Abstract
Background
Atrial fibrillation (AF) with rapid ventricular response frequently complicates the management of critically ill patients with sepsis and may necessitate the initiation of medication to avoid hemodynamic compromise. However, the optimal medication to achieve rate control for AF with rapid ventricular response in sepsis is unclear.
Research Question
What is the comparative effectiveness of frequently used AF medications (β-blockers, calcium channel blockers, amiodarone, and digoxin) on heart rate (HR) reduction among critically ill patients with sepsis and AF with rapid ventricular response?
Study Design and Methods
We conducted a multicenter retrospective cohort study among patients with sepsis and AF with rapid ventricular response (HR > 110 beats/min). We compared the rate control effectiveness of β-blockers to calcium channel blockers, amiodarone, and digoxin using multivariate-adjusted, time-varying exposures in competing risk models (for death and addition of another AF medication), adjusting for fixed and time-varying confounders.
Results
Among 666 included patients, 50.6% initially received amiodarone, 10.1% received a β-blocker, 33.8% received a calcium channel blocker, and 5.6% received digoxin. The adjusted hazard ratio for HR of < 110 beats/min by 1 h was 0.50 (95% CI, 0.34-0.74) for amiodarone vs β-blocker, 0.37 (95% CI, 0.18-0.77) for digoxin vs β-blocker, and 0.75 (95% CI, 0.51-1.11) for calcium channel blocker vs β-blocker. By 6 h, the adjusted hazard ratio for HR < 110 beats/min was 0.67 (95% CI, 0.47-0.97) for amiodarone vs β-blocker, 0.60 (95% CI, 0.36-1.004) for digoxin vs β-blocker, and 1.03 (95% CI, 0.71-1.49) for calcium channel blocker vs β-blocker.
Interpretation
In a large cohort of patients with sepsis and AF with rapid ventricular response, a β-blocker treatment strategy was associated with improved HR control at 1 h, but generally similar HR control at 6 h compared with amiodarone, calcium channel blocker, or digoxin.
Key Words: atrial fibrillation, comparative effectiveness, rate control, sepsis
Abbreviations: AF, atrial fibrillation; aOR, adjusted OR; HR, heart rate; MAP, mean arterial pressure; RVR, rapid ventricular response
FOR EDITORIAL COMMENT, SEE PAGE 1315
Atrial fibrillation (AF) occurs in nearly one-quarter of critically ill patients with sepsis and is associated with short-term and long-term morbidity and mortality.1,2 During sepsis, high circulating catecholamines may increase the risk of rapid atrioventricular-nodal conduction in AF, leading to reduced diastolic filling time and an increased risk for hemodynamic compromise.3,4 Thus, practice guidelines5 recommend medications to reduce heart rate (HR) in AF with rapid ventricular response (RVR) in patients who do not require emergent electric cardioversion. However, the optimal medication to achieve rate control for AF with RVR in sepsis is unclear. In this multicenter retrospective cohort study, we sought to compare the effectiveness of commonly used medications for AF rate and rhythm control during sepsis6 (β-blockers, calcium channel blockers, amiodarone, and digoxin) on HR reduction among critically ill patients with sepsis and AF with RVR admitted to the ICU.
Methods
Cohort
We used the eICU Collaborative Research Database,7,8 a multicenter 20% subset of patients admitted from 2014 through 2015 to 208 US hospitals participating in the Philips telehealth system (eCareManager), to identify adult patients (≥ 18 years) with sepsis and AF with RVR who were treated with an IV AF medication (metoprolol, esmolol diltiazem, verapamil amiodarone, or digoxin). We identified patients with sepsis using previously validated9 International Classification of Diseases, Ninth Revision, codes (because the eICU database does not contain reliable culture information to use Sepsis-3 definitions10). We identified the presence and timing of AF using physician documentation in the active diagnosis and treatment sections of eCareManager. AF with RVR was defined as an HR > 110 beats/min, a HR evaluated as the upper limit definition of HR control in prior trials.11,12 We limited our cohort to those patients who had an HR of ≥ 110 beats/min at the time that the AF medication was initiated. For patients with multiple admissions, we evaluated the initial admission for inclusion in the study.
Outcomes
The primary outcome of interest was the risk-adjusted rate of HR of < 110 beats/min by 1 h after administration. Secondary effectiveness outcomes included (1) the risk-adjusted rate of HR of < 110 beats/min by 6 h after administration, (2) the percent change in HR at 1 and 6 h after initial AF medication administration, and (3) the per-person average HR during the first 1 h and during the first 6 h after initial AF medication administration. Secondary safety outcomes included (1) incidence of at least one mean arterial pressure (MAP) reading of < 65 mm Hg by 6 h (hypotension that may reflect hemodynamic instability and worse outcomes in sepsis3), (2) incidence of HR of < 60 beats/min by 6 h (bradycardia), (3) incidence of a vasopressor medication started or increased in dose by 6 h, (4) incidence of initiation of at least one additional AF medication by 6 h, (5) incidence of undergoing direct current cardioversion by 6 h, (6) the proportion of patients undergoing pacemaker placement by 6 h, (7) hospital length of stay, and (8) incidence of death during hospitalization.
Exposures and Covariates
Among patients with AF with RVR, we identified the type and timing of the first IV AF medication. The AF medication types of interest were β-blockers (metoprolol and esmolol), calcium channel blockers (diltiazem and verapamil), amiodarone, and digoxin. We used an intention-to-treat treatment strategy in which the initial AF medication used was assigned as the treatment strategy selected for that patient. AF medication was included as a time-varying exposure variable.
Because different clinical characteristics may influence both the type of AF medication given and the HR response, we measured multiple potentially confounding fixed and time-varying covariates. At the time of admission, we identified each patient’s age, race, sex, use of home AF medications (amiodarone, β-blockers, calcium channel blockers, and digoxin), and history of pre-existing AF, congestive heart failure, and asthma or COPD. Within 24 h of the first AF medication administration, we identified po orders for amiodarone, β-blockers, calcium channel blockers, and digoxin. We also identified the time in hours from AF with RVR diagnosis to first AF medication administration. Time-varying covariates identified from the time of first AF medication administration included HR, MAP, hemoglobin oxygen saturation, sequential organ failure assessment score,13 vasopressor and inotrope use, blood magnesium, potassium, troponin I, WBC count level, and use of mechanical ventilation and hemodialysis. Last value carried forward imputation was used for time-varying covariates with missing entries for a given time.
Statistical Analysis
We used means to summarize continuous baseline characteristics and counts and percentages to summarize categorical baseline characteristics in patients taking AF medication with HR of ≥ 110 beats/min at the time of medication administration. These characteristics were stratified by AF medication type. Baseline was defined as the time of AF medication initiation. Because receipt of additional medications after a failed initial AF treatment may produce spurious conclusions regarding the effectiveness of the initial treatment strategy, we used competing risk models to determine subdistribution hazard ratios for each AF medication estimating the effect of each AF medication on HR response in the setting of competing risk of death and addition of a new AF medication class. Given the clinical importance of understanding short-term and medium-term rate control effectiveness, as well as nonproportional hazards after 6 h, we evaluated HR control at 1-h and 6-h time points. We included death and use of additional AF medications as competing risks. The subdistribution hazard ratios can be interpreted as the increase in the rate of AF with RVR resolution (HR < 110 beats/min) associated with the AF medication of interest among patients who showed HR of ≥ 110 beats/min at the time of AF medication or who had experienced a competing event. We calculated E values for each hazard ratio to determine the strength of association among a theoretical unmeasured confounder, initial AF medication type, and the primary outcome that the unmeasured confounder must have to bring the observed effect estimate to the null.14
For other secondary effectiveness and safety outcomes, we limited our cohort to those patients with an HR of ≥ 110 beats/min at the time of initial AF medication administration and to those patients who had available HR data at both the 1-h and 6-h time points. For each secondary effectiveness and safety outcome, we used linear models for continuous outcomes (eg, percent change in HR) and logistic regression models for dichotomous outcomes (eg, hypotension). For secondary outcome models (except bradycardia, vasopressor use, direct cardioversion, and pacemaker placement, which had low outcome rates that precluded the use of adjusted models), we adjusted for covariates (age, sex, race or ethnicity, congestive heart failure history, asthma or COPD history, HR, MAP, sequential organ failure assessment score, vasopressor dose, magnesium level, potassium level, troponin level, WBC count, hemoglobin oxygen saturation, mechanical ventilation, and hemodialysis) at the time of initial AF medication. In all primary and secondary outcome models, β-blockers were used as the reference AF medication group to which all other AF mediation effect estimates were compared.
All tests were two-sided (α = 0.05). SAS version 9.4 software (SAS Institute, Inc.) was used for statistical analyses. This study was designated by the Boston University Institutional Review Board as not human subjects research.
Results
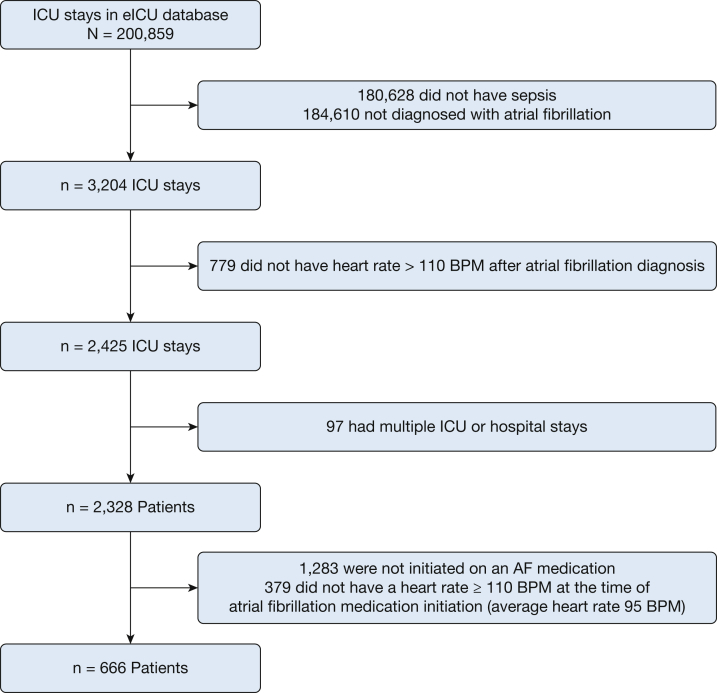
Among 2328 ICU patients with sepsis and AF with RVR, 666 initially received an AF medication and showed HR of ≥ 110 beats/min at the time of AF medication administration (Fig 1). Three hundred thirty-seven patients (50.6%) initially received amiodarone, 67 patients (10.1%) initially received a β-blocker, 225 patients (33.8%) initially received a calcium channel blocker, and 37 patients (5.6%) initially received digoxin. The average age was 72 years (SD, 12 years), and 208 patients (31.2%) died during the index hospitalization (Table 1). At the time of AF medication administration, the average HR was 128 beats/min (SD, 15 beats/min), and 246 patients (36.9%) were mechanically ventilated.
Figure 1.
Flow diagram showing patient inclusion and exclusion into the study cohort. AF = atrial fibrillation; BPM = beats per minute; eICU = eICU Collaborative Research Database.
Table 1.
Characteristics of ICU Patients With Sepsis and Treated AF With RVR
| Characteristic | Overall (N = 666) | Amiodarone (n = 337) | β-Blocker (n = 67) | Calcium Channel Blocker (n = 225) | Digoxin (n = 37) |
|---|---|---|---|---|---|
| Age, y | 72 ± 12 | 72 ± 12 | 72 ± 12 | 73 ± 12 | 75 ± 11 |
| Female sex | 362 (54.4) | 192 (57.0) | 36 (53.7) | 115 (51.1) | 19 (51.4) |
| Race or ethnicity | |||||
| Asian | 7 (1.1) | 4 (1.2) | 1 (1.5) | 2 (0.9) | 0 (0.0) |
| Black | 37 (5.6) | 19 (5.6) | 5 (7.5) | 12 (5.3) | 1 (2.7) |
| White | 559 (83.9) | 272 (80.7) | 61 (91.0) | 191 (84.9) | 35 (94.6) |
| Hispanic | 31 (4.7) | 20 (5.9) | 0 (0.0) | 11 (4.9) | 0 (0.0) |
| Other | 2 (0.3) | 2 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Unknown | 30 (4.5) | 20 (5.9) | 0 (0.0) | 9 (4.0) | 1 (2.7) |
| History of CHF | 181 (27.2) | 97 (28.8) | 16 (23.9) | 56 (24.9) | 12 (32.4) |
| History of asthma or COPD | 121 (18.2) | 58 (17.2) | 9 (13.4) | 47 (20.9) | 7 (18.9) |
| History of AF | 243 (36.5) | 114 (33.8) | 25 (37.3) | 92 (40.9) | 12 (32.4) |
| Home medications | |||||
| Amiodarone | 15 (2.3) | 11 (3.3) | 1 (1.5) | 3 (1.3) | 0 (0.0) |
| β-Blocker | 101 (15.2) | 49 (14.5) | 4 (6.0) | 44 (19.6) | 4 (10.8) |
| Calcium channel blocker | 30 (4.5) | 10 (3.0) | 2 (3.0) | 17 (7.6) | 1 (2.7) |
| Digoxin | 18 (2.7) | 6 (1.8) | 1 (1.5) | 10 (4.4) | 1 (2.7) |
| po Medication order within 24 h of first medication | |||||
| Amiodarone | 12 (1.8) | 7 (2.1) | 0 (0.0) | 5 (2.2) | 0 (0.0) |
| β-Blocker | 76 (11.4) | 29 (8.6) | 13 (19.4) | 31 (13.8) | 3 (8.1) |
| Calcium channel blocker | 5 (0.7) | 1 (0.3) | 0 (0.0) | 3 (1.3) | 1 (2.7) |
| Digoxin | 9 (1.4) | 2 (0.6) | 2 (3.0) | 5 (2.2) | 0 (0.0) |
| HR at the time of AF with RVR, beats/min | 128 ± 15 | 128 ± 15 | 132 ± 15 | 127 ± 14 | 132 ± 14 |
| Time from AF with RVR to first medication, h | 1.9 (0.5-11.9) | 1.3 (0.5-7.9) | 10.2 (1.6-22.7) | 1.8 (0.4-10.8) | 4.9 (1.3-24.9) |
| Mean arterial pressure at the time of AF with RVR, mm Hg | 78 ± 18 | 74 ± 14 | 85 ± 32 | 82 ± 16 | 78 ± 18 |
| Serum magnesium level at the time of AF with RVR, mg/dL | 2.0 ± 0.4 | 2.0 ± 0.4 | 2.0 ± 0.3 | 2.0 ± 0.4 | 2.0 ± 0.3 |
| Serum potassium level at the time of AF with RVR, mEq/L | 4.1 ± 0.6 | 4.1 ± 0.7 | 4.0 ± 0.6 | 4.0 ± 0.6 | 4.1 ± 0.6 |
| Maximum SOFA score at the time of AF with RVR | 8 ± 3 | 9 ± 4 | 7 ± 3 | 7 ± 3 | 8 ± 4 |
| Mechanically ventilated at the time of AF with RVR | 246 ± 36.9 | 146 ± 43.3 | 18 ± 26.9 | 72 (32.0) | 10 ± 27.0 |
| Vasopressor or inotrope at the time of AF with RVR | 254 ± 38.1 | 188 ± 55.8 | 16 ± 23.9 | 43 ± 19.1 | 7 ± 18.9 |
| Hospital mortality | 208 (31.2) | 132 (39.2) | 21 (31.3) | 50 (22.2) | 5 (13.5) |
| Pneumonia sepsis source | 336 (50.5) | 159 (47.2) | 33 (49.3) | 124 (55.1) | 20 (54.1) |
Data are No. (%), mean ± SD, or median (interquartile range). AF = atrial fibrillation; CHF = congestive heart failure; HR = heart rate; RVR = rapid ventricular response; SOFA = sequential organ failure assessment.
Competing Risk Models
After adjusting for covariates and accounting for competing risks of death and use of additional AF rate or rhythm control medications, the adjusted hazard ratio for HR of < 110 beats/min by 1 h was 0.50 (95% CI, 0.34-0.74; P < .001; E = 2.61) for amiodarone vs β-blocker, 0.37 (95% CI, 0.18-0.77; P = .007; E = 3.37) for digoxin vs β-blocker, and 0.75 (95% CI, 0.51-1.11; P = .15; E = 1.74) for calcium channel blocker vs β-blocker. By 6 h, the adjusted hazard ratio for HR of < 110 beats/min was 0.67 (95% CI, 0.47-0.97; P = .03; E = 1.97) for amiodarone vs β-blocker, 0.60 (95% CI, 0.36-1.004; P = .052; E = 2.20) for digoxin vs β-blocker, and 1.03 (95% CI, 0.71-1.49; P = .88; E = 1.17) for calcium channel blocker vs β-blocker.
Effectiveness Outcomes
Six hundred thirty-six patients were evaluated in the secondary effectiveness outcomes analyses having HR measurements at both 1 and 6 h. The results of the secondary effectiveness outcomes showed that patients who received a β-blocker achieved a larger reduction in HR at 1 h, but not at 6 h, after administration compared with those patients who received other AF medications (Table 2). For example, the average adjusted HR during the first 1 h after treatment among patients who received a β-blocker (115 beats/min [95% CI, 112-118 beats/min]) was lower compared with patients who received other AF medications (amiodarone, 122 beats/min [95% CI, 122-123 beats/min; P < .001]; calcium channel blocker, 122 beats/min [95% CI, 120-124 beats/min; P < .001]); and digoxin, 124 beats/min [95% CI, 119-129 beats/min; P = .002]). However, during the first 6 h, the average adjusted HR of patients who received a β-blocker (110 beats/min [95% CI, 106-114 beats/min]) was significantly lower only compared with patients who received digoxin (118 beats/min [95% CI, 112-123 beats/min; P = .03]), but not compared with patients who received amiodarone (114 beats/min [95% CI, 112-115 beats/min; P = .11]) or a calcium channel blocker (110 beats/min [95% CI, 108-112 beats/min; P = 1.00]). Compared with patients who initially received β-blocker therapy, patients who initially received amiodarone therapy (adjusted OR [aOR], 0.40; 95% CI, 0.17-0.93; P = .03) and calcium channel blocker therapy (aOR, 0.32; 95% CI, 0.13-0.78; P = .01), but not digoxin therapy (aOR 2.30; 95% CI, 0.73-7.25; P = .15), showed a lower odds of being administered at least one additional AF medication type by 6 h.
Table 2.
Secondary Effectiveness Outcomes Associated With AF With RVR During Sepsis Stratified by Initial AF Medication
| Outcome | β-Blocker (n = 61) | Amiodarone (n = 322) | Calcium Channel Blocker (n = 217) | Digoxin (n = 36) |
|---|---|---|---|---|
| Average change in HR at 1 h | ||||
| Unadjusted | −15.2 (−18.3 to −12.2) | −6.2 (−7.6 to −4.9) | −7.8 (−9.5 to −6.2) | −6.0 (−9.9 to −2.0) |
| Adjusteda | −15.3 (−18.5 to −12.1) | −6.8 (−8.3 to −5.3) | −8.0 (−9.9 to −6.1) | −4.9 (−9.8 to −0.1) |
| Average change in HR at 6 h | ||||
| Unadjusted | −15.9 (−20.0 to −11.8) | −15.0 (−16.8 to −13.3) | −19.1 (−21.3 to −17.0) | −15.9 (−21.2 to −10.6) |
| Adjusteda | −15.2 (−19.2 to −11.2) | −16.3 (−18.1 to −14.4) | −20.5 (−22.8 to −18.1) | −11.3 (−17.2 to −5.3) |
| Average HR during the first 1 h | ||||
| Unadjusted | 118 (114 to 121) | 122 (121 to 124) | 120 (118 to 122) | 126 (121 to 131) |
| Adjusteda | 115 (112 to 118) | 122 (120 to 123) | 122 (120 to 124) | 124 (119 to 129) |
| Average HR during the first 6 h | ||||
| Unadjusted | 112 (108 to 116) | 114 (113 to 116) | 110 (108 to 112) | 117 (112 to 122) |
| Adjusteda | 110 (106 to 114) | 114 (112 to 115) | 110 (108 to 112) | 118 (112 to 123) |
Data are percentage (95% CI) for change values or beats/min (95% CI). AF = atrial fibrillation; HR = heart rate; RVR = rapid ventricular response.
Adjusted for HR at the time of initial AF medication administration, age, sex, race or ethnicity, congestive heart failure and asthma or COPD history, mean arterial pressure, sequential organ failure assessment score, vasopressor or inotrope use, magnesium level, potassium level, white blood cell count, troponin I level, hemoglobin oxygen saturation, and presence of mechanical ventilation and hemodialysis.
Safety Outcomes
Safety outcomes stratified by initial AF medication type are shown in Table 3. Safety outcomes were rare—occurring in less than 10% of patients—across all AF medication treatment strategies except for hypotension (MAP < 65 mm Hg; β-blocker, 58.5%; amiodarone, 69.4%; calcium channel blocker, 56.0%; and digoxin, 51.4%) and hospital mortality (β-blocker, 27.4%; amiodarone, 37.6%; calcium channel blocker, 19.8%; and digoxin, 18.4%). Compared with patients who received β-blockers, the adjusted odds of hypotension (MAP < 65 mm Hg) were lower in patients who received digoxin (aOR, 0.20; 95% CI, 0.07-0.63; P = .006), but not in patients who received amiodarone (aOR, 0.72; 95% CI, 0.34-1.54; P = .40) or calcium channel blockers (aOR, 0.72; 95% CI, 0.34-1.56; P = .41).
Table 3.
Safety Outcomes Stratified by AF Medication Type
| Medication | β-Blocker (n = 113) | Amiodarone (n = 529) | Calcium Channel Blocker (n = 354) | Digoxin (n = 49) |
|---|---|---|---|---|
| MAP < 65 mm Hg by 6 h | 58.2 (46.4-70.0) | 69.4 (64.5-74.4) | 56.0 (49.5-62.5) | 51.4 (35.2-67.5) |
| HR < 60 beats/min by 6 h | 3.0 (1.1-7.1) | 4.5 (2.2-6.7) | 0.9 (0.3-2.1) | 5.4 (1.9-12.7) |
| Vasopressor medication started or increased in dose by 6 h | 0.0 (0.0-0.0) | 5.3 (2.9-7.7) | 0.9 (0.3-2.1) | 2.7 (2.5-7.9) |
| Direct cardioversion by 6 h | 0.0 (0.0-0.0) | 2.1 (0.6-3.6) | 0.4 (0.4-1.3) | 2.7 (2.5-7.9) |
| Pacemaker by 6 h | 1.5 (1.4-4.4) | 1.2 (0.0-2.3) | 0.4 (0.4-1.3) | 0.0 (0.0-0.0) |
| Additional AF medications by 6 h | ... | ... | ... | ... |
| None | 79.1 (69.4-88.8) | 92.3 (89.4-95.1) | 91.1 (87.4-94.8) | 75.7 (61.9-89.5) |
| One | 19.4 (9.9-28.9) | 7.1 (4.4-9.9) | 7.6 (4.1-11.0) | 24.3 (10.5-38.1) |
| Two | 1.5 (0.0-4.4) | 0.6 (0.0-1.4) | 0.9 (0.0-2.1) | 0.0 (0.0-0.0) |
| Three | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.4 (0.0-1.3) | 0.0 (0.0-0.0) |
| Hospital length of stay, median (95% CI), d | 6.3 (4.6-7.4) | 5.6 (4.4-6.6) | 6.4 (5.3-7.5) | 5.1 (3.5-8.4) |
| Hospital mortality | 31.3 (27.7-34.8) | 39.2 (34.0-44.4) | 22.2 (16.8-27.7) | 13.5 (2.5-24.5) |
Data are percentage (95% CI), unless otherwise indicated. AF = atrial fibrillation; HR = heart rate; MAP = mean arterial pressure.
The average adjusted length of stay (β-blocker [8.1 days], amiodarone [9.0 days; P = .61], calcium channel blocker [10.4 days; P = .18], and digoxin [9.4 days; P = .63]) and odds of death during hospitalization (amiodarone [aOR, 1.23; 95% CI, 0.61-2.51; P = .56], calcium channel blocker [aOR, 0.63; 95% CI, 0.30-1.34; P = .23], and digoxin [aOR, 0.33; 95% CI, 0.09-1.22; P = .10]) were not different between patients who initially received β-blocker therapy and patients who initially received other AF medications.
The numbers of patients with bradycardia (n = 21 [3.2%]), who began receiving a vasopressor medication or received an increased dose (n = 21 [3.2%]), who underwent direct cardioversion (n = 9 [1.4%]), and who underwent pacemaker placement (n = 6 [0.9%]) by 6 h were small (Table 3). Thus, we were unable to construct models to determine aORs for these safety outcomes.
Discussion
AF with RVR during sepsis is a common clinical problem; however, the comparative effectiveness of medications to achieve HR control is unclear. We performed an observational, comparative, effectiveness study comparing the ability of β-blockers, calcium channel blockers, digoxin, and amiodarone to achieve HR control during AF with RVR among critically ill patients with sepsis. Although β-blockers were associated with improved HR control at 1 h after administration, by 6 h, the difference in rate control between AF medications was minimal (all AF medications were associated with a 10%-20% reduction in HR). We also found that amiodarone, despite being the most frequently administered medication in our study, was associated with the longest times to rate control. Our results have implications for clinicians managing critically ill patients with sepsis and AF with RVR.
Our results should be viewed in the context of previous studies. In a single-center observational study, Moskowitz et al15 found that among patients admitted to the ICU (regardless of diagnosis) with AF with RVR, administration of β-blockers within 2 h of AF with RVR onset was associated with lower odds of failure (defined by the use of a second agent before the end of a RVR episode) compared with patients administered amiodarone. Although our study also identified β-blockers as the AF medication associated with the fastest time to RVR resolution, our secondary analysis also suggested that all medications achieved similar HR responses by 6 h. When comparing a β-blocker treatment strategy with other treatment strategies, patients treated with β-blockers also were more than twice as likely (19% vs 7%) to receive an additional AF medication by 6 h compared with the most frequently administered AF medications: amiodarone and calcium channel blockers. Thus, although β-blockers may achieve faster time to initial rate control that may be valuable in the short-term, it is unclear if other medications or more frequent dosing may be needed to achieve longer-term rate control. Differences in AF mechanism between the undifferentiated general ICU population in the study by Moskowitz et al15 and the sepsis-specific patients included in our study may impact the effectiveness and duration of effectiveness of specific AF medications. Unlike our previous observations6 among patients with AF during sepsis, we did not find that β-blockers are associated with lower hospital mortality compared with other AF medications. In sum, these results suggest that, in the absence of contraindications (decompensated heart failure, uncontrolled bronchospasm) and randomized control trial evidence, clinicians aiming to reduce HR rapidly among patients with sepsis and AF with RVR who do not require cardioversion should consider β-blockers as a first-line therapy. Rapid reduction in HR may be particularly beneficial in patients with new-onset AF during critical illness, given that up to 37% may experience hemodynamic compromise in association with AF.16 However, if a rapid reduction is not necessary, there seems to be less difference in the ability to achieve rate control among β-blockers, amiodarone, calcium channel blockers, and digoxin. Clinicians also should continue to monitor patients and prepare potentially to initiate additional AF medications or consider more frequent dosing or continuous infusion in the event that HR response is of short duration. Future randomized control trials are needed to make specific treatment recommendations for optimal strategies for HR control in AF during sepsis.
Our study has several strengths. Our results were robust to potential time-varying confounders and to strong unmeasured confounding. In addition, we were able to quantify the estimates of the association between AF medication and HR control, the estimate of the association between initial AF medication and the use of subsequent AF medications, and the average reduction in HR that can be expected at 1-h and 6-h time points from each medication. Knowing the risk of the need for additional AF medications is particularly valuable to clinicians, especially when the risk of hemodynamic compromise with treatment failure is high (eg, significant diastolic dysfunction) or when patients have limited venous access. Finally, the combined results from our multiple secondary effectiveness and adverse event outcomes provide clinicians with novel data that quantifies the average reductions in HR expected at different time points for commonly used medications and evaluates risks and benefits of different strategies for HR control of AF during sepsis.
Our study also has several limitations. Although characteristics of patients receiving β-blockers, calcium channel blockers, and digoxin generally were similar, patients receiving amiodarone showed higher rates of mechanical ventilation and vasopressor needs at baseline, which also may suggest a greater risk of unmeasured confounding for comparisons with amiodarone. However, the E value of 2.65 suggests that unmeasured variables associated with a 2-fold to 3-fold higher odds of both receiving amiodarone and not achieving HR control would be needed to change the results substantively. For example, we did not include attending of record in our models, a variable previously associated with receiving amiodarone for AF during sepsis (OR, 1.36).6 Thus, if the attending of record also was associated with HR control, then the attending of record could be an unmeasured confounder in our study. However, the E value of 2.61 suggests that the strength of the associations between attending of record, amiodarone use, and HR control would have to be at least 2.61 to change our conclusions substantively. The optimal HR at which to start AF medications during sepsis is unclear, and our choice of 110 beats/min, although consistent with cutpoints chosen in prior clinical trials looking at outpatient HR control, may not represent the ideal target during critical illness. Further studies of optimal HR targets for AF during critical illness are needed. However, results using continuous analysis of HR supported the HR > 110 beats/min analyses. We were not able to identify the time of resolution of AF in our cohort, and thus we were unable to compare rhythm control between medications or time to conversion to sinus rhythm, a finding of particular interest when evaluating effects of amiodarone. In addition, the use of po medication order, rather than po medication administration, in our models might have affected our results because we cannot be sure if po medications that were ordered were actually administered. Finally, future randomized controlled studies are needed to confirm the hypothesis-generating comparative effectiveness findings in our study.
Interpretation
We found that in a large US multicenter cohort of patients with sepsis and AF with RVR, a β-blocker treatment strategy was associated with improved HR control compared with amiodarone, calcium channel blocker, or digoxin treatment strategies at 1 h. However, the relative improvement in HR using a β-blocker strategy was diminished after 6 h, and we did not find evidence that a β-blocker treatment strategy improved nonhemodynamic-related outcomes (ie, hospital death).
Acknowledgments
Author contributions: All authors contributed to the study conception and design. Material preparation and data collection and analysis were performed by N. A. B., J. M. M., M. R. W., E. K. Q., and A. J. W. The first draft of the manuscript was written by N. A. B., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. N. A. B. and A. J. W. take responsibility for the integrity of the work as a whole, from inception to published article.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: D. D. M. has received honorariums, speaking and consulting fees, or grants from Flexcon, Rose Consulting, Bristol-Myers Squibb, Pfizer, Boston Biomedical Associates, Samsung, Phillips, Mobile Sense, Care Evolution, Flexcon Boehringer Ingelheim, Biotronik, Otsuka Pharmaceuticals, and Sanofi. None declared (N. A. B., J. M. R., J. M. M., M. R. W., E. K. Q., K. H. C., A. J. W.).
Footnotes
FUNDING/SUPPORT: This study was funded by the National Heart, Lung and Blood Institute of the National Institutes of Health [Grant R01HL136660].
References
- 1.Klein Klouwenberg P.M.C., Frencken J.F., Kuipers S. Incidence, predictors, and outcomes of new-onset atrial fibrillation in critically ill patients with sepsis. A cohort study. Am J Respir Crit Care Med. 2017;195(2):205–211. doi: 10.1164/rccm.201603-0618OC. [DOI] [PubMed] [Google Scholar]
- 2.Walkey A.J., Wiener R.S., Ghobrial J.M., Curtis L.H., Benjamin E.J. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA. 2011;306(20):2248–2254. doi: 10.1001/jama.2011.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varpula M., Tallgren M., Saukkonen K., Voipio-Pulkki L.-M., Pettilä V. Hemodynamic variables related to outcome in septic shock. Intensive Care Med. 2005;31(8):1066–1071. doi: 10.1007/s00134-005-2688-z. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida T., Uchino S., Sasabuchi Y. Clinical course after identification of new-onset atrial fibrillation in critically ill patients: the AFTER-ICU study. J Crit Care. 2020;59:136–142. doi: 10.1016/j.jcrc.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 5.January C.T., Wann L.S., Calkins H. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140(2):e125–e151. doi: 10.1161/CIR.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 6.Walkey A.J., Evans S.R., Winter M.R., Benjamin E.J. Practice patterns and outcomes of treatments for atrial fibrillation during sepsis: a propensity-matched cohort study. Chest. 2016;149(1):74–83. doi: 10.1378/chest.15-0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pollard T.J., Johnson A.E.W., Raffa J.D., Celi L.A., Mark R.G., Badawi O. The eICU Collaborative Research Database, a freely available multi-center database for critical care research. Sci Data. 2018;5:180178. doi: 10.1038/sdata.2018.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldberger A.L., Amaral L.A., Glass L. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation. 2000;101(23):E215–E220. doi: 10.1161/01.cir.101.23.e215. [DOI] [PubMed] [Google Scholar]
- 9.Iwashyna T.J., Odden A., Rohde J. Identifying patients with severe sepsis using administrative claims: patient-level validation of the angus implementation of the international consensus conference definition of severe sepsis. Med Care. 2014;52(6):e39–e43. doi: 10.1097/MLR.0b013e318268ac86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singer M., Deutschman C.S., Seymour C.W. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wyse D.G., Waldo A.L., DiMarco J.P. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 12.Van Gelder I.C., Groenveld H.F., Crijns H.J.G.M. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362(15):1363–1373. doi: 10.1056/NEJMoa1001337. [DOI] [PubMed] [Google Scholar]
- 13.Vincent J.L., Moreno R., Takala J. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 14.Haneuse S., VanderWeele T.J., Arterburn D. Using the e-value to assess the potential effect of unmeasured confounding in observational studies. JAMA. 2019;321(6):602–603. doi: 10.1001/jama.2018.21554. [DOI] [PubMed] [Google Scholar]
- 15.Moskowitz A., Chen K.P., Cooper A.Z., Chahin A., Ghassemi M.M., Celi L.A. Management of atrial fibrillation with rapid ventricular response in the intensive care unit: a secondary analysis of electronic health record data. Shock. 2017;48(4):436–440. doi: 10.1097/SHK.0000000000000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanji S., Williamson D.R., Yaghchi B.M., Albert M., McIntyre L. Canadian Critical Care Trials Group. Epidemiology and management of atrial fibrillation in medical and noncardiac surgical adult intensive care unit patients. J Crit Care. 2012;27(3):326.e1–326.e8. doi: 10.1016/j.jcrc.2011.10.011. [DOI] [PubMed] [Google Scholar]