Abstract
An amperometric biosensor for xanthine was designed, based on covalent immobilization of xanthine oxidase (XO) of Bacillus pumilus RL-2d onto a screen-printed multi-walled carbon nanotubes gold nanoparticle-based electrodes (Nano-Au/c-MWCNT). The carboxyl groups at the electrode surface were activated by the use of 1-Ethyl-3-(3-dimethylaminopropyl carbodiimide) (EDC) and N-hydroxysuccinimide (NHS). The working electrode was then coated with 6 μL of xanthine oxidase (0.273 U/mg protein). The cyclic voltammetry (CV) study was done for the characterization of the sensor using [K3Fe(CN)6] as an artificial electron donor. The sensitivity (S) and the limit of detection (LOD) of the biosensor were 2388.88 µA/cm2/nM (2.388 µA/cm2/µM) and 1.14 nM, respectively. The developed biosensor was used for determination of fish meat freshness.
Keywords: Amperometric biosensor, Xanthine oxidase (XO), Bacilluspumilus RL-2d
Introduction
Development of biosensors is the most important area of research with applications in encompassing medical diagnostics, environmental monitoring, and food analysis (Mousty 2010). Xanthine oxidase (XO) catalysis and inhibition has been linked with both food and pharmaceutical industries (Saleheh et al. 2018). Meat and fish are among the most consumed food of the human diet and quality of food products is a major concern to the consumers. The freshness of meat and their products is the most important criterion in the quality control for their consumption. The complex microbiological, chemical as well as physical processes lead to the loss of freshness and subsequently cause spoilage (Alomirah et al. 1998). Biochemical activities of microorganisms generally lead to loss of meat freshness. Rapid degradation of nucleotides results in the evaluation of hypoxanthine which is often used as an index of freshness of meat in the food industries. When a fish is caught and sacrificed, it loses freshness during autolysis, and the breakdown of ATP (adenosine-5-triphosphate) in the fish produces ADP (adenosine-5-di phosphate) and further decaying products, such as AMP (adenosine-5-monophosphate), IMP (inosine-5-monophosphate), HxR (inosine), Hx (hypoxanthine), X (xanthine) and U (uric acid). One of the major factors to the pleasant flavour of fresh fish is IMP. After fish dies, hypoxanthine and xanthine are accumulated in the fish meat and imparts a bitter “offtaste” (Masoud et al. 2018). Therefore, their quantification can be as an indication of the fish freshness and the conversion of hypoxanthine to xanthine through the catalytic effect of xanthine oxidase (XO) together with the production of H2O2 and reaction of O2 was found to be the rate determining step in the overall reaction sequence in fish muscle (Lawal and Adeloju 2008).
Various methods have been used for the determination of xanthine and its metabolites, such as spectrophotometry (Khajehsharif and Pourbasheer 2011), mass spectrometry (Rashed et al. 2005), high-performance liquid chromatography (HPLC) (Coopera et al. 2006), capillary electrophoresis (Mu et al. 2012), chemiluminescence based fiber-optic biosensor (Hlavay et al. 1994) and electrochemiluminescence biosensor (Lin et al. 2008), but these methods are costly and taking more time to get the results. However, the enzyme-based electrochemical sensors are one of the most important research topics in this field due to simplicity of operation, high selectivity and sensitivity (Shin et al. 2013; Basniwal et al. 2013; Sharma et al. 2012). Various methods, such as adsorption, covalent linkage, and fixing via intermediate linker molecule, have been used to immobilize enzymes onto solid substrates (Yang et al. 2006; Devi et al. 2013) but in the past few years, use of nanoparticles has revealed significant potential in biosensor’s performance in bioanalysis. The construction of biosensor uses various mediators, such as gold nanoparticles (Pingarron et al. 2008), colloidal gold (Liu et al. 2004), Prussian blue (Teng et al. 2010), cobalt pthalocyanin (Kilinc et al. 1998), Co-doped CeO2 nanoparticles (Lavanya et al. 2016), carbon nanotubes/graphene complexes (Si et al. 2018) and ferrocene (Arslan et al. 2006).
The electrophoretic analysis (Bory et al. 1996), GC/MS (Chabard et al. 1980), HPLC (Yamamoto et al. 1996), chemiluminescence (Hlavay et al. 1994) and UV spectrophotometry (Kito et al. 1983) are the most common methods for detecting and quantifying purines. However, these methods taking more time, involve costly equipment, and are labor-demanding in terms of sample preparation. On the other hand, electrochemical methods offer portability, ease, selectivity, and high sensitivity and can be applied to samples without any pretreatment. Number of chemically modified electrodes have been used for the detection of xanthine, such as polymer films (Kalimuthu and John 2009), pretreated carbon paste (Cai et al. 1994), nanoporous carbon fiber (Kathiwala et al. 2008), pre-anodized nontronite-coated carbon (Zen et al. 2002) and multi-wall carbon nanotube composite (Wang 2011)-modified electrodes. The main interfering substances, such as uric acid, ascorbic acid, glucose, and sodium benzoate, which are widely used in preservation of meat and meat products show negligible effect and Dervisevic et al. 2017 showed that the selectivity of the biosensor towards xanthine, the amperometric response to interferants is negligible.
In the present communication, an amperometric biosensor developed using screen-printed multi-walled carbon nanotubes gold nanoparticle (Nano-Au/c-MWCNT) treated with a mixture of cross-linker, i.e. NHS (N-hydroxysuccinimide) and EDC (1-Ethyl-3-(3-dimethylaminopropyl carbodiimide) and coupled with xanthine oxidase is being reported which is highly selective, sensitive and cost-effective for determination of xanthine in fish meat samples.
Materials and methods
Chemicals and reagents
1-Ethyl-3-(3-dimethylaminopropyl carbodiimide) (EDC), N-hydroxysuccinimide (NHS), potassium ferricyanide K3[Fe(CN)6] were procured from Sigma Company, USA. Xanthine was procured from HiMedia. The molecular biology-grade chemicals were used in the study. The Milli-Q water was used for the preparation of all reagents. Screen-printed carbon nanotubes gold nanoparticle-based electrodes (Nano-Au/c-MWCNT) and specific connector were purchased from DropSens, Spain. The NOVA software was used for electrochemical measurements which were carried out on µAutoLab, The Netherlands.
Fabrication of biosensor
The fabrication of enzyme-based sensor was carried out using the three-electrode system-based screen-printed electrode (nano-Au/c-MWCNT). The autoclaved Milli-Q water and PBS buffer (0.1 M, pH 7) was used for washing the electrode, respectively, and was then dried well at room temperature. After this, the carboxyl group on the nano-Au/c-MWCNT electrode surface was activated by treating with a mixture of 10 mM EDC and 10 mM NHS [(EDC, NHS acts as cross-linker, (1:1, v/v in PBS, pH 7)] for 1.5 h (Gupta et al. 2016). The PBS (pH 7) buffer was used to remove the excess of reagent on electrode surface. Then, the electrode was allowed to dry at room temperature. The working electrode was then coated with 6 μL of xanthine oxidase (0.273 U/mg protein) and incubate for 5 h at 4 °C. The xanthine oxidase activity was determined by Nitroblue tetrazolium (NBT)-based method (Monika et al. 2019). After incubation, the working electrode was washed with PBS buffer (pH 7). 1 mM potassium ferricyanide acted as an artificial electron donor and was used in the characterization of the sensor using cyclic voltammetry (CV). Scanning electron microscopy (SEM) images were taken from Hitachi-make model JSM6100 for bare as well as xanthine oxidase immobilized electrode.
Binding and characterization of biosensor with xanthine
The xanthine (substrate) of varying concentrations (10–7 µM to 10 µM) after heating at 50 °C was hybridized onto the surface of xanthine oxidase-fabricated Nano-Au/c-MWCNT. Electrochemical studies were then characterized by cyclic voltammetry and hybridization time was standardized as 10 min, followed by electrode surface washing using PBS (pH 7.4) to remove the unbound xanthine. A standard curve of varying xanthine concentrations vs. change in relative peak current (µA) reading was made. The start potential and stop potential parameters used in CV analysis are given in Table 1.
Table 1.
The start and stop potential parameters used in CV analysis
| Start potential (V) | − 0.200 V |
| Upper vertex potential (V) | − 0.080 V |
| Lower vertex potential (V) | − 0.450 V |
| Stop potential (V) | 0.8 V |
| Number of stop crossings | 2 |
| Step potential (V) | − 0.00244 V |
| Scan Rate (v/s) | 0.02000 |
Evaluation of fish freshness
Fish was procured from local market and cut into small pieces. The small pieces of fish were homogenized in a blender. The homogenized sample was mixed with double distilled water in a 1:3 (w/w) ratio. The mixture was stirred mechanically for 10 min and centrifuged at 8000 g. Then, the xanthine was extracted from homogenate by filtered through 0.2-µm membrane filter. The xanthine content in the filtrate was estimated using the biosensor operating under standard conditions. Current change (µA) was studied for each samples and the xanthine concentration was calculated from the standard reference plot.
Results
Development of xanthine oxidase based amperometric biosensor for xanthine determination
The fabrication of the nano-Au/c-MWCNT-based biosensor and immobilization of the xanthine oxidase is conceptually presented in Fig. 1. Binding of immobilized enzyme with xanthine and its electrochemical detection may be described by the following reaction mechanism:
Fig. 1.
Schematic diagram for the xanthine oxidase/screen printed multiwalled carbon nano tubes gold nanoparticle based electrode (XO/ nano-Au/c-MWCNT)
SEM (scanning electron microscopy) study for the characterization of working electrode
The morphology of the bare as well as immobilized electrode was characterized by SEM micrographs. The surface of bare electrode, i.e. Nano-Au/c-MWCNT and xanthine oxidase-fabricated Nano-Au/c-MWCNT obtained by SEM was shown in Figs. 2a, b, respectively. The surface of the bare electrode was smooth, whereas the surface of electrode after xanthine oxidase fabrication shows roughness and homogeneous immobilization of enzyme on the surface of the modified Nano-Au/c-MWCNT electrode.
Fig. 2.
a SEM image of bare electrode and b SEM image of enzyme immobilize electrode
Cyclic voltammetry (CV) studies
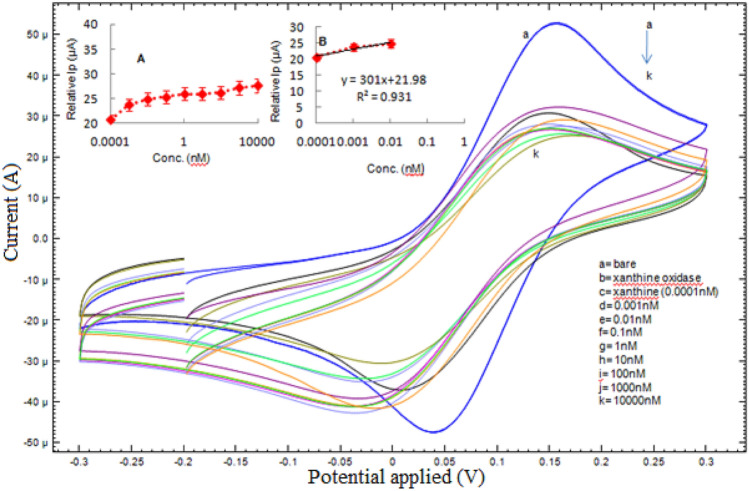
Voltammetric measurements of bare nano-Au/c-MWCNT, immobilized xanthine oxidase and after interaction with substrate (xanthine) were carried out using redox indicator, i.e. potassium ferricyanide. The peak current (Ip) in cyclic voltammetry (CV) study of immobilized XO/ nano-Au/c-MWCNT and after catalysis (Fig. 3) was reduced in comparison to bare nano-Au/c-MWCNT). The observed peak current for bare nano-Au/c-MWCNT and XO/nano-Au/c-MWCNT electrode was 52μA and 30μA, respectively. For different xanthine concentrations, i.e. 0.0001, 0.001, 0.01, 0.1, 1, 10, 100, 1000, 10,000 nM, the peak currents were 32.29, 29.22, 28.11, 27.61, 27.07, 27.02, 26.78, 25.79 and 25.36 μA, respectively. The plot as well as linear equation [Ip (μA) = 301(μA/nM) x xanthine concentration (nM) + 21.98] with regression coefficient (R2) 0.931 was shown in Fig. 3 inset B. The Sensitivity (S) of the biosensor was calculated using the formula S = m/A where, m is the slope of the linear equation and A is the area of the working gold (0.126 cm2) electrode and was calculated as 2388.88 µA/cm2/nM (2.388 µA/cm2/µM). The limit of detection (LOD) was calculated using the formula LOD = 3(σ/S) where σ is the standard deviation and S is the sensitivity and found approximately 1.14 nM.
Fig. 3.
Cyclic voltammetric studies of xanthine oxidase based amperometric biosensor using different xanthine concentrations ranging from 0.0001 to 10,000 nM at 50 mVs−1 using 5 mM K3[Fe(CN)6]. The inset A shows curve from 0.0001 to 10,000 nM with linear peak current (Ip). Inset B shows the linear plot from 0.0001 to 0.01 nM xanthine concentration for the calculation of sensitivity and LOD
Determination of Fish freshness and sensor stability
To evaluate the analytical reliability of the method, the biosensor was employed to detect xanthine in the fish samples by cyclic voltametry studies. The observed peak current for bare nano-Au/c-MWCNT and XOD/ nano-Au/c-MWCNT was 52μA and 30μA, respectively. After the addition of the fresh fish extract sample and the five-day-old fish extract sample, the peak currents observed were 29 μA (0.01 nM) and 27 μA (10 nM), respectively (Fig. 4). The stability of the developed nano-hybrid enzyme sensor was studied on storage at 4 °C using CV at regular interval of 30 days. The sensor was found stable up to 4 months at 4 °C with approx. 10% loss in original CV current (Fig. 5).
Fig. 4.
Cyclic voltammetric studies of xanthine oxidase based bio sensor for the detection of fish freshness. Sample 1: Fresh fish extract; Sample 2: Fish extract after five days
Fig. 5.

Stability of the sensor measured as % relative peak current with a regular interval of 30 days for 4 months on storage at 4 °C
Discussion
The bacterial xanthine oxidase (XO) from B. pumilus RL-2d was immobilized on nano-Au/c-MWCNT to construct a xanthine biosensor. The peak current (Ip) in the cyclic voltammetry (CV) study of immobilized XOD/ nano-Au/c-MWCNT and after catalysis was reduced in comparison to bare nano-Au/c-MWCNT) and may be due to the decrease in the surface area of nano-Au/c-MWCNT electrode at each step of fabrication. As the xanthine concentration increases, the current response decreases which can be attributed to their non-electrochemical activity, which blocks the electrochemical communication between the [Fe(CN)6]3−/4− solution and the electrode to some extent. The present electrochemical biosensor performed in terms of applied potential and the electro-catalytic oxidation response and showed a detection limit of 1.0 × 10–13 M. On the other hand, the detection limits of xanthine-based biosensors, such as XO/layered double hydroxide (Shan et al. 2009a), XO/calcium carbonate nanoparticles (Shan et al. 2009b), XO/laponite nanoparticles (Shan et al. 2009a, b, c), XO/ZnOx/polypyrrole (Devi et al. 2012), XO/glassy carbon paste (Villalonga et al. 2011), EPG/XDH (Kalimuthu et al. 2012), XOD/c-MWCNTs/Fe3O4/TCNQ/CHIT/GCE (Dalkiran et al. 2017) and XO/Poly(L-Asp)/MWCNT/GCE (Samira et al. 2019), were 1 × 10−7, 2 × 10−6, 1 × 10−8, 8 × 10−7, 4 × 10−6, 2.5 × 10−10, 2 × 10−7 and (3.5 × 10–10 M), respectively. The sensitivity of the biosensor was 2.388µA/cm2/µM while the sensitivity of XOD/CHT/PtNPs/PANI/Fe3O4/CPE biosensor (polyaniline nanoparticles/chitosan/carbon paste electrode) was 13.58 µA /µM/ cm2 (Sadeghi et al. 2014). The detection limit for the present biosensor was very low as compared to other reported xanthine biosensors and the fabricated biosensor was effectively used for the detection of fish and chicken meat freshness.
Conclusion
The xanthine oxidase (XO) of B. pumilus RL-2d was immobilized on nano-Au/c-MWCNT to construct an amperometric biosensor for detection/assay of xanthine. This electrochemical biosensor worked in terms of applied potential and the electrocatalytic oxidation and its sensitivity was 2388.88 µA/cm2/nM (2.388 µA/cm2/µM). The limit of detection (LOD) for xanthine was observed to be 1.14 nM. It was used for the analysis of fish freshness and the observed peak currents for fresh fish extract sample and the five-day-old fish extract sample were 29 μA (0.01 nM) and 27 μA (10 nM), respectively.
Acknowledgements
The authors acknowledge the University Grants Commission vide F. No. 39-274/2010 (SRF) to Nirmal Kant Sharma for financial assistance. Tek Chand Bhalla greatly acknowledges UGC, New Delhi for BSR-Faculty Fellowship (F. No. 18-1/2011 (BSR)/24th Feb 2014).
Declarations
Conflict of Interest
Authors declare no conflict of interests.
Ethical statement
The authors declare no studies with animals or human participant.
References
- Alomirah HF, Ali I, Gibbs BF, Konishi Y. Identification of proteolytic products as indicators of quality in ground and whole meat. J Food Qual. 1998;21:299–316. [Google Scholar]
- Arslan F, Yasar A, Kili E. An amperometric biosensor for xanthine determination prepared from xanthine oxidase immobilized in polypyrrole film. Artif Cells Blood Substit Immobil Biotechnol. 2006;34:113–128. doi: 10.1080/10731190500430289. [DOI] [PubMed] [Google Scholar]
- Basniwal RK, Chauhan RPS, Parvez S, Jain VK. Development of cholesterol by chronoamperometric deposition of polyaniline-Ag nanocomposites. Int J Polym Mater. 2013;62:493–498. [Google Scholar]
- Bory C, Chanting C, Boulieu R. Comparison of capillary electrophoretic and liquid chromatographic determination of hypoxanthine and xanthine for the diagnosis of xanthinuria. J Chromatogr A. 1996;730:329–331. doi: 10.1016/0021-9673(95)01086-6. [DOI] [PubMed] [Google Scholar]
- Cai X, Kalcher K, Neuhold C. Simultaneous determination of uric acid, xanthine and hypoxanthine with an electrochemically pretreated carbon paste electrode. Fresenius J Anal Chem. 1994;348:660–665. [Google Scholar]
- Chabard JL, Lartigue-Mattei C, Vedrine F, Petit J, Berger JA. Mass fragmentographic determination of xanthine and hypoxanthine in biological fluids. J Chromatogra B: Biomed Sci Appl. 1980;221:9–17. doi: 10.1016/s0378-4347(00)81002-6. [DOI] [PubMed] [Google Scholar]
- Coopera N, Khosravanb R, Erdmanna C, Fienea J, Lee JW. Quantification of uric acid, xanthine and hypoxanthine in human serum by HPLC for pharmacodynamic studies. J Chromatogr B. 2006;837:1–10. doi: 10.1016/j.jchromb.2006.02.060. [DOI] [PubMed] [Google Scholar]
- Dalkiran B, Erden PE, Kilic E. Amperometric biosensors based on carboxylated multiwalled carbon nanotubesmetal oxide nanoparticles-7,7,8,8-tetracyanoquinodimethane composite for the determination of xanthine. Talanta. 2017;167:286–295. doi: 10.1016/j.talanta.2017.02.021. [DOI] [PubMed] [Google Scholar]
- Dervisevic M, Dervisevic E, Cevik E, Senel M. Novel electrochemical xanthine biosensor based on chitosanepolypyrroleegold nanoparticles hybrid bio-nanocomposite platform. J Food Drug Anal. 2017;25:510–519. doi: 10.1016/j.jfda.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi R, Yadav S, Pundir CS. Amperometric detection of xanthine in fish meat by zinc oxide nanoparticle/chitosan/multiwalled carbon nanotube/polyaniline composite film bound xanthine oxidase. Analyst. 2012;137:754–759. doi: 10.1039/c1an15838d. [DOI] [PubMed] [Google Scholar]
- Devi R, Yadav S, Nehra R, Yada S, Pundir CS. Sensitive and selective xanthine amperometric sensors based on calcium carbonate nanoparticles. J Food Eng. 2013;115:207–214. [Google Scholar]
- Gupta S, Kaushal A, Kumar A, Kumar D. Multiwalled carbon nanotubes based immunosensor for diagnosis of celiac disease. Cell Mol Biol. 2016;62:139–141. [Google Scholar]
- Hlavay J, Haemmerli SD, Guilbault GG. Fibre-optic biosensor for hypoxanthine and xanthine based on a chemiluminescence reaction. Biosens Bioelectron. 1994;9:189–195. doi: 10.1016/0956-5663(94)80121-5. [DOI] [PubMed] [Google Scholar]
- Kalimuthu P, John SA. Simultaneous determination of ascorbic acid, dopamine, uric acid and xanthine using a nanostructured polymer film modified electrode. Talanta. 2009;80:1686–1691. doi: 10.1016/j.talanta.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Kalimuthu P, Leimkuhler S, Bernhardt PV. Low-potential amperometric enzyme biosensor for xanthine and hypoxanthine. Anal Chem. 2012;84:10359–10365. doi: 10.1021/ac3025027. [DOI] [PubMed] [Google Scholar]
- Kathiwala M, Affum AO, Perry J, Brajter-Toth A. Direct measurements of xanthine in 2000-fold diluted xanthinuric urine with a nanoporous carbon fiber sensor. Analyst. 2008;133:810–816. doi: 10.1039/b718125f. [DOI] [PubMed] [Google Scholar]
- Khajehsharif H, Pourbasheer E. Acid in real matrix by orthogonal signal correction–partial least squares. J Iran Chem Soc. 2011;8:1113–1119. [Google Scholar]
- Kilinc E, Erdem A, Gokgunnec L, Dalbasti T, Karaoglan M, Ozsoz M. Buttermilk based cobalt phthalocyanine dispersed ferricyanide mediated amperometric biosensor for the determination of xanthine. Electroanalysis. 1998;20:273–275. [Google Scholar]
- Kito M, Tawa R, Takeshima S, Hirose S. Fluorometric determination of hypoxanthine and xanthine in biological fluids by high-performance liquid chromatography using enzyme reactors. J Chromatogr B: Biomed Sci Appl. 1983;278:35–42. doi: 10.1016/s0378-4347(00)84753-2. [DOI] [PubMed] [Google Scholar]
- Lavanya N, Sekar C, Murugan R, Ravi G. An ultrasensitive electrochemical sensor for simultaneous determination of xanthine, hypoxanthine and uric acid based on Co doped CeO2 nanoparticles. Mat Sci Eng C. 2016;65:278–286. doi: 10.1016/j.msec.2016.04.033. [DOI] [PubMed] [Google Scholar]
- Lawal AT, Adeloju SB. Polypyrrole-based xanthine oxidase pootentiometric biosensor for hypoxanthine. J Appl Sci. 2008;8:2599–2605. [Google Scholar]
- Lin Z, Sun J, Chen J, Guo L, Chen Y, Chen G. Electrochemiluminescent biosensor for hypoxanthine based on the electrically heated carbon paste electrode modified with xanthine oxidase. Anal Chem. 2008;80:2826–2831. doi: 10.1021/ac702471r. [DOI] [PubMed] [Google Scholar]
- Liu Y, Nie L, Tao W, Yao S. Amperometric study of Au-colloid function on xanthine biosensor based on xanthine oxidase immobilized in polypyrrole layer. Electroanalysis. 2004;16:1271–1278. [Google Scholar]
- Masoud R, Ali B, Sajjad G, Saleheh A, Hamid RZ. Electrochemical investigation of the inhibition effect of carvacrol on xanthine oxidase activity merging with theoretical studies. Process Biochem. 2018;83:86–95. [Google Scholar]
- Monika SNK, Thakur N, Sheetal S, Bhalla TC. Xanthine oxidase of Acinetobacter calcoaciticus RL2-M4: Production, purification and characterization. Protein Expr Purif. 2019;160:36–44. doi: 10.1016/j.pep.2019.03.014. [DOI] [PubMed] [Google Scholar]
- Mousty C. Biosensing applications of clay-modified electrodes: a review. Anal Bioanal Chem. 2010;396:315–325. doi: 10.1007/s00216-009-3274-y. [DOI] [PubMed] [Google Scholar]
- Mu G, Luan F, Xu L, Hu F, Liu H, Gao Y. Determination of purines in soybean milk by capillary electrophoresis in comparison with high performance liquid chromatography. Anal Methods. 2012;4:3386–3391. [Google Scholar]
- Pingarron JM, Paloma YS, Araceli GC. Gold nanoparticle-based electrochemical biosensors. Electrochim Acta. 2008;53:5848–5866. [Google Scholar]
- Rashed MS, Saadallah AA, Rahbeeni Z, Eyaid W, Seidahmed MZ, Al-Shahwan S, Salih MAM, Osman ME, Al-Amoudi M, Al-Ahaidib L, Jacob M. Determination of urinary S-sulphocysteine, xanthine, and hypoxanthine by liquid chromatography–electrospray tandem mass spectrometry. Biomed Chromatogr. 2005;12:223–230. doi: 10.1002/bmc.439. [DOI] [PubMed] [Google Scholar]
- Sadeghi S, Fooladi E, Malekaneh M. A nanocomposite/crude extract enzyme-based xanthine biosensor. Anal Biochem. 2014;464:51–59. doi: 10.1016/j.ab.2014.07.013. [DOI] [PubMed] [Google Scholar]
- Saleheh A, Sajjad G, Ali B, Masoud R, Ali AS. An in-depth view of potential dual effect of thymol in inhibiting xanthine oxidase activity: electrochemical measurements in combination with four way PARAFAC analysis and molecular docking insights. Int J boil Macromol. 2018;119:1298–1310. doi: 10.1016/j.ijbiomac.2018.08.018. [DOI] [PubMed] [Google Scholar]
- Samira Y, Ali B, Saleheh A, Masoud R. Enzyme-based ultrasensitive electrochemical biosensor using poly(l-aspartic acid)/MWCNT bio-nanocomposite for xanthine detection: a meat freshness marker. Microchem J. 2019;149:104000. [Google Scholar]
- Shan D, Wang Y, Xue H, Cosnier S. Sensitive and selective xanthine amperometric sensors based on calcium carbonate nanoparticles. Sen Actuat B. 2009;136:510–515. [Google Scholar]
- Shan D, Wang Y, Xue H, Cosnier S, Ding SN. Xanthine oxidase/laponite nanoparticles immobilized on glassy carbon electrode: Direct electron transfer and multielectrocatalysis. Biosens Bioelectron. 2009;24:3556–3561. doi: 10.1016/j.bios.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Shan D, Wang Y, Zhu M, Xue H, Cosnier S, Wang C. Development of a high analytical performance-xanthine biosensor based on layered double hydroxides modified-electrode and investigation of the inhibitory effect by allopurinol. Biosens Bioelectron. 2009;24:1171–1176. doi: 10.1016/j.bios.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Sharma AL, Kumar P, Deep A. Highly sensitive glucose sensing with multi-walled carbon nanotubes—polyaniline composite. Polym Plast Technol Eng. 2012;51:1382–1387. [Google Scholar]
- Shin MJ, Kim JG, Shin JS. Amperometric cholesterol biosensor using layer-by-layer adsorption technique on polyaniline-coated polyester films. Int J Polym Mater Polym Biomater. 2013;62:140–144. [Google Scholar]
- Si Y, Park JW, Jung S, Hwang GS, Goh E, Lee HJ. Layer-by-layer electrochemical biosensors configuring xanthine oxidase and carbon nanotubes/graphene complexes for hypoxanthine and uric acid in human serum solutions. Biosens Bioelectron. 2018;15:265–271. doi: 10.1016/j.bios.2018.08.074. [DOI] [PubMed] [Google Scholar]
- Teng YJ, Chen C, Zhou CX, Zhao HL, Lan MB. Disposable amperometric biosensors based on xanthine oxidase immobilized in the Prussian blue modified screen-printed three electrode system. Sci China Chem. 2010;53:2581–2586. [Google Scholar]
- Villalonga R, Diez P, Gamella M, Reviejo J, Pingarron JM. Immobilization of xanthine oxidase on carbon nanotubes through double supramolecular junctions for biosensor construction. Electroanalysis. 2011;23:1790–1796. [Google Scholar]
- Wang Y. Simultaneous determination of uric acid, xanthine and hypoxanthine at poly (pyrocatechol violet)/functionalized multi-walled carbon nanotubes composite film modified electrode. Colloids Surf B. 2011;88:614–621. doi: 10.1016/j.colsurfb.2011.07.051. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Moriwaki Y, Takahashi S, Tsutsumi Z, Ji Y, Nasako Y, Hiroishi K, Higashino K. Determination of human plasma xanthine oxidase activity by high-performance liquid chromatography. J Chromatogr B: Biomed Sci App. 1996;681:395–400. doi: 10.1016/0378-4347(96)00071-0. [DOI] [PubMed] [Google Scholar]
- Yang M, Yang Y, Liu Y, Shen G, Yu R. Platinum nanoparticles–doped sol–gel/carbon nanotubes composite electrochemical sensors and biosensors. Biosens Bioelectron. 2006;21:1125–1131. doi: 10.1016/j.bios.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Zen JM, Lai YY, Yang HH, Kumar SA. Multianalyte sensor for the simultaneous determination of hypoxanthine, xanthine and uric acid based on a preanodized nontronite-coated screen-printed electrode. Sen Actuat B. 2002;84:237–244. [Google Scholar]