Abstract
Objective
Patients with platinum-resistant ovarian cancer (PROC) have a high need for reliable prognostic markers. Since significance of primary platinum resistance (PPR) versus secondary platinum resistance (SPR) was identified for patients receiving anti-angiogenic therapy, it has not been confirmed for chemotherapy only.
Methods
PROC patients from 3 prospective trials of the NOGGO study group (TOWER, NOGGO-Treosulfan, and TRIAS) were included in this meta-analysis. Exploratory Cox and logistic regression analyses were performed to correlate progression-free survival (PFS) and overall survival (OS) with the timing when platinum resistance developed.
Results
Of 477 patients, 264 (55.3%) were classified as PPR, compared to 213 (44.7%) with SPR. For patients receiving chemotherapy only, SPR was associated with a significantly longer median PFS of 3.9 compared to 3.1 months for PPR (hazard ratio [HR]=0.78; p=0.015). SPR versus PPR was confirmed to be an independent prognostic factor for better PFS in multivariate analysis (HR=0.74; p=0.029). Benefit from adding sorafenib to chemotherapy was mainly seen in PPR (HR=0.40; p<0.001) compared to SPR patients (HR=0.83; p=0.465).
Conclusions
Prognostic significance of SPR versus PPR could be elucidated for patients receiving chemotherapy only. In contrast to bevacizumab, the multi-kinase inhibitor sorafenib exhibits profound therapeutic efficacy in PPR patients indicating potential to overcome this negative prognostic impact.
Keywords: Recurrent Ovarian Cancer, Primary Platinum Resistance, Mono-chemotherapy, Anti-angiogenic Treatment, Sorafenib, Prognostic Factor
INTRODUCTION
Despite recent advances in ovarian cancer treatment, development of platinum resistance is still one of the main events worsening prognosis [1]. In platinum-resistant disease, traditional therapeutic approaches as cytoreductive surgery and platinum-based chemotherapy have failed (common definition: relapse within 6 months following last platinum treatment) so that treatment options mainly focus on chemotherapy and targeted therapies [2]. Over the past years, different drugs have proven efficacy including paclitaxel, pegylated liposomal doxorubicin (PLD), topotecan, gemcitabine, and treosulfan although the overall oncologic outcome remains poor [3,4,5,6]. In this context, addition of anti-angiogenic bevacizumab to single-agent chemotherapy led to a significant improvement in progression-free survival (PFS) in the randomized phase III AURELIA (ENGOT-ov3/AGO-OVAR 2.15) trial. However, the effect on overall survival (OS) was not significant [7]. Recently, the randomized phase II trial TRIAS investigated the combination treatment of mono-chemotherapy topotecan in combination with the oral multi-kinase inhibitor sorafenib and a subsequent maintenance treatment. Sorafenib-containing therapy was associated with significantly improved PFS (hazard ratio [HR]=0.60; 95% confidence interval [CI]=0.43–0.83; p=0.002) as well as OS (HR=0.65; 95% CI=0.45–0.93; p=0.017) over placebo so that TRIAS was the first trial in platinum-resistant ovarian cancer (PROC) with a significant improvement for both endpoints, PFS and OS [8].
A previous exploratory analysis of the AURELIA trial suggested better prognosis for patients who initially responded to chemotherapy but then developed platinum resistance (secondary platinum resistance, SPR) compared to patients who already failed to respond to first-line platinum chemotherapy (primary platinum resistance, PPR). While this effect was statistically significant and clinically relevant in bevacizumab-treated patients, it could not be confirmed in patients with chemotherapy only in this cohort [9].
To deepen the understanding of these effects in a larger group of patients receiving established mono-chemotherapy regimens, we conducted this meta-analysis of the 3 prospective trials from the North-Eastern German Society of Gynecological Oncology (NOGGO) study group focusing on patients with PROC. In addition, specific analysis of the TRIAS cohort allows a deeper understanding of the impact of PPR for patients undergoing anti-angiogenic treatment and further elucidates the considerable and firstly described OS benefit in a randomized trial for platinum-resistant disease.
MATERIALS AND METHODS
1. Patients and study design
Individual patient data included in the 3 previously performed prospective trials of the NOGGO study group from patients with PROC were analyzed (TOWER [ClinicalTrials.gov Identifier: NCT00170677], NOGGO-Treosulfan [ClinicalTrials.gov Identifier: NCT00170690], TRIAS [ClinicalTrials.gov Identifier: NCT01047891]). The datasets were provided by the principle investigators of the single studies who are considered as co-authors. Design and methods of these studies have been previously published [8,10,11]. Briefly, TOWER was an open-label, randomized, controlled multicenter phase II trial, conducted at 54 German institutions. Patients with recurrent PROC and up to 2 previous platinum-based chemotherapies were randomly assigned to 2 different intravenous topotecan schedules. Weekly administration of 4.0 mg/m2/wk, applied on days 1, 8, and 15 of a 28-day cycle was compared to conventional administration of 1.25 mg/m2/day on 5 consecutive days of a 21-day cycle [10]. NOGGO-Treosulfan was designed as an open label, randomized, controlled, multicenter phase IIIb trial, conducted at 30 German institutions. Patients with recurrent ovarian cancer and at least 2 previous lines of chemotherapy were included and randomized to either treosulfan intravenous 7,000 mg/m2 day 1 every 4 weeks or treosulfan p.o. 600 mg/m2 days 1–28 every 8 weeks [11]. TRIAS was carried out as a multicenter, placebo-controlled, randomized, phase II trial at 20 German sites. Patients with PROC previously treated with up to 2 chemotherapy lines for recurrent disease were randomly assigned to topotecan (1.25 mg/m2 days 1–5) plus either oral sorafenib 400 mg or placebo twice daily on days 6–15, repeated every 21 days for 6 cycles, followed by daily maintenance sorafenib or placebo for up to 1 year in patients without progression [8]. All studies had been registered and approved by national and/or participating-institution independent ethics committees.
2. Statistical analysis
All patients included in this analysis had platinum-resistant recurrent disease defined as disease progression after a treatment-free interval of <6 months following the last platinum-based regimen. As previously described, the definition of PPR versus SPR was applied based on the response to previous anticancer treatments [9]. Patients with progression <6 months after completing first-line platinum-based chemotherapy were classified as PPR. In contrast, patients with progression ≥6 months after completing first-line platinum-based chemotherapy but <6 months after the last platinum-based chemotherapy were defined as SPR. Seven patients being classified as SPR according to platinum-free interval were excluded as they had received repeated non-platinum containing therapies prior to inclusion into the trial not sufficiently documented as relapses.
All exploratory analyses in this pooled cohort of prospective, randomized trials were based on the intention-to-treat populations. Calculations in the current study were pre-specified before being conducted although they had not been included in the study protocols due to the retrospective nature of this approach. Cox analyses were applied to correlate the time to development of platinum resistance with PFS and OS. In this context, PFS and OS were calculated from the time of randomization. Clinicopathological variables and treatment parameters were compared by applying an independent samples t-test or Mann-Whitney U test, and a χ2 test or Fisher's exact test, as appropriate. There were no adjustments for multiple testing. In accordance with the previous AURELIA analysis [12], cancer antigen 125 (CA125), ascites, and Eastern Cooperative Oncology Group (ECOG) performance status were selected for both multivariate analyses as they describe the current clinical situation the best together with age. To account for possible imbalance of the 3 trials in the pooled cohort, ‘study group’ was also added as variable. Size of target lesion was not reliably documented in TOWER, so that this item was not tested in the pooled cohort but was considered in the TRIAS cohort. In contrast to the first-line setting, FIGO stage and histology have not been identified as prognostic factors in platinum-resistant disease so that they were not included in the multivariate testing. CA125 were included as binary measures with a cut-off set at 60 years for age well as 100 U/mL for ascites to generate representative cohorts.
RESULTS
1. PPR and its impact on prognosis in patients undergoing mono-chemotherapy
A total of 477 patients with platinum-resistant disease could be included in the current analysis. Of these, 186 patients were retrieved from TOWER, 119 from NOGGO-Treosulfan, and 172 from TRIAS. All patient characteristics are displayed in Table 1.
Table 1. Patient baseline characteristics of patients from all 3 trials.
| Characteristics | PPR (n=264) | SPR (n=213) | p-value* | |
|---|---|---|---|---|
| Study in rows | <0.001 | |||
| TOWER | 135 (72.6) | 51 (27.4) | ||
| NOGGO-Treosulfan | 33 (27.7) | 86 (72.3) | ||
| TRIAS | 96 (55.8) | 76 (44.2) | ||
| Age at randomization (yr) | ||||
| Median (range) | 64 (25–84) | 67 (27–87) | 0.018 | |
| No. of relapses | <0.001 | |||
| 1 | 214 (81.1) | 0 (0.0) | ||
| 2 | 35 (13.3) | 131 (61.5) | ||
| 3 | 8 (3.0) | 51 (23.9) | ||
| 4 | 3 (1.1) | 14 (6.6) | ||
| >4 | 4 (1.5) | 17 (8.0) | ||
| FIGO stage | 0.165 | |||
| I | 3 (1.1) | 6 (2.8) | ||
| II | 8 (3.0) | 14 (6.6) | ||
| III | 177 (67.0) | 141 (66.2) | ||
| IV | 59 (22.3) | 37 (17.4) | ||
| Missing | 17 (6.4) | 15 (7.0) | ||
| Histologic subtype | 0.180 | |||
| Serous | 188 (71.2) | 140 (65.7) | ||
| Mucinous | 13 (4.9) | 17 (8.0) | ||
| Endometrioid | 13 (4.9) | 8 (3.8) | ||
| Others | 24 (9.1) | 15 (7.0) | ||
| Missing | 26 (9.8) | 33 (15.5) | ||
| ECOG performance status | 0.754 | |||
| 0 | 99 (37.5) | 79 (37.1) | ||
| 1 | 142 (53.8) | 113 (53.1) | ||
| 2 | 21 (8.0) | 17 (8.0) | ||
| Missing | 2 (0.8) | 4 (1.9) | ||
| Presence of ascites at study inclusion | 0.009 | |||
| Yes | 121 (46.9) | 71 (34.5) | ||
| None | 122 (47.3) | 112 (54.4) | ||
| Missing | 15 (5.8) | 23 (11.2) | ||
| CA125 levels | 0.124 | |||
| <100 U/mL | 63 (23.9) | 40 (18.8) | ||
| ≥100 U/mL | 172 (65.2) | 157 (73.7) | ||
| Missing | 29 (11.0) | 16 (7.5) | ||
| Size of target lesion (only NOGGO-Treosulfan, TRIAS) | 0.206 | |||
| Non measurable | 31 (24.0) | 25 (15.4) | ||
| <5 cm | 57 (44.2) | 70 (43.2) | ||
| ≥5 cm | 27 (20.9) | 45 (27.8) | ||
| Missing | 14 (10.9) | 22 (13.6) | ||
| Platinum free interval | 0.188 | |||
| <3 months | 52 (19.7) | 40 (18.8) | ||
| ≥3 months | 212 (80.3) | 170 (79.8) | ||
| Missing | 0 (0.0) | 3 (1.4) | ||
Baseline characteristics for patients from all 3 trials (TOWER, NOGGO-Treosulfan, TRIAS; n=477) comparing patients with PPR (55.3%) opposed to patients with SPR (44.7%). Data shown are number (%) not otherwise specified.
ECOG, Eastern Cooperative Oncology Group; FIGO, Fédération Internationale de Gynécologie et d'Obstétrique; PPR, primary platinum-resistant; SPR, secondary platinum-resistant.
*p-values excluding missing, significant p-values shown in bold.
Of the overall 477 patients, 264 (55.3%) were classified as PPR, compared to 213 (44.7%) with SPR. These numbers were significantly different between the trials (p<0.001), with PPR patients in 72.6% (TOWER), 27.7% (NOGGO-Treosulfan), and 55.8% (TRIAS). Among all PPR patients, 81.1% were included when experiencing their first relapse, while 18.9% of them had 2 or more prior lines of chemotherapy with a platinum-free interval already at first relapse according to the PPR definition (p<0.001). The majority of SPR patients (61.5%) was diagnosed with their second relapse when included into the trial, while 38.5% already had 2 or more relapses when they were randomized. Median age at randomization was 64 (range 25–84) years for PPR patients compared to 67 (range 27–87) years for patients with SPR (p=0.018). Patients with PPR had a higher proportion of ascites at study inclusion (46.9%) compared to SPR patients (34.5%, p=0.009). For all other baseline characteristics, no significant differences were seen (Table 1).
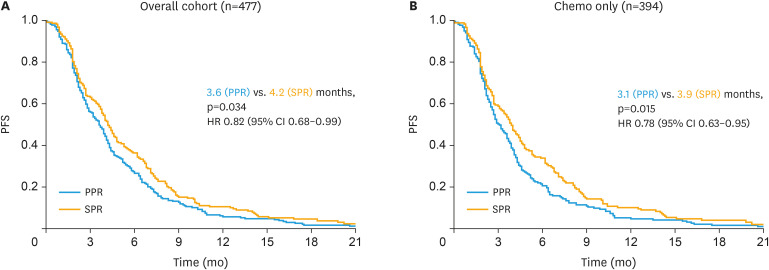
In the overall cohort (n=477), patients with SPR had a significantly improved PFS with a median of 4.2 months compared to 3.6 months in patients with PPR (HR=0.82; 95% CI=0.68–0.99; p=0.034; Fig. 1A). When excluding 83 patients who had received sorafenib as targeted therapy in the experimental arm of TRIAS to generate a chemotherapy only cohort (n=394), this effect was even more pronounced with a median PFS of 3.9 months compared to 3.1 months in this subgroup (HR=0.78; 95% CI=0.63–0.95; p=0.015; Fig. 1B). In separate consideration of the single trials, differences between SPR and PPR tend in the same direction in all trials but were non-significant for TOWER (3.8 vs. 2.9 months; p=0.940; Supplementary Fig. 1A) and NOGGO-Treosulfan (3.4 vs. 2.8 months; p=0.533, Supplementary Fig. 1B), while patients in the standard treatment arm of TRIAS exhibited a significant difference of 4.9 versus 3.8 months (p=0.012; Supplementary Fig. 1C). Same trends were noted for OS although these differences did not reach statistical significance (Supplementary Fig. 2A-E).
Fig. 1. PFS according to time to development of platinum resistance (PPR vs. SPR) in patients form all 3 trials. Kaplan-Meier curves regarding PFS for patients with PPR compared to SPR in the overall cohort from all 3 trials (A, n=477) and in the overall cohort excluding patients who received sorafenib within TRIAS (B, n=394).
CI, confidence interval; HR, hazard ratio; PFS, progression-free survival; PPR, primary platinum-resistant; SPR, secondary platinum-resistant.
Multivariate analysis for PFS in all patients excluding patients who received sorafenib within TRIAS confirmed a significantly independent prognostic impact of SPR versus PPR (HR=0.74; p=0.029; Table 2) while other suggested prognostic factors (assignment to the trial, CA125 at study inclusion, presence of ascites, ECOG) failed to reach statically significance. For OS, SPR versus PPR did not exhibit statistically significance (HR=0.85; p=0.235; Table 2) in contrast to for the presence of ascites, CA125 at study entry and ECOG.
Table 2. Multivariate analyses of prognostic factors for patients receiving chemotherapy only.
| Factors | PFS | OS | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | ||
| Platinum resistance (SPR vs. PPR) | 0.74 | 0.57–0.97 | 0.029 | 0.85 | 0.64–1.12 | 0.235 | |
| Study group vs. TRIAS | 0.804 | 0.481 | |||||
| TOWER | 1.08 | 0.81–1.44 | 0.84 | 0.62–1.13 | |||
| NOGGO | 0.97 | 0.67–1.42 | 0.95 | 0.65–1.39 | |||
| Age at randomization (≥60 vs. <60 years) | 1.01 | 0.77–1.32 | 0.964 | 1.02 | 0.76–1.35 | 0.910 | |
| CA-125 (≥100 vs. <100 IU/mL) | 1.27 | 0.97–1.66 | 0.082 | 1.34 | 1.02–1.77 | 0.037 | |
| Presence of ascites at study inclusion (yes vs. no) | 1.12 | 0.88–1.43 | 0.350 | 1.77 | 1.39–2.27 | <0.001 | |
| ECOG (1/2 vs. 0) | 1.10 | 0.85–1.43 | 0.465 | 1.40 | 1.08–1.83 | 0.012 | |
Investigation of the independent significance for prognostic factors regarding PFS and OS in the overall cohort excluding patients who received sorafenib within TRIAS (n=394).
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; PPR, primary platinum-resistant; SPR, secondary platinum-resistant.
2. Prognostic and predictive impact of PPR in patients receiving topotecan in combination and subsequent maintenance with sorafenib
In addition, the impact of PPR versus SPR on prognosis was further investigated in the overall cohort of the TRIAS trial (n=172, Supplementary Table 1).
Here, 96 patients (55.8%) were identified with PPR, opposed to 76 patients (44.2%) with SPR. In addition to the expected difference in terms of numbers of relapses (PPR with 75% first relapse compared to 100% of second or third relapse in SPR, p<0.001) due to the definition of platinum resistance, proportion of patients with a target lesion size ≥5 cm was significantly higher in SPR patients with 34.2% compared to 18.8% in PPR (p=0.042, Supplementary Table 1).
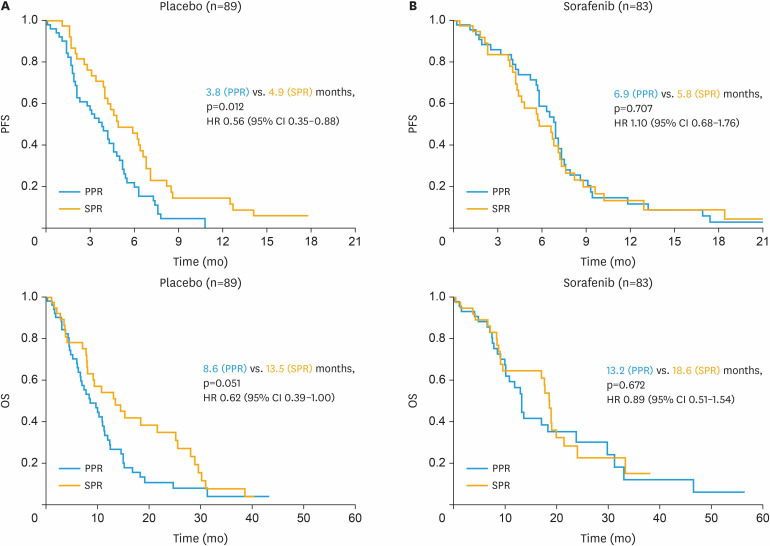
Contrarily to the already described effect in patients of the placebo arm (n=89) with a significantly improved PFS of 4.9 months for SPR versus 3.8 months for PPR patients (HR=0.56; 95% CI=0.35–0.88; p=0.012; Fig. 2A), this prognostic impact could not be seen in patients receiving sorafenib (n=83) with 5.8 (SPR) versus 6.9 (PPR) months (HR=1.10; 95% CI=0.68–1.76; p=0.707; Fig. 2B). Similar effects were noted for OS with 13.5 months compared to 8.6 months for SPR versus PPR patients receiving placebo (HR=0.62; 95% CI=0.39–1.00; p=0.051) and no significant difference in the sorafenib arm with 18.6 versus 13.2 months (HR=0.89; 95% CI=0.51–1.54; p=0.672).
Fig. 2. PFS and OS according to time to development of platinum resistance (PPR vs. SPR) in the TRIAS cohort (n=172). Kaplan-Meier curves comparing PPR with SPR regarding PFS and OS in patients receiving placebo plus chemotherapy (A) and sorafenib plus chemotherapy (B).
CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; PPR, primary platinum-resistant; SPR, secondary platinum-resistant.
In multivariate analysis for PFS in the placebo cohort, PPR versus SPR was identified as an independent, statistically significant prognostic marker (HR=0.56; p=0.028) as it was for ascites (HR=1.69; p=0.036) in contrast to the other investigated factors age, CA125, ECOG, and the presence of measurable disease (Table 3). For patients receiving sorafenib, PPR versus SPR failed to exhibit statistically prognostic significance (HR=0.79; p=0.418) while other markers as presence of ascites (HR=2.37; p=0.009), and measurable disease (HR=2.48; p=0.020), were confirmed as independent prognostic markers (Table 3).
Table 3. Multivariate analyses of prognostic factors within the TRIAS cohort.
| Factors | Placebo | Sorafenib | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Platinum resistance (SPR vs. PPR) | 0.56 | 0.34–0.94 | 0.028 | 0.79 | 0.45–1.39 | 0.418 |
| Age at randomization (≥60 vs. <60 years) | 0.76 | 0.48–1.19 | 0.230 | 1.20 | 0.71–2.01 | 0.501 |
| CA-125 (≥100 vs. <100 IU/mL) | 0.99 | 0.59–1.65 | 0.970 | 1.73 | 0.91–3.31 | 0.096 |
| Presence of ascites at study inclusion (yes vs. no) | 1.69 | 1.03–2.75 | 0.036 | 2.37 | 1.24–4.54 | 0.009 |
| ECOG (1/2 vs. 0) | 1.56 | 0.95–2.55 | 0.078 | 0.58 | 0.33–1.00 | 0.050 |
| Measurable disease (yes vs. no) | 1.11 | 0.63–1.95 | 0.712 | 2.48 | 1.15–5.33 | 0.020 |
Investigation of prognostic factors for patients receiving Placebo (A) and Sorafenib (B) regarding their independent significance for progression-free survival in the TRIAS cohort (n=172).
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; PPR, primary platinum-resistant; SPR, secondary platinum-resistant.
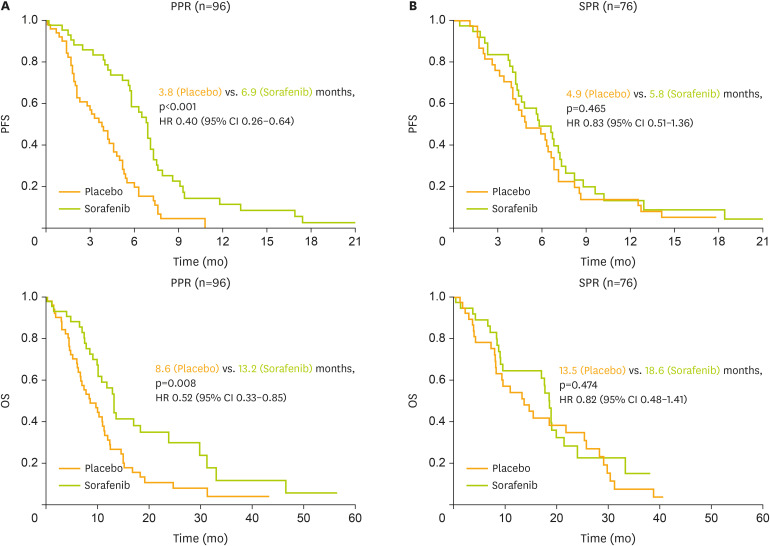
Predictive effects according to randomized treatment on outcome were compared within the SPR and PPR subgroups. While sorafenib did not have a significant impact on PFS in SPR patients with 5.8 months for sorafenib treated patients compared to 4.9 months in patients receiving placebo (HR=0.83; 95% CI=0.51–1.36; p=0.465; Fig. 3B), the PFS gain by adding sorafenib was statistically significant in PPR patients with 6.9 months compared to 3.8 months (HR=0.40; 95% CI=0.26–0.64; p<0.001, Fig. 3A). A similar direction of effect was noted regarding OS with no significant difference in SPR patients (18.6 vs. 13.5 months, HR=0.82; 95% CI=0.48–1.41; p=0.474; Fig. 3B) but a confirmed statistically significant impact on patients with PPR estimated at 13.2 months for sorafenib receiving patients in contrast to 8.6 months for patients receiving placebo (HR=0.52; 95% CI=0.33–0.85; p=0.008; Fig. 3A).
Fig. 3. PFS and OS according to treatment in the PPR and SPR subgroups of the TRIAS cohort (n=172).
Kaplan-Meier curves comparing treatment (placebo plus chemotherapy vs sorafenib plus chemotherapy) regarding PFS and OS in PPR patients (A) and in SPR patients (B).
CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; PPR, primary platinum-resistant; SPR, secondary platinum-resistant.
3. Toxicity profiles of patients with PPR compared to SPR
Focusing on the 5 adverse events (AEs) with highest frequency within each of the 3 prospective trials resulted in a list of 7 AEs (hematological events, gastrointestinal events, fatigue, alopecia, pain, infection, and dyspnea) being investigated for differences based on the timing when platinum resistance developed. For the 394 patients undergoing mono chemotherapy, similar frequencies for the different AEs were noted for both subgroups (Supplementary Fig. 3A). For patients receiving sorafenib within TRIAS, the most common AEs (hematological events, gastrointestinal events, fatigue, alopecia, and pain), were differentially expressed between the subgroups. Higher rates for alopecia (63.2% vs. 48.9%) and pain (47.4% vs. 26.7%) were noted in SPR patients when focusing on AE of all grades. Of note, grade III fatigue was almost doubled in patients with SPR compared to PPR (15.8% vs. 8.9%), while the other 4 of the 5 most frequent AEs did not exhibit significant differences (Supplementary Fig. 3B).
DISCUSSION
Patients with platinum-resistant disease are still the group of patients with the highest need for therapeutic improvements in ovarian cancer care regarding symptom control and quality of life aspects. While the timeframe in which platinum resistance develops has previously been identified as an important prognostic factor for patients undergoing anti-angiogenic treatment [9], this present meta-analysis elucidates the negative prognostic impact of PPR compared to SPR for patients undergoing mono-chemotherapy in 3 prospective trials and confirms its significance for patients in the TRIAS trial which had been investigating the multi-kinase inhibitor sorafenib.
Within this pooled analysis of 477 patients with PROC, patients receiving chemotherapy only were identified to have a significantly shorter PFS with 3.1 months in case of PPR disease compared to 3.9 months for SPR patients. OS data tends into the same direction although this difference was not statistically significant. In multivariate analyses, prognostic impact was confirmed to be statistically independent with regard to PFS. For patients receiving chemotherapy alone, this information is new and clinically relevant as it complements the traditional classification based on the platinum-free interval and is taking information on treatment responses over the clinical course into account. In the difficult treatment situation of platinum resistance, patients need to be counseled with all information available. PPR patients have the highest need for effective therapies so that innovative biologic approaches and genomic sequencing results in these cases might be necessary to support clinical decision making [13,14]. On the other hand, this information could, however, help physicians to find justifications for reducing treatment burdens towards best palliative care at some points [2]. Therefore, prospective trials in patients with platinum resistance should use PPR and SPR at least as stratification factor.
When the definition of PPR versus SPR was first implemented in the previous analysis of the AURELIA trial, it revealed a prognostic impact for patients with chemotherapy and bevacizumab but not for patients with chemotherapy alone. The missing significance in the AURELIA chemotherapy only cohort might have been diminished by low number of patients, imbalances between the standard chemotherapy regimens, and a potentially higher rate of symptomatic patients (reflected by presence of ascites in 35% PPR versus 21% SPR patients). Paclitaxel, the therapeutic agent known to have the best therapeutic index in PROC patients was more frequently chosen as chemotherapy backbone in 40% of SPR patients compared to 32% of the PPR patients [9,15]. In contrast to AURELIA, the present analysis overcomes this limitation with a more homogenously treated cohort as the randomization in 2 trials (TOWER, NOGGO-Treosulfan) was focused on treatments schedules as well as on application forms but not on different drugs, and in TRIAS topotecan was the exclusive chemotherapy backbone.
On first sight, the results focusing on the TRIAS cohorts appear to be in conflict with the previously reported results from AURELIA. Within TRIAS, the difference of PPR versus SPR is evident in patients of the placebo arm receiving single topotecan but diminished following treatment with sorafenib. While the magnitude from adding bevacizumab in AURELIA was more pronounced in SPR patients, the effect of sorafenib in TRIAS was almost exclusively seen in PPR patients with impressive improvements for PFS (HR=0.40; p<0.001) and for OS (HR=0.52; p=0.008) but not significant in SPR patients. Lower rates for pain and fatigue in PPR compared to SPR patients could eventually be considered an expression of better efficacy of sorafenib in the PPR subgroup. Therefore, sorafenib seems to diminish the prognostically negative constellation of PPR patients in the placebo cohort, so that Kaplan-Meier (KM) curves approach within the subgroup of sorafenib treated patients. Accordingly, ‘platinum resistance’ was identified as an independent prognostic factor in the placebo cohort (HR=0.56; p=0.028) but not in the sorafenib cohort (HR=0.79; p=0.418) following the presumed equalizing therapeutic effect of sorafenib in multivariate analyses. The differential effectiveness between bevacizumab and sorafenib with regard to PPR and SPR subgroups might be explained in their different biologic target structures. While anti-angiogenic bevacizumab specifically addresses the vascular endothelial growth factor receptor (VEGFR) [16], sorafenib as a multikinase inhibitor focuses on several signal cascades and therefore might have a better potential to overcome the main problem in PPR patients who never had responded to a chemotherapy [17]. In addition to further anti-angiogenic targets with CRAF, VEGFR-2, VEGFR-3, and PDGFR-β, sorafenib also addresses specific targets in the tumor cells (CRAF, BRAF, c-KIT, and FLT-3) [17]. Future analyses with targeted therapies are warranted to define their therapeutic potential and the role discriminate between PPR and SPR in this setting.
Deeper biologic understanding of the mechanisms could guide treatment decisions in these patients, especially as re-challenge of anti-angiogenic treatments has been shown to be feasible and effective [18]. In different cancer subtypes, evaluation of specific mutations or genetic alterations has already been established to identify subgroups of patients being more likely to respond to specific targeted therapies. Examples for these biomarkers include BRAF mutations in colon cancer for multi-targeting MAPK signaling, BRCA mutations in ovarian cancer for PARP inhibitors, and MSI high for the PD1 inhibitor pembrolizumab in endometrial cancer [19,20,21] which could also be a promising perspective for PPR as a clinical marker. Evaluation of toxicity data did not reveal relevant differences between the subgroups for patients receiving chemotherapy only, although notably higher rates of pain in general and grade III/IV fatigue were noted in SPR patients receiving sorafenib. These differences might be an additional surrogate marker for the pronounced efficacy of sorafenib in PPR patients with better symptom control and less AEs compared to SPR.
Despite several strengths of this meta-analyses with a high number of included patients and first description of toxicity data, some drawbacks need to be considered. Although the homogeneity of the applied treatments facilitates the interpretation of the current data, some widely accepted agents (paclitaxel, PLD) for PROC have not been investigated in the 3 trials. In this context, consideration of single trials can be misleading due to low numbers of evaluable patients so that focusing on larger trial cohorts or broader meta-analyses is warranted. In addition, biologic information accompanying clinical characteristics is urgently needed which was not feasible in AURELIA as well as in the present analysis where no patient specimens are available for translational research. Collection of biosamples should be an indispensable requirement for execution of clinical trials in the future, even if this implies more logistics for collection of blood samples or an additional biopsy in this cohort of patients. Recently presented data regarding immunotherapies in PROC has impressively demonstrated how important evaluation of biomarkers can be for the interpretation of clinical results [22].
Although sorafenib has not yet been developed for further indications in ovarian cancer, the described specific potential of overcoming PPR opens important perspectives for future applications and focused translational approaches. As elucidated in this analysis, the timing when platinum resistance develops has not only impact for patients with anti-angiogenic therapies but also for patients envisaged for chemotherapy only. Therefore, consideration of PPR should become routine in clinical assessment of ovarian cancer patients with recurrent disease. Investigation of translational aspects being responsible for this clinical characteristic could further support the acceptance in clinical care.
Footnotes
Conflict of Interest: Fabian Trillsch: grants and personal fees from AstraZeneca, Clovis, Medac, MSD, PharmaMar, Roche, and Tesaro/GSK. Sven Mahner: grants and personal fees from AstraZeneca, Clovis, Medac, Novartis, Olympus Europe, PharmaMar, Pfizer, Roche, Sensor Kinesis, Tesaro/GSK, and TEVA. Pauline Wimberger: grants and personal fees from AstraZeneca, Amgen, Clovis, Medac, MSD, Pfizer, PharmaMar, Roche, Tesaro/GSK, Novartis, Eisai, Celgene and TEVA. Jalid Sehouli: grants and personal fees from Astra Zeneca, Bayer, Eisai, Clovis, Olympus, Johnsons and Johnson, PharmaMar, Pfizer, TEVA, Tesaro/GSK, MSD, Lilly, Roche and Merck. All remaining authors have declared no conflicts of interest.
- Conceptualization: T.F., M.S., S.J.
- Formal analysis: T.F., M.S., C.B., R.M.
- Investigation: C.R., B.E.I., O.Ö.G., W.P., R.R.
- Project administration: T.F., M.S., S.J.
- Software: R.M.
- Writing - original draft: T.F., M.S., S.J.
- Writing - review & editing: C.B., R.M., C.R., B.E.I., O.Ö.G., W.P., R.R.
SUPPLEMENTARY MATERIALS
Patient baseline characteristics of patients from TRIAS (n=172)
PFS according to time to development of platinum resistance (PPR vs. SPR) in patients form all 3 trials. Kaplan-Meier curves regarding PFS for patients with PPR compared to SPR in the TOWER cohort (A, n=189), in the NOGGO-Treosulfan cohort (B, n=119), and in the TRIAS cohort excluding patients with sorafenib (C, n=89).
OS according to time to development of platinum resistance (PPR vs. SPR) in in patients form all 3 trials. Kaplan-Meier curves regarding OS for patients with PPR compared to SPR in the overall cohort from all 3 trials (A, n=477), in the overall cohort excluding patients who received sorafenib within TRIAS (B, n=394), in the TOWER cohort (C, n=189), in the NOGGO-Treosulfan cohort (D, n=119), and in the TRIAS cohort excluding patients with sorafenib (E, n=89).
Toxicity profiles of patients with PPR compared to SPR.
References
- 1.Wilson MK, Pujade-Lauraine E, Aoki D, Mirza MR, Lorusso D, Oza AM, et al. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: recurrent disease. Ann Oncol. 2017;28:727–732. doi: 10.1093/annonc/mdw663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colombo N, Sessa C, Bois AD, Ledermann J, McCluggage WG, McNeish I, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Int J Gynecol Cancer. 2019 doi: 10.1136/ijgc-2019-000308. [DOI] [PubMed] [Google Scholar]
- 3.Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME, Lacave AJ. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol. 2001;19:3312–3322. doi: 10.1200/JCO.2001.19.14.3312. [DOI] [PubMed] [Google Scholar]
- 4.Mutch DG, Orlando M, Goss T, Teneriello MG, Gordon AN, McMeekin SD, et al. Randomized phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2007;25:2811–2818. doi: 10.1200/JCO.2006.09.6735. [DOI] [PubMed] [Google Scholar]
- 5.ten Bokkel Huinink W, Gore M, Carmichael J, Gordon A, Malfetano J, Hudson I, et al. Topotecan versus paclitaxel for the treatment of recurrent epithelial ovarian cancer. J Clin Oncol. 1997;15:2183–2193. doi: 10.1200/JCO.1997.15.6.2183. [DOI] [PubMed] [Google Scholar]
- 6.Reed NS, Poole CJ, Coleman R, Parkin D, Graham JD, Kaye SB, et al. A randomised comparison of treosulfan and carboplatin in patients with ovarian cancer: a study by the Scottish Gynaecological Cancer Trials Group (SGCTG) Eur J Cancer. 2006;42:179–185. doi: 10.1016/j.ejca.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 7.Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32:1302–1308. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 8.Chekerov R, Hilpert F, Mahner S, El-Balat A, Harter P, De Gregorio N, et al. Sorafenib plus topotecan versus placebo plus topotecan for platinum-resistant ovarian cancer (TRIAS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018;19:1247–1258. doi: 10.1016/S1470-2045(18)30372-3. [DOI] [PubMed] [Google Scholar]
- 9.Trillsch F, Mahner S, Hilpert F, Davies L, García-Martínez E, Kristensen G, et al. Prognostic and predictive effects of primary versus secondary platinum resistance for bevacizumab treatment for platinum-resistant ovarian cancer in the AURELIA trial. Ann Oncol. 2016;27:1733–1739. doi: 10.1093/annonc/mdw236. [DOI] [PubMed] [Google Scholar]
- 10.Sehouli J, Stengel D, Harter P, Kurzeder C, Belau A, Bogenrieder T, et al. Topotecan weekly versus conventional 5-day schedule in patients with platinum-resistant ovarian cancer: a randomized multicenter phase II trial of the North-Eastern German Society of Gynecological Oncology Ovarian Cancer Study Group. J Clin Oncol. 2011;29:242–248. doi: 10.1200/JCO.2009.27.8911. [DOI] [PubMed] [Google Scholar]
- 11.Sehouli J, Tomè O, Dimitrova D, Camara O, Runnebaum IB, Tessen HW, et al. A phase III, open label, randomized multicenter controlled trial of oral versus intravenous treosulfan in heavily pretreated recurrent ovarian cancer: a study of the North-Eastern German Society of Gynecological Oncology (NOGGO) J Cancer Res Clin Oncol. 2017;143:541–550. doi: 10.1007/s00432-016-2307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trillsch F, Mahner S, Hilpert F, Davies L, García-Martínez E, Kristensen G, et al. Prognostic and predictive effects of primary versus secondary platinum resistance for bevacizumab treatment for platinum-resistant ovarian cancer in the AURELIA trial. Ann Oncol. 2016;27:1733–1739. doi: 10.1093/annonc/mdw236. [DOI] [PubMed] [Google Scholar]
- 13.Sultova E, Westphalen CB, Jung A, Kumbrink J, Kirchner T, Mayr D, et al. NGS-guided precision oncology in metastatic breast and gynecological cancer: first experiences at the CCC Munich LMU. Arch Gynecol Obstet. 2020 doi: 10.1007/s00404-020-05881-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massard C, Michiels S, Ferté C, Le Deley MC, Lacroix L, Hollebecque A, et al. High-throughput genomics and clinical outcome in hard-to-treat advanced cancers: results of the MOSCATO 01 trial. Cancer Discov. 2017;7:586–595. doi: 10.1158/2159-8290.CD-16-1396. [DOI] [PubMed] [Google Scholar]
- 15.Poveda AM, Selle F, Hilpert F, Reuss A, Savarese A, Vergote I, et al. Bevacizumab combined with weekly paclitaxel, pegylated liposomal doxorubicin, or topotecan in platinum-resistant recurrent ovarian cancer: analysis by chemotherapy cohort of the randomized phase III AURELIA trial. J Clin Oncol. 2015;33:3836–3838. doi: 10.1200/JCO.2015.63.1408. [DOI] [PubMed] [Google Scholar]
- 16.Presta LG, Chen H, O'Connor SJ, Chisholm V, Meng YG, Krummen L, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–4599. [PubMed] [Google Scholar]
- 17.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 18.Pfisterer J, Shannon CM, Baumann K, Rau J, Harter P, Joly F, et al. Bevacizumab and platinum-based combinations for recurrent ovarian cancer: a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21:699–709. doi: 10.1016/S1470-2045(20)30142-X. [DOI] [PubMed] [Google Scholar]
- 19.Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154–2164. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 20.Caputo F, Santini C, Bardasi C, Cerma K, Casadei-Gardini A, Spallanzani A, et al. BRAF-mutated colorectal cancer: clinical and molecular insights. Int J Mol Sci. 2019;20:5369. doi: 10.3390/ijms20215369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pujade-Lauraine E, Fujiwara K, Dychter SS, Devgan G, Monk BJ. Avelumab (anti-PD-L1) in platinum-resistant/refractory ovarian cancer: JAVELIN Ovarian 200 Phase III study design. Future Oncol. 2018;14:2103–2113. doi: 10.2217/fon-2018-0070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient baseline characteristics of patients from TRIAS (n=172)
PFS according to time to development of platinum resistance (PPR vs. SPR) in patients form all 3 trials. Kaplan-Meier curves regarding PFS for patients with PPR compared to SPR in the TOWER cohort (A, n=189), in the NOGGO-Treosulfan cohort (B, n=119), and in the TRIAS cohort excluding patients with sorafenib (C, n=89).
OS according to time to development of platinum resistance (PPR vs. SPR) in in patients form all 3 trials. Kaplan-Meier curves regarding OS for patients with PPR compared to SPR in the overall cohort from all 3 trials (A, n=477), in the overall cohort excluding patients who received sorafenib within TRIAS (B, n=394), in the TOWER cohort (C, n=189), in the NOGGO-Treosulfan cohort (D, n=119), and in the TRIAS cohort excluding patients with sorafenib (E, n=89).
Toxicity profiles of patients with PPR compared to SPR.