Abstract
Background
To compare RCB (Residual Cancer Burden) and Neo-Bioscore in terms of prognostic performance and see if adding pathological variables improve these scores.
Methods
We analysed 750 female patients with invasive breast cancer (BC) treated with neoadjuvant chemotherapy (NAC) at Institut Curie between 2002 and 2012. Scores were compared in global population and by BC subtype using Akaike information criterion (AIC), C-Index (concordance index), calibration curves and after adding lymphovascular invasion (LVI) and pre-/post-NAC TILs levels.
Results
RCB and Neo-Bioscore were significantly associated to disease-free and overall survival in global population and for triple-negative BC. RCB had the lowest AICs in every BC subtype, corresponding to a better prognostic performance. In global population, C-Index values were poor for RCB (0.66; CI [0.61–0.71]) and fair for Neo-Bioscore (0.70; CI [0.65–0.75]). Scores were well calibrated in global population, but RCB yielded better prognostic performances in each BC subtype. Concordance between the two scores was poor. Adding LVI and TILs improved the performance of both scores.
Conclusions
Although RCB and Neo-Bioscore had similar prognostic performances, RCB showed better performance in BC subtypes, especially in luminal and TNBC. By generating fewer prognostic categories, RCB enables an easier use in everyday clinical practice.
Subject terms: Breast cancer, Breast cancer, Chemotherapy
Background
Neoadjuvant chemotherapy (NAC) is currently administered to patients with locally advanced breast cancers (BC), to BC of poor prognosis (triple-negative and HER2-positive tumours, or BC with nodal involvement and/or high proliferation rates), or to early stage BC having an indication of systemic therapy.1,2 Beyond increasing breast-conserving surgery rates,3–5 NAC enables the evaluation of systemic treatments in vivo, thus making it theoretically possible to discontinue ineffective treatments.6,7 Response to NAC also carries important prognostic information. Indeed, patients with pathological complete response (pCR) after NAC were reported to have more favourable long-term outcomes,8,9 especially for HER2-positive and triple-negative BC (TNBC).10 However, a minority of tumours reach pCR after NAC. Depending on definitions of residual disease (ypT0 ypN0 or ypT0/is, respectively), pCR rates vary from 13% (IC95%12–14) to 22% (IC95%21,22).10
Hence, prognostic scores such as RCB (Residual Cancer Burden index),8 CPS (Clinical-Pathologic Scoring),11 CPS + EG (oestrogen-receptor (E) status and nuclear grade (G))11 and Neo-Bioscore12 were developed to classify BC patients into different prognostic risk categories after NAC. Thereby, patients considered as having a high risk of relapse can be candidates to further second-line treatments (TDM-1, Capecitabine) or clinical trials. Pathological variables such as lymphovascular invasion (LVI) and tumour-infiltrating lymphocytes’ (TILs) may also have a prognostic value after NAC.13,14 LVI, defined as the presence of tumour cells in lymphatic or blood vessels, was identified as a risk factor of axillary and distant metastasis,15,16 associated to higher risks of node involvement, distant metastasis and death.13,17–19 High TILs levels on BC biopsy have been associated to high pCR rates in the neoadjuvant setting and to better outcomes in the adjuvant setting.20–23
Altogether, although RCB has been suggested as the preferred score,24 its prognostic performance has not yet been compared to more recent models such as Neo-Bioscore. Furthermore, despite growing evidence of their impact, whether LVI and/or TILs improve the prognostic performance of scores after NAC remains unknown.
The objective of the present study was to compare the main models that exist to refine prognosis after NAC by evaluating their prognostic performance in a large real-life cohort of BC patients, and to determine whether adding pathological variables improved these scores.
Methods
Patients and tumours
We analysed a cohort of 750 female patients with T1–3NxM0 invasive BC (NEOREP Cohort, CNIL declaration number 1547270) treated with NAC at Institut Curie between 2002 and 2012. The cohort included unifocal, unilateral, nonrecurrent, nonmetastatic tumours, excluding T4 tumours (inflammatory, chest wall or skin invasion). All patients included in the cohort received NAC. Patients were treated according to national guidelines. Approved by the Breast Cancer Study Group of Institut Curie, the study was conducted according to institutional and ethical rules concerning research on tissue specimens and patients. Informed consent from patients was not required.
Information on clinical and tumour characteristics were retrieved from medical health records.
ER, PR and HER2 positivity determination and treatment protocol are detailed in the supplemental material. Based on immunohistochemistry surrogates, pathological subtypes were defined as follows: tumours positive for either ER or PR and negative for HER2 were classified as luminal; tumours positive for HER2 were classified as HER2-positive BC; tumours negative for ER, PR and HER2 were classified as triple-negative BC (TNBC).
RCB and Neo-Bioscore were retrospectively reviewed by a single pathologist.
Prognostic models
pCR was defined as the absence of residual invasive cancer cells in the breast and axillary lymph nodes (ypT0/is +ypN0).10
Residual cancer burden (RCB) index was calculated using the web-based calculator (available on internet),25 by considering dimensions of the primary tumour, tumour bed cellularity and axillary nodal burden.8 RCB is composed of four categories: RCB‐0 (complete pathologic response = pCR), RCB‐I (minimal residual disease), RCB‐II (moderate residual disease) and RCB‐III (extensive residual disease).
Neo-Bioscore12 derives from the CPS and CPS + EG scores. According to the American Joint Committee on Cancer (AJCC) guidelines, Neo-Bioscore is calculated by considering for each patient the pretreatment clinical stage and the post-treatment pathologic stage.26 Additional points are added in case of ER-negative disease, nuclear grade 3 tumours, and HER2-negative tumours. Neo-Bioscore is composed of eight categories (Neo-Bioscore 0–7), of increasingly poor prognosis.
LVI was defined as the presence of carcinoma cells within a finite endothelial-lined space (a lymphatic or blood vessel). Presence or absence of LVI was determined by unstained standard formalin-fixed paraffin-embedded examination. Immunostaining with vascular markers was occasionally performed to rule out invasive carcinoma with shrinkage artefact. Data concerning LVI were extracted from pathologic records by two independent researchers.
TILs levels were evaluated on pretreatment core needle biopsies and post-NAC surgical specimens for the presence of mononuclear cells infiltrate (including lymphocytes and plasma cells, excluding polymorphonuclear leukocytes), following the international TILs Working Group recommendations.27 They were evaluated in stroma, within tumour scar border, after excluding areas around ductal carcinoma in situ, tumour zones with necrosis and artefacts, and were scored continuously as the average percentage of stromal area occupied by mononuclear cells. Pre-NAC TILs were described in categories (low: <10%; intermediate: 11–59%; high ≥60%).14 Post-NAC TILs were analysed as a continuous variable, as no threshold has yet been determined.
Since RCB and Neo-Bioscore are composed of a different number of risk categories (four risk categories for RCB vs. eight risk categories for Neo-Bioscore, respectively), establishing common risk categories was necessary to compare the two scores. Hence, based on the predicted risk of events, we established three risk categories: low risk (predicted 5-year DFS > 90%, corresponding to RCB-I/pCR and Neo-Bioscore 0–3); intermediate risk (predicted 5-year DFS comprised between 70 and 90%, corresponding to RCB-II and Neo-Bioscore 4–5); and high risk (predicted 5-year DFS < 70%, corresponding to RCB-III and Neo-Bioscore 6–7).
Study endpoints
Disease-free survival (DFS) was defined as the time from surgery to death, loco-regional recurrence or distant recurrence, whichever occurred first. Overall survival (OS) was defined as the time from surgery to death. Patients for whom none of these events were recorded were censored at the date of their last known contact.
Statistical analysis
The Akaike Information Criterion (AIC)28 was used to compare prognostic performances. AIC determines the best prognostic model from a set of models by selecting the one with the highest likelihood under the constraint of the smallest number of predictors. The lowest AIC value corresponds to the best model.
Discrimination (i.e. whether the relative ranking of individual predictions is in the correct order) was evaluated using the concordance index (C-Index).29 C-Index is the probability that given two randomly selected patients, the patient with the most pejorative outcome will in fact have the most pejorative predicted outcome. A C-index value of 0.9–1.0 corresponds to an excellent discriminative power, whereas a C-Index value of 0.5 corresponds to a worthless test. Its discriminative power is considered poor from 0.6 to 0.7, fair from 0.7 to 0.8 and good from 0.8 to 0.9. C-Index can be used for censored data. A bootstrap method with 500 resample was used to determine confidence intervals.
Calibration (i.e. the relationship between outcomes observed and predicted probabilities) was evaluated with graphical representations (calibrations curves). By definition, a well-calibrated risk score or prediction rule attributes the correct probability of event to all predicted risk levels. A poorly calibrated rule under- or over-predicts the probability of events. Calibration applied on survival analysis are particular since observations are events. Well-calibrated models have an intercept α of zero and a slope β of 1. The calibration of censored data is mainly visual. In this study, 60-months survival was used for the calibration of cox models, which corresponds to our median follow-up.
All analyses were performed in global population and after stratification by BC subtype. Qualitative variables were compared by Chi-square or Fisher exact tests and quantitative ones by Student t-tests. Survival probabilities were estimated by Kaplan–Meier method, and survival curves were compared with log-rank tests. Hazard ratios and their 95% confidence intervals were calculated with the Cox proportional hazards model. Significance threshold was of 5%. Analyses were performed with R software, version 3.3.30
Results
Patient characteristics
Our cohort was composed of 750 patients. Patient characteristics are detailed in Appendix Table A1. Mean age at diagnosis was of 48.3 years. Tumour distribution by BC subtype was as follows: luminal: n = 221 (29.5%); TNBC: n = 320 (42.7%); HER2-positive: n = 209 (27.9%). At NAC completion, 281 (37.5%) patients had reached pCR. After a median follow-up of 52.8 months (CI [50.2–56.3]), 146 events were observed.
The distribution of patients into the three risk categories established is detailed Table 1. The distribution of RCB and Neo-Bioscore in global population and by BC subtype is represented in Appendix Fig. A1.
Table 1.
Distribution of RCB and Neo-Bioscore, in global population and by BC subtype.
| Scores | Global population | Luminal | TNBC | HER2-positive | Risk category |
|---|---|---|---|---|---|
| n = 750 | n = 221 | n = 320 | n = 209 | ||
| RCB | |||||
| RCB-0 | 281 (37.5) | 31 (14) | 138 (43.1) | 112 (53.6) | Low (44.5%) |
| RCB-I | 53 (7.1) | 14 (6.3) | 19 (5.9) | 20 (9.6) | |
| RCB-II | 286 (38.1) | 100 (45.2) | 123 (38.4) | 63 (30.1) | Intermediate (38.1%) |
| RCB-III | 130 (17.3) | 76 (34.4) | 40 (12.5) | 14 (6.7) | High (17.3%) |
| Neo-Bioscore | |||||
| 0 | 5 (0.7) | 0 (0) | 0 (0) | 5 (2.4) | Low |
| 1 | 54 (7.2) | 12 (5.4) | 0 (0) | 42 (20.1) | (58.8%) |
| 2 | 147 (19.6) | 54 (24.4) | 12 (3.8) | 81 (38.8) | |
| 3 | 235 (31.3) | 79 (35.7) | 96 (30) | 60 (28.7) | |
| 4 | 227 (30.3) | 73 (33) | 133 (41.6) | 21 (10) | Intermediate |
| 5 | 76 (10.1) | 3 (1.4) | 73 (22.8) | 0 (0) | (40.4%) |
| 6 | 6 (0.8) | 0 (0) | 6 (1.9) | 0 (0) | High |
| 7 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | (0.8%) |
Low-risk category = predicted 5-year DFS > 90%, Intermediate-risk category = predicted 5-year DFS comprised between 70 and 90%, High-risk categor = predicted 5-year DFS < 70%.
According to RCB, 44.5% of patients were classified in the low-risk category (37.5% for RCB-0 and 7.1% RCB-I, respectively), 38.1% were classified in the intermediate-risk category and 17.3% were classified in the high-risk category.
According to Neo-Bioscore, 58.8% of patients were classified in the low-risk category (Neo-Bioscore 0/1/2/3: 1%; 8%; 19% and 32.7%, respectively), 40.4% were classified in the intermediate-risk category, whereas only 0.8% were classified in the high-risk category.
Association between Neo-Bioscore, RCB and DFS
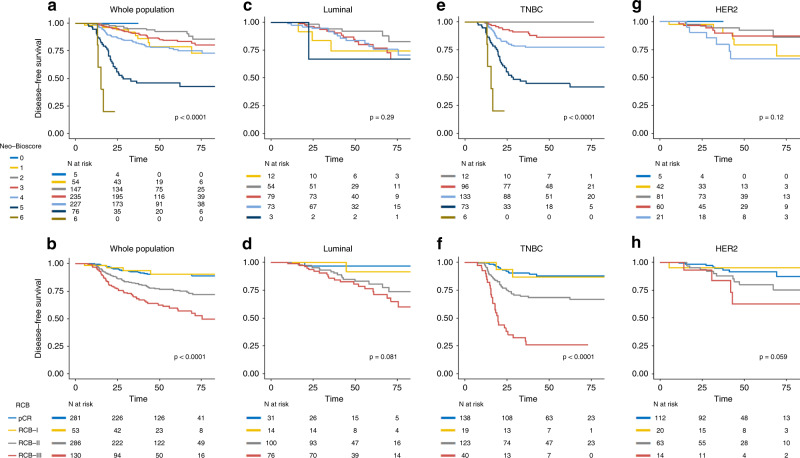
RCB and Neo-Bioscore were both significantly associated with DFS in global population (p < 0.0001) (Fig. 1a, b) and for TNBC patients (p < 0.0001) (Fig. 1e, f). A trend in favour of an association between RCB and DFS was observed for HER2-positive and luminal BC (p = 0.059 and p = 0.08, respectively) (Fig. 1c, d) (Fig. 1g, h).
Fig. 1. Association between RCB, Neo-Bioscore and disease free survival in the whole population and by pathological subtypes.
a Neo-bioscore in the whole population; b RCB in the whole population; c Neo-bioscore in the Luminal subtype; d RCB in the Luminal subtype; e Neo-bioscore in the Triple negative subtype; f RCB in the Triple negative subtype; g Neo-bioscore in the HER2-positive subtype; h RCB in the HER2-positive subtype.
RCB-0 and RCB-I curves were superimposed in all cases (in global population and in every BC subtype) and were both associated to a very good prognosis (5 y DFS rate = 90%, CI [86.5–94.4%] and 90% CI [81.7–100], respectively).
Similar results were obtained for OS (Appendix Fig. A2).
Comparison of prognostic performance
Prognostic performance
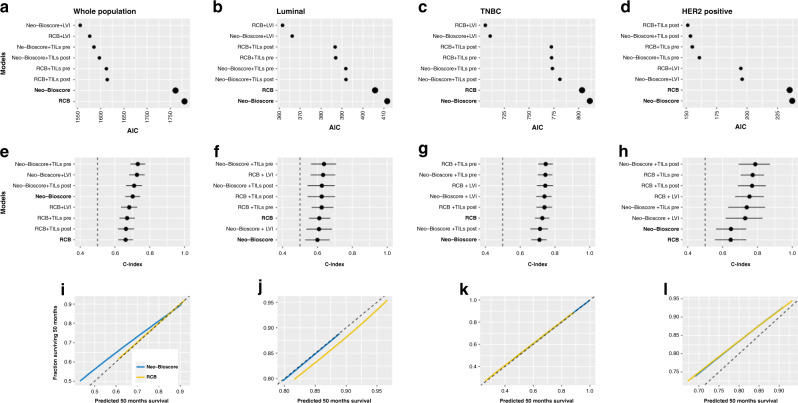
We assessed the prognostic performance of Neo-Bioscore and RCB by calculating AIC (Fig. 2a–d). In global population, Neo-Bioscore was associated to a slightly lower AIC than RCB (AIC: 1738 vs. 1756, respectively), corresponding to a better prognostic performance.
Fig. 2. AIC, C-index and calibration curves for RCB and neo-bioscore, in whole population and by pathological substypes.
a AIC in the whole population; b AIC in the luminal subtype; c AIC in the triple negative subtype; d AIC in the HER2-positive subtype; e C-index in the whole population; f C-index in the luminal subtype; g C-index in the triple negative subtype; h C-index in the HER2-positive subtype; i Calibration curves for the whole population; j Calibration curves for the luminal subtype; k Calibration curves for the triple negative subtype; l Calibration curves for the HER2-positive subtype.
However, RCB had the lowest AICs for every BC subtype (luminal: 403 vs. 408, respectively; TNBC: 800 vs. 808, respectively; HER2-positive BC: 232 vs. 234, respectively).
Discrimination
In global population, C-Index values were poor for RCB (0.66; CI [0.61–0.71]) and fair for Neo-Bioscore (0.70; CI [0.65–0.75]). C-Index values were higher in TNBC (RCB: 0.73, 9CI [0.68–0.77]; Neo-Bioscore: 0.71, CI [0.66–0.75]) and HER2-positive tumours (RCB: 0.64, CI [0.56–0.73]; Neo-Bioscore: 0.64, CI [0.56–0.73]) compared to luminal tumours (RCB: 0.61, CI [0.55–0.67], and Neo-Bioscore: 0.60, CI [0.53–0.67]) (Fig. 2e-h).
Calibration
Five-year calibration curves are represented Fig. 2i–l. Both Neo-Bioscore and RCB were well calibrated in global population and in every BC subtype. The best calibration was observed for TNBC patients. Altogether, both RCB and Neo-Bioscore were well calibrated among BC subtypes, and accurately discriminated the risk of patients. Neo-Bioscore had good performance in global population. RCB had slightly better prognostic performances when each BC subtype was evaluated separately.
Concordance between Neo-Bioscore and RCB
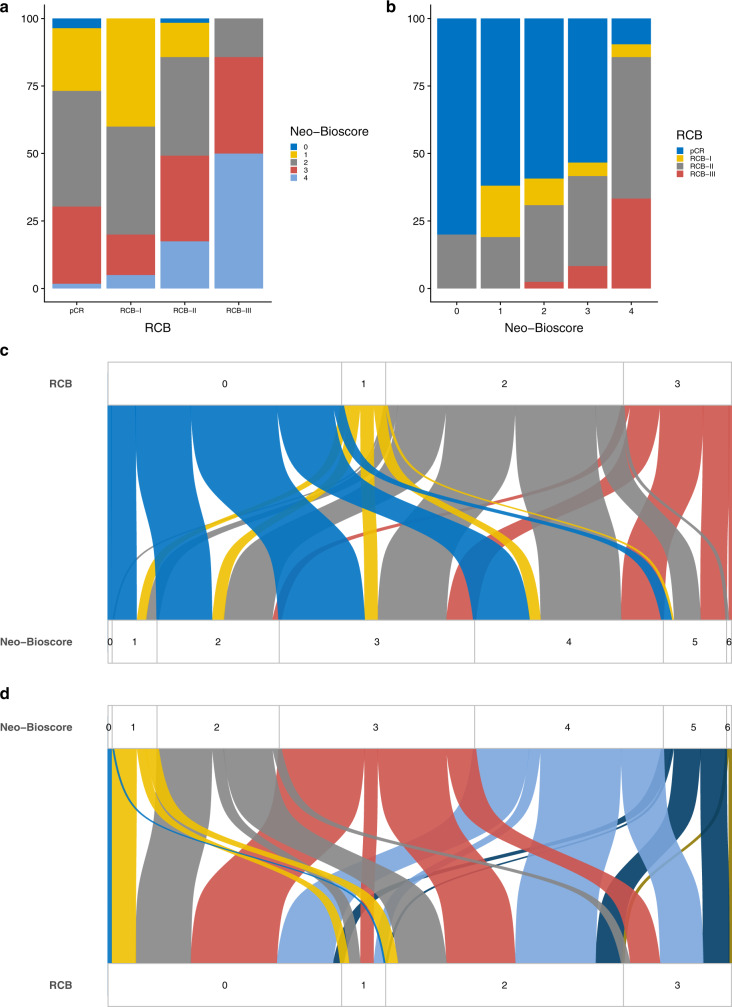
Distributions of Neo-Bioscore according to RCB, and RCB according to Neo-Bioscore, respectively, are detailed Fig. 3. Concordance between the two scores was poor. 28% of patients classified RCB-pCR (i.e. low-risk category) corresponded to intermediate and high-risk categories according to Neo-Bioscore (Neo-Bioscore 4: 23.8%, and Neo-Bioscore 5: 4.2%, respectively) (Fig. 3a, c). Conversely, 34.6% patients classified RCB-III (i.e. high-risk category) corresponded to low-risk categories according to Neo-Bioscore (Neo-Bioscore 2–3: 7.7% and 26.9%, respectively).
Fig. 3. Concordance between RCB and neo-bioscore in the global population.
a Neo-bioscore repartition according to RCB. b RCB repartition according to neo-bioscore. c Sankey plot of repartition of neo-bioscore according to RCB. d Sankey plot of repartition of RCB according to neo-bioscore.
Similarly, among the patients classified in the low-risk categories according to Neo-Bioscore (Neo-Bioscore 0–3), 49.8% corresponded to intermediate or high-risk categories according to RCB (RCB-II or III) (Fig. 3b, d).
Hence, on an individual scale, RCB and Neo-Bioscore displayed poor consistency (Fig. 3c). RCB and Neo-Bioscore were discrepant in 49.3% of cases when classifying patients into the three risk categories (low/intermediate/high). Despite good prognostic performances on a population scale, RCB and Neo-Bioscore were poorly concordant on an individual scale.
In BC subtypes, the highest concordance was observed for HER2-positive tumours (67% vs. only 30% of concordant predictions for luminal tumours) (Appendix Figs. A3, A4). 50.9% of predictions were concordant for TNBC tumours (Appendix Fig. A5). In the patient with a real event (recurrence, metastasis or death), concordance decreased to 35.5% in global population, 29.4% in HER2-positive tumours, and only 16.6% in the luminal tumours (vs. 48% in the TNBC population), underlying the difficulty of models to predict survival in high-risk groups.
Addition of histological variables
Adding pathological variables to RCB and Neo-Bioscore slightly improved their prognostic performance.
In global population, AICs of models were systematically improved by adding LVI or pre-/post-NAC TILs level (Fig. 2). The best AIC was observed for Neo-Bioscore + LVI.
When analysing by BC subtype (Fig. 2), LVI also improved AICs of both RCB and Neo-Bioscore for TNBC and luminal tumours. For HER2-positive patients, pre- and post-NAC TILs levels improved models the most.
However, adding pathological variables did not improve C-Index, neither in global population, nor in BC subtypes.
Discussion
Our study on a large cohort of BC patients treated with NAC showed that RCB and Neo-Bioscore were both significantly associated with prognosis in global population and in BC subtypes, especially for TNBC patients. Analyses were not relevant in the HER2-positive population due to the small number of events. Neo-Bioscore showed better performance in global population, whereas RCB offered better performance in pathological subtypes, notably for luminal and TNBC. In addition, our results suggest that these scores might be improved by the addition of pathological variables such as TILs or LVI. However, on an individual scale, RCB and Neo-Bioscore were highly discrepant in their predictions (~50% of consistency between risk categories).
Both scores had been individually validated in independent cohorts. An external validation of Neo-Bioscore was performed by Bergquist et al.31 on 43.320 patients from the National Cancer Database. Neo-Bioscore had a greater discriminative power compared to CPS + EG and AJCC clinical staging (5-year OS). The long-term prognostic relevance of RCB within each BC subtype was demonstrated by Symmans et al.32 in five BC cohorts (n = 1158). RCB has also been shown to be highly reproducible.33 Concerning direct comparison of the different scoring systems, Choi et al.34 evaluated seven pathologic classification systems, of which RCB and CPS + EG. RCB had the best performance compared to the other scoring systems, both for OS and distant disease-free survival (DDFS). However, Neo-Bioscore (an improvement of the CPS + EG score) was not evaluated in the latter study. Provenzano et al.24 had already suggested that RCB index was the preferred method for more detailed quantification of residual disease at NAC completion. Hence, our study adds strength to literature by being the first to our knowledge to compare the prognostic performance of RCB and Neo-Bioscore on a large real-life cohort of BC patients.
Identifying the best model to classify patients into prognostic categories after NAC is crucial, notably since scoring systems can help identify patients of poor prognosis that can be candidates to further second-line treatments or clinical trials. In addition, it appears important to determine the best prognostic score so that a unique score can be used in studies, to help improve their comparability. Indeed, different staging systems yield different estimates for future risks. Very different predictions can be obtained for the same patient. In our study, both scores showed high performance on a population scale, but were poorly concordant with one another on an individual scale. These limitations are common to numerous risk calculators and partially explains the lack of widespread use in routine practice.35 These differences could be explained by the fact that these models do not capture the same response patterns. Indeed, whereas RCB only considers tumour size variables (primary tumour dimensions, tumour bed cellularity and axillary nodal burden), Neo-Bioscore also comprises pathological characteristics such as oestrogen-receptor status, nuclear grade and HER2 status. Altogether, since prognostic predictions in global population are currently outdated, pathological subtypes should be considered as distinct entities, and their prognosis should be evaluated independently. Another explanation for these discrepancies could come from the difference (and the very large number) of categories of both models. Hence, the worst concordance rates were found in the patient who expected an event (recurrence, death or metastasis), highlighting the difficulty of numerous models to identify the high-risk groups.
In an effort to improve prognostic performance, other studies suggested considering pathological variables as prognostic elements after NAC, alone or in combination with existing models.13,20,23Von Minckwitz et al.36 evaluated Ki67, a proliferation index, as a prognostic marker on 1151 patients after NAC. Patients with high Ki67 levels (>35.1) had significantly higher recurrence and death rates compared to patients with low or intermediate Ki67 levels. Adding Ki67 to the analysis of pCR was more contributive than pCR only for luminal BC. Sheri et al.37 also showed that the addition of post-treatment Ki67 to RCB improved the prediction of long-term outcome. However, Ki67 suffers from poor reproducibility and its assessment is difficult to standardise.38 Hence, its clinical utility remains limited in routine care. TILs on pretreatment biopsy have been associated with high pCR rates in the neoadjuvant setting and with better outcomes in the adjuvant setting.14,20,21,23 In addition, their assessment is rather standardised according to guidelines of the TILs working group.27 The added value of post-NAC TILs remains to be determined. Indeed, different prognostic values have been described among BC subtypes, with higher levels being associated with a good outcome in TNBC patients with residual disease after NAC,39 but with a poor outcome in HER2-positive patients.23 Asano et al.40 suggested that RCB combined with post-NAC TILs could be a more sensitive predictor for BC recurrence after NAC, with a 0.048 risk of recurrence (hazard ratio) in global population for RCB-TILs-positive patients (p < 0.001), 0.041 in TNBC patients (p = 0.018), 0.134 in HER2-positive patients (p = 0.036) and 0.081 for luminal tumours (p = 0.002). Future models should probably integrate this information.
In conclusion, our results suggest that although RCB and Neo-Bioscore offer similar prognostic performances to classify patients at NAC completion in global population, RCB showed better performance in pathological subtypes, especially in luminal and TNBC. RCB offers the advantage of generating fewer prognostic classes compared to Neo-Bioscore, which enables an easier use in everyday real-life practice. Further studies are warranted to confirm the present data and to evaluate the prognostic performance of other pathological variables like TILs or LVI that could be included in future scores to improve their prognosis performance.
Supplementary information
Acknowledgements
None.
Author contributions
Wrote the paper: J.L., E.L. Proofreading English text: J.L. Conceived and designed the experiments: E.L., A.S.H., G.B., D.d.C., J.Y.P., F.R. Analysed the data: E.L., J.L., T.B., J.G., M.L., J.G.F., F.C. Contributed to materials/analysis tools: D.d.C., J.G.F., F.C.
Ethics approval and consent to participate
Approved by the Breast Cancer Study Group of Institut Curie, the study was conducted according to institutional and ethical rules concerning research on tissue specimens and patients and in accordance with the Declaration of Helsinki. Informed consent from patients was not required.
Data availability
All supplementary data are available from authors on reasonable request.
Competing interests
The authors declare no competing interests.
Funding information
None.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Enora Laas, Julie Labrosse
Supplementary information
Supplementary information is available for this paper at 10.1038/s41416-020-01251-3.
References
- 1.Kaufmann M, von Minckwitz G, Smith R, Valero V, Gianni L, Eiermann W, et al. International expert panel on the use of primary (Preoperative) systemic treatment of operable breast cancer: review and recommendations. J. Clin. Oncol. 2003;21:2600–2608. doi: 10.1200/JCO.2003.01.136. [DOI] [PubMed] [Google Scholar]
- 2.Kaufmann M, von Minckwitz G, Bear HD, Buzdar A, McGale P, Bonnefoi H, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: new perspectives 2006. Ann. Oncol. 2007;18:1927–1934. doi: 10.1093/annonc/mdm201. [DOI] [PubMed] [Google Scholar]
- 3.Mieog JSD, van der Hage JA, van de Velde CJH. Neoadjuvant chemotherapy for operable breast cancer. Br. J. Surg. 2007;94:1189–1200. doi: 10.1002/bjs.5894. [DOI] [PubMed] [Google Scholar]
- 4.Mauri D, Pavlidis N, Ioannidis JPA. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J. Natl Cancer Inst. 2005;97:188–194. doi: 10.1093/jnci/dji021. [DOI] [PubMed] [Google Scholar]
- 5.Jackisch C, Harbeck N, Huober J, von Minckwitz G, Gerber B, Kreipe H-H, et al. 14th St. Gallen International Breast Cancer Conference 2015: Evidence, Controversies, Consensus—Primary Therapy of Early Breast Cancer: Opinions Expressed by German Experts. Breast Care Basel Switz. 2015;10:211–219. doi: 10.1159/000433590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandão M, Reyal F, Hamy A-S, Piccart-Gebhart M. Neoadjuvant treatment for intermediate/high-risk HER2-positive and triple-negative breast cancers: no longer an « option » but an ethical obligation. ESMO Open. 2019;4:e000515. doi: 10.1136/esmoopen-2019-000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reyal F, Hamy AS, Piccart MJ. Neoadjuvant treatment: the future of patients with breast cancer. ESMO Open. 2018;3:e000371. doi: 10.1136/esmoopen-2018-000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J. Clin. Oncol. 2007;25:4414–4422. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 9.Guarneri V, Broglio K, Kau S-W, Cristofanilli M, Buzdar AU, Valero V, et al. Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. J. Clin. Oncol. 2006;24:1037–1044. doi: 10.1200/JCO.2005.02.6914. [DOI] [PubMed] [Google Scholar]
- 10.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 11.Jeruss JS, Mittendorf EA, Tucker SL, Gonzalez-Angulo AM, Buchholz TA, Sahin AA, et al. Combined use of clinical and pathologic staging variables to define outcomes for breast cancer patients treated with neoadjuvant therapy. J. Clin. Oncol. 2008;26:246–252. doi: 10.1200/JCO.2007.11.5352. [DOI] [PubMed] [Google Scholar]
- 12.Mittendorf EA, Vila J, Tucker SL, Chavez-MacGregor M, Smith BD, Symmans WF, et al. The Neo-Bioscore update for staging breast cancer treated with neoadjuvant chemotherapy: incorporation of prognostic biologic factors into staging after treatment. JAMA Oncol. 2016;2:929–36. doi: 10.1001/jamaoncol.2015.6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamy A-S, Lam G-T, Laas E, Darrigues L, Balezeau T, Guerin J, et al. Lymphovascular invasion after neoadjuvant chemotherapy is strongly associated with poor prognosis in breast carcinoma. Breast Cancer Res. Treat. 2018;169:295–304. doi: 10.1007/s10549-017-4610-0. [DOI] [PubMed] [Google Scholar]
- 14.Denkert C, Minckwitz G, von, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19:40–50. doi: 10.1016/S1470-2045(17)30904-X. [DOI] [PubMed] [Google Scholar]
- 15.Lee AHS, Pinder SE, Macmillan RD, Mitchell M, Ellis IO, Elston CW, et al. Prognostic value of lymphovascular invasion in women with lymph node negative invasive breast carcinoma. Eur. J. Cancer. 2006;42:357–362. doi: 10.1016/j.ejca.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 16.Rakha EA, Martin S, Lee AHS, Morgan D, Pharoah PDP, Hodi Z, et al. The prognostic significance of lymphovascular invasion in invasive breast carcinoma. Cancer. 2012;118:3670–3680. doi: 10.1002/cncr.26711. [DOI] [PubMed] [Google Scholar]
- 17.Liu YL, Saraf A, Lee SM, Zhong X, Hibshoosh H, Kalinsky K, et al. Lymphovascular invasion is an independent predictor of survival in breast cancer after neoadjuvant chemotherapy. Breast Cancer Res. Treat. 2016;157:555–564. doi: 10.1007/s10549-016-3837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi MK, Park YH, Kil WH, Lee JE, Nam SJ, Ahn JS, et al. Clinicopathological features of early failure of neoadjuvant chemotherapy in locally advanced breast cancer. Cancer Chemother. Pharmacol. 2014;74:521–529. doi: 10.1007/s00280-014-2542-5. [DOI] [PubMed] [Google Scholar]
- 19.Abdel-Fatah TM, Ball G, Lee AHS, Pinder S, MacMilan RD, Cornford E, et al. Nottingham Clinico-Pathological Response Index (NPRI) after neoadjuvant chemotherapy (Neo-ACT) accurately predicts clinical outcome in locally advanced breast cancer. Clin. Cancer Res. 2015;21:1052–1062. doi: 10.1158/1078-0432.CCR-14-0685. [DOI] [PubMed] [Google Scholar]
- 20.Mao Y, Qu Q, Zhang Y, Liu J, Chen X, Shen K. The value of tumor infiltrating lymphocytes (TILs) for predicting response to neoadjuvant chemotherapy in breast cancer: a systematic review and meta-analysis. PLoS ONE. 2014;9:e115103. doi: 10.1371/journal.pone.0115103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J. Clin. Oncol. 2010;28:105–113. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 22.Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J. Clin. Oncol. 2015;33:983–991. doi: 10.1200/JCO.2014.58.1967. [DOI] [PubMed] [Google Scholar]
- 23.Hamy A-S, Pierga J-Y, Sabaila A, Laas E, Bonsang-Kitzis H, Laurent C, et al. Stromal lymphocyte infiltration after neoadjuvant chemotherapy is associated with aggressive residual disease and lower disease-free survival in HER2-positive breast cancer. Ann. Oncol. 2017;28:2233–2240. doi: 10.1093/annonc/mdx309. [DOI] [PubMed] [Google Scholar]
- 24.Provenzano E, Bossuyt V, Viale G, Cameron D, Badve S, Denkert C, et al. Standardization of pathologic evaluation and reporting of postneoadjuvant specimens in clinical trials of breast cancer: recommendations from an international working group. Mod. Pathol. 2015;28:1185–1201. doi: 10.1038/modpathol.2015.74. [DOI] [PubMed] [Google Scholar]
- 25.Residual Cancer Burden Calculator [Internet]. http://www3.mdanderson.org/app/medcalc/index.cfm?pagename=jsconvert3n (2018).
- 26.Giuliano AE, Connolly JL, Edge SB, Mittendorf EA, Rugo HS, Solin LJ, et al. Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017;67:290–303. doi: 10.3322/caac.21393. [DOI] [PubMed] [Google Scholar]
- 27.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015;26:259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akaike H. A new look at the statistical model identification. IEEE Trans. Autom. Control. 1974;19:716–723. doi: 10.1109/TAC.1974.1100705. [DOI] [Google Scholar]
- 29.Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 30.R: The R Project for Statistical Computing [Internet]. https://www.r-project.org/. (2018).
- 31.Bergquist JR, Murphy BL, Storlie CB, Habermann EB, Boughey JC. Incorporation of treatment response, tumor grade and receptor status improves staging quality in breast cancer patients treated with neoadjuvant chemotherapy. Ann. Surg. Oncol. 2017;24:3510–3517. doi: 10.1245/s10434-017-6010-4. [DOI] [PubMed] [Google Scholar]
- 32.Symmans WF, Wei C, Gould R, Yu X, Zhang Y, Liu M, et al. Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J. Clin. Oncol. 2017;35:1049–1060. doi: 10.1200/JCO.2015.63.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peintinger F, Sinn B, Hatzis C, Albarracin C, Downs-Kelly E, Morkowski J, et al. Reproducibility of residual cancer burden for prognostic assessment of breast cancer after neoadjuvant chemotherapy. Mod. Pathol. 2015;28:913–920. doi: 10.1038/modpathol.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi M, Park YH, Ahn JS, Im Y-H, Nam SJ, Cho SY, et al. Assessment of pathologic response and long-term outcome in locally advanced breast cancers after neoadjuvant chemotherapy: comparison of pathologic classification systems. Breast Cancer Res. Treat. 2016;160:475–489. doi: 10.1007/s10549-016-4008-4. [DOI] [PubMed] [Google Scholar]
- 35.Laas E, Mallon P, Delomenie M, Gardeux V, Pierga J-Y, Cottu P, et al. Are we able to predict survival in ER-positive HER2-negative breast cancer? A comparison of web-based models. Br. J. Cancer. 2015;112:912–917. doi: 10.1038/bjc.2014.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Minckwitz G, Schmitt WD, Loibl S, Müller BM, Blohmer JU, Sinn BV, et al. Ki67 measured after neoadjuvant chemotherapy for primary breast cancer. Clin. Cancer Res. 2013;19:4521–4531. doi: 10.1158/1078-0432.CCR-12-3628. [DOI] [PubMed] [Google Scholar]
- 37.Sheri A, Smith IE, Johnston SR, A’Hern R, Nerurkar A, Jones RL, et al. Residual proliferative cancer burden to predict long-term outcome following neoadjuvant chemotherapy. Ann. Oncol. 2015;26:75–80. doi: 10.1093/annonc/mdu508. [DOI] [PubMed] [Google Scholar]
- 38.Focke CM, Bürger H, van Diest PJ, Finsterbusch K, Gläser D, Korsching E, et al. Interlaboratory variability of Ki67 staining in breast cancer. Eur. J. Cancer. 2017;84:219–227. doi: 10.1016/j.ejca.2017.07.041. [DOI] [PubMed] [Google Scholar]
- 39.Dieci MV, Criscitiello C, Goubar A, Viale G, Conte P, Guarneri V, et al. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann. Oncol. 2014;25:611–618. doi: 10.1093/annonc/mdt556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asano Y, Kashiwagi S, Goto W, Takada K, Takahashi K, Hatano T, et al. Prediction of survival after neoadjuvant chemotherapy for breast cancer by evaluation of tumor-infiltrating lymphocytes and residual cancer burden. BMC Cancer. 2017;17:888. doi: 10.1186/s12885-017-3927-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All supplementary data are available from authors on reasonable request.