Abstract
Microbial plant interaction plays a major role in the sustainability of plants. The understanding of phytomicrobiome interactions enables the gene-editing tools for the construction of the microbial consortia. In this interaction, microbes share several common secondary metabolites and terpenoid metabolic pathways with their host plants that ensure a direct connection between the microbiome and associated plant metabolome. In this way, the CRISPR-mediated gene-editing tool provides an attractive approach to accomplish the creation of microbial consortia. On the other hand, the genetic manipulation of the host plant with the help of CRISPR-Cas9 can facilitate the characterization and identification of the genetic determinants. It leads to the enhancement of microbial capacity for more trait improvement. Many plant characteristics like phytovolatilization, phytoextraction, phytodesalination and phytodegradation are targeted by these approaches. Alternatively, chemical communications by PGPB are accomplished by the exchange of different signal molecules. For example, quorum-sensing is the way of the cell to cell communication in bacteria that lead to the detection of metabolites produced by pathogens during adverse conditions and also helpful in devising some tactics towards understanding plant immunity. Along with quorum-sensing, different volatile organic compounds and N-acyl homoserine lactones play a significant role in cell to cell communication by microbe to plant and among the plants respectively. Therefore, it is necessary to get details of all the significant approaches that are useful in exploring cell to cell communications. In this review, we have described gene-editing tools and the cell to cell communication process by quorum-sensing based signaling. These signaling processes via CRISPR- Cas9 mediated gene editing can improve the microbe-plant community in adverse climatic conditions.
Keywords: Phytomicrobiome, Plant-microbe interactions, Quorum-sensing, Cell communication, Microbial community
Introduction
Phytomicrobiome or plant microbiome represents the microbial communities that colonize with the various plant parts. These microorganisms include fungi, bacteria and archaea that collectively play a key role in the productivity, growth and health of the plant [1]. The phytomicrobiome may be rhizospheric (associated with roots), epiphytic (on the surface of plant parts) and endophytic (inner side of plant parts) [2]. It is reported that both soil microbes and mycorrhizal fungi can change the carbon composition into the soil by the exudation process of carbon. This exudation process increases the microbial activity and helps in the enhancement of more carbon decomposition. The rate of mineralization and soil decomposition increases by the interaction between microbes, mycorrhizal fungi and host plants [3]. A large number of inorganic and organic compounds synthesizes by microbes that help in encouraging root colonization. The microbial interaction can be of both types i.e. endophytic and epiphytic with their host plants. This type of interaction affects the plants either negatively or positively by several interactions such as amensalism, commensalism, and mutualism [4]. Endophytic bacterial colonization is the best example of microbe-plant interaction in which bacteria live within the host plant without causing any damage [5]. They colonize in the intercellular spaces and enter into the cell by cellulose degradation or local rupture of the roots. Different types of endophyte have been reported from different crops like cotton, sorghum, rice, wheat, maize, potato, sorghum and Arabidopsis. There are various studies conducted on microbe-plant interaction that express the regulation of cellular activity and signaling mechanisms [6, 7]. A better understanding of microbe-plant interaction can accomplish by various technique viz. gene-editing process and their cell to cell communication [8, 9]. Rhizospheric colonization exchanges different types of signals between bacteria and plants that can be beneficial, neutral or detrimental to the plants [10].
The ambition of phytomicrobiome engineering is to increase the significant outcomes of the plant including ISR, mineralization of organic matter and nutrient cycling during salinity and drought stress conditions [11]. The phytomicrobiome interactions depend upon various climatic conditions, soil type and plant species [12, 13]. Another major aspect of microbiome engineering is to harness all these variations during unfavorable conditions. This review also focuses on the several concepts of synthetic biology tools and their plant-microbe interactions by various signaling processes. By these approaches, stress response mechanisms during the biotic and abiotic conditions and their symbiosis process can also be understood.
Synthetic Biology for the Modification of Microbes
From the last few decades, various gene-editing tools have been designed and used for the enhancement of crop productivity by the modification of the local microbial community. Genetic engineering technique plays a major role in agriculture through recombinant DNA technology [14, 15]. The advancement of RNAi (RNA interference) has become the recent development in biotechnological genetic tools. It tends to the modification of targeted genes at the expression level. Along with RNAi, short-interfering and microRNAs (miRNA) also play a key role in gene expression. RNAi can manipulate and influence the essential traits of plants like branching, height, size and inflorescence. For example, gene OsDWARF4, can stimulate erect leaf structure in small plants by the RNAi manipulation process [16]. Also, the suppression of gene GA 20-oxidase (OsGA20ox2) can convert long rice (QX1 variety) into dwarf one. It was reported that the OsSPL14 gene is the target of miR156 gene in rice plants.The high rate expression of the OsSPL14 gene can lead to high grain yield with lower tiller number. Hence, miRNA and its targeted gene OsSPL14 are utilized for modifying the whole plant structure for the superior rice variety [17]. It is reported that the interaction of the OsmiR444a with D14 and OsMADS57 gene helps in controlling tillering in rice plants [18, 19]. The suppression of genes ipt and iaaM by RNAi mediated process can lead to the reduction in tumor formation in Agrobacterium tumefaciens. In Agrobacterium tumefaciens, the miR393 gene regulates TIR1 auxin receptors to reduce the infection by Pseudomonas syringae. Arabidopsis plants show high bacterial resistance by overexpression of miR393 gene [20]. Along with miR393, two other miRNAs, miR825 and miR398 cause a reduction in bacterial infections by down regulation. The expression of miR398 targets (CSD1 and CSD2) superoxide dismutases and causes up-regulation of bacterial infection under stress condition. The initiation process of RNAi occurs by the activation of ribonuclease protein Dicer [21]. After this process, some specific proteins bind with this siRNA and form a complex, which is integrated into RISC (RNA- induced silencing complex). The complementary mRNA sequence binds with the siRNA strand and results in the inhibition of the translation process and gene expression [22]. RNAi is used for the anti-pathogen activity in the agriculture field by the silencing process of targeted genes. A combinatory overview of modern technologies towards improving bioinoculants is illustrated in Fig. 1. It is reported that RNAi has the potential to engineer the local microbial community and provides resistance against pathogens. The gene-editing process becomes much easier with the development of CRISPR-Cas9 technology. During this process, the engineered and modified sgRNA (sequence-specific single guide RNA) forms a complex with Cas9. The genome of a cell can be edited with the removal or insertion on a specific location [23, 24]. Several reports have been demonstrated that the gene-editing approach helps in the enhancement of resistivity to various pathogens. By the approach of molecular tools, the desired phenotype and genotype can also be improved for the defence against pathogen and also for nutrient mobilization [11, 25, 26]. The information about some synthetic biology approaches and tools for improving microbial consortia is presented in Table 1. An assortment of recent approaches by gene-editing tools and their utilization method is depicted in Fig. 2. The engineering pathways help in the improvement of food production efficiency through the desired food ingredient. On the other hand, the engineering process involves those genes that led to the synthesis of unsaturated fatty acid by de novo process [36]. A lot of products that are traditionally derived from plants are now produced by engineered microbes. For example, resveratrol (heart health source) and stevia sweetener are now produced by a genetically engineered approach. The CRISPR-Cas9 technology, under synthetic biology, provides a better resistance mechanism against fungus and also responsible for turn off allergic reactions that affect the yield on large scale [37]. It is also reported that some aliphatic polyamines are found in microbes that help in the growth and development of plants. The most plentiful polyamines are spermidine and putrescine that play a major role in the signaling and differentiation of the cells [38, 39].
Fig. 1.
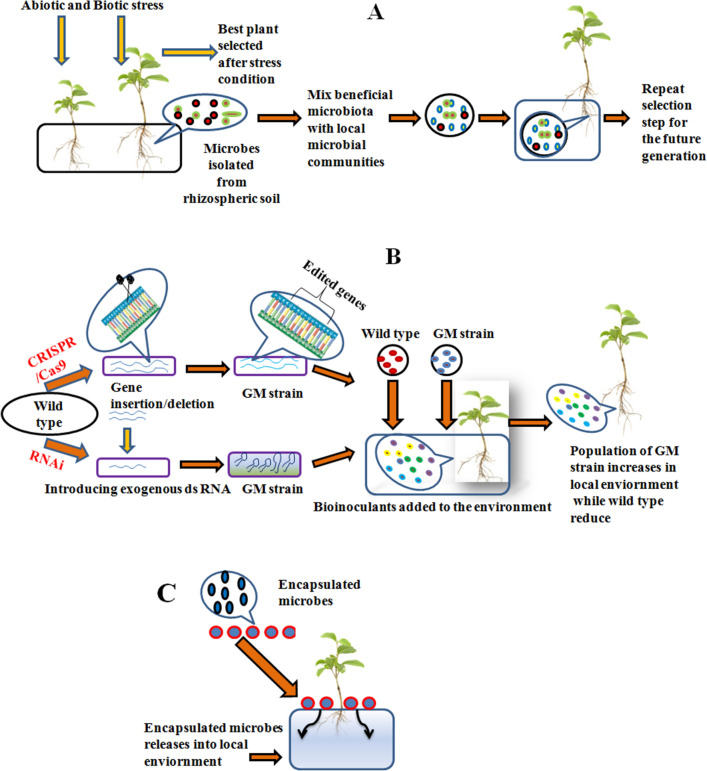
Combinatory overview of modern technologies towards improving bioinoculants
Table 1.
Synthetic biology tools and their subsequent approaches for improving microbial consortia
| Synthetic biology tools | Microbes used | Synthetic biology approaches | Application | Other description | References |
|---|---|---|---|---|---|
| Engineered syntrophies and computational models | S. cerevisiae and E. Coli |
Pathway separation, population control and DOL |
Bioproduction of medicines, protein and biofuels | DOL, engineered signaling and pathway separation are the most usable approach | [27, 28] |
| Computational models and intercellular signaling | E. Coli |
DOL and Spatial organization |
Logic memory/computing |
Logic gates are usually connected with signaling molecules and distributed between consortium members |
[29, 30] |
| Engineered syntrophies, computational models and exogenous controllers |
CoculturedS. Cerevisiae or E. coli with a microbe having activity against pollutant |
Pathway separation |
Deterioration of pollutants |
Intensify the degradation process of pollutants, polymers and complex substrates |
[31] |
| Exogenous controllers and intercellular signaling | B. subtilis and E. Coli | DOL | Biosensing | Detection of metabolites and small molecules | [32, 33] |
| Intercellular signaling and exogenous controllers | B. subtilis and E. Coli |
DOL and Spatial organization |
Functionalized biomaterials |
Communication in synthetic consortia and production of functionalized biomaterials |
[34, 35] |
Fig. 2.
An assortment of recent approaches by gene editing tools and their utilization method in fields. In this a showing the strategies for improving microbial inoculants for formulation, b revealed the RNAi technology with the CRISPR/Cas method and c depicted the encapsulation process of microbes and their delivery method
Quorum Sensing and Communication Signals
In quorum sensing, the cell-cell communication process mediates by the tiny diffusible extracellular signaling molecules. In this process, gram-positive bacteria and gram-negative bacteria use peptide-signaling molecules and acylated homoserine lactones (AHLs) respectively that act as autoinducers [40]. These signals can activate various transcription factors at a specific population concentration of bacteria and help in the induction of genes in the bacterial community. Pantoea stewartii, Pseudomonas syringae and Pseudomonas aureofaciens rhizobacterial species utilize acylated homoserine lactones to control the microbial-plant interaction [41]. In Azospirillum lipoferum, Quorum sensing molecules are strain-specific and adapted to rhizospheric plant roots. Azospirillum brasilense supports both the process of chemokinesis and chemotaxis for chemo-effectors by enhancing the bacterial-root interaction. Microbial-plant interactions such as phytostimulation, biocontrol, biofertilization and rhizoremediation are QS dependent [42]. It is reported that various rhizobacterial species are capable of forming microcolonies on the roots of the plant and in induction of QS by high bacterial cell density. The secretion of mucigel by plant roots helps in the retardation of the diffusion of N-acyl homoserine lactones and in the coverage of bacteria which is required for quorum sensing. Mainly two N-acyl homoserine lactone systems RhlI/R and LasI/R are involved in the regulation of PGPR traits in Pseudomonas aeruginosa [43, 44]. Several health benefits could obtain by an effective signaling system [45, 46]. It is reported that root microflora and plants established a significant symbiotic relationship through a useful communication network [47, 48]. The rhizospheric plant roots are surrounded by an immense amount of microbial community that consists of hundreds to thousands of different species diversity [49]. Signaling pathways over the molecular level can control the microbial population in a better way by establishing a flourishing relationship between microflora and plants [50]. Many efforts have been made for knowing the molecular mechanism of communication network but still no adequate information to get the stability and evolutionary origins of the rhizosphere communication system. Table 2 represents the potential of gene editing tools for enhancing phytomicrobiome interactions. The association of Arbuscular mycorrhizal and PGPR-legume root symbioses existed by several signals of small diffusible molecules that is produced by both microbe and plants. The root hairs provide a favorable biological surface for colonization, migration and chemotaxis to microbial communities [56]. A well-known symbiotic pathway is activated by signals that are produced by fungi and rhizospheric bacteria in the form of LCOs (lipo-chitooligosaccharides). These signals are understood via LysM (lysine-motif) that occur on the membrane of plants that help in the activation of CSP and regulation of rhizospheric microorganisms. It is reported that LysM receptor found in both non- legume plants and legume plants and receives signals from AM fungi (Myc-LCO signals) and rhizobia (nod factor signals) [5]. Numerous evidence demonstrated that the signaling pathway between roots of the host plant and AM fungi is much flourish and engaged for understanding the evolution of symbiosis [57]. Microbial communities and plants use various signaling pathways to convey useful information concerning their internal situation and colonization [3, 58]. In the symbiotic system of AM (arbuscular mycorrhiza) strigolactones molecule plays a major role. It is a plant hormone and terpenoid which is the byproduct of carotenoid metabolism [59]. It regulates the metabolic cycle of AM fungus and supports its growth towards the root [60]. Several types of strigolactones are produced by different plants for the attraction of AM fungi [61]. Strigolactones helps in the germination of arbuscular mycorrhiza fungal spores and bring out a series of signal molecule viz. lipo-chitooligosaccharides and chitooligosaccharides. These types of molecules trigger a set of various interactions in the plant root system. As a result of this, the cytosolic concentration of calcium increases and help in the induction of gene expression of mycorrhizal fungi that directs the penetration system. Plant roots promote the fungi for the initiation of arbuscular growth and hypopodium formation [62]. The plant hormone, auxin is synthesized by both PGPRs and plants via multiple pathways. Certain reports demonstrated that the IAA (indole-3-acetic acid) is a natural auxin that act as signaling molecules in the microbial community [63]. As a result of this IAA affects gene expression in several microbes and precedes reciprocal signaling molecule in microbe-plant interactions. IAA act as a controller of gene expression in IpdC gene of Azospirillum brasilense. IAA also has been observed as an inhibitory signaling molecule for the viral gene Agrobacterium tumefaciens [64]. Besides this, the level of auxin in microbe-plant interaction affects nodule formation and its differentiation [65]. Apart from this, chemical signaling has various consequences that affect microbe-plant interactions. The Als produced during interspecies communication can change the activities of its competitors. By disturbing this activity, beneficial microbes defend the host plant by inhibiting the activity of pathogens. In many cases, microbes produce VOCs and use enzymes like acylase and lactonase for the degradation of AHLs184 that disrupt bacterial AHL. Sometimes, microbes show a wide range of interactions from symbiotic to antagonistic and affect the immunity of plant [66]. Induced systemic resistance is controlled by these interconnected signaling pathways and their components. Plants frequently maintain both inducible and constitutive defense mechanisms by the process of hormonal signaling pathways. The phytohormones like JA, ethylene, auxins, ABA, SA and their derivatives play a vital role in the regulation of the immune system. According to the trophic relation of microbes, signaling molecules like JA and SA show a different type of immune response with their host plant. For instance, biotrophic microbes acquire nutritional requirements from living cells whereas necrotrophic obtain from dead plant cells by the production of phytotoxins. On the other hand, hemi-biotrophic microbes take nutrients from both types of cells i.e., from living or dead state. In hemi-biotrophic and biotrophic type only the SA defense pathway exists while in the necrotrophic JA pathway is effectual against pathogens. Consequently, signaling molecules enable both inter and intra microbial communication with the host plant and sustaining the colonization and symbiotic associations within plants [67].
Table 2.
Potential of gene editing tools for enhancing the phytomicrobiome interactions
| Gene editing Tools | Traits present | Application | References |
|---|---|---|---|
| Cpf1 (gene replacement, gene knock-out, multiplex GE) and Cas9 | Metabolic engineering | Exploration the metabolome pathways of novel plant | [51] |
| Cpf1 (gene replacement, gene knock-out, multiplex GE) and Cas9 | Signaling molecules helps in Improvement of stress resistance | Enhances nutrients uptake | [52] |
| Cpf1 (gene replacement, gene knock in/out,) and Cas9 | Improves nodulation in non-leguminous plants | Nutrient uptake and plant growth promotion | [52] |
| RNA editing tool and Cas13 | In plant disease resistance | Identify pathogens | [53] |
| Base editing, Cpf1 (gene replacement, gene knock-out, multiplex GE) and Cas9 | In plant disease resistance | Plant disease resistance against pest | [53] |
| EvolvR | Phytomicrobiome interactions | In allele generation | [54] |
| Lineage tracing and Genotyping | Phytomicrobiome interactions | In identification of genes that involves in phytomicrobiome interactions | [55] |
Biofilm Production and Defence Induction by Microbes for Plant Growth
Biofilms are communities of microbial cells that are covered by extracellular polymeric substances (EPS). The formation of biofilm is initiated by the following factors like oxygen supply, osmolarity, pH and temperature [68]. Efficient root colonization by EPS-producing microbes helps in the circulation of essential nutrients to the host plant and prevents it attacking from foreign pathogens. Mainly PGPR is known for EPS characteristics that help in the formation of polymeric substances. Pseudomonas and Rhizobium species produce a variety of nucleic acids, organic molecules, proteins and polysaccharides that helps in the biofilm matrix. Biofilms developed in vitro are also used as biofilm biofertilizers. Furthermore, polysaccharides formed by rhizobial species also act as an active signal for defense response during any infection [69]. Many PGPR-EPS also bind Na+cations and help in the alleviation of salt stress by reducing Na+ content. It is also reported that the co-inoculation of biofilm microbial strain also helps in the induction of biocontrol mechanism.
Along with biofilm production, it is also reported that many species of microbes show endosymbiotically association and triggers the expression of plant genes that are responsible for different stress responses. Many studies showed that the Trichoderma that act as endosymbiont shows a good association with woody root plants and help in alleviating different abiotic stress condition and enhances the nitrogen-fixing efficiency of plants [70]. SA and JA mediated SAR and ISR responses are triggered by the higher dose of Trichoderma. Peptaibols and Xylanase are produced by various Trichoderma spp. that elicit a good immune response in plants and help in crop enhancement [71]. Recently, a hybrid enzyme polyketide synthases were identified in maize plants that involve in defence mechanisms. Epl1/Sm1 is an educer and secreted as cysteine-rich protein produced by Trichoderma spp .In maize crops, the deletion of the Trichoderma gene elicits the ISR response. On the other hand, it is also reported that a variety of secondary metabolites are produced by Trichoderma. Nonribosomal polyketides and peptides signify chief components of secondary metabolites. The pks4 gene from Trichoderma reesei was identified that involve in the synthesis of aurofusarin. The deletion of the gene in Trichoderma reesei results in the vanish of pigmentation and variation of teleomorphic structures. Besides this, the deletion of pks4 also influences the expression of PKS-encoding genes in Trichoderma reesei by affecting the stress resistance and defense mechanism.
Conclusion
Phytomicrobiome interactions play a significant role in the sustainability of plant health. These interactions result in beneficial, favorable or detrimental for plant growth and productivity. Hence, to control this complex process, signals or cell to cell communication play a crucial role between microbiome and plant. In this present review, gene-editing tools, cell-to-cell communication and their approaches have been described to understand the various factors that control microbiome congregation. Next step after this signaling process, new omics tools will certainly help in cultivating the microbiome for a better understanding of the engineering process. Furthermore, the biofilm production and polyketides gene identified in Trichoderma are also described with their application in defence induction for plant growth. By the engineering efforts, microbiome interactions will attain a sustainably, purposefully and presumably goal to engineer a better phytomicrobiome.
Acknowledgements
The author, TC acknowledges MaharshiDayanand University, Rohtak, India for University Research Scholarship (URS). PS acknowledges the Department of Science and Technology, New Delhi, Govt. of India, FIST grant (Grant No. 1196 SR/FST/LS-I/2017/4) and Department of Biotechnology, Government of India (Grant No. BT/PR27437/BCE/8/1433/2018).
Compliance with Ethical Standards
Conflict of interest
The authors don’t have any conflict of interest.
References
- 1.Singh A, Kumari R, Yadav AN, Mishra S, Sachan A, Sachan SG (2020) Tiny microbes, big yields: microorganisms for enhancing food crop production for sustainable development. In: Rastegari AA, Yadav AN, Yadav N (eds) New and future developments in microbial biotechnology and bioengineering, Elsevier, pp. 1–15. 10.1016/B978-0-12-820526-6.00001-4
- 2.Quiza L, St-Arnaud M, Yergeau E. Harnessing phytomicrobiome signaling for rhizosphere microbiome engineering. Front Plant Sci. 2015;6:507. doi: 10.3389/fpls.2015.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalia VC (2017) Microbial applications, biomedicine, agriculture and industry bioremediation & bioenergy. Springer. 10.1007/978-3-319-52669-0
- 4.Chaudhary T, Shukla P. Bioinoculants for bioremediation applications and disease resistance: innovative perspectives. Indian J Microbiol. 2019;59:1–8. doi: 10.1007/s12088-019-00783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olanrewaju OS, Ayangbenro AS, Glick BR, Babalola OO. Plant health: feedback effect of root exudates-rhizobiome interactions. Appl Microbiol Biotechnol. 2019;103:1155–1166. doi: 10.1007/s00253-018-9556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imam J, Singh PK, Shukla P. Plant microbe interactions in post genomic era: perspectives and applications. Front Micro-biol. 2016;7:1488. doi: 10.3389/fmicb.2016.01488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imam J, Shukla P, Mandal P. Microbial interactions in plants: perspectives and applications of proteomics. Curr Protein PeptSci. 2017;18:956–965. doi: 10.2174/1389203718666161122103731. [DOI] [PubMed] [Google Scholar]
- 8.Kumar V, Baweja M, Singh PK, Shukla P. Recent developments in systems biology and metabolic engineering of plant-microbe interactions. Front Plant Sci. 2016;7:1421. doi: 10.3389/fpls.2016.01421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ritchie MD, Holzinger ER, Li R, Pendergrass SA, Kim D. Methods of integrating data to uncover genotype–phenotype interactions. Nat Rev Genet. 2015;16:85–97. doi: 10.1038/nrg3868. [DOI] [PubMed] [Google Scholar]
- 10.Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica 1–15. 10.6064/2012/963401 [DOI] [PMC free article] [PubMed]
- 11.Meena KK, Sorty AM, Bitla UM, Choudhary K, Gupta P, Pareek A, Singh DP, Prabha R, Sahu PK, Gupta VK, Singh HB. Abiotic stress responses and microbe-mediated mitigation in plants: the omics strategies. Front Plant Sci. 2017;8:172. doi: 10.3389/fpls.2017.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uroz S, Courty PE, Oger P (2019) Plant symbionts are engineers of the plant-associated microbiome. Trends plant sci. 10.1016/j.tplants.2019.06.008 [DOI] [PubMed]
- 13.Chaudhary T, Shukla P (2020) Commercial bioinoculant development: techniques and challenges. In: Shukla P (eds) Microbial enzymes and biotechniques. Springer, Singapore, pp 57–7010.1007/978-981-15-6895-4_4
- 14.Wang W, Vinocur B, Altman A. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. PLANTA. 2003;218:1–14. doi: 10.1007/s00425-003-1105-5. [DOI] [PubMed] [Google Scholar]
- 15.Kumar P, Patel SKS, Lee JK, Kalia VC. Extending the limits of Bacillus for novel biotechnological applications. Biotechnol Adv. 2013;31:1543–1561. doi: 10.1016/j.biotechadv.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Trautman P, Crawford J. Linking biosynthetic gene clusters to their metabolites via pathway-targeted molecular networking. Curr Top Med Chem. 2016;16:1705–1716. doi: 10.2174/1568026616666151012111046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou M, Luo H. MicroRNA-mediated gene regulation: potential applications for plant genetic engineering. Plant Mol Biology. 2013;83:59–75. doi: 10.1007/s11103-013-0089-1. [DOI] [PubMed] [Google Scholar]
- 18.Guo S, Xu Y, Liu H, Mao Z, Zhang C, Ma Y, Zhang Q, Meng Z, Chong K. The interaction between OsMADS57 and OsTB1 modulates rice tillering via DWARF14. Nat Commun. 2013;4:1–12. doi: 10.1038/ncomms2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamthan A, Chaudhuri A, Kamthan M, Datta A (2015) Small RNAs in plants: recent development and application for crop improvement. Front Plant Sci 6:208. 10.3389/fpls.2015.00208 [DOI] [PMC free article] [PubMed]
- 20.Su P, Zhao L, Li W, Zhao J, Yan J, Ma X, Li A, Wang H, Kong L. Integrated metabolo-transcriptomics and functional characterization reveals that the wheat auxin receptor TIR1 negatively regulates defense against Fusarium graminearum. J Integr Plant Biol. 2020 doi: 10.1111/jipb.12992. [DOI] [PubMed] [Google Scholar]
- 21.Ali M, Javaid A, Naqvi SH, Batcho A, Kayani WK, Lal A, Sajid IA, Nwogwugwu JO (2020) Biotic stress triggered small RNA and RNAi defense response in plants. Mol Biol Rep 1–12. 10.1007/s11033-020-05583-4 [DOI] [PubMed]
- 22.Yer EN, Baloglu MC, Ayan S. Identification and expression profiling of all Hsp family member genes under salinity stress in different poplar clones. Gene. 2018;678:324–336. doi: 10.1016/j.gene.2018.08.049. [DOI] [PubMed] [Google Scholar]
- 23.Ali Z, Abul-Faraj A, Li L, Ghosh N, Piatek M, Mahjoub A, et al. Efficient virus-mediated genome editing in plants using the CRISPR/Cas9 system. Mol Plant. 2015;8:1288–1291. doi: 10.1016/j.molp.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Basu S, Rabara RC, Negi S, Shukla P. Engineering PGPMOs through gene editing and systems biology: a solution for phytoremediation? Trends Biotechnol. 2018;36:499–510. doi: 10.1016/j.tibtech.2018.01.01. [DOI] [PubMed] [Google Scholar]
- 25.Gupta SK, Shukla P. Gene editing for cell engineering: trends and applications. Crit Rev Biotechnol. 2017;37:672–684. doi: 10.1080/07388551.2016.1214557. [DOI] [PubMed] [Google Scholar]
- 26.Chaudhary T, Shukla P. Bioinoculant capability enhancement through metabolomics and systems biology approaches. Brief Funct Genom. 2019;18:159–168. doi: 10.1093/bfgp/elz011. [DOI] [PubMed] [Google Scholar]
- 27.Zhou K, Qiao K, Edgar S, Stephanopoulos G (2015) Distributing a metabolic pathway among a microbial consortium enhances production of natural products. Nat Biotechnol 33: 377. 10.1038/nbt.3095 [DOI] [PMC free article] [PubMed]
- 28.Bernstein HC, Paulson SD, Carlson RP. Synthetic Escherichia coli consortia engineered for syntrophy demonstrate enhanced biomass productivity. J Biotechnol. 2012;157:159–166. doi: 10.1016/j.jbiotec.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macia J, Manzoni R, Conde N, Urrios A, de Nadal E, Sole R, Posas F (2016) Implementation of complex biological logic circuits using spatially distributed multicellular consortia. PLoS Comput Biol. 10.1371/journal.pcbi.1004685 [DOI] [PMC free article] [PubMed]
- 30.Urrios A, Macia J, Manzoni R, Conde N, Bonforti A, de Nadal E, Posas F, Solee R. A synthetic multicellular memory device. ACS Synth Biol. 2016;5:862–873. doi: 10.1021/acssynbio.7b00463. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H, Pereira B, Li Z, Stephanopoulos G. Engineering Escherichia coli coculture systems for the production of biochemical products. Proc Natl Acad Sci. 2015;112:8266–8271. doi: 10.1073/pnas.1506781112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiu Y, Jang S, Jones JA, Zill NA, Linhardt RJ, Yuan Q, Jung GY, Koffas MA. Naringenin-responsive riboswitch‐based fluorescent biosensor module for Escherichia coli co‐cultures. Biotechnol Bioeng. 2017;114:2235–2244. doi: 10.1002/bit.26340. [DOI] [PubMed] [Google Scholar]
- 33.Meyer A, Pellaux R, Potot S, Becker K, Hohmann HP, Panke S, Held M. Optimization of a whole-cell biocatalyst by employing genetically encoded product sensors inside nanolitre reactors. Nat Chem. 2015;7:673. doi: 10.1038/nchem.2301. [DOI] [PubMed] [Google Scholar]
- 34.Gilbert ES, Walker AW, Keasling JD. A constructed microbial consortium for biodegradation of the organophosphorus insecticide parathion. Appl Microbiol Biotechnol. 2003;61:77–81. doi: 10.1007/s00253-002-1203-5. [DOI] [PubMed] [Google Scholar]
- 35.Hong SH, Hegde M, Kim J, Wang X, Jayaraman A, Wood TK. Synthetic quorum-sensing circuit to control consortial biofilm formation and dispersal in a microfluidic device. Nat Commun. 2012;3:1–8. doi: 10.1038/ncomms1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim HJ, Jeong H, Lee SJ. Synthetic biology for microbial heavy metal biosensors. Anal Bioanal Chem. 2018;410:1191–1203. doi: 10.1007/s00216-017-0751-6. [DOI] [PubMed] [Google Scholar]
- 37.El-Sayed AS, Abdel-Ghany SE, Ali GS (2017) Genome editing approaches: manipulating of lovastatin and taxol synthesis of filamentous fungi by CRISPR/Cas9 system. Appl Microbiol101:3953–3976. 10.1007/s00253-017-8263-z [DOI] [PubMed]
- 38.Andersen MM, Landes X, Xiang W, Anyshchenko A, Falhof J, Osterberg JT, et al. Feasibility of new breeding techniques for organic farming. Trends Plant Sci. 2015;20:426–434. doi: 10.1016/j.tplants.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 39.Kusano T, Berberich T, Tateda C, Takahashi Y. Polyamines: essential factors for growth and survival. Planta. 2008;228:367–381. doi: 10.1007/s00425-008-0772-7. [DOI] [PubMed] [Google Scholar]
- 40.Kalia VC, Purohit HJ. Quenching the quorum sensing system: potential antibacterial drug targets. Crit Rev Microbiol. 2011;37:121–140. doi: 10.3109/1040841X.2010.532479. [DOI] [PubMed] [Google Scholar]
- 41.He S, Guo L, Niu M, Miao F, Jiao S, Hu T, Long M. Ecological diversity and co-occurrence patterns of bacterial community through soil profile in response to long-term switchgrass cultivation. Sci Rep. 2017;7:1–10. doi: 10.1038/s41598-017-03778-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Umaru FF, Owuama CI (2018) Application of plant-microbe interactions in contaminated agroecosystem management. In: Kumar V, Kumar M, Prasad R (eds) Phytobiont and ecosystem restitution. Springer, Singapore, pp 63–100. 10.1007/978
- 43.Kalia VC. Quorum sensing inhibitors: an overview. Biotechnol Adv. 2013;31:224–245. doi: 10.1016/j.biotechadv.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Kalia VC (2015) Microbes: the mostfriendly beings? In: Kalia VC (ed) Quorum sensing vs quorum quenching: a battle with no end in sight. Springer India, pp 1–5. 10.1007/978-81-322-1982-8_1
- 45.Park H, Lee K, Yeo S, Shin H, Holzapfel WH (2017) Autoinducer-2 quorum sensing influences viability of Escherichia coli O157: H7 under osmotic and in vitro gastrointestinal stress conditions. Front Microbiol 8:1077. 10.3389/fmicb.2017.01077 [DOI] [PMC free article] [PubMed]
- 46.Mokkonen M, Lindstedt C. The evolutionary ecology of deception. Biol Rev. 2016;91:1020–1035. doi: 10.1111/brv.12208. [DOI] [PubMed] [Google Scholar]
- 47.Yu K, Pieterse CM, Bakker PA, Berendsen RL. Beneficial microbes going underground of root immunity. Plant cell environ. 2019;42:2860–2870. doi: 10.1111/pce.13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joshi N, Nautiyal P, Papnai G. Unravelling diverse roles of strigolactones in stimulating plant growth and alleviating various stress conditions: a review. J Pharmacogn Phytochem. 2019;8:396–404. doi: 10.3389/fpls.2016.00434. [DOI] [Google Scholar]
- 49.McKinley VL. Effects of land use and restoration on soil microbial communities. In: Hurst C, editor. Understanding terrestrial microbial communities. Cham: Springer; 2019. pp. 173–242. [Google Scholar]
- 50.Mukherjee D (2019) Microbial interventions in soil and plant health for improving crop efficiency. In: Singh D, Prabha R (eds) Microbial interventions in agriculture and environment. Springer, Singapore pp 17-47. 10.1007/978-981-32-9084-6$4
- 51.Shelake RM, Pramanik D, Kim JY. Exploration of plant-microbe interactions for sustainable agriculture in CRISPR era. Microorganisms. 2019;7:269. doi: 10.3390/microorganisms7080269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marwein R, Debbarma J, Sarki YN, Baruah I, Saikia B, Boruah HPD, Velmurugan N, Chikkaputtaiah C (2019) Genetic engineering/Genome editing approaches to modulate signaling processes in abiotic stress tolerance. In: Maragioglio N (ed) Plant Signaling molecules. Woodhead Publishing, pp 63–82. 10.1016/B978-0-12-816451-8.00002-2
- 53.Langner T, Kamoun S, Belhaj K. CRISPR crops: plant genome editing toward disease resistance. Annu Rev Phytopathol. 2018;56:479–512. doi: 10.1146/annurev-phyto-080417-050158. [DOI] [PubMed] [Google Scholar]
- 54.Friesen ML, Friel CA. Legumes modulate allocation to rhizobial nitrogen fixation in response to factorial light and nitrogen manipulation. Front Plant Sci. 2019;10:316. doi: 10.3389/fpls.2019.01316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cooper JE. Early interactions between legumes and rhizobia: disclosing complexity in a molecular dialogue. J Appl Microbiol. 2007;103:1355–1365. doi: 10.1111/j.1365-2672.2007.03366.x. [DOI] [PubMed] [Google Scholar]
- 56.Martinez-Camara R, Montejano-Ramirez V, Moreno-Hagelsieb G, Santoyo G, Valencia-Cantero E (2019) The volatile organic compound dimethylhexadecylamine affects bacterial growth and swarming motility of bacteria. Folia Microbiol 1–10.10.1007/s12223-019-00756-6 [DOI] [PubMed]
- 57.Vaishnav A, Hansen AP, Agrawal PK, Varma A, Choudhary DK (2017) Biotechnological perspectives of legume–rhizobium symbiosis. In: Hansen A, Choudhary D, Agrawal P, Varma A (eds) Rhizobium biology and biotechnology. Soil biology, vol 50. Springer, Cham, pp 247–256
- 58.Whiteside MD, Werner GD, Caldas VE, van’tPadje A, Dupin SE, Elbers B, Bakker M, Wyatt GA, Klein M, Hink MA, Postma M. Mycorrhizal fungi respond to resource inequality by moving phosphorus from rich to poor patches across networks. Curr Biol. 2019;29:2043–2050. doi: 10.1016/j.cub.2019.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fiorilli V, Wang JY, Bonfante P, Lanfranco L, Al-Babili S. Apocarotenoids: old and new mediators of the arbuscular mycorrhizal symbiosis. Front Plant Sci. 2019;10:1186. doi: 10.3389/fpls.2019.01186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rochange S, Goormachtig S, Lopez-Raez JA, Gutjahr C. The role of strigolactones in plant–microbe interactions. In: Koltai H, Prandi C, editors. Strigolactones-biology and applications. Cham: Springer; 2019. pp. 121–142. [Google Scholar]
- 61.Schouteden N, De Waele D, Panis B, Vos CM. Arbuscular mycorrhizal fungi for the biocontrol of plant-parasitic nematodes: a review of the mechanisms involved. Front Microbiol. 2015;6:1280. doi: 10.3389/fmicb.2015.01280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van’t Padje A, Whiteside MD, Kiers ET. Signals and cues in the evolution of plant–microbe communication. Curr Opin Plant Biol. 2016;32:47–52. doi: 10.1016/j.pbi.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 63.Spaepen S, Vanderleyden J. Auxin and plant-microbe interactions. Cold Spring Harb. 2011;3:001438. doi: 10.1101/cshperspect.a001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu P, Nester EW (2006) Indoleacetic acid, a product of transferred DNA, inhibits vir gene expression and growth of Agrobacterium tumefaciens C58. PNAS 103:4658–4662. 10.1073/pnas.0600366103 [DOI] [PMC free article] [PubMed]
- 65.Remans R, Beebe S, Blair M, Manrique G, Tovar E, Rao I, Croonenborghs A, Torres-Gutierrez R, El-Howeity M, Michiels J, Vanderleyden J. Physiological and genetic analysis of root responsiveness to auxin-producing plant growth-promoting bacteria in common bean (Phaseolus vulgaris L.) Plant Soil. 2008;302:149–161. doi: 10.1007/s11104-007-9462-7. [DOI] [Google Scholar]
- 66.Chagas FO, de Cassia Pessotti R, Caraballo-Rodriguez AM, Pupo MT. Chemical signaling involved in plant–microbe interactions. Chem Soc Rev. 2018;47:1652–1704. doi: 10.1039/c7cs00343a. [DOI] [PubMed] [Google Scholar]
- 67.Seneviratne G, Weerasekara M, L M A W, Kumar esan D, Zavahir JS (2017) Microbial signaling in plant-microbe interactions and its role on sustainability of agroecosystems. In: Singh JK, Seneviratne G (eds) Agro-environmental sustainability, vol 1. Springer, Cham, pp 1–17. 10.1007/978-3-319-49724-2_1
- 68.Liu Z, Hong CJ, Yang Y, Dai L, Ho CL (2020) Advances in bacterial biofilm management for maintaining microbiome homeostasis. Biotechnol J 1900320. 10.1002/biot.201900320 [DOI] [PubMed]
- 69.Saha I, Datta S, Biswas D (2020) Exploring the role of bacterial extracellular polymeric substances for sustainable development in agriculture. Curr Microbiol 1–16. 10.1007/s00284-020-02169-y [DOI] [PubMed]
- 70.Tian L, Lin X, Tian J, Ji L, Chen Y, Tran LSP, Tian C. Research advances of beneficial microbiota associated with crop plants. Int J Mol Sci. 2020;21:1792. doi: 10.3390/ijms21051792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hermosa R, Viterbo A, Chet I, Monte E. Plant-beneficial effects of Trichoderma and of its genes. Microbiology. 2012;158:17–25. doi: 10.1099/mic.0.052274-0. [DOI] [PubMed] [Google Scholar]
- 72.Atanasova L, Knox BP, Kubicek CP, Druzhinina IS, Baker SE. The polyketide synthase gene pks4 of Trichoderma reesei provides pigmentation and stress resistance. Eukaryot Cell. 2013;12:1499–1508. doi: 10.1128/EC.00103-13. [DOI] [PMC free article] [PubMed] [Google Scholar]