Abstract
Objective:
Evaluate the effect of 70% isopropyl alcohol- impregnated central venous catheter caps on ambulatory central line-associated bloodstream infections (CLABSI) in pediatric hematology/oncology patients.
Design:
24 month, cluster-randomized, 2 period, crossover clinical trial
Setting:
15 pediatric healthcare institutions, including 16 pediatric hematology/oncology clinics
Participants:
All patients with an external central line followed at one of the 16 hematology/oncology clinics
Intervention:
Usual ambulatory central line care per each institution with use of 70% isopropyl alcohol- impregnated caps at home compared to usual ambulatory central line care per each institution without use of 70% isopropyl alcohol- impregnated caps.
Results:
Of the 16 participating clinics, 15 clinics completed both assignment periods. As assigned, there was no reduction in CLABSI incidence in clinics using 70% isopropyl alcohol-impregnated caps (1.23 per 1000 days) compared with standard practices (1.38 per 1000 days; adjusted incidence rate ratio [aIRR] 0.83, 95%CI 0.63, 1.11). In the per protocol population, there was a reduction in any positive blood culture incidence in clinics using 70% isopropyl alcohol-impregnated caps (1.51 per 1000 days) compared with standard practices (1.88 per 1000 days; aIRR 0.72, 95%CI 0.52, 0.99). There were no reported adverse events.
Conclusions:
Isopropyl alcohol-impregnated central line caps did not lead to a statistically significant reduction in CLABSI rates in ambulatory hematology/oncology patients. In the per protocol analysis, there was a statistically significant decrease in any positive blood cultures. Larger trials are needed to elucidate the impact of 70% isopropyl alcohol-impregnated caps in the ambulatory setting.
INTRODUCTION:
Childhood survival after a cancer diagnosis has significantly increased over the past 25 years in the United States.1,2 One reason is intensive chemotherapy regimens delivered through implanted and non-implanted central venous catheters.1,2 Unfortunately, these same central venous catheters pose risk to immune-compromised children for developing healthcare associated infections such as central line-associated bloodstream infections (CLABSI). National efforts have reduced CLABSI in hospitalized patients, but relatively little work has targeted CLABSI prevention in the home setting.
In some high-risk populations, more CLABSI occur in the outpatient setting (ambulatory CLABSI), than in the inpatient setting. For example, there are 1.8–2.9 times as many ambulatory CLABSI in pediatric hematology/oncology patients as compared to inpatient hematology/pediatric oncology CLABSI.3,4 Ambulatory CLABSI often result in a hospitalization and sometimes catheter removal and replacement. The home setting has unique attributes that hamper translation of known best central line practices in the inpatient setting to prevent CLABSI in the ambulatory setting. For example, caregivers handling the central line at home are often patients and families as opposed to medically-trained nurses and physicians, and catheters in the home setting are subject to realities not encountered in hospitals, such as patients playing games outside, routinely showering, and interacting with pets. A recent survey of patients and families in a tertiary care pediatric hematology/oncology clinic found significant gaps in adherence to best practices at home for central line care, contradictory maintenance care training between hospital providers and home care providers, and significant gaps in education and awareness of risk among patients and families.5 Additionally and similar to inpatient CLABSI efforts, initial studies suggest CLABSI in the home setting are also preventable.6
Isopropyl alcohol impregnated protective caps guard central venous catheters from external contamination. In 2013 when this trial was proposed, many hospitals were using these caps on central venous lines, but data were limited on whether they prevented CLABSI either on inpatient or in ambulatory settings.7–11 Our objective was to determine if 70% isopropyl alcohol impregnated protective caps reduced CLABSI in ambulatory pediatric hematology/oncology patients.
METHODS:
Trial Design:
We designed the Community Central Line Prevention Trial (CCLIP), an investigator-initiated, unmasked, cluster-randomized, 2 period, crossover trial to evaluate the effect of 70% isopropyl alcohol impregnated protective caps on ambulatory bloodstream infections in pediatric hematology/oncology patients with external central lines between November 2015 and May 2018. Each of 16 clinic sites had one control (B) and one intervention (A) period and was randomized to one of the two sequences: either AB or BA. The intervention was delivered at the clinic level, such that all eligible patients in a clinic during a given period would receive the same assignment. After an initial 12 month period, clinics crossed to the alternative assignment for another 12 month period. For sites crossing from intervention to control (AB), there was a planned 3-month washout period. Sites reported that limited quantities of caps were dispensed to families, so the 3-month washout period was reduced to 2-months, still providing a sufficient wash-out period. A staggered study initiation was planned. The final protocol and amendments and the final statistical analysis plan are available in Supplement A. This study is registered with ClinicalTrials.gov (Identifier: NCT02351258).
Participants:
Hospitals participating in the Children’s Hospital Association Hematology/Oncology Inpatient CLABSI Quality Transformation Collaborative and other hospitals with Pediatric Hematology/Oncology programs were invited. Eligibility criteria for clinics included 1) agree to randomization, 2) agree to not use intervention during the control period, and 3) obtain IRB approval. All patients who visited a pediatric hematology/oncology clinic and had an external central line (e.g. Hickman, Broviac, central PICC, non-tunneled central lines) were eligible for participation in this trial. Three institutions were using 70% isopropyl alcohol impregnated protective caps for central lines in ambulatory pediatric hematology/oncology patients prior to start of this trial and agreed to discontinue cap use for the 12-month Control period of the trial to be eligible.
Ethical Considerations and Institutional Review Board:
The Johns Hopkins University Institutional Review Board (IRB) served as the central IRB and approved JHU as the coordinating center and a participating site. Each participating site received approval from their Institutional Review Board (Supplemental B Table B1). Some sites required informed consent and others waived consent.
Treatment Allocation:
This study tested two standards of care for preventing bloodstream infections. Randomization occurred at the institution level. The goal of randomization was to achieve balance between 2 study arms with respect to the ambulatory external central line day volume and the annual number of patients at each clinic site who underwent a hematopoietic cell transplant (HCT). Central line day volume was estimated as the number of ambulatory external central line days based on data provided by sites from May and June 2015. Both central line day volume the annual number of patients at each clinic site who underwent a HCT were divided into 2 strata: below versus above the median. In addition to these two covariates, attention was taken to ensure that the study arms were also balanced on ambulatory bloodstream infection rate for all patients with an external central line assessed for a two month period (May and June 2015). Stratified block randomization was used to generate the study arm assignments separately for each of the four randomization strata of central line day volume and annual number of patients undergoing HCT.12 Period 1 assignment was concealed from hospitals until they agreed to participate. During the study period, the investigators and caregivers were not masked.
Intervention:
In the intervention arm, 70% isopropyl alcohol impregnated protective caps were placed on external central lines in pediatric hematology/oncology patients who were not admitted to the hospital. Children at sites participating in the Children’s Hospital Association Hematology/Oncology Inpatient CLABSI Quality Transformation Collaborative received the best practice central line Maintenance Care Bundle (Supplement B Figure B1) from health care workers during outpatient clinic visits and other sites provided their institutional central line maintenance care bundle. Fourteen institutions were provided 3M™ Curos™ Disinfecting Cap for Needleless Connectors (3M, St. Paul, MN). At the first ambulatory visit during the intervention period, each site provided families with Disinfecting Cap and instructions for use (see Supplement B Figure B2). One institution used the SwabCap™ (ICU Medical, Inc. San Clemente, CA) 70% isopropyl alcohol impregnated protective caps, a product the hospital had been using prior to start of this trial. In the control period, patients received institutional central line maintenance care bundle per institutional policy and practices. If during the Intervention period of the trial an individual participant was admitted to the hospital, the use of the 70% isopropyl alcohol impregnated protective cap was discontinued and care of the central line during the hospitalization was per individual institutional inpatient policy. Upon discharge from that hospital admission, that individual participant returned to the assigned intervention.
Study Outcomes:
The primary outcome was ambulatory central line-associated bloodstream infections (CLABSI) defined using Centers for Disease Control and Prevention’s (CDC) National Healthcare Safety Network (NHSN) 2016 surveillance criteria for CLABSI.13 We defined an ambulatory CLABSI as a CLABSI that occurred while in the outpatient setting and was more than 48 hours after hospital discharge or within 48 hours of hospital admission. Ambulatory central line days were tracked by each site using a standardized data collection form (see Supplement B Figure B3). The primary outcome measurement was the incidence of ambulatory CLABSI per 1000 ambulatory external central line days.
Secondary outcomes included ambulatory secondary bloodstream infections (secondary BSI) as determined using CDC NHSN criteria, ambulatory single positive blood cultures (SPBC) that grew an organism on the CDC NHSN’s list of common commensal organisms14, ambulatory CLABSI in patients with mucosal barrier injury (MBI-CLABSI), and positive blood cultures that included CLABSI, MBI-CLABSI, secondary BSI, and SPBC combined. Each participating institution’s study team worked with their infection prevention team to determine which ambulatory positive blood cultures met definitional criteria for CLABSI, secondary BSI, SPBC, or MBI-CLABSI. Each hospital’s infection prevention team monitored ambulatory bacteremias in pediatric hematology/oncology patients with external catheters, prospectively applied NHSN criteria to distinguish CLABSIs, MBI-CLABSIs, secondary BSIs and SPBC, and provided a list of outcomes to the study team. Study team members used standard NHSN methodology to collect ambulatory external central line days. After completion of the trial, we updated the outcomes to align with the current CDC NHSN definitions instead of those in place at the time the trial was proposed. Initially, the protocol proposed that bloodstream infections (BSIs) that met the CDC NHSN’s laboratory-confirmed mucosal barrier injury criteria would be included as secondary bloodstream infections (secondary BSIs). Consistent with current CDC NHSN criteria, we updated the statistical analysis plan to include Mucosal Barrier Injury central line-associated bloodstream infections (MBI-CLABSI) as a stand-alone secondary outcome.
Data collection:
Each participating site submitted monthly data to the coordinating center using REDCap, including the number of primary and secondary outcomes, the number of ambulatory external central line days, and the number and type of microorganisms associated with each outcome event. No patient-level information was collected. Sites were queried after each outcome to determine if the patient was adherent with the assignment at time of the event. Each team assessed non-adherence by asking the family of a child with an ambulatory positive blood culture whether protective caps were used on central lines at the time of event in the control period (non-adherent) or whether protective caps were not used at the time of event in the intervention period (non-adherent). We conducted an annual site survey to determine patient demographics and infection prevention practices at the participating clinics.
Sample Size:
The required sample size was estimated using baseline CLABSI incidence data from January 2013 to simulate two 12 month periods, with a crossover, for a total study period of 24 months. Monthly ambulatory external central line days were simulated from a uniform distribution on the range 20% lower and 20% greater than those reported in January 2013. The monthly number of infections were sampled from a Poisson distribution. For each simulated study, we fit a generalized linear mixed effect Poisson regression model that included an indicator for treatment period, order of treatment, an offset for ambulatory external central line days, random intercept for unit and robust variance estimate. We considered four cases based upon the proportion of up to 24 clinics that would participate (24 clinics expressed interest at time of grant submission): 100%, 75%, 67% and 50% participation rate. Sixteen clinics enrolling for 24 months would provide >80% power with a Type I error rate of 5% to detect a treatment effect of 0.50 for ambulatory CLABSI and a treatment effect of 0.60 for all positive blood cultures.
Statistical analysis:
The primary analysis followed the principles of intention-to-treat (ITT). The analysis sample included monthly events and line-day data from all randomized clinics. The per protocol (PP) sample included monthly events and ambulatory external central line days from all clinics, excluding events that occurred in patients that were not adherent with the assignment. Because central line days were not collected at the patient-level, for the PP sample, sensitivity analyses removed varying number of days from the denominator for the site and month that the non-adherent event occurred. At-risk days could not be removed for patients that did not develop an event. The treatment effect was determined using a mixed effects Poisson regression model, specifically, the monthly number of CLABSI or the monthly number of any positive blood culture modeled as a function of the dichotomous treatment assignment, with inclusion of an offset representing the monthly number of ambulatory external central line days, adjustment for time period (i.e. 1st vs. 2nd 12-month time period), and a random intercept for unit to account for the correlation of monthly rates of infections over time within the same unit. Planned secondary analyses included 1) adjusted model including the combined number of HCT patients dichotomized at the median value and the use of chlorhexidine impregnated disc in ambulatory patients. We assessed the distributions of other potential confounding site-level variables over time using annual survey data, such as prior use of an antibiotic or antiseptic impregnated catheter, antibiotic catheter locks, chlorhexidine impregnated central line dressings, and chlorhexidine bathing, volume of patients, admissions, beds, central line days or HCT patients using Kruskal-Wallis and Fisher’s exact tests. We also tested for “order effect”, specifically whether there is differential treatment effect by time period. Above analyses were performed for the ITT and PP groups. Data were analyzed using STATA statistical software program (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC).
RESULTS:
Study Population:
Of 24 hospitals participating in the Children Hospital Association’s Hematology/Oncology Inpatient CLABSI Quality Transformation Collaborative and the other contacted hospitals with Pediatric Hematology/Oncology programs, 16 hospitals encompassing 17 pediatric hematology/oncology clinics agreed to participate (Figure 1). One institution withdrew in the first few months following randomization due to poor enrollment. Fifteen hospitals encompassing 16 pediatric hematology/oncology clinics completed both 12 month arms of the trial. One hospital randomized to intervention in period 1 was unable to recruit patients in the control period but was included in the ITT analysis despite contributing no information to period 2. The patient population characteristics and ambulatory pediatric hematology/oncology clinic characteristics and practices are shown in Table 1. Of the 16 clinics, 12 (75%) used chlorhexidine impregnated disc at the central line insertion site in ambulatory patients, and only 1 site (6%) reported chlorhexidine gluconate bathing in ambulatory patients prior to the trial. The patient and unit characteristics and practices did not change over the study period.
FIGURE 1:
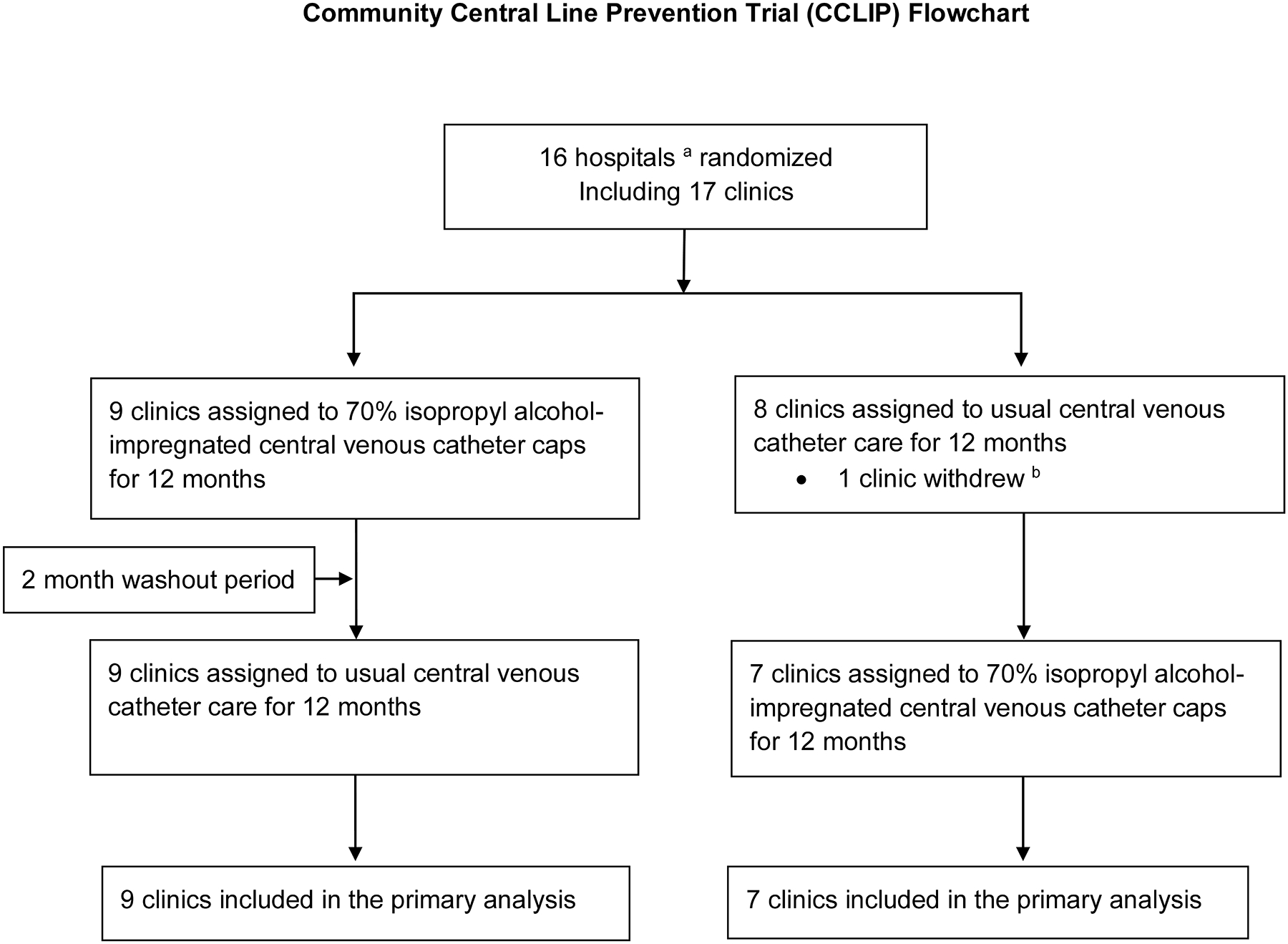
Community Central Line Infection Prevention (CCLIP) Trial Flowchart. This diagram describes the recruitment and enrollment of the CCLIP Trial from November 2015 to May 2018. a Unit of randomization was the hospital, so two clinics at the same hospital were randomized to the same arm; b unable to recruit patients into trial
Table 1.
Clinic and patient population characteristics and practices of ambulatory pediatric oncology clinics and patient populations
| 2015 (Baseline) | 2016 | 2017 | p-value | |
|---|---|---|---|---|
| Site Characteristic | N=16 | N=16 | N=16 | |
| Female, % (IQR) | 46 (45–52) | 47 (43–49) | 47 (44–48) | 0.65 a |
| Age, % (IQR) | ||||
| <2 years | 15 (10–19) | 9 (5–17) | 9 (5–14) | 0.35 a |
| 2–5 years | 19 (15–22) | 19 (17–28) | 18 (14–22) | 0.71 a |
| 6–11 years | 26 (24–29) | 27 (22–30) | 27 (24–29) | 0.97 a |
| ≥ 12 years | 41 (36–46) | 43 (32–46) | 44 (39–48) | 0.75 a |
| Number of inpatient pediatric oncology beds, median (IQR) | 25 (20–34) | 25 (20–34) | 25 (20–34) | 1.00 a |
| Number of annual pediatric oncology admissions, median (IQR) | 1,091 (727–1,354) | 896 (655–1,236) | 884 (752–1,170) | 0.96 a |
| Number of pediatric oncology ambulatory visits, median (IQR) | 8,552 (6,790–11,858) | 8,731 (4,176–11,763) | 8,483 (6,115–10,877) | 0.93 a |
| Number of total inpatient days, median (IQR) | 5,640 (4,664–8,423) | 5,563 (4,491–9,527) | 5,537 (4,544–9,226) | 0.99 a |
| Number of total HCT patients, median (IQR) | 32 (10–49) | 30 (19–48) | 32 (13–51) | 0.80 a |
| External Central Line Practices for Ambulatory Patients | ||||
| Use antimicrobial/antiseptic impregnated catheters % sites (N) | 0 (0) | 0 (0) | 0 (0) | |
| Use antibiotic/non-antibiotic locks % sites (N) | 19 (3) | 12 (2) | 12 (2) | 1.00 b |
| Use of chlorhexidine impregnated disc % sites (N) | 75 (12) | 62 (10) | 62 (10) | 0.81 b |
| Use chlorhexidine gluconate baths % sites (N) | 6 (1) | 0 (0) | 0 (0) | 1.00 b |
| External Central Line Practices for Inpatients | ||||
| Prior to this trials - Percentage of patients using alcohol impregnated catheter caps prior to the trial % sites (N) | ||||
| 0 | 38 (6) | |||
| 1–49% | 12 (2) | |||
| 50–100% | 50 (8) | |||
Kruskal-Wallis test;
Fisher’s exact test
Primary Outcome (CLABSI):
In the ITT analysis, there were 232 CLABSI, 123 CLABSI during control and 109 during intervention periods. The crude CLABSI incidence was 1.38 per 1,000 ambulatory external central line days (range 0 – 2.30, 95% CI 1.08, 1.77) in control clinics and 1.23 per 1,000 ambulatory external central line days (range 0 – 3.95, 95% CI 0.94, 1.60) in intervention clinics. In 9 of 16 clinics, the crude incidence of CLABSI was lower during the intervention period compared with the control period, regardless of whether alcohol impregnated caps were assigned in study period 1 or 2 (Figure 2 and Supplement B Table B2).
FIGURE 2:
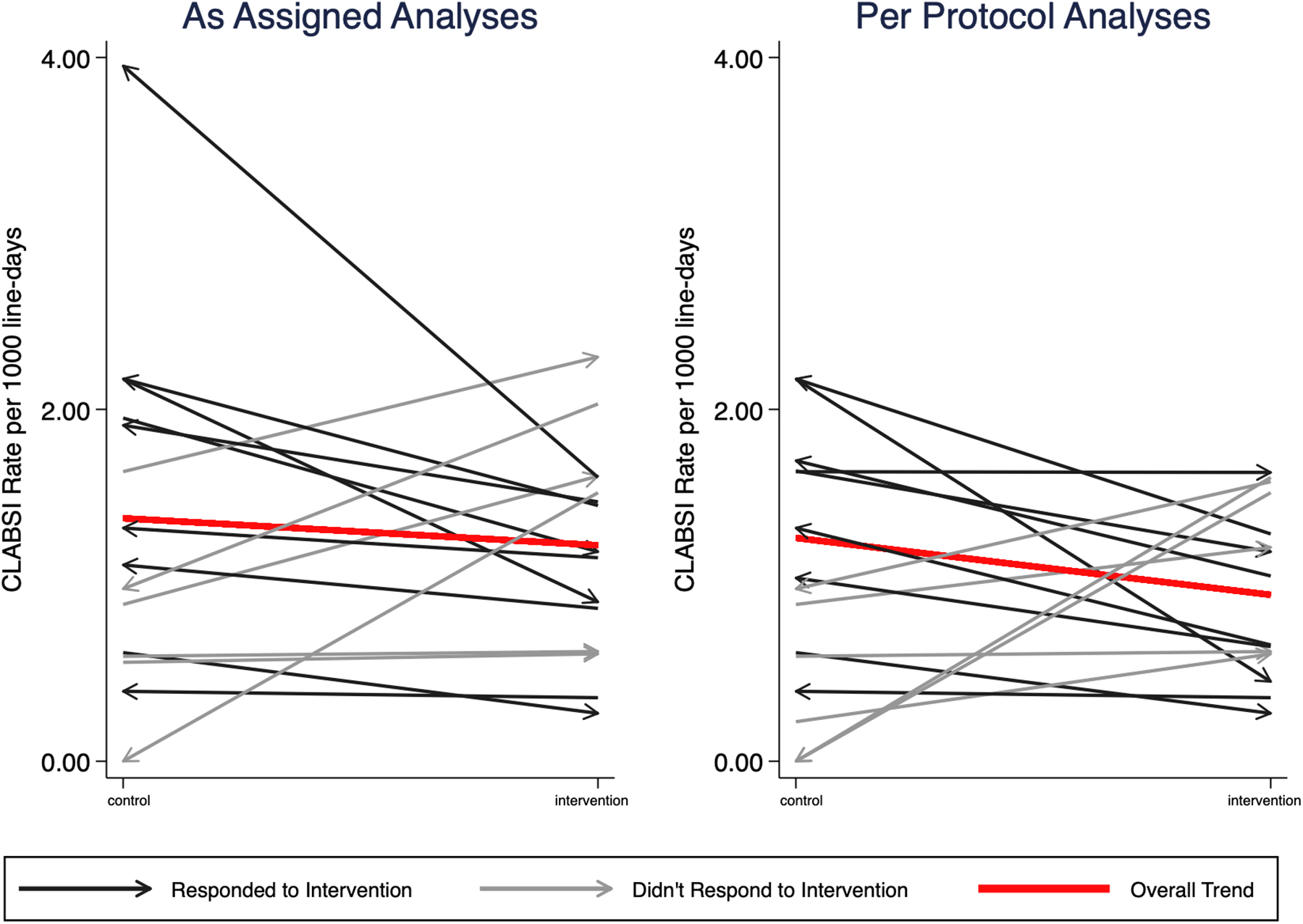
Crude incidence of central line associated bloodstream infections (CLABSI). Each line represents one clinic. The slope shows the change in incidence of CLABSI between the intervention and control periods. The red line represents the change in overall crude incidence during intervention and control periods. Black lines represent clinics that had a decrease in CLABSI during the intervention period and blue lines represent clinics that did not have a decrease in CLABSI during intervention periods. Arrows indicate the assignment change from period one to period two, such that an arrow pointing to the intervention side indicates that the unit was assigned to control in period 1 and crossed over to intervention in period 2.
During intervention periods (clinics assigned to using alcohol-impregnated caps), clinics had a 14% lower incidence of CLABSI that did not achieve statistical significance (incidence rate ratio [IRR] 0.86, 95%CI 0.63,1.18, p=0.35) compared to control periods (clinics assigned to no caps). (Table 2) After adjusting for the number of HCT patients and the use of chlorhexidine impregnated discs around the central line in ambulatory patients, during intervention periods, the difference in the incidence of CLABSI of 17% remained not statistically significant (IRR 0.83, 95%CI 0.63,1.11, p=0.22) compared to control periods.
Table 2.
Summary of Primary and Secondary (MBI-CLABSI, SBSI, SPBC, Any positive blood culture) Outcomes
| Events in Control Clinics | Events in Intervention Clinics | Crude Control IR per 1000 at-risk days (95%CI) | Crude Intervention IR per 1000 at-risk days (95%CI) | Crude IRR (95% CI) | p-value | Adjusted IRRa (95% CI) | p-value | |
|---|---|---|---|---|---|---|---|---|
| ITT Analyses | ||||||||
| CLABSI, as assigned (primary outcome) | 123 | 109 | 1.38 (1.08 to 1.77) | 1.23 (0.94 to 1.60) | 0.86b (0.63 to 1.18) | 0.35 | 0.83 (0.63 to 1.11) | 0.22 |
| MBI-CLABSI, as assigned | 16 | 10 | 0.18 (0.08 to 0.40) | 0.11 (0.05 to 0.24) | 0.58c (0.22 to 1.54) | 0.27 | 0.54 (0.17 to 1.75) | 0.30 |
| Secondary BSI, as assigned | 11 | 0 | 0.12 (0.06 to 0.26) | 0 | 0 | - | - | - |
| Single positive blood culture, as assigned | 36 | 51 | 0.40 (0.23 to 0.70) | 0.57 (0.35 to 0.95) | 1.37b,c (0.77 to 2.42) | 0.28 | 1.38c (0.76 to 2.48) | 0.29 |
| Any positive blood culture, as assigned (includes CLABSI, MBI-CLABSI, SBSI, SPBC) | 186 | 170 | 2.09 (1.67 to 2.62) | 1.91 (1.43 to 2.56) | 0.85b (0.62 to 1.17) | 0.31 | 0.81 (0.61 to 1.07) | 0.14 |
| Per Protocol Analysis | ||||||||
| CLABSI, as assigned | 113 | 84 | 1.27 (0.95 to 1.69) | 0.95 (0.72 to 1.25) | 0.73b (0.49 to 1.07) | 0.11 | 0.71 (0.48 to 1.04) | 0.08 |
| MBI-CLABSI, as assigned | 14 | 8 | 0.16 (0.06 to 0.39) | 0.09 (0.04 to 0.20) | 0.52 (0.19 to 1.46) | 0.21 | 0.48 (0.15 to 1.60) | 0.23 |
| Secondary BSI, as assigned | 11 | 0 | 0.12 (0.06 to 0.26) | 0 | 0 | - | - | - |
| Single positive blood culture, as assigned | 29 | 42 | 0.33 (0.19 to 0.57) | 0.47 (0.28 to 0.80) | 1.39b,c (0.76 to 2.57) | 0.29 | 1.35 (0.73 to 2.49) | 0.34 |
| Any positive blood culture, as assigned (includes CLABSI, MBI-CLABSI, SBSI, SPBC) | 167 | 134 | 1.88 (1.47 to 2.39) | 1.51 (1.14 to 2.00) | 0.75b (0.53 to 1.06) | 0.100 | 0.72 (0.52 to 0.99) | 0.045 |
CLABSI - central line-associated bloodstream infection; MBI-CLABSI – mucosal barrier injury central line-associated bloodstream infection; SBSI – secondary bloodstream infection; SPBC - single positive blood culture; IR – Incidence rate; IRR – Incidence rate ratio
Primary outcome highlighted in yellow
adjusted for the year-specific total number of HCT patients and the use of chlorhexidine impregnated disc
no statistically significant interaction between intervention and period
the planned model did not converge, a generalized linear model with unit-correlated robust variance was used instead
After excluding events that occurred in patients found non-adherent with assignment, there were 197 CLABSI; 113 CLABSI during control and 84 during intervention periods. During intervention periods, clinics had a 27% non-statistically significant reduction (IRR 0.73, 95%CI 0.49, 1.07, p=0.11) in the incidence of CLABSI compared to control periods. After adjusting for the number of HCT patients and the use of chlorhexidine impregnated discs around the central line in ambulatory patients, during intervention periods, clinics had a 29% non-statistically significant reduction (IRR 0.71, 95%CI 0.48, 1.04, p=0.08) in the incidence of CLABSI compared to control periods (Table 2 and Supplement B Table B3).
Secondary Outcomes (MBI-CLABSI, secondary BSI, SPBC, and any positive blood culture):
In the ITT analysis, there were no statistically significant differences in MBI-CLABSI, SPBC and any positive blood culture comparing intervention and control periods. There were more secondary BSI in the control group. Consistent with the primary outcome analysis, the treatment effect for the secondary outcomes was similar in the per protocol (PP) analysis (Table 2). In the PP analytic group, there were 301 positive cultures in patients that complied with assignment, 167 in clinics assigned to control and 134 in clinics assigned to intervention. During intervention periods, clinics had a 25% non-statistically significant reduction (IRR 0.75, 95%CI 0.53, 1.06, p=0.10) in the incidence of positive cultures compared to control periods (Supplement B Figure B4). After adjusting for prespecified variables above, during intervention periods, clinics had a 28% reduction (IRR 0.72, 95%CI 0.52, 0.99, p=0.045) in the incidence of positive cultures compared to control periods.
Microbiology of outcomes:
For the 232 CLABSI, there were 286 organisms isolated. The most common organisms included coagulase-negative Staphylococci (n=50), Staphylococcus aureus (n=35), and Enterobacter cloacae (n=21) (Table 3 and Supplement B Table B4). There were no differences in the distribution of type of organism causing CLABSI by treatment arm (Gram positive organisms causing CLABSI in the control and intervention periods 47.3% and 45.00%, respectively, p=0.69). Similarly, there were no differences in the distribution of Gram positive and Gram negative organisms causing any positive blood cultures comparing intervention and control periods.
Table 3.
Microorganisms isolated from patients with central line-associated bloodstream infections (CLABSI) and any positive blood culture
| Intervention | Control | p-valuea | Intervention | Control | p-valuea | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Microorganisms, number | 140 | 146 | 202 | 217 | ||||||
| Gram positive | 63 | 45.00% | 69 | 47.26% | 0.69 | 113 | 55.94% | 116 | 53.46% | 0.66 |
| Staphylococcus species, coagulase negative | 25 | 17.86% | 25 | 17.12% | 42 | 20.79% | 43 | 19.82% | ||
| Staphylococcus aureus | 21 | 15.00% | 14 | 9.59% | 25 | 12.38% | 18 | 8.29% | ||
| Enterococcus faecalis | 3 | 2.14% | 10 | 6.85% | 4 | 1.98% | 10 | 4.61% | ||
| Other | 14 | 10.00% | 20 | 13.70% | 42 | 20.79% | 45 | 20.74% | ||
| Gram negative | 75 | 53.57% | 72 | 49.32% | 87 | 43.07% | 95 | 43.78% | ||
| Enterobacter cloacae | 16 | 11.43% | 5 | 3.42% | 18 | 8.91% | 8 | 3.69% | ||
| Escherichia coli | 6 | 4.29% | 13 | 8.90% | 9 | 4.46% | 19 | 8.76% | ||
| Klebsiella pneumonia | 9 | 6.43% | 8 | 5.48% | 9 | 4.46% | 13 | 5.99% | ||
| Other | 44 | 31.4% | 46 | 31.51% | 51 | 25.25% | 55 | 25.35% | ||
| Candida species | 1 | 0.71% | 3 | 2.05% | 1 | 0.50% | 3 | 1.38% | ||
| Otherb | 1 | 0.71% | 2 | 1.37% | 1 | 0.50% | 3 | 1.38% |
Fisher’s exact test of association, looking at the difference in distribution of the type of microorganism by treatment arm
Mycobacterium species (n=3), other, not otherwise specified (n=1)
ADVERSE EVENTS:
There were no reported adverse events.
DISCUSSION:
Our trial evaluating use of 70% isopropyl alcohol impregnated protective caps in ambulatory pediatric hematology/oncology patients did not show a statistically significant reduction in CLABSI across 16 clinic sites in an intention to treat analysis. Alcohol impregnated protective caps did lead to a statistically significant 28% reduction in the per protocol analysis for the secondary outcome of all positive blood cultures. This outcome includes single positive blood cultures that may represent blood culture contamination, but many single positive blood cultures are treated none the less and lead to antibiotic exposure. Pediatric hematology/oncology patients more commonly get CLABSI at home than while in the hospital, but few evidence-based strategies exist to protect ambulatory patients from CLABSI. Larger trials are needed to elucidate whether 70% isopropyl alcohol-impregnated caps have a clinically meaningful effect on CLABSI in the ambulatory setting.
Intense and ever growing pressures on inpatient CLABSI rates from pay for performance efforts to public reporting, has created a substantial body of literature over the last 15 years on the preventability of inpatient CLABSI and the associated costs. Few studies, however, focus on the epidemiology and preventability of ambulatory CLABSI. Ambulatory CLABSI often result in a hospital admission, so the lack of attention and resources to prevent these infections is inconsistent with their impact on patients.15 Prior studies suggest that alcohol impregnated protective caps defend central venous catheters from bacterial colonization and possibly infections, but most data on the impact of these caps exist from studies of inpatient adults.16 Our trial did not show a statistically significant reduction in CLABSI in ambulatory pediatric hematology/oncology patients, a high-risk group often with intensive central line needs at home. Externalized catheters make up only a small proportion of ambulatory catheters in this population and may represent a underlying diagnosis that requires more intensive chemotherapy and care.4,17 Our findings may reflect unique challenges to ambulatory CLABSI prevention, including the relatively uncontrolled home environment compared to the hospital environment, as well as the skill level of central line caretakers, namely patients and families compared to nurses and doctors.
Further compounding meaningful research on CLABSI in any setting is the intense focus on CLABSI from payment and reporting efforts such that institutions are at times throwing the proverbial ‘kitchen sink’ at central line prevention without careful attention to the evidence behind interventions nor cost of these interventions to patients. In our trial, a significant recruitment barrier was either the institution or the individual patient’s unwillingness to stop using alcohol impregnated protective caps and scientifically evaluate this CLABSI prevention strategy. In our analysis of events in patients who were adherent to assignment, alcohol impregnated protective caps did lead to a 28% reduction of all positive blood cultures. While the cost of a single alcohol impregnated cap or antibiotic disc may seem insignificant, their repeated use on all patients with central lines likely adds significant additional costs to health care expenditure with far from conclusive evidence of true impact on CLABSI reduction. Therefore, further research must explore the clinical and economic impact of isopropyl alcohol- impregnated central line caps in the ambulatory setting.
The biologic argument for alcohol-impregnated caps is to provide a protective barrier over the hub of a central line and reduce the risk of introducing organisms into the lumen of the central line with each line entry. We hypothesized that a reduction in catheter hub contamination would reduce positive blood cultures and CLABSI, but did not expect this intervention to impact secondary BSIs or MBI-CLABSI. Contrary to our expectations, there were more secondary BSIs in the control group, but patient-level data were not available to confirm appropriate classification. Given the frequent use of alcohol-impregnated caps, further research is warranted to ensure no unintended harm is done to patients.
Strengths of this trial include the pragmatic trial design with a crossover and inclusion of 15 hospitals from across the country. Readers should consider several limitations. First, this was a pragmatic trial that did not collect patient level data, preventing adjusted analyses for confounders such other concurrent interventions (e.g. lock therapy) and adherence. Recording whether the patient was adherent with assignment at the time of each outcome event allowed for an as treated analysis, but non-adherent patients who did not have an event could not be identified and excluded. Because ambulatory external central line days were not available at the patient level, assumptions were made about the at-risk time of patients for the as assigned analysis. Second, adherence in clinic and at home with best central line care practices were not available, so whether this intervention would have been more or less effective in the setting of best central line care practices remains unknown. Third, although this study investigated two accepted standards of care for maintaining catheters and posed minimal risk to patients, the interpretation of various IRBs led to sites differences with regard to informed consent and the percent of patients enrolled into the trial. Fourth, because randomization occurred at the clinic level, patients that did not agree to participate still contributed data to the as-treated group. Fifth, there was unanticipated difficulty in recruiting sites to bolster power such that institutions were unwilling to stop using alcohol-impregnated caps in their ambulatory population despite no data at the time to support efficacy. Sixth, children with existing central lines were enrolled and the intervention was held when children were admitted to the hospital, so catheter contamination may have occurred before the intervention was introduced, in clinic, or during hospitalization. Seventh, this study only included ambulatory pediatric hematology/oncology patients and may not be generalize to other populations.
In conclusion, isopropyl alcohol-impregnated caps did not lead to a statistically significant reduction in CLABSI rates in ambulatory hematology/oncology patients; however, there was a statistically significant reduction in positive blood cultures in the per protocol analysis. Further research is needed to understand the clinical and economic impact of alcohol-impregnated caps in the ambulatory setting.
Supplementary Material
ACKNOWLEDGEMENTS:
We would like to thank Brian Dyer (Johns Hopkins University School of Public Health) for statistical support, Annie Voskertchian (Johns Hopkins University) for regulatory support and study coordination. We would also like to thank the following site leads for their support of this study: Jamie Jordan, PA-C, Denise Lahoski, MSN, RN, CPHON, Tiffany Tidmore, BSN, RN (Akron Children’s Hospital, Akron, OH); Amir Mian, MD (Arkansas Children’s Hospital); Jennifer Davila, MD, Siobhan Polese, NP (Children’s Hospital at Montefiore, Bronx, NY); Flori Legette, ND, RN (Children’s Hospital Colorado, Aurora, CO); Joseph Chewning, MD (Children’s of Alabama, Birmingham, AL); Linda Formby, RN, Michelle Cooper, RN (Medical University of South Carolina Children’s Hospital, Charleston, SC); Randal Olshefski, MD, Mindy Bibart, MSN, RN, CPHON (Nationwide Children’s Hospital, Columbus, OH); E. Anders Kolb, MD (Alfred DuPont Hospital for Children, Wilmington, DE); Kristina Bryant, MD, Kerry McGowan, MD, Alexa Cheerva, MD (Norton Children’s Hospital, Louisville, KY); Timothy Porea, MD, Janet DeJean, MSN, RN, CPON, Robbie Norville, MSN, RN, CPON (Texas Children’s Hospital, Houston, TX); William Slayton, MD (University of Florida Shands Children’s Hospital, Gainesville, FL).
Financial support. This project was supported by grant number R01HS022870 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare
Research and Quality. The study was designed, conducted, and analyzed by the authors. AHRQ had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The commercial sponsor (3M, St. Paul, MN) provided in kind product but had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Funding Source: This study was funded by the Agency for Healthcare Research and Quality (R01 HS022870). Alcohol impregnated caps were supplied in kind by 3M™ Curos.
Abbreviations:
- CLABSI
Central line-associated bloodstream infections
- MBI
mucosal barrier injury
- CCLIP
Community Central Line Prevention Trial
- IRB
Institutional Review Board
- HCT
hematopoietic cell transplant
- NHSN
National Healthcare Safety Network
- MBI-CLABSI
CLABSI in patients with mucosal barrier injury
- BSI
bloodstream infections
- SPBC
single positive blood cultures
- CDC
Centers for Disease Control and Prevention
- ITT
intention-to-treat
- PP
per protocol
Footnotes
Registration: ClinicalTrials.gov; NCT02351258;
Financial Disclosure: A.M.M. received prior grant support from Sage Products, Inc. and received consulting fees for BD both unrelated to this work, and receives grant support from NIH, CDC, and AHRQ unrelated to this work. J.G.C is a member of CDC’s Healthcare Infection Control Practice Advisory Committee (HICPAC). Other authors report no conflicts.
Conflicts of Interest: A.M.M. received prior grant support from Sage Products, Inc. and received consulting fees for BD both unrelated to this work, and receives grant support from NIH, CDC, and AHRQ unrelated to this work. J.G.C is a member of CDC’s Healthcare Infection Control Practice Advisory Committee (HICPAC). Other authors report no conflicts.
REFERENCES
- 1.Pulte D, Gondos A, Brenner H. Trends in 5- and 10-year survival after diagnosis with childhood hematologic malignancies in the United States, 1990–2004. J Natl Cancer Inst 2008;100:1301–9. [DOI] [PubMed] [Google Scholar]
- 2.Pulte D, Gondos A, Brenner H. Trends in survival after diagnosis with hematologic malignancy in adolescence or young adulthood in the United States, 1981–2005. Cancer 2009;115:4973–9. [DOI] [PubMed] [Google Scholar]
- 3.Rinke ML, Milstone AM, Chen AR, et al. Ambulatory pediatric oncology CLABSIs: epidemiology and risk factors. Pediatr Blood Cancer 2013;60:1882–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hord JD, Lawlor J, Werner E, et al. Central Line Associated Blood Stream Infections in Pediatric Hematology/Oncology Patients With Different Types of Central Lines. Pediatr Blood Cancer 2016;63:1603–7. [DOI] [PubMed] [Google Scholar]
- 5.Rinke ML, Chen AR, Milstone AM, et al. Bringing central line-associated bloodstream infection prevention home: catheter maintenance practices and beliefs of pediatric oncology patients and families. Jt Comm J Qual Patient Saf 2015;41:177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rinke ML, Bundy DG, Chen AR, et al. Central line maintenance bundles and CLABSIs in ambulatory oncology patients. Pediatrics 2013;132:e1403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sweet MA, Cumpston A, Briggs F, Craig M, Hamadani M. Impact of alcohol-impregnated port protectors and needleless neutral pressure connectors on central line-associated bloodstream infections and contamination of blood cultures in an inpatient oncology unit. Am J Infect Control 2012;40:931–4. [DOI] [PubMed] [Google Scholar]
- 8.Wright MO, Tropp J, Schora DM, et al. Continuous passive disinfection of catheter hubs prevents contamination and bloodstream infection. Am J Infect Control 2013;41:33–8. [DOI] [PubMed] [Google Scholar]
- 9.Merrill KC, Sumner S, Linford L, Taylor C, Macintosh C. Impact of universal disinfectant cap implementation on central line-associated bloodstream infections. Am J Infect Control 2014;42:1274–7. [DOI] [PubMed] [Google Scholar]
- 10.Patel PA, Boehm S, Zhou Y, et al. Prospective observational study on central line-associated bloodstream infections and central venous catheter occlusions using a negative displacement connector with an alcohol disinfecting cap. Am J Infect Control 2017;45:115–20. [DOI] [PubMed] [Google Scholar]
- 11.Ramirez C, Lee AM, Welch K. Central Venous Catheter Protective Connector Caps Reduce Intraluminal Catheter-Related Infection. Journal of the Association for Vascular Access 2012;17:210–3. [Google Scholar]
- 12.Moulton LH. Covariate-based constrained randomization of group-randomized trials. Clin Trials 2004;1:297–305. [DOI] [PubMed] [Google Scholar]
- 13.National Healthcare Safety Network (NHSN) Patient Safety Component Manual, January 2016 Centers for Disease Control and Prevention, 2016. (https://www.cdc.gov/nhsn/pdfs/validation/2016/pcsmanual_2016.pdf). (Accessed January 20, 2016, [Google Scholar]
- 14.National Healthcare Safety Network (NHSN) Patient Safety Component Manual Master Organism List, January 2016 Centers for Disease Control and Prevention, 2016. (http://www.cdc.gov/nhsn/XLS/master-organism-Com-Commensals-Lists.xlsx). (Accessed January 20, 2016, [Google Scholar]
- 15.Wong Quiles CI, Gottsch S, Thakrar U, Fraile B, Billett AL. Health care institutional charges associated with ambulatory bloodstream infections in pediatric oncology and stem cell transplant patients. Pediatr Blood Cancer 2017;64:324–9. [DOI] [PubMed] [Google Scholar]
- 16.Voor In ‘t Holt AF, Helder OK, Vos MC, et al. Antiseptic barrier cap effective in reducing central line-associated bloodstream infections: A systematic review and meta-analysis. Int J Nurs Stud 2017;69:34–40. [DOI] [PubMed] [Google Scholar]
- 17.Dandoy CE, Kelley T, Gaur AH, et al. Outcomes after bloodstream infection in hospitalized pediatric hematology/oncology and stem cell transplant patients. Pediatr Blood Cancer 2019;66:e27978. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


