Abstract
Purpose
To evaluate the effectiveness and safety of lidocaine on postoperative quality of recovery and lung protection of patients undergoing thoracoscopic radical resection of lung cancer.
Patients and Methods
Seventy ASA II–III patients undergoing thoracoscopic radical resection of lung cancer were randomly assigned into either the lidocaine group (Group L) or control group (Group C). Patients in Group L received lidocaine with a 1.5 mg/kg bolus before induction of anesthesia, followed by 2.0 mg/kg/h until the end of the operation while the patients in Group C received volume-matched normal saline at the same rate. The main outcome was the quality of recovery-40 score (QoR-40 score) at 24 h postoperatively. The peak airway pressure (Ppeak) and plateau airway pressure (Pplat), the partial pressure of oxygen in arterial blood (PaO2), partial pressure of carbon dioxide in arterial blood (PaCO2), alveolar-arterial oxygen gradient (A-aDO2), oxygenation index (OI), time to first flatus and defecation, intraoperative hemodynamics and opioid consumption were also recorded.
Results
There were no statistically difference at patients’ baseline characteristics. The QoR-40 score of Group L was significantly higher than that of Group C at 24 h after surgery (P=0.014). Ppeak, Pplat, and A-aDO2 of Group L were significantly lower than those of Group C (P<0.001, P<0.001, P=0.025, respectively) after the ventilation recovery of both lungs, and the PaO2 and OI of the Group L were significantly higher than those of Group C (P=0.027, P=0.027, respectively). Time to first flatus and defecation in Group L was significantly lower compared with Group C (P=0.037, P=0.025, respectively).
Conclusion
Intravenous lidocaine can improve the quality of recovery of patients undergoing thoracoscopic radical resection of lung cancer, while also providing lung protection, favorable postoperative analgesia, a reduction in the time to first flatus and defecation after surgery.
Keywords: lidocaine, quality of recovery, lung-protective effects, radical resection of lung cancer
Introduction
At present, surgery is the main treatment option for early-stage lung cancer, as it can prolong survival and improve the quality of life of patients. In recent years, the progress in the pathophysiology of the perioperative period has shown that a variety of factors such as pain, response to stress, organ dysfunction, nausea, vomiting, intestinal obstruction, hypoxemia symptoms, and immobilization can lead to increased postoperative morbidity and prolongation of the hospital stay and recovery period.1 One lung ventilation (OLV) is often required during thoracoscopic radical resection of lung cancer, eventually leading to an imbalance in the ventilation/blood flow and an increase in the intrapulmonary shunt rate. OLV over 2 h can cause acute lung injury.2 Studies have shown that the intravenous use of lidocaine in outpatient surgery3 and laparoscopic bariatric surgery4 can significantly improve the quality of postoperative recovery and reduce the consumption of opioids in the perioperative period. Lidocaine is a common clinical amide local anesthetic, with a wide range of anti-inflammatory and analgesic effects. Animal experiments have shown that lidocaine can reduce the inflammatory response during surgery, increase lung compliance, and relax airway smooth muscle, thereby playing a role in lung protection.5,6 However, the role of lidocaine in improving the quality of recovery and lung protection effects in patients undergoing thoracoscopic radical resection of lung cancer is still unclear. This study intends to explore the effect of intravenous use of lidocaine during thoracoscopic radical resection of lung cancer on the patients’ postoperative quality recovery and lung protection.
Patients and Methods
General Information
This study was a single-center, prospective, double-blind, randomized controlled trial. The protocol was approved by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (XYFY2020-KL160-01) and was registered at the Chinese Clinical Trial Registry (ChiCTR1900026910). This study complies with the Declaration of Helsinki and adheres to CONSORT guidelines. All patients and their families signed an informed consent form. Between January 2020 and June 2020, patients who underwent thoracoscopic radical resection of lung cancer in the Department of Thoracic Surgery of our hospital were evaluated for eligibility. In this study, the types of operation include sleeve lobectomy, wedge lobectomy resection, segment resection, and lobectomy, while pneumonectomy was not included in the study. All operations were performed by uniportal VATS according to the different location of the tumor and the standardized treatment according to the guidelines. The operation was performed by Hao Zhang group, Department of Thoracic Surgery, Affiliated Hospital of Xuzhou Medical University. Two surgeons, Chief Physician Hao Zhang and Attending Doctor Teng Sun, worked together to complete more than 1000 thoracic surgeries. Residents are not included. All male and female patients aged between 45 and 65 with an ASA grade II–III, were included in the study. The exclusion criteria were: a previous history of other malignant tumors, it is impossible to determine whether this lung cancer is primary; use of radiotherapy, chemotherapy, anti-inflammatory drugs, analgesics, and hormone drugs before surgery; tumor metastasis to other organs; allergies to local anesthetics; liver dysfunction (defined as serum total bilirubin ≥ 2 mg/dl); renal dysfunction (defined as glomerular filtration rate < 90 mL/min/1.73m2); sinus bradycardia; atrioventricular block of more than a second degree; chronic pain; mental illness, communication disorder; refusal to receive lidocaine infusion. Patients were excluded from the analysis if the: surgery was canceled; converted to thoracotomy; if local anesthetic poisoning or adverse reactions occurred during surgery; if they were admitted to the intensive care unit (ICU) following surgery; surgery took more than 6 h and if intraoperative blood transfusion was required. The eligible patients were randomly divided into Group L and Group C by a computer-generated random allocation sequence (1:1). The patients in Group L were given a lidocaine bolus of 1.5 mg/kg before induction anesthesia which was then maintained at a rate of 2.0 mg/kg/h until the end of the operation. The patients in Group C were injected using a load and pump with an equal volume of normal saline. The grouping information was kept in a sealed envelope by a nurse who did not participate in the experiment. Patients, anesthesiologists, and surgeons were blinded in the grouping of the experiment. After the patient entered the operating room, another anesthesiologist opened the sealed envelope and prepared related drugs according to the assigned patient group. The anesthesiologist did not participate in the follow-up data collection and data analysis. The data were then analyzed by a statistician who was not aware of the assigned patient group.
Anesthesia Protocol
After the patient entered the room, ECG, BP, SpO2, and PETCO2 were routinely monitored, and the contralateral upper extremity venous access was opened. The contralateral radial artery was punctured and catheterized under local anesthesia (heparin anticoagulation is retained the blood samples are collected and arterial invasive blood pressure is monitored). The Anesthesia index (AI) was used to monitor the depth of anesthesia, and the TOF was used to monitor the degree of muscle relaxation. Anesthesia induction consisted of an intravenous injection of midazolam 0.06 mg/kg, sufentanil 0.5 μg/kg, etomidate 0.3 mg/kg, cisatracurium besylate 0.15 mg/kg and remifentanil 1 μg/kg. When the TOF value dropped to zero, an F37 or F35 double-lumen bronchial tube was inserted through the mouth using a fiberoptic bronchoscope positioning for males and females, respectively, but the specific size of double-lumen bronchial tube is adjusted according to the actual situation of patients. After tracheal intubation, mechanical ventilation was performed. Inhaled oxygen concentration was maintained at 60% and oxygen flow at 2 L/min, VT6–8 mL/kg, RR 10–14 times/min, inhalation–expiration ratio 1:2, RR 12–16 times/min in OLV. Other ventilation parameters remained unchanged and PETCO2 was maintained at 35–45 mmHg. Maintenance of anesthesia was achieved via an intravenous pump injection of propofol 4–12 mg/kg/h, remifentanil 0.2–0.3 μg/kg/h, and cisatracurium 0.06–0.12 mg/kg/h. The propofol infusion rate was adjusted to maintain the depth of anesthesia index value between 40 and 60 and to maintain the heart rate (HR) and mean arterial pressure (MAP) fluctuation range within 20% of the baseline value. Intraoperative intravenous infusion of Sodium Potassium Magnesium Calcium and Glucose Injection which consisted of crystalloid solution (multiple electrolytes of sodium, potassium, magnesium, calcium, glucose) and succinyl gelatin in a volume ratio of 2:1. Propofol was stopped 5 min before the end of the operation. Intravenous atropine injection of 0.25–0.50 mg was given while intraoperative HR was below 50 beats/min, and esmolol 10–20 mg was administered when the HR was higher than 100 beats/min. When MAP exceeded 20% of the baseline, if AI was higher than 60, propofol infusion rate should be increased to maintain AI at 40–60. If AI is between 40 and 60, MAP is still 20% higher than the baseline, a remifentanil bolus of 0.5 μg/kg is given every 2 min to deepen the depth of anesthesia and maintain hemodynamic stability. To avoid the inhibition of hypoxic pulmonary vasoconstriction by sevoflurane, induction, and maintenance should avoid the use of sevoflurane. If this was not sufficient to control MAP, intravenous urapidil 15–25 mg was administered. Conversely, an intravenous injection of ephedrine 3–6 mg or phenylephrine 40–80 μg was administered if MAP was reduced by more than 20% of the preoperative level.
Arterial blood samples were collected intermittently during the operation for blood gas analysis to maintain a stable internal environment. When hypoxemia (SpO2 < 90%) occurred during the operation, fiberoptic bronchoscopy was performed to confirm that the double-lumen endotracheal tube was well aligned. If there was no improvement in the sputum suction, positive end-expiratory pressure ventilation, and other measures, double lung ventilation was performed and the occurrence of hypoxemia was recorded during the operation. Postoperative patient-controlled intravenous analgesia (PCA) was performed with drug formulations (sufentanil 2 μg/kg, dezocine 10 mg, tropisetron 10 mg, normal saline diluted to 100 mL) using a set lock time of 15 min, a background infusion dose to 2 mL/h, and the PCA was set at 0.5 mL of/times to maintain the visual analogue scale (VAS) below or equal to 3 points. Cisatracurium was stopped before the end of the operation. All narcotic drugs were also stopped at the end of the operation, and the patient was taken to post-anesthesia care unit (PACU) with a tube. More precisely, neostigmine 0.04–0.07 mg/kg was given when the second twitch response (T2) of TOF spontaneously recovered, and the maximum dose was 5 mg. Atropine and neostigmine were injected slowly with the same syringe, atropine dose was generally half of neostigmine, and the depth of muscle relaxation was continuously monitored in the process. When the consciousness, cough, and swallowing reflex of the patient were recovered, the head was lifted from the bed surface for 5 s, the tidal volume was more than 5 mL/kg, TOF ratio (T4/T1) was >0.9 and the respiratory rate was less than 20 times/min, patient was extubated and after a 30-min observation, the patient was sent back to the ward.
Primary and Secondary Outcomes
Primary Outcome Measure
The primary outcome measure of this study was the QoR-40 score. This was measured 24 h after surgery using the QoR-40 questionnaire. The questionnaire consists of 40 questions assessing 5 aspects of the patient’s recovery including the degree of physical comfort, pain, physical independence, psychological support, and emotional state. Each domain is quantified using a 5-point rating scale whereby 1 indicates never, 2 sometimes, 3 frequently, 4 most, and 5 always. The QoR-40 score ranges from 40 to 200, with 200 being the best and 40 being the worst. All patients received the questionnaire assessment 24 h postoperatively.
Secondary Outcomes Measures
One milliliter of radial artery blood was collected for arterial blood gas analysis. The respiratory mechanics’ indexes including Ppeak, Pplat, pulmonary dynamic compliance (Cdyn), and pulmonary static compliance (Cst). A-aDO2 and OI were recorded at 4 different time points: intubation (T1), 30 min OLV (T2), 60 min OLV (T3), and following the recovery of the ventilation of both lungs for 20 min (T4). The consumption of opioids during the perioperative period and the consumption of remedial analgesics (including NSAIDs) within 24 h after the operation were recorded.
Safety Evaluation
The safety evaluation included an analysis of the hemodynamic changes at each time point during the operation. The incidence of postoperative adverse reactions, including nausea, vomiting, postoperative shivering, and hypoxemia, and the recovery time for gastrointestinal function after surgery, including the time to first exhaust and defecation, were also evaluated.
Sample Size Calculation
This study design involved a parallel randomized controlled study, whereby the QoR-40 score was compared between the intervention Group L and the control Group C. According to previous literature reports, an average increase of 10 points in the total QoR-40 scores indicates that the quality of clinical recovery has improved. On this basis, we assumed that the QoR-40 score of Group L was 10 points higher than that of Group C at 24 h postoperatively, with a standard deviation of 13, α of 0.05, and β of 0.2. Assuming a 20% patient dropout rate, we calculated that a sample size of 35 patients per group is required. Seventy patients were finally included (n=70).
Statistical Analysis
SPSS statistical software (version 25.0; SPSS Inc., IBM, Chicago, IL, USA) was used for statistical data analysis; The measurement data used the Shapiro–Wilk test to determine the normality of the data distribution, and the Levene method was used to test the homogeneity of variance. The measurement data that meet the normal distribution were expressed as the mean± standard deviation (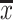 ± s) or represented by the median (M) and the interquartile range (IQR) which meets the non-normal distribution; When the data were normally distributed and homogeneity of variance, two independent sample t-tests were used for comparison between groups, and repeated measures analysis of variance was used for different time points within the group. Mann–Whitney U-test was used for non-normally distributed data; Using chi-square test or Fisher exact probability method for counting data; P < 0.05 considered the difference to be statistically significant.
± s) or represented by the median (M) and the interquartile range (IQR) which meets the non-normal distribution; When the data were normally distributed and homogeneity of variance, two independent sample t-tests were used for comparison between groups, and repeated measures analysis of variance was used for different time points within the group. Mann–Whitney U-test was used for non-normally distributed data; Using chi-square test or Fisher exact probability method for counting data; P < 0.05 considered the difference to be statistically significant.
Results
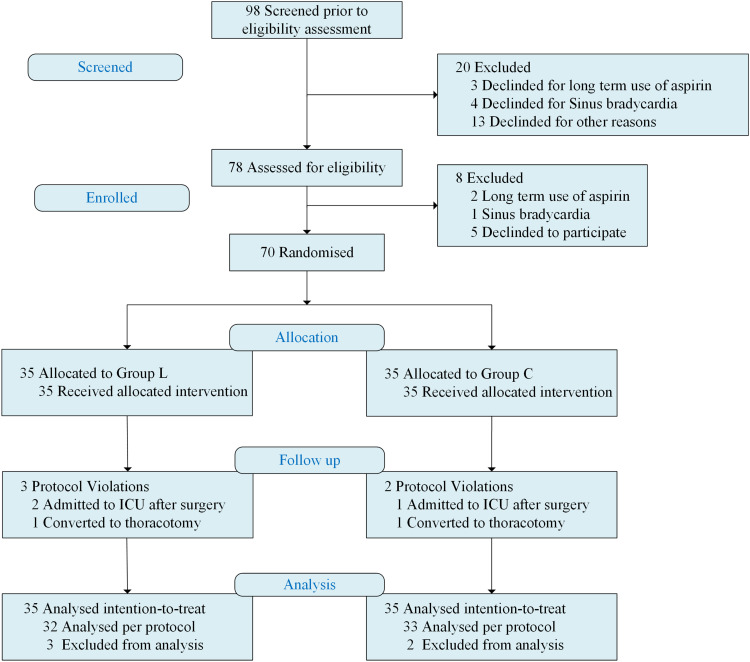
A total of 98 patients were screened to participate in the trial, of which 20 were excluded. Three of these patients were excluded as they were treated with aspirin for a long time, and another 4 patients were excluded as they suffered from sinus bradycardia. After being enrolled, 8 more patients were excluded. Two of these patients were excluded as they were treated with aspirin for a long time, and 1 patient was excluded as newly discovered sinus bradycardia. Thirty-five people were randomly assigned to each group. In Group L, two cases were admitted to ICU after the operation, and one case was converted to thoracotomy during operation. One case in Group C was admitted to ICU after the operation, and one case was converted to thoracotomy during operation. Using intention-to-treat, a total of 70 cases were finally included for statistical analysis (Figure 1).
Figure 1.
CONSORT flow diagram with study overview and recruitment profile.
Notes: Group L, lidocaine group; Group C, control group.
Abbreviations: CONSORT, Consolidated Standards for Reporting of Trials; ICU, intensive care unit.
There was no statistically significant difference in the patient characteristics including gender, age, height, weight, body mass index (BMI), ASA classification, anesthesia time, operation time, tidal volume, FEV1/FVC, total lung volume, diabetes mellitus, hypertension, obstructive sleep apnea-hypopnea syndrome (OSAHS), and other general conditions between the two groups (Table 1).
Table 1.
Comparison of General Information of the Two Groups of Patients (n=70)
| Group C | Group L | P | |
|---|---|---|---|
| Gender (M/F) | 13/22 | 16/19 | 0.467 |
| Age (yr) | 57.20±5.75 | 58.09±5.20 | 0.502 |
| Height(m) | 1.63±0.07 | 1.64±0.08 | 0.580 |
| Body weight (kg) | 63.09±9.17 | 65.34±10.48 | 0.340 |
| BMI (kg/m2) | 23.72±3.40 | 24.08±2.86 | 0.633 |
| ASA (II/III) | 28/7 | 29/6 | 0.467 |
| Anesthesia time (min) | 198.00±36.63 | 190.86±34.83 | 0.920 |
| Operation time (min) | 168.11±33.50 | 161.26±32.40 | 0.786 |
| VT (L) | 0.64±0.09 | 0.66±0.09 | 0.906 |
| FEV1/FVC (%) | 0.78(0.73–0.79) | 0.80(0.75–0.81) | 0.215 |
| TLC (L) | 5.24±1.13 | 5.09±06 | 0.578 |
| DM | 30/5 | 32/3 | 0.710 |
| Hypertension | 26/9 | 28/7 | 0.569 |
| OSAHS | 1/34 | 1/34 | 0.754 |
Notes: Data are presented as mean ± SD or numbers (%). Group C= the control group, Group L= the lidocaine group.
Abbreviations: BMI, body mass index; ASA, American Society of Anesthesiologists; VT, tidal volume; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; TLC, total lung capacity; DM, diabetes mellitus; OSAHS, obstructive sleep apnea-hypopnea syndrome.
Compared with Group C, the overall QoR-40 score of Group L increased significantly at 24 h postoperatively, and the difference was statistically significant (P=0.014). When analyzing each questionnaire domain, a statistically significant higher improvement in Group L was noted for the physical comfort, physical independence, and pain at 24 h after surgery when compared with Group C (P=0.026; P=0.039; P=0.041, respectively) (Table 2).
Table 2.
QoR-40 Score (n=70)
| Group C | Group L | P | |
|---|---|---|---|
| Physical comfort | 49(48.40–49.55) | 51(50.40–51.48) | 0.026 |
| Physical independence | 15(15.04–15.81) | 17(16.19–17.12) | 0.039 |
| Emotional state | 36(35.21–36.11) | 37(36,21–37.11) | 0.215 |
| Psychological support | 33(32.33–33.04) | 33(32.44–33.41) | 0.337 |
| Pain | 30(29.59–30.41) | 33(32.59–33.41) | 0.041 |
| Total | 163(161.58–163.90) | 170(168.90–171.16) | 0.014 |
Notes: Data are presented as median (interquartile range). Group C= the control group, Group L= the lidocaine group.
The PaO2, PaCO2, A-aDO2, OI, Ppeak, Pplat, Cst, Cdyn of the two groups at T1-T3 were not statistically significant. Compared with Group C, PaO2, and OI of Group L increased significantly at T4 (P=0.027, P=0.027, respectively). Compared with Group C, Ppeak, Pplat and A-aDO2 of Group L decreased significantly at T4 (P<0.001, P<0.001, P=0.025, respectively) (Table 3).
Table 3.
Comparison of the Respiratory Index and Respiratory Mechanics Parameters Between the Two Groups of Patients (n=70)
| T1 | T2 | T3 | T4 | ||
|---|---|---|---|---|---|
| PaO2 | Group C | 398.01±70.78 | 171.64±81.70 | 183.36±78.46 | 402.2±77.72 |
| (mmHg) | Group L | 416.61±77.85 | 192.60±89.87 | 205.49±86.30 | 446.24±85.50 |
| P | 0.299 | 0.311 | 0.266 | 0.027 | |
| PaCO2 | Group C | 39.09±8.23 | 39.01±2.80 | 38.31±1.69 | 38.92±2.24 |
| (mmHg) | Group L | 39.95±3.01 | 39.87±2.93 | 39.13±1.78 | 39.77±2.35 |
| P | 0.229 | 0.216 | 0.053 | 0.128 | |
| A-aDO2 | Group C | 265.14±71.61 | 491.61±81.36 | 480.77±77.81 | 261.14±78.18 |
| (mmHg) | Group L | 220.45±78.71 | 469.56±89.52 | 457.60±885.62 | 216.06±85.97 |
| P | 0.278 | 0.285 | 0.240 | 0.025 | |
| OI | Group C | 398.01±70.78 | 171.64±81.70 | 183.36±78.46 | 402.2±77.72 |
| (mmHg) | Group L | 441.61±77.85 | 192.60±89.87 | 205.49±86.30 | 446.24±85.50 |
| P | 0.299 | 0.311 | 0.266 | 0.027 | |
| Ppeak | Group C | 15(14.04–15.68) | 23(21.99–24.12) | 23(22.95–24.77) | 18(16.69–17.46) |
| (cmH2O) | Group L | 17(16.19–17.87) | 23(22.67–24.70) | 22(20.95–24.96) | 15(14.23–17.12) |
| P | 0.067 | 0.389 | 0.208 | <0.001 | |
| Pplat | Group C | 15.00(12.96–14.64) | 22(20.89–22.94) | 22(20.60–22.72) | 17(12.75–13.94) |
| (cmH2O) | Group L | 16(15.15–16.96) | 21(20.90–23.70) | 23(21.70–23.56) | 16(14.73–16.47) |
| P | 0.075 | 0.191 | 0.166 | <0.001 | |
| Cst | Group C | 45.6±15.52 | 24.34±5.75 | 24.89±3.81 | 48.71±16.58 |
| (mL/cmH2O) | Group L | 37.51±12.04 | 22.37±4.64 | 22.66±4.6 | 39.03±13.76 |
| P | 0.058 | 0.119 | 0.093 | 0.057 | |
| Cdyn | Group C | 40.11±12.22 | 22.89±5.38 | 22.74±5.25 | 42.29±11.54 |
| (mL/cmH2O) | Group L | 36.06±9.54 | 21.40±4.41 | 21.09±4.05 | 37.09±9.52 |
| P | 0.054 | 0.211 | 0.144 | 0.066 |
Notes: The data are presented as means ± SD or median (interquartile range). Group C= the control group, Group L= the lidocaine group.
Abbreviations: PaO2, partial pressure of oxygen in arterial blood; PaCO2, partial pressure of carbon dioxide in arterial blood; A-aDO2, alveolar-arterial oxygen gradient; OI, oxygenation index; Ppeak, peak airway pressure; Pplat, plateau airway pressure; Cst, static lung compliance; Cdyn, dynamic lung compliance.
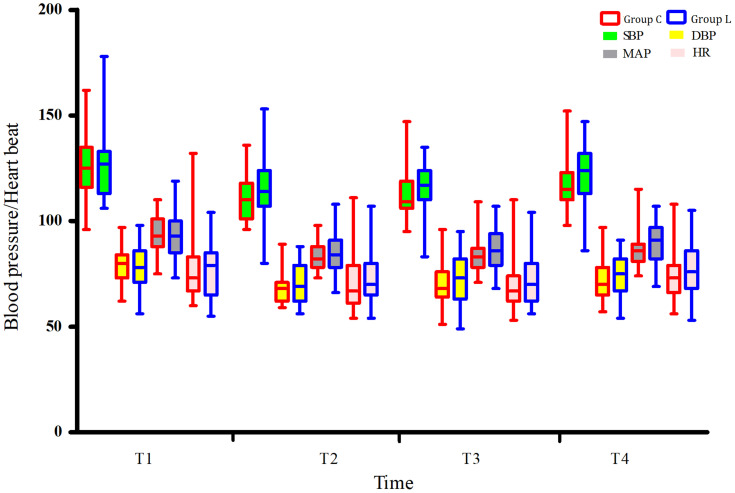
The hemodynamics of the two groups of patients was not statistically significant at T1-T4 (Figure 2).
Figure 2.
Comparison of intraoperative hemodynamics between the two groups.
Notes: Group L, lidocaine group; Group C, control group.
Abbreviations: T1, intubation; T2, 30 min after OLV; T3, 60 min after OLV; T4, 20 min after the recovery of the ventilation of both lungs.
Compared with Group C, the intraoperative consumption of remifentanil and postoperative consumption of sufentanil in Group L was significantly reduced (P<0.001, P=0.046, respectively). Compared with Group C, the time to flatus and defecation after the operation was significantly shorter in Group L (P=0.037, P=0.025, respectively) (Table 4).
Table 4.
Perioperative Data of the Two Groups of Patients (n=70)
| Group C | Group L | P | |
|---|---|---|---|
| Intraoperative consumption of remifentanil (mg) | 1.87±0.44 | 1.29±0.28 | <0.001 |
| Postoperative consumption of sufentanil (μg) | 40(36.58–40.45) | 40 (36.77–40.89) | 0.046 |
| Intraoperative consumption of propofol (mg) | 943.6±247.13 | 988±198.35 | 0.168 |
| Time to first flatus (h) | 18.8±3.5 | 12.3±1.5 | 0.037 |
| Time to first defecation (h) | 28.7±4.7 | 22.3±3.2 | 0.025 |
Notes: The data are presented as the means ± SD or the medians (interquartile ranges). Group C= the control group, Group L= the lidocaine group.
The incidence of postoperative hypoxemia, PONV, and postoperative shivering in the two groups was not statistically significant. Compared with Group C, the number of patients using remedial analgesics in group L was significantly lower compared with Group C (P=0.045) (Table 5).
Table 5.
Comparative Examples of Postoperative Remedial Analgesics and Adverse Reactions Between the Two Groups of Patients [n (%)]
| Group C | Group L | P | |
|---|---|---|---|
| Remedial analgesics | 9 (25.7) | 2 (6.7) | 0.045 |
| PONV | 13 (37.1) | 9 (25.7) | 0.303 |
| Postoperative shivering | 6 (17.1) | 5 (14.3) | 0.743 |
| Hypoxemia | 7 (20.0) | 4 (11.4) | 0.513 |
Notes: The data are presented as n (%). PONV, postoperative nausea, and vomiting. Group C= the control group, Group L= the lidocaine group.
Discussion
The QoR-407 questionnaire is a reliable multi-dimensional assessment tool used to assess the patient’s health status after surgery and anesthesia. Several studies have shown that the QoR-40 questionnaire can be used to assess the postoperative quality of recovery. Currently, there is no “gold standard” for postoperative analgesia after thoracic surgery, and perioperative multimodal analgesia is the recommended method for enhanced recovery after surgery (ERAS).8 Inadequate postoperative analgesia may cause severe postoperative pain, stress, circulatory, and respiratory dysfunction, increase the risk of developing perioperative complications, and directly affect the patient’s emotional state and daily life. The pain can become chronic and affects the quality and outcome of the postoperative recovery of patients. This study found that the use of intraoperative intravenous lidocaine can significantly improve the quality of the patients’ postoperative recovery as shown by the improvement in the total QoR-40 score particularly in the three sub-aspects of physical comfort, physical independence, and pain. Furthermore, the application of lidocaine significantly reduced the consumption of opioids in the operation, shortened the time of first flatus and defecation after the operation, reduced the use of remedial analgesia, and accelerated the recovery of gastrointestinal function. However, the influence of lidocaine on the quality of recovery and the analgesic effect is still controversial for different surgical methods. Previous, studies have shown that the use of lidocaine during surgery can significantly relieve postoperative pain, especially in open or laparoscopic abdominal surgery. Cui9 applied lidocaine in thoracic surgery and found that the pain score of patients was significantly reduced 24 h after surgery. Lidocaine was also used in thyroid surgery,10 but the acute postoperative pain and the quality of postoperative recovery did not improve. Martin11 reported that the use of intravenous lidocaine in total hip replacement surgery did not result in a significant reduction in the use of opioids and postoperative pain scores within 24 h. The variation between these studies may be related to the surgical site and the dose of lidocaine applied during the operation. Abdominal surgery and thoracic surgery lead to different levels of visceral damage, while thyroid surgery and total hip replacement surgery are inflicting mostly physical pain. This may be because lidocaine is better at relieving visceral pain than physical pain.
Damage to organs such as pleura and lung tissue can put the body in a state of stress, and excessive stress response seriously affects the prognosis of patients. During intraoperative OLV, airway pressure increases, lung compliance decreases, and the non-ventilated lung completely collapses, while still receiving part of the cardiac output from the right ventricle, resulting in an intrapulmonary shunt. Although the ventilation volume and pulmonary blood flow of the ventilated lung are increased, the VA/Q ratio cannot be completely normalized. In OLV, the intrapulmonary shunt flow can reach 20–40%.12 Increased intrapulmonary shunt rate leads to pulmonary venous blood adulteration, which can produce hypoxemia. The results of this test showed that the PO2 and OI of the L group were significantly increased after the patient resumed bilateral lung ventilation for 20 min, and A-aDO2, Ppeak, and Pplat were significantly reduced. A-aDO2 and OI had a good correlation with the intrapulmonary shunt rate, suggesting that lidocaine reduces the shunt rate in the lungs to a certain extent. Various mechanisms have been proposed on how lidocaine can reduce lung damage. In an animal study,13 it was demonstrated that lidocaine can play a lung-protective effect by reducing endothelial cell damage caused by the inflammatory response, while also reducing the expression of adhesion molecules, and shedding enzymes. Another study showed5 that lidocaine can reduce lung injury caused by ischemia/reperfusion injury.
Ho14 found that after a bolus of 1.5 mg/kg with 1.0 mg/kg/h continuous infusion of lidocaine for 48 h, the average plasma concentration of lidocaine was 1.6 μg/mL. El-Tahan15 found that after receiving a bolus 1.5 mg/kg before induction of anesthesia in patients undergoing cesarean section, 1.5 mg/kg/h was continuously pumped with lidocaine, and the blood concentration rises to (2.05±0.42) μg/mL; Sahmeddini16 with a bolus of 1.5 mg/kg followed by continuous infusion of lidocaine at 2.0 mg/kg/h until the end of the operation, the blood concentration of lidocaine is 1.0–2.0 μg/mL during the entire operation period. The currently recommended regimen for intravenous application of lidocaine is 1.0–2.0 mg/kg for continuous infusion after a bolus of 1.0–2.0 mg/kg/h. At this time, the blood concentration of lidocaine was far lower than the concentration of 5.0 μg/mL that produces toxic reactions and is therefore considered to be safe.17 During this trial, none of the patients had lidocaine-related adverse events. Furthermore, in this trial, there was no significant difference in the hemodynamics between the two groups of patients at various time points, which would not significantly affect the patient’s intraoperative hemodynamics. Therefore, this dose of lidocaine can be safely used in ordinary patients.
There are still some limitations to this trial. This trial only included patients between 45 and 65 years of age, and the incidence of lung cancer is gradually becoming “younger”. The sample size was small, and therefore a multi-center, large-sample prospective randomized controlled clinical trial is required to confirm the result. Moreover, this trial did not explore the dose–effect relationship between lidocaine and postoperative recovery quality and lung protection.
Conclusion
Our study suggested that lidocaine can improve the quality of postoperative recovery in patients undergoing thoracoscopic radical resection of lung cancer, provides a lung protection effect, improves the postoperative analgesia, reduces the application of opioids during the operation, and promotes the recovery of gastrointestinal function of patients. It can therefore be used in clinical practice.
Acknowledgments
No commercial funding was received.
Data Sharing Statement
The individual participant’s data that underlying the results reported in this article would be accessed with approval from the corresponding author after 6 months of publication. The study protocol, statistical analysis plan and clinical study report will also be available.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Holbek BL, Horsleben Petersen R, Kehlet H, et al. Fast-track video-assisted thoracoscopic surgery: future challenges. Scand Cardiovasc J. 2016;50(2):78–82. doi: 10.3109/14017431.2015.1114665 [DOI] [PubMed] [Google Scholar]
- 2.Umari M, Falini S, Segat M, et al. Anesthesia and fast-track in video-assisted thoracic surgery (VATS): from evidence to practice. J Thorac Dis. 2018;10(Suppl4):S542–SS54. doi: 10.21037/jtd.2017.12.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Oliveira GS Jr, Fitzgerald P, Streicher LF, et al. Systemic lidocaine to improve postoperative quality of recovery after ambulatory laparoscopic surgery. Anesth Analg. 2012;115(2):262–267. doi: 10.1213/ANE.0b013e318257a380 [DOI] [PubMed] [Google Scholar]
- 4.De Oliveira GS Jr, Duncan K, Fitzgerald P, et al. Systemic lidocaine to improve quality of recovery after laparoscopic bariatric surgery: a randomized double-blinded placebo-controlled trial. Obes Surg. 2014;24(2):212–218. doi: 10.1007/s11695-013-1077-x [DOI] [PubMed] [Google Scholar]
- 5.Das KC, Misra HP. Amelioration of postischemic reperfusion injury by antiarrhythmic drugs in isolated perfused rat lung. Environ Health Perspect. 1994;102 Suppl 10(Suppl10):117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson ME, Berney C, Behan AL, et al. The effect of intravenous lidocaine infusion on bronchoalveolar lavage cytology in equine recurrent airway obstruction. J Vet Intern Med. 2012;26(6):1427–1432. doi: 10.1111/j.1939-1676.2012.01010.x [DOI] [PubMed] [Google Scholar]
- 7.Myles PS. Measuring quality of recovery in perioperative clinical trials. Curr Opin Anaesthesiol. 2018;31(4):396–401. doi: 10.1097/ACO.0000000000000612 [DOI] [PubMed] [Google Scholar]
- 8.Beverly A, Kaye AD, Ljungqvist O, et al. Essential elements of multimodal analgesia in Enhanced Recovery After Surgery (ERAS) guidelines. Anesthesiol Clin. 2017;35(2):e115–e143. doi: 10.1016/j.anclin.2017.01.018 [DOI] [PubMed] [Google Scholar]
- 9.Cui W, Li Y, Li S, et al. Systemic administration of lidocaine reduces morphine requirements and postoperative pain of patients undergoing thoracic surgery after propofol-remifentanil-based anaesthesia. Eur J Anaesthesiol. 2010;27(1):41–46. [DOI] [PubMed] [Google Scholar]
- 10.Choi KW, Nam KH, Lee JR, et al. The effects of intravenous lidocaine infusions on the quality of recovery and chronic pain after robotic thyroidectomy: a Randomized, Double-Blinded, Controlled Study. World J Surg. 2017;41(5):1305–1312. doi: 10.1007/s00268-016-3842-1 [DOI] [PubMed] [Google Scholar]
- 11.Martin F, Cherif K, Gentili ME, et al. Lack of impact of intravenous lidocaine on analgesia, functional recovery, and nociceptive pain threshold after total hip arthroplasty. Anesthesiology. 2008;109(1):118–123. doi: 10.1097/ALN.0b013e31817b5a9b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bender SP, Anderson EP, Hieronimus RI, et al. One-lung ventilation and acute lung injury. Int Anesthesiol Clin. 2018;56(1):88–106. doi: 10.1097/AIA.0000000000000172 [DOI] [PubMed] [Google Scholar]
- 13.Rancan L, Simón C, Sánchez Pedrosa G, et al. Glycocalyx degradation after pulmonary transplantation surgery. Eur Surg Res. 2018;59(3–4):115–125. doi: 10.1159/000489492 [DOI] [PubMed] [Google Scholar]
- 14.Ho MLJ, Kerr SJ, Stevens J, et al. Intravenous lidocaine infusions for 48 h in open colorectal surgery: a prospective, randomized, double-blinded, placebo-controlled trial. Korean J Anesthesiol. 2018;71(1):57–65. doi: 10.4097/kjae.2018.71.1.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Tahan MR, Warda OM, Diab DG, et al. A randomized study of the effects of perioperative i.v. lidocaine on hemodynamic and hormonal responses for cesarean section. J Anesth. 2009;23(2):215–221. doi: 10.1007/s00540-009-0738-3 [DOI] [PubMed] [Google Scholar]
- 16.Sahmeddini MA, Khosravi MB, Farbood A, et al. Comparison of perioperative systemic lidocaine or systemic ketamine in acute pain management of patients with opioid use disorder after orthopedic surgery. J Addict Med. 2019;13(3):220–226. doi: 10.1097/ADM.0000000000000483 [DOI] [PubMed] [Google Scholar]
- 17.Dunn LK, Durieux ME. Perioperative use of intravenous lidocaine. Anesthesiology. 2017;126(4):729–737. doi: 10.1097/ALN.0000000000001527 [DOI] [PubMed] [Google Scholar]