Abstract
Aim
We investigated whether perioperative urine pH was associated with contrast-associated acute kidney injury (CA-AKI) in patients undergoing emergency percutaneous coronary intervention (PCI).
Methods
The study enrolled 1109 consecutive patients undergoing emergency PCI. Patients were divided into three groups based on perioperative urine pH (5.0–6.0, 6.5– 7.0, 7.5–8.5). The primary endpoint was the development of CA-AKI, defined as an absolute increase ≥ 0.3 mg/dL or a relative increase ≥ 50% from baseline serum creatinine within 48 h after contrast medium exposure.
Results
Overall, 181 patients (16.3%) developed contrast-associated acute kidney injury. The incidences of CA-AKI in patients with urine pH 5.0–6.0, 6.5–7.0, and 7.5–8.5 were 19.7%, 9.8%, and 23.3%, respectively. After adjustment for potential confounding factors, perioperative urine pH 5.0–6.0 and 7.5–8.5 remained independently associated with CA-AKI [odds ratio (OR)1.86, 95% confidence interval (CI) 1.25–2.82, P = 0.003; OR 2.70, 95% CI 1.5–4.68, P < 0.001, respectively]. The association was consistent in subgroups of patients stratified by several CA-AKI risk predictors. However, the risk of CA-AKI associated with urine pH 7.5–8.5 was stronger in patients with worse renal function (estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73m2) (HR 5.587, 95% CI 1.178–30.599 vs. HR 2.487, 95% CI 1.331–4.579; overall interaction P < 0.05).
Conclusion
The urine pH and CA-AKI may underlie the V-shape relationship.
Keywords: Urine pH, Contrast-associated acute kidney injury, Emergency percutaneous coronary intervention
Introduction
Contrast-associated acute kidney injury (CA-AKI), as a common complication after coronary intervention, is the third leading cause of hospital acquired acute kidney injury [1]. It increases length of hospitalization, medical expenses and risk of dialysis and major adverse cardiovascular events (MACE) [2].
Many strategies have been tested to prevent CA-AKI with more or less success, and hydration is recommended by current guidelines. Infusion of sodium bicarbonate instead of sodium chloride was ever advocated for urinary alkalization and reducing the generation of free radicals [3]. Some studies found administration of sodium bicarbonate was effective in preventing CA-AKI [4, 5], but some others drew different conclusions [6, 7], even harmful [8].
Recent studies showed urine pH was an independent risk factor of AKI [9, 10]. However, the predictive value of urine pH on the development of CA-AKI in patients undergoing emergency PCI remains unclear. Therefore, in current study, we sought to explore the relationship between urine pH and CA-AKI in patients undergoing emergency PCI.
Materials and methods
Patients
This is a prospective observational study conducted in Fujian Provincial Hospital in China, from January 2012 to December 2018. A total of 1210 consecutive patients undergoing emergency PCI were enrolled. Exclusion criteria were as follows: (1) lack of data on pre-procedural or post-procedural serum creatinine (SCr) levels (n = 60); lack of data on perioperative urine pH (n = 20); cancer with expectation of life less than 1 year (n = 6); end-stage renal disease (eGFR < 15 mL/min/1.73m2) or long-term dialysis treatment (n = 6); intra-vascular administration of contrast medium within the last 7 days postoperatively (n = 5); died within 24 h after admission (n = 4). Finally, 1109 patients were included in the analysis. The study was approved by an institutional review committee and the subjects gave informed consent.
Protocol
The concentration of urine pH was measured for each patient during the perioperative period of PCI. SCr was measured at admission and daily for the 2 days after contrast exposure. We also measured white blood cell count (WBC), platelet (PLT), hemoglobin, cholesterol, glucose concentrations (GLU), troponin I, N-terminal pro-B-type natriuretic peptide (NT-pro-BNP) and other parameters before PCI procedure. Experienced interventional cardiologists performed PCI and used medications according to standard clinical practice. Nonionic, low-osmolar contrast media (either Iopamiron or Ultravist, both 370 mg I/mL) was used in procedural and 0.9% normal saline (NS) at a rate of 1 mL/kg/h was administered intravenously approximately 12 h during perioperative period (0.5 mL/kg/h if patients with heart failure). The protocol fulfilled the requirements of the Declaration of Helsinki and was approved by the ethics committee of the Fujian Provincial Hospital, China (ethics approval number: K2019-07-011).
Definitions and follow-up
The primary endpoint was CA-AKI, defined as an absolute increase ≥ 0.3 mg/dL or a relative increase ≥ 50% from baseline serum creatinine within 48 h of contrast medium exposure [11, 12]. Additional end points were long-term mortality. The eGFR was calculated using the modified modification of diet in renal disease equation: 186.3 × SCr-1.154 × (age in years) -0.203 × 1.212 (if patient was black) × 0.742 (if patient was female) [13]. Peri-hypotension was defined as within 24-h periprocedural period, systolic blood pressure (SBP) < 80 mmHg for at least 1 h and medications or intra-aortic balloon pump (IABP) was needed [14]. All patients were followed for more than 1 year. Follow-up events were carefully monitored and recorded by trained nurses through outpatient visits or post-discharge telephone contacts with patients or their relatives.
Statistical analysis
All data were analyzed with R version 4.0.2. We compared the baseline characteristics among 2 groups divided by CA-AKI. Normally distributed continuous variables are expressed as mean ± standard deviation (SD). The Student’s t test, Wilcoxon rank sum test or one way-analysis of variance was performed to determine the differences among groups. Categorical variables were compared by Chi-square test or Fisher exact test. The P for trend was determined with a Wilcoxon type test for trend across ordered groups. Kaplan–Meier curve were used to compute the cumulative incidence of mortality stratified by urine pH levels.
After testing for proportional hazard assumptions, logistic analysis was used to examine the association of urine pH 5.0–6.0 and urine pH 7.5–8.5 (vs. urine pH 6.5–7.0) with CA-AKI in models adjusted as follows: model 1 adjusted for traditional risk factors for CA-AKI (age > 75 years, diabetes mellitus, eGFR < 60 mL/min/1.73m2, and LVEF < 40%); and model 2 adjusted for variables in model 1 plus the variables with P value < 0.05 in the univariate statistical results including atrial fibrillation (AF), perioperative hypotension, LgNT-pro-BNP, GLU, WBC. Subgroup analysis in the study participants stratified by the several CA-AKI risk factors was also examined and the P values for interaction were calculated in each subgroup. A 2-sided P value < 0.05 was considered significant.
Results
Baseline characteristics
This study included 1109 consecutive patients, of whom 181 (16.3%) developed CA-AKI. Table 1 shows the univariate analysis of the baseline and procedural characteristics between the patients with and without CA-AKI. Patients who developed CA-AKI were older, more likely to have AF and worse renal function, had higher baseline of NT-pro-BNP, WBC, GLU, and had a higher percentage of perioperative hypotension, but lower left ventricular ejection fraction (LVEF).
Table 1.
Baseline variables between non-AKI group and AKI group
| Non-AKI | AKI | P value | |
|---|---|---|---|
| (n = 928) | (n = 181) | ||
| Demographics | |||
| Age, years | 62.46 ± 12.20 | 66.60 ± 12.46 | < 0.001 |
| Age > 75 years [n (%)] | 156 (16.8) | 53 (29.3) | < 0.001 |
| Sex, female [n (%)] | 126 (13.6) | 33 (18.2) | 0.129 |
| Systolic blood pressure (mmHg) | 123.01 ± 22.49 | 118.96 ± 27.53 | 0.065 |
| Diastolic blood pressure (mmHg) | 73.41 ± 15.55 | 71.35 ± 19.27 | 0.179 |
| Medical history | |||
| Hypertension [n (%)] | 531 (57.2) | 116 (64.1) | 0.103 |
| Type of ACS | 0.254 | ||
| STEMI [n (%)] | 775 (83.5) | 160 (88.4) | |
| NSTEMI [n (%)] | 111 (12.0) | 15 (8.3) | |
| UA, n (%) [n (%)] | 42 (4.5) | 6 (3.3) | |
| Diabetes [n (%)] | 324 (34.9) | 79 (43.7) | 0.032 |
| Atrial fibrillation [n (%)] | 53 (5.7) | 32 (17.7) | < 0.001 |
| Anemia [n (%)] | 211 (22.7) | 40 (22.1) | 0.928 |
| Medical therapy during hospitalization | |||
| Statin use [n (%)] | 920 (99.1) | 178 (98.3) | 0.401 |
| CCB use [n (%)] | 113 (12.2) | 23 (12.7) | 0.940 |
| Antiplatelet agents use [n (%)] | 891 (96.0) | 168 (92.8) | 0.089 |
| Laboratory measurements | |||
| NT-proBNP (pg/mL) | 1095.96 ± 2777.96 | 2360.510 ± 4711.11 | 0.001 |
| WBC (109/L) | 11.36 ± 3.79 | 12.78 ± 4.57 | < 0.001 |
| HGB (g/L) | 141.75 ± 18.09 | 140.25 ± 19.47 | 0.339 |
| PLT (1012/L) | 228.11 ± 69.88 | 226.39 ± 59.65 | 0.733 |
| Cholesterol (mmol/L) | 4.8 0 ± 1.21 | 4.72 ± 1.22 | 0.457 |
| LDL-C(mmol/L) | 3.24 ± 1.06 | 3.15 ± 1.05 | 0.343 |
| eGFR (mL/min/1.73 m2) | 100.37 ± 28.90 | 92.34 ± 36.24 | 0.005 |
| eGFR < 60 mL/min/1.73 m2 [n (%)] | 63 (6.8) | 31 (17.1%) | < 0.001 |
| LVEF | 55.10 ± 7.10 | 49.65 ± 9.91 | < 0.001 |
| LVEF < 40% [n (%)] | 28 (3.0%) | 23 (12.7) | < 0.001 |
| Procedure performed | |||
| Perioperative hypotension [n (%)] | 235 (25.3) | 77 (42.5) | < 0.001 |
| Contrast volume (mL) | 168.83 ± 47.18 | 175.54 ± 49.24 | 0.093 |
| Iso-osmolar contrast media use [n (%)] | 294 (31.7) | 49 (27.1) | 0.255 |
Risk factors of CA-AKI
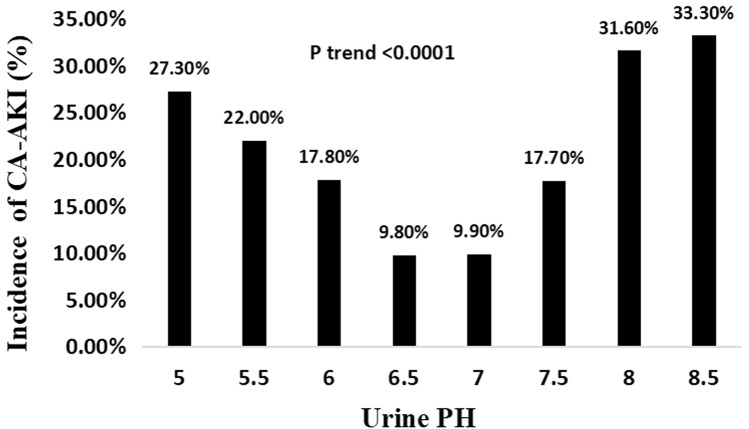
The incidence of CA-AKI was 27.30%, 22.00%, 17.80%, 9.80%, 9.90%, 17.70%, 31.60% and 33.30% in patients with perioperative urine pH 5, 5.5, 6, 6.5, 7, 7.5, 8, 8.5, respectively (Fig. 1). In multivariable-adjusted logistic proportional hazard models, compared with urine pH 6.5–7.0, urine pH 5.0–6.0 and urine pH 7.5–8.5 were both significantly associated with increased risk of CA-AKI, independent of demographics and clinical risk factors (Table 2). In model 1, after adjusted for traditional risk factors including age > 75 years, diabetes mellitus, eGFR < 60 mL/min/1.73 m2, and LVEF < 40%, perioperative urine pH 5.0–6.0 and 7.5–8.5 were both significantly correlated with CA-AKI, and the odds ratios (OR) values were 2.03 (95% CI 1.38–3.02, P < 0.001) and 2.62 (95% CI 1.53–4.45, P < 0.001), respectively. In model 2, after adjusting for variables in model 1 plus other variables including AF, perioperative hypotension, LgNT-pro-BNP, GLU and WBC, perioperative urine pH 5.0–6.0 and 7.5–8.5 remained associated with increased risk of CA-AKI, the OR values were 1.86 (95% CI 1.25–2.82, P = 0.003) and 2.70 (95% CI 1.55–4.68, P < 0.001), respectively.
Fig. 1.
Incidence of CA-AKI
Table 2.
Associations between urine pH levels and CA-AKI
| Participants (n) | Events (n) | Rate (%) | Model 1a
OR (95% CI) |
P value | Model 2b
OR (95% CI) |
P value | ||
|---|---|---|---|---|---|---|---|---|
| Urine PH | 6.5–7 | 427 | 42 | 9.8 | 1.00 (Ref.) | 1.00 (Ref.) | ||
| 5–6 | 553 | 109 | 19.7 | 2.03 (1.38–3.02) | < 0.0001 | 1.86 (1.25–2.82) | 0.003 | |
| 7.5–8.5 | 129 | 30 | 23.3 | 2.62 (1.53–4.45) | < 0.0001 | 2.70 (1.55–4.68) | < 0.0001 |
CI confidence interval, HR hazard ratio
aModel 1 adjusted for age > 75 years, diabetes mellitus, eGFR < 60 mL/min/1.73m2, and LVEF < 40%
bModel 2 adjusted for variables in model 1 plus atrial fibrillation, perioperative hypotension, LgNT-pro-BNP, GLU, WBC
Subgroup analysis based on CA-AKI risk predictors
Figure 2 shows subgroup analyses stratified by several CA-AKI risk predictors. The associations between urine pH and CA-AKI are consistent among these subgroups. There was no effect modification of age > 75 years, diabetes mellitus, hypertension, AF or LVEF < 40% on the association between urine pH and risk of CA-AKI. However, there was an effect modification by eGFR: the risk of CA-AKI associated with urine pH 7.5–8.5 was stronger in patients with worse renal function (eGFR < 60 mL/min/1.73 m2) than in those with eGFR≧60 mL/min/1.73 m2. (HR 5.587, 95% CI 1.178–30.599 vs. HR 2.487, 95% CI 1.331–4.579; overall interaction P < 0.05).
Fig. 2.
Forest and interaction
CA-AKI, urine pH level and long-term outcomes
The median follow-up period was 559 days (interquartile range 371–912 days). Compared with patients without CA-AKI, the Kaplan–Meier curve showed that patients with CA-AKI had higher rate of all-cause long-term mortality (P < 0.001) (Fig. 3). However, there was no significantly difference in all-cause long-term mortality among three different groups stratified by urine pH levels (Fig. 4).
Fig. 3.
Mortality between CA-AKI
Fig. 4.
Mortality between urine pH
Discussion
To our knowledge, this study is first to observe a V-shape relationship between urine pH and CA-AKI. Perioperative urine pH was found to be associated with the development of CA-AKI. Compared with the relatively normal urine pH value (between 6.5 and 7), both acid urine (5–6) and alkaline urine (7.5–8.5) were significantly associated with increased risk of CA-AKI.
CA-AKI, as the third leading cause for hospital acquired AKI, was known to increase the co-morbidities, in-hospital mortality and length of hospitalization [5]. Due to various factors including hemodynamic instability, insufficient hydration and cardiac dysfunction, the risk of CA-AKI was higher in patients undergoing emergency PCI than those undergoing elective PCI [15]. In addition to hydration and contrast agent dose reduction, pharmacological strategies such as furosemide [8], N-acetylcysteine [9], statin [10], ascorbic acid [11] and sodium bicarbonate [4, 12] were also evaluated. However, no effective treatment for CA-AKI in high-risk patients has been defined.
Sodium bicarbonate was supposed to be useful in the prevention of CA-AKI for urine alkalization and reducing the creation of free oxygen radicals. However, the results were not consistent.
In 2004, Gregory et al. [4] first proved the effectiveness of preventing contrast-associated renal failure by hydration with sodium bicarbonate instead of normal saline(NS) before contrast exposure(13.6% vs. 1.7%, P = 0.02). Subsequently, some studies showed the intravenous sodium bicarbonate administration reduced the risk of CA-AKI in patients at medium to high risk [5] or undergoing emergency PCI [16, 17]. Furthermore, a meta-analysis [18] demonstrated that hydration with bicarbonate reduced the incidence of CA-AKI compared with NS. However, some later trials showed different results [7, 19, 20], and even suggested that bicarbonate may increase the risk of CA-AKI. A retrospective cohort study at Mayo Clinic [8] showed that urine alkalinization by bicarbonate alone was associated with increased risk of CA-AKI compared with placebo, and a prospective randomized trial conducted by Theresia Klima et al. [21] drew similar conclusion in patients with renal insufficiency. The inconsistency of the results may be associated with following factors: (1) urine alkalization was not achieved in all studies. (2) The definitions of CA-AKI were different in some studies. (3) Some studies included low-risk patients with intact baseline kidney function but some included patients with moderate-to-severe renal dysfunction [20, 22, 23]. In 2018, PRESERVE trial [24] included 4993 patients and showed there was no benefit of intravenous sodium bicarbonate over intravenous sodium chloride for the prevention of CA-AKI among patients at high risk for renal complications who were undergoing angiography. Urine alkalization was confirmed by the higher urine pH observed in the sodium bicarbonate group compared with sodium chloride group (6.7 ± 0.8 vs. 6.0 ± 0.8, P < 0.001). It seemed that there was no additional benefit of bicarbonate in preventing CA-AKI compared to sodium chloride.
However, previous studies including PRESERVE trial did not study the relationship between urine PH and CA-AKI. It is unclear whether the inconsistent conclusion of sodium bicarbonate in preventing CA-AKI was affected by different urine pH levels.
Our study first found a V-shape relationship between urine pH and CA-AKI in patients undergoing emergency PCI. The result showed both acid urine (5–6) and alkaline urine (7.5–8.5) were significantly associated with increased risk of CA-AKI. Recent studies have shown acid urine was an independent risk factor of CA-AKI. Darko Markota et al.[9] found patients with a urine pH < 6 had a more than tenfold higher risk of CA-AKI. Jun-qing Yang et al. also found [10] urine pH < 6 was associated with higher risk of CA-AKI in T2DM patients undergoing elective contrast media exposure. It may be explained by the increased level of free radical formation in an acid medium compared with the higher PH of normal extracellular fluid [25]. Our studies supported the urine pH < 6.5 was associated with higher risk of CA-AKI. More importantly, our study showed a higher urine pH (7.5–8.5) was also an independent risk factor of CA-AK, which was not reported in previous studies. Based on the V-shape relationship between urine pH and CA-AKI observed in our study, it is possible that excessive urine alkalization may promote renal injury, which may be the reason why previous trials drew different conclusions.
In addition, subgroup analysis showed the V-shape association between urine pH and CA-AKI was consistent in patients stratified by several CA-AKI risk predictors, especially in those with worse renal function. It suggested patients with renal insufficiency may have higher risk of CA-AKI when they had a higher or lower perioperative urine pH. However, it was CA-AKI not urine PH that associated with higher long-term mortality in our study, which need more large studies to confirm in the future.
The mechanism by which patients with neutral perioperative urine pH have minimum risk of CA-AKI is multifactorial. Within an acid environment-free radical formation is promoted for activated Harber–Weiss reaction (HWR), resulting in an increased renal oxidant injury and occurrence of CA-AKI [26, 27]. In addition, superoxide generated by ischemia might react with medullary nitric oxide to form the potent oxidant peroxynitrite [28]. At physiologic concentrations, bicarbonate scavenges reactive species especially peroxynitrite generated from nitric oxide [29]. On the other hand, a higher urine pH may reflect the dysfunction of renal tubular cells which secrete H + and reabsorb bicarbonate physiologically [30, 31]. Meanwhile, reactive oxygen species (ROS) like peroxymonocarbonate (HCO4-) can be activated by sodium bicarbonate as described by Richardson et al. [32], which reveals that excessive alkalization may promote renal injury.
Limitations
There are several limitations in our study. First, this study was a single-center, observational study, which potentially may limit the generalizability of the findings. Second, for the restricted length of hospitalization in patients with emergency PCI, assessments for detecting CA-AKI in our study are limited to the first 48 h and some actual CA-AKI patients may be missed. Third, patients in our study were not routinely treated with sodium bicarbonate strategy. Fourth, data about blood gas analysis and volume of fluid administered before procedure are lacking, which may influence the incidence of CA-AKI. Fifth, data about AKI stage were limited in our study. Despite these limitations, our results provided useful insights into the correlation of baseline urine pH with the incidence of CA-AKI.
Conclusion
In conclusion, urine pH showed a V-shape association with the risk of CA-AKI in patients undergoing emergency PCI. Excessive acid or alkaline urine was associated the increased risk of CA-AKI. As the test of urine pH is common and simple, it could be used to identify high-risk patients and guide the sodium bicarbonate strategy for preventing CA-AKI.
Acknowledgements
This study was funded by a grant from the Joint Funds for the innovation of science and Technology, Fujian province (Grant number: 2018Y9097), high-level hospital foster grants from Fujian Provincial Hospital, Fujian province, China (Grant number: 2020HSJJ05), Fujian provincial health technology project (Grant number: 2019-ZQN-10), National Natural Science Foundation of China General Program (Grant number: 81873495), Natural Science Foundation of Fujian Province (Grant number: 2018J01242), and Fujian Provincial Health Commission Youth Key Talents Project (Key Category, Grant number: 2014-ZQN-ZD-2).
Compliance with ethical standards
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
The protocol fulfilled the requirements of the Declaration of Helsinki and was approved by the ethics committee of the Fujian Provincial Hospital, China (ethics approval number: K2019-07–011) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consents were obtained from all participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hanchuan Chen and Chen He have contributed equally to this work.
Contributor Information
Kaiyang Lin, Email: lky7411@sina.com.
Yansong Guo, Email: ysguo1234@126.com.
References
- 1.Weisbord SD, Mor MK, Resnick AL, Hartwig KC, Sonel AF, Fine MJ, et al. Prevention, incidence, and outcomes of contrast-induced acute kidney injury. Arch Intern Med. 2008;168(12):1325–1332. doi: 10.1001/archinte.168.12.1325. [DOI] [PubMed] [Google Scholar]
- 2.Subramanian S, Tumlin J, Bapat B, Zyczynski T. Economic burden of contrast-induced nephropathy: implications for prevention strategies. J Med Econ. 2007;10(2):119–134. doi: 10.3111/200710119134. [DOI] [PubMed] [Google Scholar]
- 3.Stacul F, van der Molen AJ, Reimer P, Webb JA, Thomsen HS, Morcos SK, et al. Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol. 2011;21(12):2527–2541. doi: 10.1007/s00330-011-2225-0. [DOI] [PubMed] [Google Scholar]
- 4.Merten GJ, Burgess WP, Gray LV, Holleman JH, Roush TS, Kowalchuk GJ, et al. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA. 2004;291(19):2328–2334. doi: 10.1001/jama.291.19.2328. [DOI] [PubMed] [Google Scholar]
- 5.Briguori C, Airoldi F, D'Andrea D, Bonizzoni E, Morici N, Focaccio A, et al. Renal Insufficiency Following Contrast Media Administration Trial (REMEDIAL): a randomized comparison of 3 preventive strategies. Circulation. 2007;115(10):1211–1217. doi: 10.1161/CIRCULATIONAHA.106.687152. [DOI] [PubMed] [Google Scholar]
- 6.Brar SS, Shen AY, Jorgensen MB, Kotlewski A, Aharonian VJ, Desai N, et al. Sodium bicarbonate vs sodium chloride for the prevention of contrast medium-induced nephropathy in patients undergoing coronary angiography: a randomized trial. JAMA. 2008;300(9):1038–1046. doi: 10.1001/jama.300.9.1038. [DOI] [PubMed] [Google Scholar]
- 7.Valette X, Desmeulles I, Savary B, Masson R, Seguin A, Sauneuf B, et al. Sodium bicarbonate versus sodium chloride for preventing contrast-associated acute kidney injury in critically Ill patients: a randomized controlled trial. Crit Care Med. 2017;45(4):637–644. doi: 10.1097/CCM.0000000000002267. [DOI] [PubMed] [Google Scholar]
- 8.From AM, Bartholmai BJ, Williams AW, Cha SS, Pflueger A, McDonald FS. Sodium bicarbonate is associated with an increased incidence of contrast nephropathy: a retrospective cohort study of 7977 patients at mayo clinic. Clin J Am Soc Nephrol CJASN. 2008;3(1):10–18. doi: 10.2215/CJN.03100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markota D, Markota I, Starcevic B, Tomic M, Prskalo Z, Brizic I. Prevention of contrast-induced nephropathy with Na/K citrate. Eur Heart J. 2013;34(30):2362–2367. doi: 10.1093/eurheartj/eht009. [DOI] [PubMed] [Google Scholar]
- 10.Yang JQ, Ran P, Chen JY, He YT, Li LW, Tan N, et al. Development of contrast-induced acute kidney injury after elective contrast media exposure in patients with type 2 diabetes mellitus: effect of albuminuria. PLoS ONE. 2014;9(9):e106454. doi: 10.1371/journal.pone.0106454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davenport MS, Perazella MA, Yee J, Dillman JR, Fine D, Mcdonald RJ, et al. Use of intravenous iodinated contrast media in patients with kidney disease: consensus Statements from the American College of Radiology and the National Kidney Foundation. Kidney Med. 2020;2(1):85–93. doi: 10.1016/j.xkme.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Coresh J, Balk EM, Kausz AT, Levin A, Steffes MW, et al. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139(2):137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 14.Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44(7):1393–1399. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Lin L, Li Y, Li H, Wu DX, Zhao JB, et al. Relationship between the urine flow rate and risk of contrast-induced nephropathy after emergent percutaneous coronary intervention. Medicine. 2015;94(50):e2258. doi: 10.1097/MD.0000000000002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Recio-Mayoral A, Chaparro M, Prado B, Cozar R, Mendez I, Banerjee D, et al. The reno-protective effect of hydration with sodium bicarbonate plus N-acetylcysteine in patients undergoing emergency percutaneous coronary intervention: the RENO Study. J Am Coll Cardiol. 2007;49(12):1283–1288. doi: 10.1016/j.jacc.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 17.Masuda M, Yamada T, Mine T, Morita T, Tamaki S, Tsukamoto Y, et al. Comparison of usefulness of sodium bicarbonate versus sodium chloride to prevent contrast-induced nephropathy in patients undergoing an emergent coronary procedure. Am J Cardiol. 2007;100(5):781–786. doi: 10.1016/j.amjcard.2007.03.098. [DOI] [PubMed] [Google Scholar]
- 18.Navaneethan SD, Singh S, Appasamy S, Wing RE, Sehgal AR. Sodium bicarbonate therapy for prevention of contrast-induced nephropathy: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53(4):617–627. doi: 10.1053/j.ajkd.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 19.Thayssen P, Lassen JF, Jensen SE, Hansen KN, Hansen HS, Christiansen EH, et al. Prevention of contrast-induced nephropathy with N-acetylcysteine or sodium bicarbonate in patients with ST-segment-myocardial infarction: a prospective, randomized, open-labeled trial. Circ Cardiovasc Interv. 2014;7(2):216–224. doi: 10.1161/CIRCINTERVENTIONS.113.000653. [DOI] [PubMed] [Google Scholar]
- 20.Solomon R, Gordon P, Manoukian SV, Abbott JD, Kereiakes DJ, Jeremias A, et al. Randomized trial of bicarbonate or saline study for the prevention of contrast-induced nephropathy in patients with CKD. Clin J Am Soc Nephrol CJASN. 2015;10(9):1519–1524. doi: 10.2215/CJN.05370514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klima T, Christ A, Marana I, Kalbermatter S, Uthoff H, Burri E, et al. Sodium chloride vs. sodium bicarbonate for the prevention of contrast medium-induced nephropathy: a randomized controlled trial. Eur Heart J. 2012;33(16):2071–2079. doi: 10.1093/eurheartj/ehr501. [DOI] [PubMed] [Google Scholar]
- 22.Schiffl H. Sodium bicarbonate infusion for prevention of acute kidney injury: no evidence for superior benefit, but risk for harm. Int Urol Nephrol. 2015;47(2):321–326. doi: 10.1007/s11255-014-0820-0. [DOI] [PubMed] [Google Scholar]
- 23.Maioli M, Toso A, Leoncini M, Gallopin M, Tedeschi D, Micheletti C, et al. Sodium bicarbonate versus saline for the prevention of contrast-induced nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. J Am Coll Cardiol. 2008;52(8):599–604. doi: 10.1016/j.jacc.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 24.Weisbord SD, Gallagher M, Jneid H, Garcia S, Cass AT, et al. Outcomes after angiography with sodium bicarbonate and acetylcysteine. N Engl J Med. 2018;378(7):603–614. doi: 10.1056/NEJMoa1710933. [DOI] [PubMed] [Google Scholar]
- 25.Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-B. [DOI] [PubMed] [Google Scholar]
- 26.Heyman SN, Rosen S, Khamaisi M, Idee JM, Rosenberger C. Reactive oxygen species and the pathogenesis of radiocontrast-induced nephropathy. Invest Radiol. 2010;45(4):188–195. doi: 10.1097/RLI.0b013e3181d2eed8. [DOI] [PubMed] [Google Scholar]
- 27.Katholi RE, Woods WT, Jr, Taylor GJ, Deitrick CL, Womack KA, Katholi CR, et al. Oxygen free radicals and contrast nephropathy. Am J Kidney Dis. 1998;32(1):64–71. doi: 10.1053/ajkd.1998.v32.pm9669426. [DOI] [PubMed] [Google Scholar]
- 28.Fry BC, Edwards A, Layton AT. Impacts of nitric oxide and superoxide on renal medullary oxygen transport and urine concentration. Am J Physiol-Renal Physiol. 2015;308(9):F967–F980. doi: 10.1152/ajprenal.00600.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barreto R. Prevention of contrast-induced nephropathy: the rational use of sodium bicarbonate. Nephrol Nurs J. 2007;34(4):417–421. [PubMed] [Google Scholar]
- 30.Valles PG, Batlle D. Hypokalemic distal renal tubular acidosis. Adv Chronic Kidney Dis. 2018;25(4):303–320. doi: 10.1053/j.ackd.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Alexander RT, Bitzan M. Renal tubular acidosis. Pediatr Clin N Am. 2019;66(1):135–157. doi: 10.1016/j.pcl.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Richardson DE, Regino CA, Yao H, Johnson JV. Methionine oxidation by peroxymonocarbonate, a reactive oxygen species formed from CO2/bicarbonate and hydrogen peroxide. Free Radic Biol Med. 2003;35(12):1538–1550. doi: 10.1016/j.freeradbiomed.2003.08.019. [DOI] [PubMed] [Google Scholar]