Abstract
Eosinophilia with pulmonary involvement is characterized by the presence of peripheral blood eosinophilia, typically ≥500 cells/mm3, by pulmonary symptoms and physical examination findings that are nonspecific, and by radiographic evidence of pulmonary disease and is further supported by histopathologic evidence of tissue eosinophilia in a lung or pleura biopsy specimen and/or increased eosinophils in BAL fluid, usually >10%. Considering that there are a variety of underlying causes of eosinophilia with pulmonary manifestations and overlapping clinical, laboratory, and radiologic features, it is essential to approach the evaluation of eosinophilia with pulmonary findings systematically. In this review, we will describe a case presentation and discuss the differential diagnosis, a directed approach to the diagnostic evaluation and supporting literature, the current treatment strategies for pulmonary eosinophilia syndromes, and the levels of evidence underlying the recommendations, where available. Overall, optimal management of eosinophilic lung disease presentations are directed at the underlying cause when identifiable, and the urgency of treatment may be guided by the presence of severe end-organ involvement or life-threatening complications. When an underlying cause is not easily attributable, management of eosinophilia with pulmonary involvement largely relies on eosinophil-directed interventions, for which biologic therapies are increasingly being used.
Key Words: asthma, eosinophilia, eosinophilic granulomatosis with polyangiitis hypereosinophilic, syndrome
Abbreviations: ABPA, allergic bronchopulmonary aspergillosis; AEC, absolute eosinophil count; AEP, acute eosinophilic pneumonia; AERD, aspirin-exacerbated respiratory disease; ANCA, antinuclear antibody and antineutrophil cytoplasmic antibody; CEP, chronic eosinophilic pneumonia; EGPA, eosinophilic granulomatosis with polyangiitis; GPA, granulomatosis with polyangiitis; HES, hypereosinophilic syndrome; MPA, microscopic polyangiitis; PCR, polymerase chain reaction
A 44-year-old woman with a history of asthma, allergic rhinitis, and chronic sinusitis was seen in the ED for several months of progressive dyspnea with exertion, sinus congestion, and wheezing. She denied weight loss, fevers, chills, hemoptysis, sick contacts, smoking, or vaping. She traveled to Costa Rica as a young adult. She was well until four years ago when she experienced shortness of breath, cough, and sinus infections. CT scans of the sinuses demonstrated pan-sinusitis with polyps, and she was referred for polypectomy. A pulmonologist diagnosed her with asthma and started daily inhaled glucocorticoids based on symptoms and obstructive spirometry. Over the next year, she had recurrent episodes of coughing and sinusitis that required short courses of systemic glucocorticoids for symptomatic relief.
Physical Examination Findings
The patient was afebrile with a heart rate of 96 beats/min, a respiratory rate of 22 breaths/min, pulse oximetry of 88% on ambient air, and BMI of 28 kg/m2. Decreased breath sounds were noted at bilateral lung bases. No clubbing or peripheral edema was noted. Hypoxemia resolved with supplemental oxygen. The remainder of the examination was unrevealing.
Diagnostic Studies
A CBC count with differential revealed a WBC count of 9,000 with 56% eosinophils (absolute eosinophil count [AEC] of 5,040 cells/mm3). A comprehensive metabolic profile was normal. Urinalysis was negative for protein, cells, and casts. Chest radiography revealed bilateral pulmonary opacities, and noncontrast chest CT scans showed bilateral pleural effusions, peripheral lung opacities, and a small pericardial effusion. Antibiotics were started for suspected pneumonia, and she was admitted. A peripheral blood smear did not show blasts or dysplasia. An echocardiogram showed an ejection fraction of 60% and moderate pericardial effusion. Lactate dehydrogenase level was normal. Serum protein electrophoresis and immunoglobulins were unrevealing, except for an elevation in IgE at 1,634 international units/mL. Vitamin B12 was elevated at 1,050 ng/mL. Serum tryptase was normal. Antinuclear antibody and antineutrophil cytoplasmic antibody (ANCA) were negative. Serology for Strongyloides was negative. Bronchoscopy showed normal anatomy, and BAL was negative for bacterial growth, acid-fast bacilli staining, Coccidioides polymerase chain reaction (PCR), and Aspergillus PCR but was significant for 32% eosinophils. Aspergillus IgG and IgE, galactomannan (Aspergillus antigen), and Fungitell assay results were negative. A bone marrow biopsy showed hypercellularity (70%) with trilineage hematopoiesis, eosinophilia (24%), but no increased blasts or dysplasia. Further testing for molecular and cytogenetic abnormalities were sent for analysis.
Clinical Course
She received ivermectin followed by prednisone 1 mg/kg/d; over the next week, she reported improvement in symptoms. Her AEC was 100 cells/mm3, but she complained of continued dyspnea and fatigue. Troponin-I was elevated at 2.26 ng/mL, and repeat echocardiography showed an ejection fraction of 40%. Cardiac catheterization showed no significant coronary artery disease, but left ventricular end diastolic pressure was elevated at 40 mm Hg. Karyotype was normal and cytogenetic/fluorescence in situ hybridization analysis for PDGFRA, PDGFRB, FGFR1, JAK2, and nested PCR for FIP1L1-PDGFRA fusion was negative. She was discharged with a prednisone taper and outpatient evaluation with cardiac MRI. On follow-up in clinic, she complained of improved but persistent shortness of breath on prednisone 20 mg daily.
Eosinophilia with pulmonary involvement may be associated with diverse causes (Table 1). In this review, we discuss the differential diagnosis, literature supporting the diagnostic evaluation for these conditions in the context of the case presentation, treatment approaches for pulmonary eosinophilia syndromes, and levels of evidence underlying the recommendations, where available.
Table 1.
Differential Diagnosis of Pulmonary Infiltrates With Eosinophilia
| Eosinophilic granulomatosis with polyangiitis |
| Other vasculitides |
| Granulomatosis with polyangiitis |
| Microscopic polyangiitis |
| Hypereosinophilic syndromes |
| Overlap |
| Myeloid |
| Lymphoid-variant |
| Idiopathic |
| Aspirin-exacerbated respiratory disease |
| Chronic eosinophilic pneumonia |
| Acute eosinophilic pneumonia |
| Eosinophilic bronchitis |
| Allergic bronchopulmonary aspergillosis |
| Infectious causes of pulmonary eosinophilia |
| Helminthic infections |
| Strongyloidiasis |
| Schistosomiasis |
| Fascioliasis |
| Opisthorchiasis |
| Clonorchiasis |
| Gnathostomiasis |
| Paragonimiasis |
| Echinococcosis |
| Coccidioidomycosis |
| Toxocariasis |
| Non-helminthic infections |
| Ectoparasites |
| Fungal infections |
| Mycobacterial infections |
| HIV |
| Drug-induced pulmonary eosinophilic syndromes |
| Anticonvulsants |
| Antibacterial sulfonamides |
| Allopurinol |
| Vancomycin |
| Minocycline |
Differential Diagnosis
Eosinophilic Granulomatosis With Polyangiitis
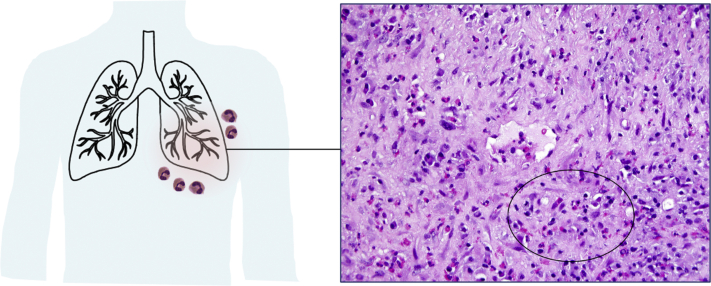
Eosinophilic granulomatosis with polyangiitis (EGPA) is high on the differential diagnosis based on the findings of asthma, eosinophilia, sinus disease, and cardiomyopathy. EGPA is a multisystem disorder characterized by asthma, chronic rhinosinusitis, and eosinophilia. It is classified as a vasculitis of small-to-medium-sized vessels; the most common organs to be affected are the lung and skin; however, any organ system may be involved. Accompanying features include blood eosinophilia >1,500 cells/mm3 or >10% of leukocyte count and ANCA, typically with a myeloperoxidase perinuclear staining pattern, in 30% of patients with pulmonary involvement.1,2 ANCA-negative EGPA is less likely to have renal involvement but may have more frequent cardiac manifestations,1, 2, 3 reflecting the heterogeneity of EGPA presentation. Chest radiography commonly shows transient and patchy opacities. Tissue biopsy from the most accessible disease site (most frequently of skin, lung, pleura, or peroneal nerve) is encouraged to provide for a definitive diagnosis. Major histopathologic findings include eosinophilic infiltrates, intra- or peri-vascular eosinophilia with vasculitis, interstitial and perivascular necrotizing granulomas, and areas of necrosis in affected organs. If a patient’s condition is stable, biopsy should be considered before glucocorticoid therapy is started, which may limit histopathologic diagnosis. A diagnosis of EGPA is defined as a history or presence of asthma, a blood eosinophil level of at least 10% constituting the leukocyte count or an AEC of >1,000 cells/mm3, and the presence of two or more of the following criteria: histopathologic evidence of eosinophilic vasculitis, perivascular eosinophilic infiltration, or eosinophil-rich granulomatous inflammation; neuropathy; pulmonary infiltrates; sinonasal abnormality; cardiomyopathy; glomerulonephritis; alveolar hemorrhage; palpable purpura; or ANCA positivity.4 Although a biopsy was not obtained in this patient, the symptoms and extrapulmonary involvement suggest that EGPA is the most appropriate diagnosis in this case (Fig 1).
Figure 1.
Transbronchial biopsy in eosinophilic granulomatosis with polyangiitis. A vessel is shown with a surrounding inflammatory infiltrate rich in plasma cells and eosinophils (oval) (H&E; original magnification, 40X).
Hypereosinophilic Syndromes
EGPA may not always be distinguishable easily from hypereosinophilic syndrome (HES). HES are heterogeneous disorders characterized by persistent blood eosinophilia >1,500 cells/mm3 and evidence of end-organ damage or dysfunction.5 In the authors’ experience, overlap HES encompasses clinically described conditions (eg, EGPA without biopsy-proven vasculitis or eosinophilic GI disorders) in which features cannot be distinguished from HES.6 Other HES clinical subtypes have been described.7 Myeloid HES involves presumed clonal eosinophilic involvement, such as in FIP1L1/PDGFRA.8 Lymphoid-variant HES is HES with demonstrable clonal or a phenotypically aberrant lymphocyte population.9 When no cause is determined, idiopathic HES is diagnosed. In general, pulmonary symptoms are present in 25% of HES cases.5 With regards to the diagnostic evaluation of a patient suspected to have HES, it is important to assess the organ involved and the severity of illness to guide the urgency of evaluation.10 The clinical presentation (eg, the most likely underlying HES clinical subtype) should guide testing,7 and a thorough search for alternate causes of hypereosinophilia should be explored. The HES subtypes that most commonly present with pulmonary eosinophilia are overlap and idiopathic HES, although myeloid and lymphoid-variant HES may also present rarely with pulmonary eosinophilia.
Aspirin-Exacerbated Respiratory Disease
Given the patient’s triad of asthma, chronic rhinosinusitis with polyposis, and blood eosinophilia, aspirin-exacerbated respiratory disease (AERD) must be considered. AERD is a syndrome characterized by severe asthma, eosinophilic chronic rhinosinusitis, nasal polyps, aspirin or nonsteroidal antiinflammatory drug intolerance; the diagnosis is confirmed by aspirin challenge.11 Blood eosinophilia is common, and AECs have been found to be significantly higher in patients with AERD than aspirin-tolerant asthma and healthy control subjects; however, wide variability in AEC limits its applicability as a biomarker in diagnosing AERD.12 The patient’s findings of asthma and eosinophilia with sinus disease confirmed on CT scan are consistent with AERD, but the presence of extrapulmonary manifestations is more suggestive of a systemic process.
Chronic Eosinophilic Pneumonia
Chronic eosinophilic pneumonia (CEP) could be considered in this patient because she has had symptoms lasting more than 2 weeks; however, her extrapulmonary manifestations are more suggestive of EGPA. CEP and acute eosinophilic pneumonia (AEP) share similarities in radiologic findings and response to glucocorticoids. CEP affects women twice as much as men, nonsmokers, individuals 30-60 years of age, and those with atopic conditions.13 CEP presents sub-acutely with cough, fever, dyspnea, weight loss, wheezing, and/or night sweats and is more likely to result in chronicity or relapse compared to AEP. Asthma precedes or accompanies illness in 50%, and BAL eosinophilia > 25% is suggestive of CEP.14
AEP
AEP is an idiopathic eosinophilic pneumonia associated with the sudden, rapid development of an acute febrile illness characterized by nonproductive cough, dyspnea and nonspecific radiographic changes, or acute respiratory failure in a previously healthy individual, which can closely resemble ARDS. AEP is unlikely, given the patient’s duration of symptoms and presentation. A recent history of cigarette smoking or exposure to smoke, fine sand, dust, or e-cigarette use15 may be elicited. Eosinophilia is rare at presentation but can develop, and histologic findings include diffuse alveolar damage, hyaline membranes, and interstitial eosinophils. If a diagnosis of AEP is considered, bronchoscopy with evaluation of BAL fluid for eosinophils may be informative because most patients with AEP respond well to glucocorticoid therapy. There are no formal diagnostic criteria, but the modified criteria of Philit et al16 have been used for diagnosis of AEP.
Allergic Bronchopulmonary Aspergillosis
A diagnosis of allergic bronchopulmonary aspergillosis (ABPA) should be considered in individuals with asthma (eg, in the patient described). ABPA is a complex hypersensitivity response to Aspergillus colonization with subsequent immune dysregulation that leads to pulmonary infiltrates, mucus plugs, IgE elevation, and blood and sputum eosinophilia.17 Symptoms include episodes of bronchial obstruction, fever, malaise, expectoration of mucus plugs, and hemoptysis. Laboratory findings include blood eosinophilia (typically >500 cells/mm3) and elevated total IgE (generally >1,000 international units/mL). High-resolution chest CT scans may show proximal bronchiectasis with upper lobe predominance and bronchial wall-thickening. Pulmonary function tests demonstrate an obstructive pattern; if there is progression to bronchiectasis and/or fibrosis, a mixed obstructive and restrictive pattern may be seen. Criteria for diagnosis of ABPA include (1) asthma, (2) the presence of allergic sensitization to Aspergillus spp via skin prick testing and/or specific IgE, and (3) total IgE >417 international units/mL; proximal bronchiectasis is suggestive but not required.17 Trending total IgE may be useful in monitoring for relapses.18 In the patient described, ABPA would be considered, given the patient’s preexisting asthma, peripheral eosinophilia, and elevated total IgE, but the negative Aspergillus specific IgE and chest CT findings are not consistent with ABPA.
Other Vasculitides
Other vasculitides, such as granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA), can affect the lungs. GPA is a necrotizing vasculitis that involves small-to-medium-sized vessels and produces granulomatous inflammation of the upper and lower respiratory tracts and necrotizing, pauci-immune glomerulonephritis. ANCA is present in >80% of patients with GPA in contrast to EGPA. MPA is a necrotizing vasculitis that primarily affects capillaries, venules, or arterioles and commonly manifests as necrotizing glomerulonephritis and/or pulmonary capillaritis. Granulomatous inflammation is typically absent, and ANCA is present in >90% of patients with MPA. Although the vasculitis present in MPA, GPA, and EGPA may be pathologically indistinguishable, the degree of eosinophilia and presence of asthma can help distinguish EGPA from GPA and MPA.
Eosinophilic Bronchitis
Eosinophilic bronchitis is defined as eosinophilic inflammation in the bronchial tree with no evidence of airway hyperresponsiveness. Sputum eosinophilia (≥3%) is characteristic.19 Eosinophilic bronchitis should be considered in a patient with chronic cough without evidence of airway hyperresponsiveness and sputum eosinophilia. A cross-sectional study demonstrated that patients with eosinophilic bronchitis (n = 16) had higher blood eosinophil counts (mean, 350 cells/mm3) compared with healthy subjects (n = 10).20
Infectious Causes of Pulmonary Eosinophilia
Depending on the clinical and travel history elicited, infectious causes of eosinophilia with pulmonary involvement should be considered. In helminth infections with transpulmonary passage, eosinophilic pulmonary infiltrates and respiratory symptoms may be present transiently due to larval migration through the lungs. Pulmonary invasion and hematogenous seeding of larvae or eggs can lead to an impressive eosinophilic pulmonary response. Larvae may be recovered from respiratory secretions or stool; however, the sensitivity of stool testing may limit its utility in all settings. Testing for helminths should be guided by exposure history, whether endemic or resulting from travel to an area where exposure occurred.21 The most sensitive screening tests are enzyme immunoassays for specific serologic condition. Strongyloidiasis is endemic worldwide and should be considered in all those who present with pulmonary eosinophilia, regardless of travel or exposure history because asymptomatic carriage is frequent.21 Additional, common causes of pulmonary eosinophilia include Schistosomiasis, Fascioliasis, Opisthorchiasis, Clonorchiasis, Gnathostomiasis, Paragonimiasis, Echinococcosis, Coccidioidomycosis, and Toxocariasis.3,21,22 Importantly, therapy should be aimed at eradication of the culprit helminth, with attention given to treatment of suspected Strongyloides spp infection prior to initiation of glucocorticoids. Additional infectious causes to consider include ectoparasites, fungal infections, mycobacterial infections, and HIV.
Drug-Induced Pulmonary Eosinophilic Syndromes
Eosinophilia with pulmonary symptoms has also been reported following ingestion or inhalation of medications or toxins23 with drug-induced hypersensitivity syndrome or drug reaction with eosinophilia and systemic symptoms being the more serious clinical presentations. In drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms, pulmonary symptoms may precede other symptoms or develop later. The most common features are interstitial infiltrates in 50%, acute respiratory distress syndrome in 31%, and cough and shortness of breath in 72% of cases at time of presentation.24 Commonly implicated medications include anticonvulsants, antibacterial sulfonamides, allopurinol, vancomycin, and minocycline.25
Treatment
Optimal management of eosinophilic lung disease presentations are directed at the underlying cause when identifiable, and the urgency of management may be guided by the presence or absence of end-organ manifestations.
Vasculitides That Include EGPA
The use of mepolizumab, an anti- IL-5 monoclonal antibody, in patients with EGPA, demonstrated fewer relapses and a higher proportion of patients in remission than did placebo as well as improved symptoms and sparing of glucocorticoids,4,26 which led to the first US Food and Drug Administration-approved drug for EGPA. Prior to US Food and Drug Administration approval of mepolizumab, the EGPA Consensus Task Force put forth the following recommendations3 with the use of GRADE guidelines27 for treatment of EGPA: (1) Glucocorticoids are recommended to achieve remission (prednisone 1 mg/kg/d) (level A evidence). (2) Patients with life- and/or organ-threatening manifestations should be treated with glucocorticoids and an additional immunosuppressant (eg, cyclophosphamide) to induce remission (level B evidence). (3) Maintenance therapy with azathioprine or methotrexate is recommended for life- and/or organ-threatening manifestations (level C evidence). (4) Glucocorticoids alone may be suitable for patients without life- and/or organ-threatening manifestations, and additional immunosuppression can be considered for patients who cannot be tapered below prednisone 7.5 mg/d after 3 to 4 months of therapy or patients with recurrent disease (level C evidence). Induction therapy with prednisone 1 mg/kg/d for 2 to 3 weeks followed by gradual tapering over several months to the minimal effective dose, or when possible, until withdrawal has been recommended.3 Continuing the lowest appropriate glucocorticoid dose to control symptoms3 and, in the authors’ experience, to maintain AEC ideally <1,000 cells/mm3 is suggested, as is continued regular monitoring for steroid-related complications (eg, bone densitometry, glucose monitoring, and ocular examinations). Though immunosuppressants for ANCA-associated vasculitides are used in patients with EGPA, the addition of azathioprine to glucocorticoids for induction of remission was not superior to glucocorticoids alone.28,29 Notably, there are no current biomarkers of EGPA that distinguish between active and inactive disease, including AEC,30 although many clinicians continue to use a combination of clinical parameters, AEC, and inflammatory markers to guide treatment decisions. Because up to 84% of patients require long-term prednisone therapy,2 emerging data on the use of additional eosinophil-targeted therapies show promise. Ongoing clinical trials include a phase 2 clinical trial of benralizumab, an anti-IL-5-receptor-α monoclonal antibody, for EGPA31 and a phase 3 trial that is comparing benralizumab and mepolizumab.32
Other vasculitides, such as GPA or MPA, with pulmonary involvement are treated with high-dose glucocorticoids and additional immunosuppressive medications, depending on the indication.
Hypereosinophilic Syndromes
Although systemic glucocorticoid therapy is effective in many patients with HES, treatment responses vary by subtype.33 With increased availability of biologic therapies for asthma, the number of clinical trials for HES is also growing. Mepolizumab has been shown to be effective and well-tolerated in glucocorticoid-responsive HES, treatment-refractory HES,34, 35, 36 and lymphoid-variant HES.37 A phase 3 trial of mepolizumab in adolescent and adult patients met its primary end point and demonstrated that 50% fewer patients experienced flares when treated with mepolizumab, compared with placebo.38 Additionally, benralizumab for PDGFRA-negative HES in a phase 2 study demonstrated reduction in AEC and clinical and hematologic responses in 89% of patients that was sustained for 48 weeks in 74% of them.39 A phase 3 trial to evaluate benralizumab in HES40 is planned. Alemtuzumab is an anti-cluster-of-differentiation-52 monoclonal antibody that can be used in refractory cases,41 although side-effects limit its long-term use.42 Other agents that have been used with efficacy include JAK inhibitors ruxolitinib and tofacitinib in small numbers,43 and a phase 2 trial to evaluate imatinib and ruxolitinib in myeloid and/or steroid-refractory HES is ongoing.44
Treatment strategies for myeloid/lymphoid neoplasms with eosinophilia and rearrangements of PDGFRA/B, FGFR1, or PCM1-JAK2 include imatinib for PDGFRA/B-rearranged disease, ALL- or AML-type chemotherapy for FGFR1-rearranged disease followed by transplantation or FGFR1 inhibitors, and JAK inhibitors for PCM1-JAK2 disease. Ruxolitinib has been used in small series with a PCM1-JAK2 or a BCR-JAK2 fusion gene45,46 and is being evaluated in other HES subtypes, as described earlier. Notably, patients presenting with lymphoid-variant HES are at an increased risk for progression to lymphoma9; thus, close follow up and regular monitoring is warranted.
Aspirin-Exacerbated Respiratory Disease
The mainstay of treatment for AERD includes aspirin desensitization followed by maintenance therapy with daily high-dose aspirin. Few prospective studies have been performed in AERD; however, aspirin desensitization appears to improve symptoms and reduce glucocorticoid requirement.47 Intranasal ketorolac challenge and desensitization followed by oral aspirin challenge is a shorter, safe alternative approach to standard oral aspirin desensitization.48 Omalizumab, an anti-IgE monoclonal antibody, has been shown to reduce systemic glucocorticoid use and improve symptoms.49 In 22 patients, mepolizumab led to improvement in nasal congestion, anosmia, and asthma control.50 Dupilumab, an anti-IL-4-receptor-α monoclonal antibody, was shown to improve sinus symptoms in AERD51 and was approved by the US Food and Drug Administration for chronic rhinosinusitis with nasal polyposis.52
Eosinophilic Pneumonias and Bronchitis
The mainstay of treatment is oral glucocorticoids with the goal of inducing remission and reducing relapse. In 62 patients with CEP, response to oral glucocorticoids was observed in all; however, 70% of them required therapy beyond a year.14 Inhaled glucocorticoids may assist with tapering systemic therapy. Omalizumab and anti-IL-5 therapy for CEP has been described in case reports.53 Importantly, there is the possibility of unmasking EGPA in these patients.54
Like CEP, oral glucocorticoids effectively treat AEP and eosinophilic bronchitis. Eosinophilic bronchitis responds well to inhaled glucocorticoids.
ABPA
Overall, treatment of ABPA is centered on prolonged oral glucocorticoid therapy with slow tapering. Case series and small trials have examined different glucocorticoid regimens.55 Because prolonged use of oral glucocorticoids may be associated with significant side-effects, glucocorticoid-sparing therapies have been examined. Efficacy of oral antifungals remains controversial but may serve as adjunctive therapy for some patients,56,57 but their use may be limited due to their interactions with glucocorticoid metabolism. Rare use of biologics such as mepolizumab58 and omalizumab have been described.59 A phase III trial of dupilumab in ABPA is planned.60
Summary
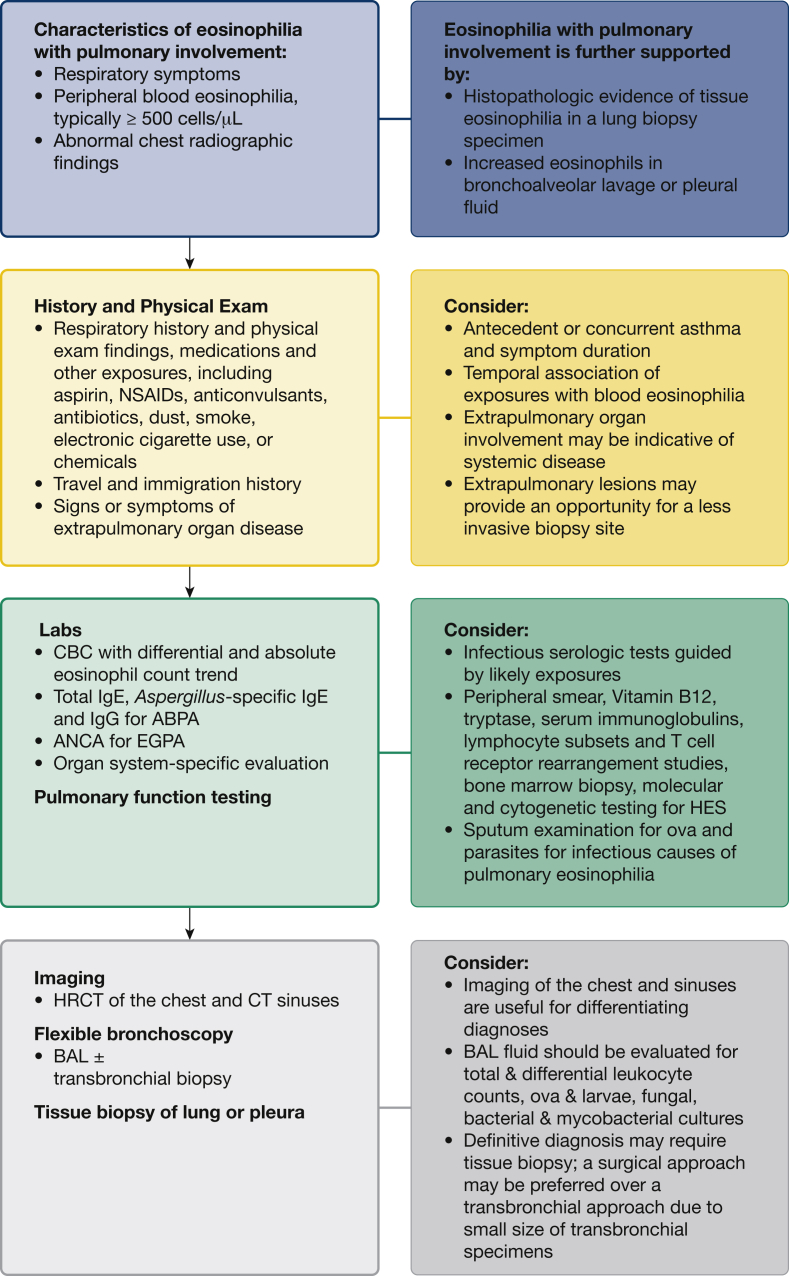
In the evaluation of a patient presenting with eosinophilia with pulmonary involvement, a thorough history and physical examination will guide the diagnostic work up. Attention should be given to medication and other exposures and temporal association with eosinophilia, travel and immigration history, physical findings, and extrapulmonary organ involvement. The initial evaluation should include a CBC count with differential and examination of the peripheral smear to evaluate for blasts or dysplasia. Blood eosinophilia helps to support certain diagnoses.61 Additional work up should be directed at suspected causes (Fig 2). High resolution chest CT scanning can help differentiate among several causes,62 including CEP, AEP and ABPA, although EGPA and HES are not easily distinguishable by imaging (Video 1). Clinical or laboratory evidence of end-organ involvement or dysfunction warrants organ system-specific evaluation, when possible.
Figure 2.
The diagnostic approach to eosinophilia with pulmonary involvement. ABPA = allergic bronchopulmonary aspergillosis; ANCA = antinuclear antibody and antineutrophil cytoplasmic antibody; EGPA = eosinophilic granulomatosis with polyangiitis; HES = hypereosinophilic syndrome; HRCT = high resolution CT; IgE = immunoglobulin E; IgG = immunoglobulin G.
The evaluation of eosinophilia with pulmonary manifestations can be extensive, given the broad differential diagnosis and overlapping clinical, laboratory, and radiologic features. A systematic approach is prudent. When an underlying cause is not identified easily, management of eosinophilia with pulmonary involvement largely relies on eosinophil-directed treatment. With emerging reports on disease improvement with biologic therapies in some of these diseases, evidence of the role of eosinophils and their implication in pulmonary complications are becoming increasingly apparent. More studies are needed to demonstrate the glucocorticoid-sparing ability and long-term safety of biologic treatments.
Acknowledgments
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: We thank the patients who consented for participation in our clinical trials (NCT00001406) as well as Les Folio, DO, MPH, and Lester Davis for help with production of the video.
Additional information: The Video can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: Supported in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Supplementary Data
References
- 1.Cottin V., Bel E., Bottero P., Dalhoff K. Revisiting the systemic vasculitis in eosinophilic granulomatosis with polyangiitis (Churg-Strauss): a study of 157 patients by the Groupe d’Etudes et de Recherche sur les Maladies Orphelines Pulmonaires and the European Respiratory Society Taskforce on eosinophilic granulomatosis with polyangiitis (Churg-Strauss) Autoimmun Rev. 2017;16(1):1–9. doi: 10.1016/j.autrev.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 2.Comarmond C., Pagnoux C., Khellaf M. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis Study group cohort. Arthritis Rheumatism. 2013;65(1):270–281. doi: 10.1002/art.37721. [DOI] [PubMed] [Google Scholar]
- 3.Groh M., Pagnoux C., Baldini C. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss) (EGPA) Consensus Task Force recommendations for evaluation and management. Eur J Intern Med. 2015;26(7):545–553. doi: 10.1016/j.ejim.2015.04.022. [DOI] [PubMed] [Google Scholar]
- 4.Wechsler M.E., Akuthota P., Jayne D. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med. 2017;376(20):1921–1932. doi: 10.1056/NEJMoa1702079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogbogu P.U., Bochner B.S., Butterfield J.H. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol. 2009;124(6):1319–1325.e3. doi: 10.1016/j.jaci.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams K.W., Ware J., Abiodun A., Holland-Thomas N.C., Khoury P., Klion A.D. Hypereosinophilia in children and adults: a retrospective comparison. J Allergy Clin Immunol Pract. 2016;4(5):941–947.e1. doi: 10.1016/j.jaip.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simon H.U., Rothenberg M.E., Bochner B.S. Refining the definition of hypereosinophilic syndrome. J Allergy Clin Immunol. 2010;126(1):45–49. doi: 10.1016/j.jaci.2010.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khoury P., Desmond R., Pabon A. Clinical features predict responsiveness to imatinib in platelet-derived growth factor receptor-alpha-negative hypereosinophilic syndrome. Allergy. 2016;71(6):803–810. doi: 10.1111/all.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roufosse F., Cogan E., Goldman M. Lymphocytic variant hypereosinophilic syndromes. Immunol Allergy Clin North Am. 2007;27(3):389–413. doi: 10.1016/j.iac.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Gotlib J. World Health Organization-defined eosinophilic disorders: 2017 update on diagnosis, risk stratification, and management. Am J Hematol. 2017;92(11):1243–1259. doi: 10.1002/ajh.24880. [DOI] [PubMed] [Google Scholar]
- 11.Makowska J., Lewandowska-Polak A., Kowalski M.L. Hypersensitivity to aspirin and other NSAIDs: diagnostic approach in patients with chronic rhinosinusitis. Curr Allergy Asthma Rep. 2015;15(8):47. doi: 10.1007/s11882-015-0552-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Comhair S.A.A., Bochenek G., Baicker-McKee S. The utility of biomarkers in diagnosis of aspirin exacerbated respiratory disease. Respir Res. 2018;19(1):210. doi: 10.1186/s12931-018-0909-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki Y., Suda T. Eosinophilic pneumonia: a review of the previous literature, causes, diagnosis, and management. Allergol Int. 2019;68(4):413–419. doi: 10.1016/j.alit.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Marchand E., Reynaud-Gaubert M., Lauque D., Durieu J., Tonnel A.B., Cordier J.F. Idiopathic chronic eosinophilic pneumonia: a clinical and follow-up study of 62 cases. Medicine. 1998;77(5):299–312. doi: 10.1097/00005792-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Kligerman S., Raptis C., Larsen B. Radiologic, pathologic, clinical, and physiologic findings of electronic cigarette or vaping product use-associated lung injury (EVALI): evolving knowledge and remaining questions. Radiology. 2020;294(3):491–505. doi: 10.1148/radiol.2020192585. [DOI] [PubMed] [Google Scholar]
- 16.Philit F., Etienne-Mastroianni B., Parrot A., Guerin C., Robert D., Cordier J.F. Idiopathic acute eosinophilic pneumonia: a study of 22 patients. Am J Respir Crit Care Med. 2002;166(9):1235–1239. doi: 10.1164/rccm.2112056. [DOI] [PubMed] [Google Scholar]
- 17.Patel G., Greenberger P.A. Allergic bronchopulmonary aspergillosis. Allergy Asthma Proc. 2019;40(6):421–424. doi: 10.2500/aap.2019.40.4262. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal R., Aggarwal A.N., Sehgal I.S., Dhooria S., Behera D., Chakrabarti A. Utility of IgE (total and Aspergillus fumigatus specific) in monitoring for response and exacerbations in allergic bronchopulmonary aspergillosis. Mycoses. 2016;59(1):1–6. doi: 10.1111/myc.12423. [DOI] [PubMed] [Google Scholar]
- 19.Lai K., Chen R., Peng W., Zhan W. Non-asthmatic eosinophilic bronchitis and its relationship with asthma. Pulmonol Pharmacol Ther. 2017;47:66–71. doi: 10.1016/j.pupt.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Zhang R., Luo W., Liang Z. Eotaxin and IL-4 levels are increased in induced sputum and correlate with sputum eosinophils in patients with nonasthmatic eosinophilic bronchitis. Medicine. 2017;96(13) doi: 10.1097/MD.0000000000006492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Connell E.M., Nutman T.B. Eosinophilia in infectious diseases. Immunol Allergy Clin North Am. 2015;35(3):493–522. doi: 10.1016/j.iac.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunst H., Mack D., Kon O.M., Banerjee A.K., Chiodini P., Grant A. Parasitic infections of the lung: a guide for the respiratory physician. Thorax. 2011;66(6):528–536. doi: 10.1136/thx.2009.132217. [DOI] [PubMed] [Google Scholar]
- 23.Bartal C., Sagy I., Barski L. Drug-induced eosinophilic pneumonia: a review of 196 case reports. Medicine. 2018;97(4):e9688. doi: 10.1097/MD.0000000000009688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taweesedt P.T., Nordstrom C.W., Stoeckel J., Dumic I. Pulmonary manifestations of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: a systematic review. Biomed Res Int. 2019;2019:7863815. doi: 10.1155/2019/7863815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peter J.G., Lehloenya R., Dlamini S. Severe delayed cutaneous and systemic reactions to drugs: a global perspective on the science and art of current practice. J Allergy Clin Immunol Pract. 2017;5(3):547–563. doi: 10.1016/j.jaip.2017.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinfeld J., Bradford E.S., Brown J. Evaluation of clinical benefit from treatment with mepolizumab for patients with eosinophilic granulomatosis with polyangiitis. J Allergy Clin Immun. 2019;143(6):2170–2177. doi: 10.1016/j.jaci.2018.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrews J.C., Schunemann H.J., Oxman A.D., GRADE guidelines: 15 Going from evidence to recommendation-determinants of a recommendation’s direction and strength. J Clin Epidemiol. 2013;66(7):726–735. doi: 10.1016/j.jclinepi.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Puéchal X., Pagnoux C., Baron G. Non-severe eosinophilic granulomatosis with polyangiitis: long-term outcomes after remission-induction trial. Rheumatology (Oxford) 2019;58(12):2107–2116. doi: 10.1093/rheumatology/kez139. [DOI] [PubMed] [Google Scholar]
- 29.Puéchal X., Pagnoux C., Baron G. Adding azathioprine to remission-induction glucocorticoids for eosinophilic granulomatosis with polyangiitis (Churg-Strauss), microscopic polyangiitis, or polyarteritis nodosa without poor prognosis factors: a randomized, controlled trial. Arthritis Rheumatol. 2017;69(11):2175–2186. doi: 10.1002/art.40205. [DOI] [PubMed] [Google Scholar]
- 30.Grayson P.C., Monach P.A., Pagnoux C. Value of commonly measured laboratory tests as biomarkers of disease activity and predictors of relapse in eosinophilic granulomatosis with polyangiitis. Rheumatology. 2014;54(8):1351–1359. doi: 10.1093/rheumatology/keu427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Institutes of Health . National Institutes of Health; Bethesda, MD: 2017. Benralizumab in the treatment of eosinophilic granulomatosis with polyangiitis (EGPA) study (BITE). NCT03010436. ClinicalTrials.gov.https://clinicaltrials.gov/ct2/show/NCT03010436 Updated May 9, 2017. [Google Scholar]
- 32.National Institutes of Health . National Institutes of Health; Bethesda, MD: 2019. Efficacy and safety of benralizumab in EGPA compared to mepolizumab (MANDARA). NCT04157348. ClinicalTrials.gov.https://clinicaltrials.gov/ct2/show/NCT04157348 Updated December 8, 2020. [Google Scholar]
- 33.Khoury P., Abiodun A.O., Holland-Thomas N., Fay M.P., Klion A.D. Hypereosinophilic syndrome subtype predicts responsiveness to glucocorticoids. J Allergy Clin Immunol Pract. 2018;6(1):190–195. doi: 10.1016/j.jaip.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuang F.L., Fay M.P., Ware J. Long-term clinical outcomes of high-dose mepolizumab treatment for hypereosinophilic syndrome. J Allergy Clin Immunol Pract. 2018;6(5):1518–1527.e5. doi: 10.1016/j.jaip.2018.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rothenberg M.E., Klion A.D., Roufosse F.E. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med. 2008;358(12):1215–1228. doi: 10.1056/NEJMoa070812. [DOI] [PubMed] [Google Scholar]
- 36.Schwarz C., Muller T., Lau S., Parasher K., Staab D., Wahn U. Mepolizumab-a novel option for the treatment of hypereosinophilic syndrome in childhood. Pediatr Allergy Immunol. 2018;29(1):28–33. doi: 10.1111/pai.12809. [DOI] [PubMed] [Google Scholar]
- 37.Roufosse F., de Lavareille A., Schandené L. Mepolizumab as a corticosteroid-sparing agent in lymphocytic variant hypereosinophilic syndrome. J Allergy Clin Immun. 2010;126(4):828–835.e3. doi: 10.1016/j.jaci.2010.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Institutes of Health . National Institutes of Health; Bethesda, MD: 2016. Efficacy and safety study of mepolizumab in subjects with severe hypereosinophilic syndrome (HES). NCT02836496. ClinicalTrials.gov.https://ClinicalTrials.gov/show/NCT02836496 Updated February 21, 2020. [Google Scholar]
- 39.Kuang F.L., Legrand F., Makiya M. Benralizumab for PDGFRA-negative hypereosinophilic syndrome. N Engl J Med. 2019;380(14):1336–1346. doi: 10.1056/NEJMoa1812185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National Institutes of Health . National Institutes of Health; Bethesda, MD: 2019. A phase 3 study to evaluate the efficacy and safety of benralizumab in patients with hypereosinophilic syndrome (HES) (NATRON). NCT04191304. ClinicalTrials.gov.https://clinicaltrials.gov/ct2/show/NCT04191304 Updated November 30, 2020. [Google Scholar]
- 41.Wagner L.A., Speckart S., Cutter B., Gleich G.J. Treatment of FIP1L1/PDGFRA-negative hypereosinophilic syndrome with alemtuzumab, an anti-CD52 antibody. J Allergy Clin Immunol. 2009;123(6):1407–1408. doi: 10.1016/j.jaci.2009.01.069. [DOI] [PubMed] [Google Scholar]
- 42.Strati P., Cortes J., Faderl S., Kantarjian H., Verstovsek S. Long-term follow-up of patients with hypereosinophilic syndrome treated with Alemtuzumab, an anti-CD52 antibody. Clin Lymphoma Myeloma Leuk. 2013;13(3):287–291. doi: 10.1016/j.clml.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.King B., Lee A.I., Choi J. Treatment of hypereosinophilic syndrome with cutaneous involvement with the JAK inhibitors tofacitinib and ruxolitinib. J Invest Dermatol. 2017;137(4):951–954. doi: 10.1016/j.jid.2016.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Institutes of Health . National Institutes of Health; Bethesda, MD: 2002. Tyrosine kinase inhibition to treat myeloid hypereosinophilic syndrome. NCT00044304. ClinicalTrials.gov.https://clinicaltrials.gov/ct2/show/NCT00044304 Updated December 31, 2020. [Google Scholar]
- 45.Rumi E., Milosevic J.D., Selleslag D. Efficacy of ruxolitinib in myeloid neoplasms with PCM1-JAK2 fusion gene. Ann Hematol. 2015;94(11):1927–1928. doi: 10.1007/s00277-015-2451-7. [DOI] [PubMed] [Google Scholar]
- 46.Schwaab J., Knut M., Haferlach C. Limited duration of complete remission on ruxolitinib in myeloid neoplasms with PCM1-JAK2 and BCR-JAK2 fusion genes. Ann Hematol. 2015;94(2):233–238. doi: 10.1007/s00277-014-2221-y. [DOI] [PubMed] [Google Scholar]
- 47.Larivee N., Chin C.J. Aspirin desensitization therapy in aspirin-exacerbated respiratory disease: a systematic review. Int Forum Allergy Rhinol. 2020;10(4):450–464. doi: 10.1002/alr.22520. [DOI] [PubMed] [Google Scholar]
- 48.Lee R.U., White A.A., Ding D. Use of intranasal ketorolac and modified oral aspirin challenge for desensitization of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2010;105(2):130–135. doi: 10.1016/j.anai.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 49.Jean T., Eng V., Sheikh J. Effect of omalizumab on outcomes in patients with aspirin-exacerbated respiratory disease. Allergy Asthma Proc. 2019;40(5):316–320. doi: 10.2500/aap.2019.40.4241. [DOI] [PubMed] [Google Scholar]
- 50.Tuttle K.L., Buchheit K.M., Laidlaw T.M., Cahill K.N. A retrospective analysis of mepolizumab in subjects with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol Pract. 2018;6(3):1045–1047. doi: 10.1016/j.jaip.2018.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laidlaw T.M., Mullol J., Fan C. Dupilumab improves nasal polyp burden and asthma control in patients with CRSwNP and AERD. J Allergy Clin Immunol Pract. 2019;7(7):2462–2465.e1. doi: 10.1016/j.jaip.2019.03.044. [DOI] [PubMed] [Google Scholar]
- 52.Bachert C., Han J.K., Desrosiers M. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394(10209):1638–1650. doi: 10.1016/S0140-6736(19)31881-1. [DOI] [PubMed] [Google Scholar]
- 53.Domingo C., Pomares X. Can omalizumab be effective in chronic eosinophilic pneumonia? Chest. 2013;143(1):274. doi: 10.1378/chest.12-2035. [DOI] [PubMed] [Google Scholar]
- 54.Kaya H., Gumus S., Ucar E. Omalizumab as a steroid-sparing agent in chronic eosinophilic pneumonia. Chest. 2012;142(2):513–516. doi: 10.1378/chest.11-1881. [DOI] [PubMed] [Google Scholar]
- 55.Agarwal R., Aggarwal A.N., Dhooria S. A randomised trial of glucocorticoids in acute-stage allergic bronchopulmonary aspergillosis complicating asthma. Eur Respir J. 2016;47(2):490–498. doi: 10.1183/13993003.01475-2015. [DOI] [PubMed] [Google Scholar]
- 56.Agarwal R., Dhooria S., Singh Sehgal I. A randomized trial of itraconazole vs prednisolone in acute-stage allergic bronchopulmonary aspergillosis complicating asthma. Chest. 2018;153(3):656–664. doi: 10.1016/j.chest.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 57.Stevens D.A., Schwartz H.J., Lee J.Y. A randomized trial of itraconazole in allergic bronchopulmonary aspergillosis. N Engl J Med. 2000;342(11):756–762. doi: 10.1056/NEJM200003163421102. [DOI] [PubMed] [Google Scholar]
- 58.Matsumoto N., Shigekusa T., Matsuo A., Tsubouchi H., Yanagi S., Nakazato M. Allergic bronchopulmonary aspergillosis complicated by eosinophilic chronic rhinosinusitis successfully treated with mepolizumab. Respirol Case Rep. 2019;7(7) doi: 10.1002/rcr2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aydın Ö., Sözener Z.Ç., Soyyiğit Ş. Omalizumab in the treatment of allergic bronchopulmonary aspergillosis: one center’s experience with 14 cases. Allergy Asthma Proc. 2015;36(6):493–500. doi: 10.2500/aap.2015.36.3909. [DOI] [PubMed] [Google Scholar]
- 60.National Institutes of Health . National Institutes of Health; Bethesda, MD: 2020. A study to evaluate the efficacy and safety of dupilumab in participants with allergy bronchopulmonary aspergillosis (ABPA) (LIBERTY ABPA AIRED). NCT04442269. clinicalTrials.gov.https://clinicaltrials.gov/ct2/show/NCT04442269 Updated November 6, 2020. [Google Scholar]
- 61.Burris D., Rosenberg C.E., Schwartz J.T. Pediatric hypereosinophilia: characteristics, clinical manifestations, and diagnoses. J Allergy Clin Immunol Pract. 2019;7(8):2750–2758.e2. doi: 10.1016/j.jaip.2019.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bernheim A., McLoud T. A review of clinical and imaging findings in eosinophilic lung diseases. AJR Am J Roentgenol. 2017;208(5):1002–1010. doi: 10.2214/AJR.16.17315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.