Libutti SK, Norton JA, White DE, Jensen RT, Alexander HR. Management and outcome of patients with sporadic gastrinoma arising in the duodenum. Ann Surg. 2003. July;238(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen RT, Cadiot G, Brandi ML, De Herder WW, Kaltsas G, Komminoth P, Scoazec JY, Salazar R, Sauvanet A, Kianmanesh R. ENETS consensus guidelines for the management of patients with digestive neuroendocrine neoplasms: functional pancreatic endocrine tumor syndromes. Neuroendocrinology. 2012;95(2):98–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito T, Igarashi H, Jensen RT. Pancreatic neuroendocrine tumors: clinical features, diagnosis and medical treatment: advances. Best Practice Res Clin Gastroenterol. 2012. December 1;26(6):737–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu LC, Goggins MG, Fishman EK. Diagnosis and detection of pancreatic cancer. Cancer J. 2017;23(6):333–42. [DOI] [PubMed] [Google Scholar]
- 20.Brentnall TA, Bronner MP, Byrd DR, Haggitt RC, Kimmey MB. Early diagnosis and treatment of pancreatic dysplasia in patients with a family history of pancreatic cancer. Ann Intern Med. 1999; 131(4):247–55. [DOI] [PubMed] [Google Scholar]
- 21.Canto MI, Goggins M, Hruban RH, Petersen GM, Giardiello FM, Yeo C, Fishman EK, Brune K, Axilbund J, Griffin C, Ali S, Richman J, Jagannath S, Kantsevoy SV, Kalloo AN. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol. 2006;4(6):766–81; quiz 665. [DOI] [PubMed] [Google Scholar]
- 22.Canto MI, Goggins M, Yeo CJ, Griffin C, Axilbund JE, Brune K, Ali SZ, Jagannath S, Petersen GM, Fishman EK, Piantadosi S, Giardiello FM, Hruban RH. Screening for pancreatic neoplasia in high-risk individuals: an EUS-based approach. Clin Gastroenterol Hepatol. 2004;2(7):606–21. [DOI] [PubMed] [Google Scholar]
- 23.Capurso G, Signoretti M, Valente R, Arnelo U, Lohr M, Poley JW, Delle Fave G, Del Chiaro M. Methods and outcomes of screening for pancreatic adenocarcinoma in high-risk individuals. World J Gastrointest Endosc. 2015;7(9):833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider R, Slater EP, Sina M, Habbe N, Fendrich V, Matthäi E, Langer P, Bartsch DK. German national case collection for familial pancreatic cancer (FaPaCa): ten years experience. Fam Cancer. 2011. June 1;10(2):323–30. [DOI] [PubMed] [Google Scholar]
- 25.Verna EC, Hwang C, Stevens PD, Rotterdam H, Stavropoulos SN, Sy CD, Prince MA, Chung WK, Fine RL, Chabot JA, Frucht H. Pancreatic cancer screening in a prospective cohort of high-risk patients: a comprehensive strategy of imaging and genetics. Clin Cancer Res. 2010. October 15;16(20):5028–37. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Yang J, Li H, Wu Y, Zhang H, Chen W. Tumor markers CA19-9, CA242 and CEA in the diagnosis of pancreatic cancer: a meta-analysis. Int J Clin Exper Med. 2015;8(7):11683. [PMC free article] [PubMed] [Google Scholar]
- 27.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. New England J Med. 2015. October 22;373(17):1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, Golstein P. A new member of the immunoglobulin superfamily—CTLA-4. Nature. 1987. July;328(6127):267. [DOI] [PubMed] [Google Scholar]
- 29.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996. March 22;271(5256):1734–36. [DOI] [PubMed] [Google Scholar]
- 30.Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH Jr, Lao CD, Linette GP. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015. April 1;16(4):375–84. [DOI] [PubMed] [Google Scholar]
- 31.Wu AA, Jaffee E, Lee V. Current status of immunotherapies for treating pancreatic cancer. Curr Oncol Rep. 2019;21(7):60. [DOI] [PubMed] [Google Scholar]
- 32.Foley K, Kim V, Jaffee E, Zheng L. Current progress in immunotherapy for pancreatic cancer. Cancer Lett. 2016;381(1):244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J. Pembrolizumab versus ipilimumab in advanced melanoma. New Engl J Med. 2015. June 25;372(26):2521–32. [DOI] [PubMed] [Google Scholar]
- 34.Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I, Rosenberg SA. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010. October 1;33(8):828–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas AM, Santarsiero LM, Lutz ER, Armstrong TD, Chen YC, Huang LQ, Laheru DA, Goggins M, Hruban RH, Jaffee EM. Mesothelin-specific CD8+ T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exper Med. 2004. August 2;200(3):297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Bernstorff W, Voss M, Freichel S, Schmid A, Vogel I, Jöhnk C, Henne-Bruns D, Kremer B, Kalthoff H. Systemic and local immunosuppression in pancreatic cancer patients. Clin Cancer Res. 2001. March 1;7(3 Suppl):925s–32s. [PubMed] [Google Scholar]
- 37.Zheng L, Foley K, Huang L, Leubner A, Mo G, Olino K, Edil BH, Mizuma M, Sharma R, Le DT, Anders RA. Tyrosine 23 phosphorylation-dependent cell-surface localization of annexin A2 is required for invasion and metastases of pancreatic cancer. PLoS One. 2011. April 29;6(4):e19390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci. 2002. September 17;99(19):12293–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kakimi K, Karasaki T, Matsushita H, Sugie T. Advances in personalized cancer immunotherapy. Breast Cancer. 2017;24(1):16–24. [DOI] [PubMed] [Google Scholar]
- 40.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. New Engl J Med. 2012. June 28;366(26):2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Couzin-Frankel J Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342(6165):1432–33. [DOI] [PubMed] [Google Scholar]
- 42.Hurwitz AA, Foster BA, Kwon ED, Truong T, Choi EM, Greenberg NM, Burg MB, Allison JP. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60(9):2444–48. [PubMed] [Google Scholar]
- 43.Kwon ED, Hurwitz AA, Foster BA, Madias C, Feldhaus AL, Greenberg NM, Burg MB, Allison JP. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc Natl Acad Sci U S A. 1997;94(15):8099–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174(3):561–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenberg SA. Cancer vaccines based on the identification of genes encoding cancer regression antigens. Immunol Today. 1997;18(4):175–82. [DOI] [PubMed] [Google Scholar]
- 46.Weber LW, Bowne WB, Wolchok JD, Srinivasan R, Qin J, Moroi Y, Clynes R, Song P, Lewis JJ, Houghton AN. Tumor immunity and autoimmunity induced by immunization with homologous DNA. J Clin Invest. 1998;102(6):1258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Allen TE, Levy CL, Yellin M. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007. November 15;13(22):6681–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kirkwood JM, Lee S, Moschos SJ, Albertini MR, Michalak JC, Sander C, Whiteside T, Butterfield LH, Weiner L. Immunogenicity and antitumor effects of vaccination with peptide vaccine+/− granulocyte-monocyte colony-stimulating factor and/or IFN-α2b in advanced metastatic melanoma: Eastern Cooperative Oncology Group Phase II Trial E1696. Clin Cancer Res. 2009. February 15;15(4):1443–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Minor DR, Chin K. Kashani-Sabet M. Infliximab in the treatment of anti-CTLA4 antibody (ipilimumab) induced immune-related colitis. Cancer Biother Radiopharm. 2009;24(3):321–25. [DOI] [PubMed] [Google Scholar]
- 50.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, Hou TZ. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011. April 29;332(6029):600–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L. Immunostimulatory monoclonal antibodies for cancer therapy. Nature Rev Cancer. 2007. February;7(2):95. [DOI] [PubMed] [Google Scholar]
- 52.O’Day ST, Hamid O, Urba WJ Targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4) A novel strategy for the treatment of melanoma and other malignancies. Cancer: Interdisc Int J Am Cancer Soc. 2007. December 15;110(12):2614–27. [DOI] [PubMed] [Google Scholar]
- 53.Robert C, Ghiringhelli F. What is the role of cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma? Oncologist. 2009;14(8):848–61. [DOI] [PubMed] [Google Scholar]
- 54.Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, Davidson N. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. New England J Med. 2011. June 30;364(26):2517–26. [DOI] [PubMed] [Google Scholar]
- 55.Banerjee K, Kumar S, Ross KA, Gautam S, Poelaert B, Nasser MW, Aithal A, Bhatia R, Wannemuehler MJ, Narasimhan B, Solheim JC. Emerging trends in the immunotherapy of pancreatic cancer. Cancer Lett. 2018. March 28;417:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clark CE, Beatty GL, Vonderheide RH. Immunosurveillance of pancreatic adenocarcinoma: insights from genetically engineered mouse models of cancer. Cancer Lett. 2009. June 28;279(1):1–7. [DOI] [PubMed] [Google Scholar]
- 57.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011. October 1;60(10):1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kurahara H, Shinchi H, Mataki Y, Maemura K, Noma H, Kubo F, Sakoda M, Ueno S, Natsugoe S, Takao S. Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. J Surg Res. 2011. May 15;167(2):e211–19. [DOI] [PubMed] [Google Scholar]
- 59.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. T Immunol. 2004. January 15;172(2):989–99. [DOI] [PubMed] [Google Scholar]
- 60.Chen CH, Wang TL, Hung CF, Yang Y, Young RA, Pardoll DM, Wu TC. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer Res. 2000. February 15;60(4):1035–42. [PubMed] [Google Scholar]
- 61.Gomez B, He L, Tsai YC, Wu TC, Viscidi RP, Hung CF. Creation of a Merkel cell polyomavirus small T antigen-expressing murine tumor model and a DNA vaccine targeting small T antigen. Cell Biosci. 2013. July;3(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pallas DC, Shahrik LK, Martin BL, Jaspers S, Miller TB, Brautigan DL, Roberts TM. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell. 1990. January 12;60(1):167–76. [DOI] [PubMed] [Google Scholar]
- 63.Shuda M, Kwun HJ, Feng H, Chang Y, Moore PS. Human Merkel cell polyomavirus small T antigen is an oncoprotein targeting the 4E-BP1 translation regulator. J Clin Investig. 2011. August 15;121(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zeng Q, Gomez BP, Viscidi RP, Peng S, He L, Ma B, Wu TC, Hung CF. Development of a DNA vaccine targeting Merkel cell polyomavirus. Vaccine. 2012. February 8;30(7):1322–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gomez BP, Wang C, Viscidi RP, Peng S, He L, Wu TC, Hung CF. Strategy for eliciting antigen-specific CD8+ T cell-mediated immune response against a cryptic CTL epitope of merkel cell polyomavirus large T antigen. Cell Biosci. 2012. October;2(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maeng H, Terabe M, Berzofsky JA. Cancer vaccines: translation from mice to human clinical trials. Current Op Immunol. 2018. April 1;51:111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sonpavde G, Kantoff PW. Immunotherapy for castration-resistant prostate cancer. Urol Clin North Am. 2012;39(4):465–81. [DOI] [PubMed] [Google Scholar]
- 68.Lunardi S, Muschel RJ, Brunner TB. The stromal compartments in pancreatic cancer: are there any therapeutic targets? Cancer Lett. 2014;343(2):147–55. [DOI] [PubMed] [Google Scholar]
- 69.Altenschmidt U, Kahl R, Moritz D, Schnierle BS, Gerstmayer B, Wels W, Groner B. Cytolysis of tumor cells expressing the Neu/erbB-2, erbB-3, and erbB-4 receptors by genetically targeted naive T lymphocytes. Clin Cancer Res. 1996. June 1;2(6):1001–8. [PubMed] [Google Scholar]
- 70.Ho WY, Blattman JN, Dossett ML, Yee C, Greenberg PD. Adoptive immunotherapy: engineering T cell responses as biologic weapons for tumor mass destruction. Cancer Cell. 2003. May 1;3(5):431–37. [DOI] [PubMed] [Google Scholar]
- 71.Irving BA, Weiss A. The cytoplasmic domain of the T cell receptor ζ chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991. March 8;64(5):891–901. [DOI] [PubMed] [Google Scholar]
- 72.Letourneur F, Klausner RD. T-cell and basophil activation through the cytoplasmic tail of T-cell-receptor zeta family proteins. Proc Natl Acad Sci U S A. 1991;88 (20):8905–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sadelain M CAR therapy: the CD19 paradigm. J Clin Invest. 2015;125(9):3392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3(1):35–45. [DOI] [PubMed] [Google Scholar]
- 75.Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, Yang JC, Phan GQ, Hughes MS, Sherry RM, Raffeld M. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. T Clin Oncol. 2015. February 20;33(6):540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD. Chimeric antigen receptor T cells for sustained remissions in leukemia. New Engl J Med. 2014. October 16;371(16):1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, Milone MC. Chimeric antigen receptor–modified T cells for acute lymphoid leukemia. New England J Med. 2013. April 18;368(16):1509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor–modified T cells in chronic lymphoid leukemia. New Engl J Med. 2011. August 25;365(8):725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak Ö, Brogdon JL, Pruteanu-Malinici I, Bhoj V, Landsburg D, Wasik M. Chimeric antigen receptor T cells in refractory B-cell lymphomas. New Engl J Med. 2017. December 28;377(26):2545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Turtle CJ, Hanafi LA, Berger C, Hudecek M, Pender B, Robinson E, Elawkins R, Chaney C, Cherian S, Chen X, Soma L. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor–modified T cells. Sci Trans Med. 2016. September 7;8(355):355ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guo S, Contratto M, Miller G, Leichman L, Wu J. Immunotherapy in pancreatic cancer: Unleash its potential through novel combinations. World J Clin Oncol. 2017;8(3):230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Looi CK, Chung FF, Leong CO, Wong SF, Rosli R, Mai CW. Therapeutic challenges and current immunomodulatory strategies in targeting the immunosuppressive pancreatic tumor microenvironment. J Exp Clin Cancer Res. 2019;38(1):162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lutz ER, Kinkead H, Jaffee EM, Zheng L. Priming the pancreatic cancer tumor microenvironment for checkpoint-inhibitor immunotherapy. Oncoimmunology. 2014;3(11):e962401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Palla AR, Doll D. Immunotherapy in Merkel cell carcinoma: role of Avelumab. Immunotargets Ther. 2018;7:15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vonderheide RH. The immune revolution: a case for priming not checkpoint. Cancer Cell. 2018;33(4):563–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hidalgo M, Cascinu S, Kleeff J, Labianca R, Lohr JM, Neoptolemos J, Real FX, Van Laethem JL, Heinemann V. Addressing the challenges of pancreatic cancer: future directions for improving outcomes. Pancreatology. 2015;15(1):8–18. [DOI] [PubMed] [Google Scholar]
- 87.Lu C, Talukder A, Savage NM, Singh N, Liu K. JAK-STAT-mediated chronic inflammation impairs cytotoxic T lymphocyte activation to decrease anti-PD-1 immunotherapy efficacy in pancreatic cancer. Oncoimmunology. 2017;6(3):e1291106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rahma OE, Duffy A, Liewehr DJ, Steinberg SM, Greten TF. Second-line treatment in advanced pancreatic cancer: a comprehensive analysis of published clinical trials. Ann Oncol. 2013. May 12;24(8):1972–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schwartz DM, Bonelli M, Gadina M, O’shea JJ Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nature Rev Rheumatol. 2016. January;12(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wan CK, Andraski AB, Spolski R, Li P, Kazemian M, Oh J, Samsel L, Swanson PA, McGavern DB, Sampaio EP, Freeman AF. Opposing roles of STAT1 and STAT3 in IL-21 function in CD4+ T cells. Proc Natl Acad Sci. 2015. July 28;112(30):9394–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Winograd R, Byrne KT, Evans RA, Odorizzi PM, Meyer AR, Bajor DL, Clendenin C, Stanger BZ, Furth EE, Wherry EJ, Vonderheide RH. Induction of T-cell immunity overcomes complete resistance to PD-1 and CTLA-4 blockade and improves survival in pancreatic carcinoma. Cancer Immunol Res. 2015. April 1;3(4):399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen L, Han X. Anti–PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Investigation. 2015. September 1;125(9):3384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bellone G, Novarino A, Vizio B, Brondino G, Addeo A, Prati A, Giacobino A, Campra D, Fronda GR, Ciuffreda L. Impact of surgery and chemotherapy on cellular immunity in pancreatic carcinoma patients in view of an integration of standard cancer treatment with immunotherapy. Int J Oncol. 2009. June 1;34(6):1701–15. [DOI] [PubMed] [Google Scholar]
- 94.Bellone G, Turletti A, Artusio E, Mareschi K, Carbone A, Tibaudi D, Robecchi A, Emanuelli G, Rodeck U. Tumor-associated transforming growth factor-β and interleukin-10 contribute to a systemic Th2 immune phenotype in pancreatic carcinoma patients. Am J Pathol. 1999. August 1;155(2):537–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nature Med. 2002. August;8(8):793. [DOI] [PubMed] [Google Scholar]
- 96.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti–CTLA-4 antibodies. J Exper Med. 2009. August 3;206(8):1717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Seo YD, Pillarisetty VG. T-cell programming in pancreatic adenocarcinoma: a review. Cancer Gene Ther. 2017. March;24(3):106. [DOI] [PubMed] [Google Scholar]