Abstract
OBJECTIVE:
Polypharmacy and anticholinergic burden are the indicators for the evaluation of the quality of pharmacotherapy in older adults. The aim of this study was to consider which anticholinergic burden scales are more related with polypharmacy among older patients.
METHODS:
Four hundred and twenty older adults were evaluated retrospectively in this cross-sectional study. The patient’s demographic data, comorbidities, the drugs, and number of drugs were recorded. Anticholinergic burden scales were calculated by a tool named anticholinergic burden calculator.
RESULTS:
The participants’ mean age was 73.08±8.71. The prevalence of polypharmacy was 32.14%. The highest relationship with polypharmacy was observed for drug burden index (DBI) (odds ratio 10.87, p<0.001).
CONCLUSION:
Our study demonstrated that polypharmacy and DBI scores were more related than other anticholinergic burden scales in older adults.
Keywords: Anticholinergic burden scales, Elderly, Polypharmacy
Highlight key points.
Polypharmacy and anticholinergic burden are both common problems in older adults.
The prevalence of polypharmacy was 32.14% in this study.
The highest relationship between polypharmacy and anticholinergic burden scales was with Drug Burden Index (DBI) score.
The world’s population of older people is growing especially in developing regions [1]. As populations grow older, developing multiple chronic diseases and associated high consumption of drugs among older adults also increases [2]. In this case, it brings major health problems in older adults such as polypharmacy [3], inappropriate drug use [4, 5], and exposure to anticholinergic burden [6]. Polypharmacy results in adverse clinical events, such as depression, cognitive impairment, urine continence problems, malnutrition, and loss of functionality [7, 8]. For that reason, the assessment of medications of older people is a major and essential part of comprehensive geriatric assessment (CGA).
Older people are frequently exposed to medicines that have anticholinergic properties [9]. The term anticholinergic burden means to point out the cumulative exposure to multiple medicines with anticholinergic properties concomitantly. The anticholinergic burden effect have a wide spectrum of side effects such as dizziness, blurred vision, urinary retention, constipation, confusion, and delirium [10, 11]. Higher anticholinergic burden increase the hospitalization risk, functional loss, cognitive decline, morbidity, and mortality [11, 12]. There are several anticholinergic risk scales that have been validated. Anticholinergic risk scale (ARS) [13], anticholinergic drug scale (ADS) [14], anticholinergic cognitive burden scale (ACB) [15], chew scale [16], and drug burden index (DBI) [17] are among these scales. Anticholinergic burden scales differ in their rationale, use and association with clinical outcomes [18] and have weak agreement between each other as they contain different medicines so that comparing the results is so hard [9, 19].
CGA is a time-consuming assessment which can be difficult to spare time for the evaluation of anticholinergic burden of an elderly with all anticholinergic burden scales during a busy clinical day. Understanding the relationship with polypharmacy and anticholinergic burden may help clinicians determine which anticholinergic burden scale can be chosen as a part of CGA. As far as we know, there is no clear evidence about the relationship between these two concepts, polypharmacy and anticholinergic burden. Therefore, the present study aimed to evaluate the relationship between polypharmacy and anticholinergic burden scales among older patients.
MATERIALS AND METHODS
This study included 420 outpatients aged 60 and over. Patients’ medical records were inspected retrospectively. Approval was provided from the Dokuz Eylul University Non-Interventional Research Ethics Committee. This study had no exclusion criteria.
Patients’ Characteristics
Socio-demographic variables (age, gender, and education), self-reported comorbidities (hypertension, peripheral vascular disease, diabetes, coronary artery disease, cerebrovascular disease, congestive heart failure, hyperlipidemia, thyroid disease, chronic obstructive pulmonary disease, and osteoporosis), and history of falls were evaluated. The Charlson comorbidity index (CCI) was used to evaluate patients’ comorbidity status.
The Evaluation of Polypharmacy and Anticholinergic Burden
All drugs used by the patients were evaluated with anticholinergic burden scales and number of drugs was recorded. Polypharmacy was considered as taking five or more medications daily [20]. Anticholinergic burden is characterized as using medications with anticholinergic adverse effects [21]. Anticholinergic burden scales are the medication lists which classify drugs in accordance with the anticholinergic potency of these drugs. The summation of anticholinergic activity scores of the drugs listed in each scale reflects the potential for negative outcomes [22].
We used ten previously published lists (briefly mentioned below) to determine the anticholinergic burden of the drugs that regularly used. ACB categorizes drugs on a score of 0–3, according to their anticholinergic effects. ACB score 0 represents no anticholinergic effect, ACB score 1 means possible, and ACB scores 2 and 3 show definite anticholinergic effects [15, 23]. ARS [13] consists of 49 drugs with anticholinergic effects and the scale ranks drugs for anticholinergic potency such as score of 0 means no or low risk; score of 1 or 2 means moderate risk; and score of 3 or higher means high anticholinergic potential. Chew’s list [16] consists of 107 drugs with anticholinergic activities which were determined in vitro and scores range from 0 to +++; score of 0/+ shows no or minimal anticholinergic activity (AA) and classified as 0.5. ADS [14] is the largest scale which includes 536 drugs and scores of the scale range from 0 (means no known anticholinergic effects) to 3 (means remarkably anticholinergic effects). Anticholinergic activity scale (AAS) [24] is based on serum anticholinergic activity of 107 drugs commonly prescribed for the elderly with Parkinson’s disease and scores of the scale range from 0 to 4. Anticholinergic load scale (ALS) [25] is also based on serum anticholinergic activity. The scoring ranges of the Clinician-rated anticholinergic scale (CrAS) [26] and Duran’s scale [27] are 0 (no anticholinergic effect) to 3 (strong anticholinergic effect). DBI [17] is a formula to determine the anticholinergic potency by calculating the ratio between the prescribed dose and the sum of the minimum effective dose and the prescribed dose of the drug and consider the daily dose and the minimum recommended daily dose of the drugs. Anticholinergic burden classification (ABC) [28] is based on the review of serum anticholinergic activity of 27 drugs and scores of the scale range from 0 to 3. Ten anticholinergic burden scales described above were administered for each of the patients’ medications by a tool named Anticholinergic Burden Calculator. Anticholinergic Burden Calculator is a web portal software program (www.anticholinergicscales.es/) [29].
Statistical Analysis
Descriptive statistics are presented as mean ± standard deviations (SDs) or percentages. Nominal variables were analyzed with Pearson χ2-test or Fisher exact test. Normally and non-normally distributed continuous variables were assessed with t-test and Mann–Whitney U-test, respectively. Age-adjustment analysis was performed with binary logistic regression analysis (P2-value). Then, the relationships between polypharmacy and each anticholinergic burden scale were analyzed with the binary logistic regression. P<0.05 was considered statistically significant. All statistical analyses were carried out with SPSS version 22.0.
RESULTS
A total of 420 patients older than 60 years were included in the study, 72% of whom were female in polypharmacy group and 63% in non-polypharmacy group. 135 (32.14%) patients were in polypharmacy group and 285 (67.86%) patients were in non-polypharmacy group. Mean age (SD) was 73.08 (8.71). The mean number of drugs (SD) was 8.1 (2.89) and 1.98 (1.5) in polypharmacy and non-polypharmacy group, respectively. The participants’ characteristics, comorbid diseases, and CCI are shown in Table 1. There was statistical significance between the two groups according to age, education years, number of drugs, and all of the self-reported comorbid diseases except congestive heart failure (p<0.05 for all). Furthermore, CCI and frequency of falls between the two groups were statistically significant (p<0.001). After the age adjustment analysis, education years and the self-reported comorbid diseases except peripheral artery disease were still significant between the two groups (p<0.05).
TABLE 1.
Characteristics and comorbid diseases of the study population (n=420)
| Characteristic | Polypharmacy (n=135) | Non-polypharmacy (n=285) | P1 | P2 |
|---|---|---|---|---|
| Age, mean±SD | 76.85±7.87 | 71.5±8.57 | <0.001 | – |
| Education years, mean±SD | 6.3±4.61 | 8.35±4.75 | <0.001 | 0.008 |
| Female, % | 72 | 63 | 0.13 | – |
| Number of drugs, mean±SD | 8.1±2.89 | 1.98±1.5 | <0.001 | – |
| Comorbid diseases, % | ||||
| Hypertension | 77.8 | 42 | <0.001 | <0.001 |
| Ischemic heart disease | 27.8 | 5.3 | <0.001 | <0.001 |
| Congestive heart failure | 2.6 | 3.2 | 1.0 | – |
| Cerebrovascular disease | 11.1 | 3.2 | 0.003 | 0.016 |
| Peripheral artery disease | 10.3 | 4.2 | 0.003 | 0.118 |
| Diabetes mellitus | 39.3 | 10.2 | <0.001 | <0.001 |
| Hyperlipidemia | 23.1 | 11 | 0.003 | 0.002 |
| Dementia | 23.9 | 9.1 | <0.001 | 0.004 |
| COPD | 22.4 | 3.5 | <0.001 | <0.001 |
| Thyroid disease | 25.6 | 12.7 | 0.003 | 0.002 |
| Osteoporosis | 38.2 | 17.7 | <0.001 | <0.001 |
| Falls | 45.3 | 22.3 | <0.001 | <0.001 |
| CCI, mean±SD | 1.34±1.02 | 0.53±0.86 | <0.001 | <0.001 |
SD: Standard deviation; CCI: Charlson comorbidity index; COPD: Chronic obstructive pulmonary disease; SD: Standard deviation; P1: P-values for comparison of two groups; P2: P-values for comparison of polypharmacy and non-polypharmacy group after adjusted for age.
The highest relationship with polypharmacy was observed for DBI (OR 10.87, p<0.001). It is found that each unit increase in DBI increased the risk of polypharmacy 10.86 times. ARS, ALS, CrAS, and Duran scale showed moderate relation with polypharmacy (with odds ratio [ORs] 3.11; 3.17; 3.3; and 3.46, respectively). The relationship between anticholinergic burden scales and polypharmacy is shown in Table 2. The odds ratios of polypharmacy according to the anticholinergic burden scales are shown in Figure 1. The scores of the anticholinergic burden scales according to the polypharmacy and non-polypharmacy are shown in Table 3.
TABLE 2.
The relationship between anticholinergic burden scales and polypharmacy
| Scale | Odds ratios | 95% CI | p |
|---|---|---|---|
| ACB | 2.32 | 1.764–3.046 | <0.001 |
| ARS | 3.11 | 1.919–5.032 | <0.001 |
| Chew | 1.90 | 1.541–2.345 | <0.001 |
| ADS | 2.35 | 1.785–3.082 | <0.001 |
| AAS | 1.44 | 1.109–1.871 | 0.006 |
| ALS | 3.17 | 2.163–4.646 | <0.001 |
| CrAS | 3.3 | 2.229–4.871 | <0.001 |
| Duran | 3.46 | 2.173–5.519 | <0.001 |
| ABC | 2.14 | 1.417–3.238 | <0.001 |
| DBI | 10.87 | 5.446–21.680 | <0.001 |
All adjusted for age. AAS: Anticholinergic activity scale; ABC: Anticholinergic burden classification; ACB: Anticholinergic cognitive burden scale; ADS: Anticholinergic drug scale; ALS: Anticholinergic load scale; ARS: Anticholinergic risk scale; CI: Confidence interval; CrAS: Clinician-rated anticholinergic scale; DBI: Drug burden index.
FIGURE 1.
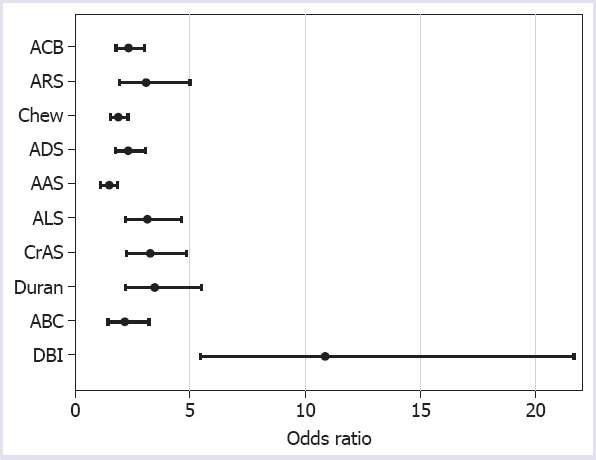
The odds ratios of polypharmacy according to the anticholinergic burden scales.
TABLE 3.
The scores of the anticholinergic burden scales
| Scale | Polypharmacy | Non-polypharmacy |
|---|---|---|
| ACB | 0.99±1.13 | 0.1±0.47 |
| ARS | 0.42±0.74 | 0.06±0.36 |
| Chew | 0.78±1.11 | 0.2±0.62 |
| ADS | 1.04±1.1 | 0.11±0.43 |
| AAS | 0.42±0.88 | 0.07±0.44 |
| ALS | 0.76±0.91 | 0.09±0.38 |
| CrAS | 0.77±0.95 | 0.08±0.36 |
| Duran | 0.6±0.98 | 0.06±0.3 |
| ABC | 0.33±0.92 | 0.02±0.25 |
| DBI | 1.36±1.21 | 0.04±0.18 |
AAS: Anticholinergic activity scale; ABC: Anticholinergic burden classification; ACB: Anticholinergic cognitive burden scale; ADS: Anticholinergic drug scale; ALS: Anticholinergic load scale; ARS: Anticholinergic risk scale; CrAS: Clinicianrated anticholinergic scale; DBI: Drug burden index. All data are expressed as means±standard deviations.
DISCUSSION
The results of the present study revealed that the highest relationship with polypharmacy was with DBI, while the lowest relationship was with AAS.
As a geriatric syndrome, polypharmacy is prevalent in the elderly and closely related with falls, mood disorders, cognitive, and functional decline [7, 20, 30]. Likewise, anticholinergic burden put older patients at increased risk of negative clinical outcomes such as falls, delirium, hospitalization, functional decline, and negative impact on cognitive functions [11, 12, 27]. Anticholinergic burden and polypharmacy lead in adverse outcomes and functional limitation of older adults and these two concepts are often in close relationship.
The prevalence of polypharmacy has been reported to range from 20% to 40% [31]. In this study, the prevalence of polypharmacy was 32.14%. This finding is similar to the previous studies that found the prevalence 30.5% in the United Kingdom [32] and 26.7% in Western countries such as Germany [33]. On the other hand, the prevalence of polypharmacy in the elderly was reported as 54.5% [2] and 55.3% [20] in the studies from Turkey, which is higher than the other European countries.
Several studies have assessed the concordance between anticholinergic burden scales [9, 34–36] and concordance between anticholinergics among potentially inappropriate medications and anticholinergic burden scales [37]. To the best of our knowledge, there are no studies which identified the relationship between polypharmacy and anticholinergic burden scales. We believe that to clarify the relationship between polypharmacy and anticholinergic burden, which are both related to the health status of older adults, will provide future researchers to better viewpoint for the factors affecting the functionality of the older adults exposed to polypharmacy and/or anticholinergic burden.
In the present study, DBI has the highest relationship with polypharmacy. DBI is an evidence-based tool used to evaluate a patient’s cumulative exposure to anticholinergic and sedative medicines [17]. Recently, it was reported that number of medicines and the DBI scores were positively associated with evaluation of the level of polypharmacy [38]; on the other hand, population and methodology of this study are different from ours.
ARS, ALS, CrAS, and Duran scale showed moderate relationship with polypharmacy (OR range from 3.11 to 3.46); ACB, Chew, ADS, and ABC showed lower relationship with polypharmacy (OR range from 1.90 to 2.35) whereas the lowest relationship was found for AAS. We may have achieved these results because there are different drugs included in each anticholinergic scale. The number of common drugs in the scales has been reported to be 29 (ADS, ARS, and ACB) while 20 drugs remained if DBI and Summated Anticholinergic Medications Scale were included in the study [34]. ADS and ACB have an acceptable relation in view of the fact that the structure of both scales has identical data and both of them include similar anticholinergic medicines [35].
The large sample size and the usage of ten anticholinergic burden scales are the strengths of the present study. As far as we know, there is no study, which investigates the relationship between polypharmacy and anticholinergic burden assessed by ten different scales; however, there are still some limitations. These are that this study is retrospective, a single-center, and observational study.
Conclusion
The current study has demonstrated that polypharmacy and DBI scores were more related than other anticholinergic burden scales. Although polypharmacy and anticholinergic burden are two different concepts, they are in close relationship. For a better understanding of polypharmacy and anticholinergic burden, further research is required to evaluate which one is more effective on the functionality and health status of older adults.
Footnotes
Ethics Committee Approval: The Dokuz Eylul University Non-Interventional Research Ethics Committee granted approval for this study (date: 13.09.2018, number: 2018/22-06).
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
Authorship Contributions: Concept – ATI; Design – ATI; Supervision – PS; Data collection and/or processing – SKO, OD, AEA, SEK; Analysis and/or interpretation – OD; Literature review – SKO; Writing – SKO, ATI; Critical review – PS, ATI.
REFERENCES
- 1.United Nations. World population prospects. [Accessed Feb 12 2021]. 2017 revision. Available at: https://population.un.org/wpp/Publications/Files/WPP2017_KeyFindings.pdf .
- 2.Ates Bulut E, Soysal P, Isik AT. Frequency and coincidence of geriatric syndromes according to age groups:single-center experience in Turkey between 2013 and 2017. Clin Interv Aging. 2018;13:1899–905. doi: 10.2147/CIA.S180281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jyrkkä J, Enlund H, Korhonen MJ, Sulkava R, Hartikainen S. Patterns of drug use and factors associated with polypharmacy and excessive polypharmacy in elderly persons:results of the Kuopio 75+study:a cross-sectional analysis. Drugs Aging. 2009;26:493–503. doi: 10.2165/00002512-200926060-00006. [DOI] [PubMed] [Google Scholar]
- 4.Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the Beers criteria for potentially inappropriate medication use in older adults:results of a US consensus panel of experts. Arch Intern Med. 2003;163:2716–24. doi: 10.1001/archinte.163.22.2716. [DOI] [PubMed] [Google Scholar]
- 5.American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616–31. doi: 10.1111/j.1532-5415.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudd KM, Raehl CL, Bond CA, Abbruscato TJ, Stenhouse AC. Methods for assessing drug-related anticholinergic activity. Pharmacotherapy. 2005;25:1592–601. doi: 10.1592/phco.2005.25.11.1592. [DOI] [PubMed] [Google Scholar]
- 7.Wilson M, Mair A, Dreischulte T, Witham MD. NHS Scotland Model of Care Polypharmacy Working Group. Prescribing to fit the needs of older people-the NHS Scotland Polypharmacy Guidance 2nd edition. J R Coll Physicians Edinb. 2015;45:108–13. doi: 10.4997/JRCPE.2015.204. [DOI] [PubMed] [Google Scholar]
- 8.Onder G, Landi F, Fusco D, Corsonello A, Tosato M, Battaglia M, et al. Recommendations to prescribe in complex older adults:results of the CRIteria to assess appropriate Medication use among Elderly complex patients (CRIME) project. Drugs Aging. 2014;31:33–45. doi: 10.1007/s40266-013-0134-4. [DOI] [PubMed] [Google Scholar]
- 9.Salahudeen MS, Hilmer SN, Nishtala PS. Comparison of anticholinergic risk scales and associations with adverse health outcomes in older people. J Am Geriatr Soc. 2015;63:85–90. doi: 10.1111/jgs.13206. [DOI] [PubMed] [Google Scholar]
- 10.Cancelli I, Beltrame M, Gigli GL, Valente M. Drugs with anticholinergic properties:cognitive and neuropsychiatric side-effects in elderly patients. Neurol Sci. 2009;30:87–92. doi: 10.1007/s10072-009-0033-y. [DOI] [PubMed] [Google Scholar]
- 11.Fox C, Smith T, Maidment I, Chan WY, Bua N, Myint PK, et al. Effect of medications with anti-cholinergic properties on cognitive function, delirium, physical function and mortality:a systematic review. Age Ageing. 2014;43:604–15. doi: 10.1093/ageing/afu096. [DOI] [PubMed] [Google Scholar]
- 12.Kumpula EK, Bell JS, Soini H, Pitkälä KH. Anticholinergic drug use and mortality among residents of long-term care facilities:a prospective cohort study. J Clin Pharmacol. 2011;51:256–63. doi: 10.1177/0091270010368410. [DOI] [PubMed] [Google Scholar]
- 13.Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med. 2008;168:503–13. doi: 10.1001/archinternmed.2007.106. [DOI] [PubMed] [Google Scholar]
- 14.Carnahan RM, Lund BC, Perry PJ, Pollock BG, Culp KR. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden:associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46:1481–6. doi: 10.1177/0091270006292126. [DOI] [PubMed] [Google Scholar]
- 15.Boustani M, Campbell N, Munger S, Maidment I, Fox C. Impact of anticholinergics on the aging brain:a review and practical application. Aging Health. 2008;4:311–20. [Google Scholar]
- 16.Chew ML, Mulsant BH, Pollock BG, Lehman ME, Greenspan A, Mahmoud RA, et al. Anticholinergic activity of 107 medications commonly used by older adults. J Am Geriatr Soc. 2008;56:1333–41. doi: 10.1111/j.1532-5415.2008.01737.x. [DOI] [PubMed] [Google Scholar]
- 17.Hilmer SN, Mager DE, Simonsick EM, Cao Y, Ling SM, Windham BG, et al. A drug burden index to define the functional burden of medications in older people. Arch Intern Med. 2007;167:781–7. doi: 10.1001/archinte.167.8.781. [DOI] [PubMed] [Google Scholar]
- 18.Welsh TJ, van der Wardt V, Ojo G, Gordon AL, Gladman JRF. Anticholinergic drug burden tools/scales and adverse outcomes in different clinical settings:a systematic review of reviews. Drugs Aging. 2018;35:523–38. doi: 10.1007/s40266-018-0549-z. [DOI] [PubMed] [Google Scholar]
- 19.Lertxundi U, Domingo-Echaburu S, Hernandez R, Peral J, Medrano J. Expert-based drug lists to measure anticholinergic burden:similar names different results. Psychogeriatrics. 2013;13:17–24. doi: 10.1111/j.1479-8301.2012.00418.x. [DOI] [PubMed] [Google Scholar]
- 20.Unutmaz GD, Soysal P, Tuven B, Isik AT. Costs of medication in older patients:before and after comprehensive geriatric assessment. Clin Interv Aging. 2018;13:607–13. doi: 10.2147/CIA.S159966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tune LE. Anticholinergic effects of medication in elderly patients. J Clin Psychiatry. 2001;62(Suppl 21):11–4. [PubMed] [Google Scholar]
- 22.Villalba-Moreno AM, Alfaro-Lara ER, Pérez-Guerrero MC, Nieto-Martín MD, Santos-Ramos B. Systematic review on the use of anticholinergic scales in poly pathological patients. Arch Gerontol Geriatr. 2016;62:1–8. doi: 10.1016/j.archger.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Campbell N, Maidment I, Fox C, Khan B, Boustani M. The 2012 update to the anticholinergic cognitive burden scale. J Am Geriatr Soc. 2013;61:S142–3. [Google Scholar]
- 24.Ehrt U, Broich K, Larsen JP, Ballard C, Aarsland D. Use of drugs with anticholinergic effect and impact on cognition in Parkinson's disease:a cohort study. J Neurol Neurosurg Psychiatry. 2010;81:160–5. doi: 10.1136/jnnp.2009.186239. [DOI] [PubMed] [Google Scholar]
- 25.Sittironnarit G, Ames D, Bush AI, Faux N, Flicker L, Foster J, et al. AIBL research group. Effects of anticholinergic drugs on cognitive function in older Australians:results from the AIBL study. Dement Geriatr Cogn Disord. 2011;31:173–8. doi: 10.1159/000325171. [DOI] [PubMed] [Google Scholar]
- 26.Han L, Agostini JV, Allore HG. Cumulative anticholinergic exposure is associated with poor memory and executive function in older men. J Am Geriatr Soc. 2008;56:2203–10. doi: 10.1111/j.1532-5415.2008.02009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durán CE, Azermai M, Vander Stichele RH. Systematic review of anticholinergic risk scales in older adults. Eur J Clin Pharmacol. 2013;69:1485–96. doi: 10.1007/s00228-013-1499-3. [DOI] [PubMed] [Google Scholar]
- 28.Ancelin ML, Artero S, Portet F, Dupuy AM, Touchon J, Ritchie K. Non-degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs:longitudinal cohort study. BMJ. 2006;332:455–9. doi: 10.1136/bmj.38740.439664.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Villalba-Moreno Á, Alfaro-Lara ER, Sánchez-Fidalgo S, Nieto-Martín MD, Santos-Ramos B. Development of the anticholinergic burden calculator web tool. Farm Hosp. 2017;41:647–8. doi: 10.7399/fh.10799. [DOI] [PubMed] [Google Scholar]
- 30.Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57–65. doi: 10.1517/14740338.2013.827660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Best O, Gnjidic D, Hilmer SN, Naganathan V, McLachlan AJ. Investigating polypharmacy and drug burden index in hospitalised older people. Intern Med J. 2013;43:912–8. doi: 10.1111/imj.12203. [DOI] [PubMed] [Google Scholar]
- 32.Slater N, White S, Venables R, Frisher M. Factors associated with polypharmacy in primary care:a cross-sectional analysis of data from The English Longitudinal Study of Ageing (ELSA) BMJ Open. 2018;8:e020270. doi: 10.1136/bmjopen-2017-020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Junius-Walker U, Theile G, Hummers-Pradier E. Prevalence and predictors of polypharmacy among older primary care patients in Germany. Fam Pract. 2007;24:14–9. doi: 10.1093/fampra/cml067. [DOI] [PubMed] [Google Scholar]
- 34.Naples JG, Marcum ZA, Perera S, Gray SL, Newman AB, Simonsick EM, et al. Health, Aging and Body Composition Study. Concordance between Anticholinergic Burden Scales. J Am Geriatr Soc. 2015;63:2120–4. doi: 10.1111/jgs.13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pont LG, Nielen JT, McLachlan AJ, Gnjidic D, Chan L, Cumming RG, et al. Measuring anticholinergic drug exposure in older community-dwelling Australian men:a comparison of four different measures. Br J Clin Pharmacol. 2015;80:1169–75. doi: 10.1111/bcp.12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayer T, Meid AD, Saum KU, Brenner H, Schöttker B, Seidling HM, et al. Comparison of nine instruments to calculate anticholinergic load in a large cohort of older outpatients:association with cognitive and functional decline, falls, and use of laxatives. Am J Geriatr Psychiatry. 2017;25:531–40. doi: 10.1016/j.jagp.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Park KH, Yang YM, Yoo JC, Choi EJ. Comparative analysis of anticholinergics prescribed to elderly patients at a Korean long-term care facility according to beers criteria 2003 2012, and 2015 and Anticholinergic-Burden Rating Scales:A cross-sectional retrospective study. Clin Interv Aging. 2019;14:1963–74. doi: 10.2147/CIA.S224434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ong GJ, Page A, Caughey G, Johns S, Reeve E, Shakib S. Clinician agreement and influence of medication-related characteristics on assessment of polypharmacy. Pharmacol Res Perspect. 2017;5:e00321. doi: 10.1002/prp2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]


