We read with great interest the report by Shaaban et al. on the UK Sloane Project.1 This unique prospective cohort of DCIS patients provides an unprecedented view on the real-world management and long-term follow-up of this pre-invasive disease. The Sloane Project provides an immense amount of valuable data.1 Although Shaaban and colleagues have extensively discussed their observations, we would like to focus on an interesting finding that was slightly neglected in the discussion, likely because it seems very banal at first glance. We use this particular observation to launch a new research hypothesis by emphasising the similarities between recurrence after breast-conserving surgery (BCS) for DCIS and intrahepatic recurrence after partial hepatectomy for hepatocellular carcinoma (HCC).2
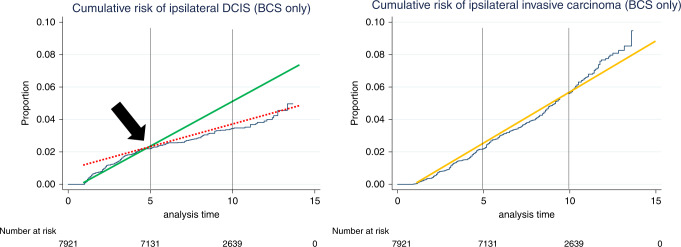
The observation of interest is the following: the median time to ipsilateral in situ recurrences amounts to 37 months, whereas the median time to ipsilateral invasive recurrences amounts to 62 months.1 Ergo, the former takes only around 60% of the latter. After long follow-up, the number of in situ recurrences (225 or 35% of all ipsilateral recurrences) is substantially lower than the number of invasive recurrences (413 or 65% of all ipsilateral recurrences),1 which contrasts with the previously reported ‘fifty-fifty distribution’ in older trials.3–5 Additionally, the number of in situ recurrences is substantially higher in the first five years after BCS.1 This real-world observation confirms the findings of NSABP-B24, wherein the rate of in situ recurrence diminished after 5 years, whereas the rate of invasive recurrences was constant over time.3 At around five years, there is a clear tipping point in the curve of in situ recurrence in the report of Shaaban and colleagues, which we reproduced here with added lines to illustrate the tilting angle of the curve (Fig. 1, left panel). Contrariwise, the slope of the curve of invasive recurrences does not change (Fig. 1, right panel). Although we are no professional biostatisticians who can objectify the degree of changed slopes; we merely aimed to visualise our hypothesis. A similar curve of ipsilateral in situ recurrences is provided in Figure 2 of the report on the SweDCIS trial.5
Fig. 1. Graphs reproduced from Shaaban et al. (Br. J. Cancer 2020; in press), illustrating the cumulative risk of ipsilateral DCIS (left panel) and of ipsilateral invasive carcinoma (right panel) after BCS only.
The in-situ recurrence curve shows a changed slope after 5 years of follow-up (black arrow), illustrated by the full green line (first 5 post-operative years) and the dashed red line (>5 years of follow-up). The invasive recurrence curve shows a steady increase without changed slope, illustrated by the full orange line (right panel).
This difference in median time to each type of recurrence yields more information for future management of DCIS patients than might initially be suspected. This observation is not new, yet substantially undervalued. Wallis et al. already reported a significant difference in mean time to in situ versus invasive recurrence for 700 DCIS patients diagnosed in 1988–1999 in the West Midlands NHS Breast Screening Programme: 15 versus 60 months respectively (with median follow-up of 183 months).6 Similarly, Rakovitch et al. reported a median time to in situ and invasive recurrence of 2.5 years and 5.7 years, respectively.7
Unfortunately, most reports only mention the median time to overall ipsilateral recurrence, without differentiating between the histological type. Interestingly, Groen et al. showed that the median time to invasive recurrence does not significantly differ between low-grade and high-grade DCIS (5,3 versus 5,6 years), nor between BCS alone versus BCS and radiotherapy (5,1 versus 5,9 years).8
It is clear from the work by Shaaban et al. and others that the risk of in situ recurrence is highest in the first five post-operative years, and thereafter, disease-specific survival curves show a changed slope. We believe that these observations, together with the fact that DCIS patients with invasive recurrence have a significantly worse disease-free and overall survival in both the real-world and randomised trial setting,3,4,9 are sufficiently strong arguments to demand separate reports on the median time to in situ versus invasive recurrence in all future studies on prognostic markers in DCIS. Moreover, future DCIS studies should explicitly differentiate between short-term (<5 years) versus long-term (>5 years) recurrence risk. This is extremely important, as the number of in situ recurrences is higher than the number of invasive recurrences in studies with shorter median overall follow-up, such as the report by Rudloff et al. (median follow-up of 5,6 years, with 122 in situ and 80 invasive recurrences).10
Therefore, the report by Shaaban et al. might become a landmark paper for future DCIS studies, just as the work by Portolani et al. was a landmark paper for studies on prognostic markers for intrahepatic recurrence of HCC.2 Intrahepatic recurrences in the first two years after partial hepatectomy are considered as real recurrences (i.e. intrahepatic metastases) and therefore, vascular invasion is a prognostic marker for HCC recurrence in the first two years. After >2 years, most neoplasms are new primary HCC, and therefore, vascular invasion is not prognostic for recurrence anymore, but cirrhosis is. Evidently, this statement is not absolute: before 2 years, some HCC will be new primaries, and after 2 years, some HCC are yet ‘real’ recurrences, but overall, the disease-free survival curve shows a clearly changed slope at 2 years of follow-up.2
Although recurrence after BCS for DCIS is not related to in-breast metastasis, we would like to use this analogy to establish an important change in DCIS reports, by respecting this 5-year threshold. We hypothesise that, analogous to HCC, a large but yet unknown number of so-called ipsilateral invasive recurrences are in fact new independent breast neoplasms. We postulate that early recurrence (i.e. <5 postoperative years) is more likely to be related to the primary DCIS. Most ipsilateral recurrences after BCS are probably outgrowths of initially incompletely removed DCIS,11 and this presumption is supported by the correlation between margin status and recurrence risk.1,4,5,10
Future research, combining histopathological, clinical-radiological, and molecular information, should determine to what extent so-called ipsilateral recurrences (either in situ or invasive) are clonally related to the initial DCIS. Only such a ‘holistic’ study will allow to discern ‘true’ recurrences from new, metachronous mammary neoplasms. This might have a major impact on the clinical management of DCIS patients.11
Acknowledgements
Not applicable.
Author contributions
M.R.V.B.: writing—first draft. L.L.: writing—review and editing. C.G.: writing—review and editing.
Ethics approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Data availability
Not applicable.
Competing interests
The authors declare no competing interests.
Funding information
M.R. Van Bockstal received a postdoctoral mandate (grant number 2019-089) from the not-for-profit organisation Foundation against Cancer (Brussels, Belgium), and is supported by the “Fonds dr. Gaëtan Lagneaux” of the Fondation Saint-Luc (Brussels, Belgium). C. Galant is supported by the “Fonds dr. Gaëtan Lagneaux” of the Fondation Saint-Luc (Brussels, Belgium).
Footnotes
The original online version of this article was revised: Due to an Additional Information error.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/20/2021
A Correction to this paper has been published: 10.1038/s41416-021-01520-9
References
- 1.Shaaban, A. M., Hilton, B., Clements, K., Provenzano, E., Cheung, S., Wallis, M. G. et al. Pathological features of 11,337 patients with primary ductal carcinoma in situ (DCIS) and subsequent events: results from the UK Sloane Project. Br. J. Cancer, 10.1038/s41416-020-01152-5 (2020). [DOI] [PMC free article] [PubMed]
- 2.Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann. Surg. 2006;243:229–235. doi: 10.1097/01.sla.0000197706.21803.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wapnir IL, Dignam JJ, Fisher B, Mamounas EP, Anderson SJ, Julian TB, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J. Natl Cancer Inst. 2011;103:478–488. doi: 10.1093/jnci/djr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donker M, Litiere S, Werutsky G, Julien JP, Fentiman IS, Agresti R, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma In Situ: 15-year recurrence rates and outcome after a recurrence, from the EORTC 10853 randomized phase III trial. J. Clin. Oncol. 2013;31:4054–4059. doi: 10.1200/JCO.2013.49.5077. [DOI] [PubMed] [Google Scholar]
- 5.Warnberg F, Garmo H, Emdin S, Hedberg V, Adwall L, Sandelin K, et al. Effect of radiotherapy after breast-conserving surgery for ductal carcinoma in situ: 20 years follow-up in the randomized SweDCIS Trial. J. Clin. Oncol. 2014;32:3613–3618. doi: 10.1200/JCO.2014.56.2595. [DOI] [PubMed] [Google Scholar]
- 6.Wallis MG, Clements K, Kearins O, Ball G, Macartney J, Lawrence GM. The effect of DCIS grade on rate, type and time to recurrence after 15 years of follow-up of screen-detected DCIS. Br J Cancer. 2012;106:1611–1617. doi: 10.1038/bjc.2012.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rakovitch E, Nofech-Mozes S, Hanna W, Narod S, Thiruchelvam D, Saskin R, et al. HER2/neu and Ki-67 expression predict non-invasive recurrence following breast-conserving therapy for ductal carcinoma in situ. Br. J. Cancer. 2012;106:1160–1165. doi: 10.1038/bjc.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groen EJ, Hudecek J, Mulder L, van Seijen M, Almekinders MM, Alexov S, et al. Prognostic value of histopathological DCIS features in a large-scale international interrater reliability study. Breast Cancer Res. Treat. 2020;183:759–770. doi: 10.1007/s10549-020-05816-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson AM, Clements K, Cheung S, Pinder SE, Lawrence G, Sawyer E, et al. Management and 5-year outcomes in 9938 women with screen-detected ductal carcinoma in situ: the UK Sloane Project. Eur. J. Cancer. 2018;101:210–219. doi: 10.1016/j.ejca.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 10.Rudloff U, Jacks LM, Goldberg JI, Wynveen CA, Brogi E, Patil S, et al. Nomogram for predicting the risk of local recurrence after breast-conserving surgery for ductal carcinoma in situ. J. Clin. Oncol. 2010;28:3762–3769. doi: 10.1200/JCO.2009.26.8847. [DOI] [PubMed] [Google Scholar]
- 11.Van Bockstal MR, Agahozo MC, Koppert LB, van Deurzen CHM. A retrospective alternative for active surveillance trials for ductal carcinoma in situ of the breast. Int. J. Cancer. 2020;146:1189–1197. doi: 10.1002/ijc.32362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.