Abstract
Introduction
Europe was the epicentre of the COVID-19 pandemic in March 2020, with the highest number of cases and deaths between March and April. In May, the infection numbers registered a fall followed by a second new rise, not proportionally reflected by an increase in the number of deaths. We aimed to investigate the relationship between disease prevalence and infection fatality rate (IFR), and the number of intensive care unit (ICU) and hospital admissions over time, to develop a predictive model, as well as appraising the potential contributing factors underpinning this complex relationship.
Methods
A prospective epidemiological study using data from six countries collected between 10 March and 4 September 2020. Data on the number of daily hospital and ICU admissions with COVID-19 were gathered, and the IFR and the prevalence were calculated. Trends over time were analysed. A linear regression model was used to determine the association between the fatality rates and the number of admissions.
Findings
The prediction model confirmed the linear association between the fatality rates and the numbers of ICU and hospital admissions. The exception was during the peak of the COVID-19 pandemic when the model underestimated the fatalities indicating that a substantial number of deaths occurred outside of the hospitals. The fatality rates decreased in all countries from May until September regardless of the trends in prevalence, differences in healthcare systems or strategic variations in handling the pandemic.
Interpretation
The observed gradual reduction in COVID-19 fatality rates over time despite varying disease prevalence and public health measures across multiple countries warrants search for a biological explanation. While our understanding of this novel virus grows, hospital and ICU admission rates remain effective predictors of patient outcomes which can be used as early warning signs for escalation of public health measures.
Keywords: epidemiology, COVID-19, international health services, public health, infection control
Strengths and limitations of this study.
Comprehensive data on mortality, hospital and intensive care unit admissions were gathered from six countries from March to September 2020 on a daily basis.
Our data were verified from multiple sources for each country to ensure accuracy and consistency.
The analysis was adjusted for the number of COVID-19 tests performed to remove the confounding influence of variations in test numbers over time and between countries.
Different countries use different testing technology which may have different diagnostic accuracy.
There were variations in reporting between countries especially when multiple tests were done on the same individual.
Introduction
COVID-19 was first reported in Wuhan (Hubei province, China) on 31 December 2019 and has emerged as a new zoonotic infectious disease, leading the WHO to declare, in early March, a global health emergency.1 The SARS-CoV-2, which is similar to other previously described coronaviruses, that is, SARS-CoV-1 and Middle East respiratory syndrome coronavirus (MERS-CoV), was identified as the pathogenic agent of COVID-19.1 Initial studies have shown the SARS-CoV-2 to have higher transmissibility, but lower pathogenicity than that of SARS-CoV-1 and MERS-CoV.1 2 About 81% of the COVID-19 symptomatic patients develop mild symptoms, such as headache, dry cough and fatigue. However, more severe cases can develop respiratory distress, sepsis, severe neurologic symptoms and multiorgan failure.2 On 13 March 2020, the WHO declared Europe the epicentre of the pandemic with more reported cases and deaths than the rest of the world combined, apart from China. In Europe, a record number of new cases and deaths caused by COVID-19 occurred between March and beginning of April. This urged most of the European countries to adopt national lockdown measures in March, with the highest stringency levels worldwide.1 The number of new cases and deaths consequently registered a fall, although by the end of May the distribution of new cases began to rise again. However, the trend in deaths continued downwards, indicating that the increase in cases was not leading to proportional increased mortality.2
To better understand these divergent trends, we analysed the data from five of the most severely affected European countries (Spain, Italy, France, Germany and the UK). Additionally, we studied data from the USA given the impact of COVID-19 on this country and its significantly different healthcare system from those in Europe. Using the data available, we estimated and compared the distribution of the infection fatality rates (IFRs) over time and the prevalence for each country. We included in our study the numbers of intensive care unit (ICU) and hospital admissions and developed a predictive model for outcomes using these two parameters. We also discussed the potential explanations for the observed trends.
Methods
Search strategy
Data on COVID-19 for each country were acquired from the Statistics and Research Coronavirus Pandemic section on Our World in Data website3 as the first step. All data were then further verified with the official publicly available sources: for Spain, from the Spanish Ministry of Health daily reports4 and the Science and Innovation Institute Carlos III,5 which made available datasets for public use about the number of tests and both hospital and ICU admission numbers; for Italy, from the Italian Ministry of Health,6 with detailed datasets published by the Presidency of the Council of Ministers—Department of Civil Protection7; for Germany, from data published in the daily epidemiological bulletin from the Robert Koch Institute8; for France, from datasets accessed from the French Public Health website9; for the UK, from datasets from the official governmental website10, and for the USA, from the Centre of Disease Control and Prevention COVID-19 Data Tracker website and US Department of Health and Human Services. Where contradictory information was found for a given variable, ministry of health or official data were given priority over other sources.
The process for COVID-19 case reporting underwent continuous change, and case notifications developed into more standardised procedures from May, when surveillance platforms, such as SiViES in Spain, NHS Test and Trace in the UK, SI-DEP in France and the internationally adopted contact tracing measures, were implemented. Consistent data were available from 10 March and were collected from this date until 4 September 2020. For most countries, the number of tests refers to the number of reverse transcriptase polymerase chain reaction (RT-PCR) tests performed. The RT-PCR is widely used as the reference standard for the diagnosis of COVID-19. The WHO published its first guidance on laboratory testing on 17 January11 and further released a more comprehensive document on 19 March.12
Serological tests have also been used as an alternative or complement to RT-PCR in the diagnosis of acute infection. In some countries such as the USA, serological tests have also been included in the total number of tests,13 while others have reported their results separately as in the UK.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Data variables
The variables included in our data analysis were the number of COVID-19 cases (new and cumulative), the number of deaths (new and cumulative), the number of tests (per day and cumulative), the daily number of confirmed COVID-19 hospitalised individuals and the daily number of individuals admitted in the ICU diagnosed with COVID-19. The data collected were homogenous for each country except for Spain, where the numbers displayed for ICU and hospital admissions were cumulative values; therefore, the analysis was performed without the linear regression.
The number of daily tests included in our calculations represents the tests that were reported during that day. Delays in case notification were up to 9 days,14 and retrospective corrections were conducted regularly in all countries and amended in the subsequent epidemiological bulletins.14–16 The approach for reporting multiple tests done on the same individual was not uniform for all countries, and detailed information on how this was addressed was inconsistent; when available, the algorithm consisted of first positive or negative RT-PCR test being declared if there were similar results, and the first positive test declared if the results were contradictory.14 As a result, overestimation of the number of individuals that were tested in each country can vary.
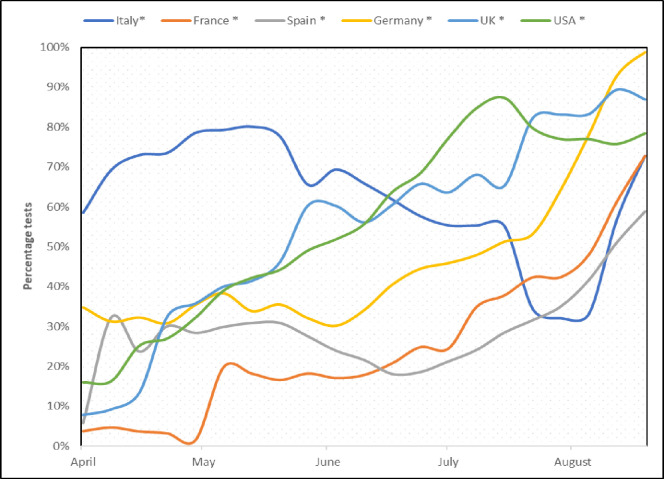
Worldwide testing capacity has improved with time and this was reflected in the daily number of tests performed. We estimated the prevalence as a proportion of positive individuals from the total tested, and this was adjusted for the number of tests, as a correction for testing fluctuations (figure 1). Among the measures used to assess the proportion of individuals with fatal outcomes, IFR was preferred over the case fatality rate and was calculated as the proportion of new deaths from the disease out of the estimated number of infected individuals, based on WHO definition17:
Figure 1.
Monthly test rate changes for all countries, expressed in percentages from the maximum recorded value.
Multiple methods have been described for the calculation of the IFRs; some studies have included the RT-PCR positive tests, while others have used the seroprevalence results. A systematic review of the published data on IFRs concluded that there was a high heterogeneity among the estimates of IFRs, the calculation of which remains a challenging task.18 Also, estimates made on seroprevalence surveys are likely to deliver slightly lower fatality rates when compared with those that are inferred from other forms of testing.18 We have based our calculations on the number of RT-PCR tests given the more consistent availability of these data across the countries studied.
Statistical analysis
Data analysis was carried out using IBM SPSS. Parametric tests were applied, and Pearson’s correlation was calculated to determine the strength of the association between the IFR and the number of ICU and hospital admissions. The three parameters were examined using a multivariate linear regression, and an IFR prediction model was developed based on the results. Sample size was considered adequate to support the regression. A stepwise model was built for each country, with the regression equation calculated based on the results:
Infection Fatality Rate=intercept+(b1×X)+(b2×Y)
Where the analysis revealed better estimates for univariate regression, the best predictor was included in the model. If a bivariate regression was calculated, the model was examined for collinearity. The strength of the association in the model was assessed by calculating the effect size using Cohen’s f. The linear regression was not validated in order to preserve the sample size. The epidemic curves including the course estimate of the IFR (observed and mean of predicted) and prevalence were plotted, and demographic characteristics were summarised for each country.
A final analysis of data heterogeneity has been performed using the method proposed by Wang et al 19 for the determination of spatial stratified heterogeneity (q) and its probability density function (F). The q statistic has been used as a tool for the assessment of the within and between countries heterogeneity. Data for each variable have been compared among the six countries during three consecutive periods corresponding to equally distributed time intervals from March until September. The variables included in the analysis were the numbers of daily new tests and deaths, the ICU and hospital admissions, and the IFR and prevalence.
Results
We developed the regression models based on the estimated values of the IFR and the prevalence. The fatality rate and the regression mean curves are displayed in figures 2 and 3 and their trends compared with the estimated prevalence.
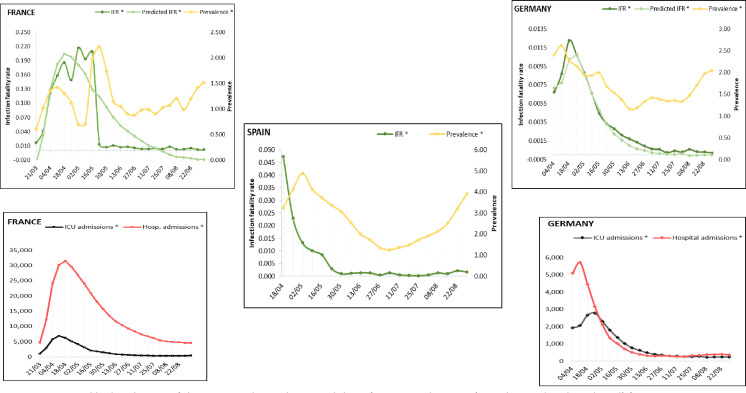
Figure 2.
Weekly distribution of the estimated prevalence and the infection fatality rate (IFR, observed and predicted) for Germany, France and Spain. Weekly distribution of intensive care unit (ICU) and hospital admissions for Germany and France.
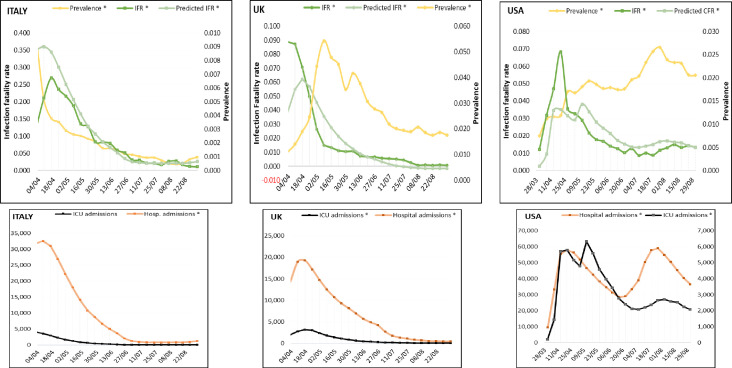
Figure 3.
Weekly distribution of prevalence and infection fatality rate (IFR, observed and predicted) for Italy, the UK and the USA. Weekly distribution of intensive care unit (ICU) and hospital admissions in the same order.
The analysis for Germany showed a strong positive association with IFR for both ICU admissions (r(157)=0.912, p<0.001) and hospital admissions (r(154)=0.771, p<0.001). The number of ICU admissions was included as best predictor, and the regression showed the highest value for the determination coefficient (R2=0.830) with the univariate model. Table 1 summarises the descriptive statistics and analysis results. The high effect size (f=1.7) validates the linear association between the two variables. The strong prediction model results in the overlapping of the fatality rate curves during the entire time frame (figure 2).
Table 1.
Linear regression analysis for each country, with results of the regression for ICU and/or hospital admissions.
| Germany | ||||
| Predictor | b (95% CI) | SE b | β | P value |
| Intercept | −0.001 (−0.001 to 222.0E−6) | 197.0E−6 | <0.05 | |
| ICU admissions | 4.23E−06 (4.0E−6 to 5.0E−6) | 1.5419E−07 | 0.911 | <0.001 |
| France | ||||
| Predictor | b (95% CI) | SE b | β | P value |
| Intercept | −0.053 (−0.094 to –0.011) | 0.021 | <0.05 | |
| Hospital admissions | 8.41E−06 (6.0E−6 to 11.0E−6) | 1.0E−6 | 0.452 | <0.001 |
| Italy | ||||
| Predictor | b (95% CI) | SE b | β | P value |
| Intercept | 424.0E−6 (90.0E−6 to 758.0E−6) | 169.0E−6 | <0.05 | |
| ICU admissions | −2.27E−06 (−3.0E−6 to –1.0E−6) | 4.81E−07 | −1.145 | <0.001 |
| Hospital admissions | 4.13E−07 (3.079E−7 to 5.1834E−7) | 5.33E−08 | 1.886 | <0.001 |
| UK | ||||
| Predictor | b (95% CI) | SE b | β | P value |
| Intercept | −0.005 (−0.008 to –0.002) | 0.002 | <0.05 | |
| Hospital admissions | 3.28E−06 (3.0E−6 to 4.0E−6) | 1.75E−07 | 0.834 | <0.001 |
| USA | ||||
| Predictor | b (95% CI) | SE b | β | P value |
| Intercept | 459.0E−6 (−0.001 to 0.002) | 0.001 | 0.616 | |
| ICU admissions | 2.19E−06 (2.0E−6 to 3.0E−6) | 2.49E−07 | 0.572 | <0.001 |
Note: Germany, R2=0.830; France, R2=0.205; Italy, R2=0.634; the UK, R2=0.696 and the USA, R2=0.327.
The results were included in the prediction model equation of the IFR.
β, standardised beta coefficient; b, unstandardised beta coefficient; ICU, intensive care unit; SE b, standard error for b.
For France, a moderate association was found between the IFR and the ICU admissions (r(169)=0.400, p<0.001) as well as the hospital admissions (r(169)=0.452, p<0.001). The correlation coefficients accounted for a medium but statistically significant effect size. The number of hospital admissions was the best predictor (R2=0.205, f=0.5) (table 1). When plotted, the modest prediction strength of the number of the hospital admissions in France was more evident from 16 May and explained the gap between the rapid decrease of the IFR within a short interval and the gradual normalisation of both ICU and hospital admissions (figure 2).
Data from Italy showed a strong association between the IFR and ICU (r(159)=0.703, p<0.001) and the hospital admissions (r(159)=0.763, p<0.001). The bivariate regression showed the highest determination coefficient (R2=0.634, f=1.3), and both variables were included in the equation (table 1). Except for the interval between 4 April and 2 May, corresponding with the peak of the ICU and hospital admissions, all parameters decreased at comparable rates, consistent with the prediction of the model (figure 3).
Analogous results were found in the UK, with significant correlation of both ICU(r(154)=0.843, p<0.001) and hospital admissions (r(154)=0.834, p<0.001) with IFR. The number of hospital admissions was included in the model and the regression found a good predictive strength (R2=0.696) and a high effect size (f=1.4) (table 1). When compared with the observed values, the regression underestimated the IFR until 20 April, although the interval corresponded to the period with the highest number of hospital admissions, after which the curves diverged again, as the fatality rates dropped faster than the number of hospitalised individuals.
In the USA, a moderate but significant association was found for the ICU admissions (r(160)=0.572, p<0.001) and a modest one with the hospital admissions (r(160)=0.333, p<0.001) and the IFR. The number of ICU admissions was included in the regression, but the strength of the prediction model was relatively low with a moderate effect size (R2=0.327, f=0.7) (table 1). The intercept contribution to the model was not significant and was excluded from the equation. Notably, the hospital admissions curve revealed a second peak in August that was not reflected in a significant increase in ICU admissions as was recorded in April and instead corresponded with the highest estimated prevalence. This finding opposed the assumption of a parallel distribution between the numbers of ICU and hospital admissions generally observed in the previous months. Thus, the regression curve predicted lower fatality rates until May and higher values until September. Another notable finding was that the estimated prevalence continued to increase from March until August and only started declining gradually towards September (figure 3).
According to our calculations, France recorded the highest fatality rate (May) among all countries (0.216% vs 0.204%, 95% CI 0.135 to 0.334) and also the highest ICU daily occupancy (7,019), followed by the UK (April) (0.089% vs 0.062%, 95% CI 0.049 to 0.074) and Spain (April) (0.047%). The highest fatality rates for the USA (0.026% vs 0.015%, 95% CI 0.014 to 0.021), Germany (0.012% vs 0.010%, 95% CI 0.010 to 0.013) and Italy (0.006% vs 0.008%, 95% CI 0.001 to 0.012) occurred in April. The fatality rates decreased with more than 90% in all countries until plateauing around June, with only small fluctuations towards September.
The estimates for prevalence showed the highest value in Spain (4.88%) in May, preceded by Italy (2.76%) in April (table 2). The largest interval between the first reported cases and the peak of the prevalence (2.22%) was registered in France. The prevalence had a continuous decline in Italy (2.76%) and the UK (0.05%) throughout the entire period, and in September, the UK had the lowest prevalence (0.01%) among all countries. In the USA, the prevalence continued to increase from April (0.02%) until August (0.07%), with a gradual decline in September (0.05%). From June in Germany and France and July in Spain (figure 2), the prevalence curves showed a gradual upturn with increasing values until September. At the point of upturn, the prevalence figures had declined in Spain by 76% (to 1.25%), in France by 61% (to 0.88%) and in Germany by 54% (to 1.16%) compared with the peak. Figures 2 and 3 depict the different trends of both prevalence and IFR and highlight the changes in their association when compared with the first and most affected months. All countries experienced a significant decrease of the fatality rates in May, which remained low from June until September, regardless of the course of prevalence.
Table 2.
Summary of the upper and lower values of the estimated infection fatality rate (IFR), prevalence, ICU and hospital admissions, and demographic characteristics of each country
| Germany | Hospital beds/1000 | 8 | Population | 83 783 945 | ||||
| IFR per 10 000 population | Prevalence | ICU admissions | Hospital admissions | |||||
| 7 June 2020 | 2.17 | 14 June 2020 | 1.16 | 9 August 2020 | 222 | 12 July 2020 | 252 | |
| 19 April 2020 | 122.60 | 12 April 2020 | 2.61 | 26 April 2020 | 2777 | 12 April 2020 | 5704 | |
| France | Hospital beds/1000 | 5.98 | Population | 65 273 512 | ||||
| IFR per 10 000 population | Prevalence | ICU admissions | Hospital admissions | |||||
| 13 June 2020 | 18.65 | 21 March 2020 | 0.61 | 01 August 2020 | 358 | 29 August 2020 | 4579 | |
| 2 May 2020 | 2160.00 | 23 May 2020 | 2.22 | 11 April 2020 | 7019 | 18 April 2020 | 31 446 | |
| Italy | Hospital beds/1000 | 3.18 | Population | 60 461 828 | ||||
| IFR per 10 000 population | Prevalence | ICU admissions | Hospital admissions | |||||
| 2 September 2020 | 2.79 | 19 August 2020 | 0.02 | 6 August 2020 | 42 | 30 July 2020 | 773 | |
| 16 April 2020 | 67.31 | 2 April 2020 | 2.76 | 2 April 2020 | 3976 | 9 April 2020 | 32 615 | |
| UK | Hospital beds/1000 | 2.54 | Population | 67 886 004 | ||||
| IFR per 10 000 population | Prevalence | ICU admissions | Hospital admissions | |||||
| 31 July 2020 | 0.58 | 3 April 2020 | 0.01 | 28 August 2020 | 68 | 4 September 2020 | 447 | |
| 3 April 2020 | 887.57 | 29 May 2020 | 0.05 | 17 April 2020 | 3243 | 17 April 2020 | 19 221 | |
| USA | Hospital beds/1000 | 2.77 | Population | 331 002 647 | ||||
| IFR per 10 000 population | Prevalence | ICU admissions | Hospital admissions | |||||
| 17 July 2020 | 32.67 | 31 March 2020 | 0.02 | 31 March 2020 | 211 | 31 March 2020 | 9480 | |
| 14 April 2020 | 255.70 | 28 July 2020 | 0.07 | 12 May 2020 | 6323 | 28 July 2020 | 59 026 | |
| Spain | Hospital beds/1000 | 2.97 | Population | 46 754 783 | ||||
| IFR per 10 000 population | Prevalence | |||||||
| 26 July 2020 | 2.14 | 5 July 2020 | 1.25 | |||||
| 19 April 2020 | 473.39 | 3 May 2020 | 4.88 | |||||
ICU, intensive care unit.
When examined for heterogeneity, the analysis has shown that there is significant heterogeneity within the data records of each country and for all variables, with higher q statistic values reflecting the within-country and not the between countries heterogeneity for the variable analysed (Fα calculated for α=0.05). Overall, the analysis shows an increasing within-country heterogeneity of the data towards September for the numbers of daily new deaths, ICU and hospital admissions, whereas for the number of daily tests, prevalence and IFR, the last period shows a trend towards less heterogenous data. The increased q statistic values towards September for the explanatory variables, and decreased for the outcome variables, are in accordance with the maintained low IFR across all countries during the time interval between July and September.
Discussion
This study was aimed at assessing the pattern of change in prevalence and estimated IFR of COVID-19 over time using data from six countries as well as establishing a predictive model for fatality based on hospital and ICU admissions. Our findings show that at the peak of the pandemic, the model underestimated IFR based on hospital and ICU admissions and that the predictive value increased gradually thereafter until September. One plausible explanation here could be the surge of cases at the peak which generally exceeded the capacity to accommodate and treat by the public health services, leading to fatalities outside the hospitals in venues such as residential and nursing homes. Once healthcare capacities were improved, hospital and/or ICU admissions became much better predictors of IFR, providing a useful tool to foresee outcomes. Our findings also show a reduction in IFR over time across all countries regardless of variations and differences in prevalence, healthcare systems and COVID-19 management strategies (figures 2 and 3), prompting discussion on possible explanations for the apparent reduced aggressiveness of the virus. Before exploring these further, however, a note needs to be added on the potential confounding effect of COVID-19 test availability on our observation. In the early stages of the pandemic, the lack of diagnostic resources and the need to prioritise tests were recognised as one of the major challenges.20 Consequently, testing among the symptomatic individuals prevailed over the detection of asymptomatic cases. The gradual increase in the number of daily tests (figure 1), enabling testing of asymptomatic/mildly symptomatic patients, can lead to underestimation of the IFR. To address this, therefore, our data have been adjusted for the number of tests.
Testing and public health explanations
Since the beginning of the COVID-19 pandemic, laboratories have used the RT-PCR assays as gold standard, but diagnostic development landscape is dynamic and moving rapidly towards antigen rapid detection tests.21 Seroepidemiological surveys are now widely used to quantify the extent of SARS-CoV-2 transmission in the population. Many of these studies are small or based on non-random sampling of participants and thus cannot provide precise estimates for the general population. Multiple surveys worldwide are currently ongoing, however preliminary data have been made available with seroprevalence estimates for various countries.22–24 As previously mentioned, the detection of asymptomatic SARS-CoV-2 infections might explain the apparent reduced pathogenicity of COVID-19. Several studies estimated a third of all infected individuals to be asymptomatic. A meta-analysis which included prediction models put the percentage of asymptomatic cases at 9.2%–69%.25 In our study, however, the pattern of reduced IFR regardless of prevalence over time was maintained even when data were adjusted for the increased number of tests.
In terms of public health measures, the first preventive steps were taken early in March, with a rapid progression towards national lockdown by the end of the month. A systematic review which included data from previous SARS-CoV-1 and MERS-CoV outbreaks concluded that despite the limited evidence in favour of quarantine to control SARS-CoV-2, the available studies supported the benefits of public health measures.26 In Europe, the lockdown did impact the viral transmission rate, and this was reflected in the general decline in the number of new cases and deaths, as well as the number of hospitalised individuals. The governmental strategies varied between countries, with high stringency levels generally maintained in the USA and the UK, while others adopted a more permissive policy from May.1 Despite the variations in the public health policy and patterns of prevalence, the IFR has continued to remain low thereafter. Therefore, the theory that slowing the spread of COVID-19 reduces the fatality rates by preventing hospitals from being overrun and thus allowing better and lifesaving care would not solely explain the persistence of low mortality rates.
The demographic characteristics of the affected population are also relevant and have been constantly changing, with a shift towards an increased incidence among the younger age groups. In France, this has been observed from July, with the highest incidence corresponding to 15–44 years olds. In Spain, the median age in July was 44, 38 in August and 39 in September. In Germany, the median age in July was 36, 32 in August with a slight increase to 35 in September. The median age in Italy decreased from 40 in July to 28 in August and 40 towards September. In the USA, the median age declined from 46 in May to 37 in July and 38 in August. In the UK, case positivity was the highest among older age groups until September; thereafter, the highest incidence was seen among individuals aged 15–44 years. In spite of the increased relative prevalence among the younger age groups, overall since July, the prevalence has been increasing in all age groups without a significant proportional increase in IFR, suggesting that other factors may also play an important role here.
Biological explanations
The relationship between the viral load and the likelihood of developing the disease has only been partly explored. As a result of the public health measures such as social distancing or wearing face masks, the individuals are likely to be exposed to lower viral loads. This may not decrease the spread of the virus across the affected population but has potentially an impact on the ability of the immune system to respond and the subsequent disease evolution in the infected individuals. Currently there is only limited evidence regarding reduced viral loads in asymptomatic versus symptomatic individuals, as well as reduced seroconversion among the asymptomatic population,27 28 to suggest a positive association between viral load and disease severity.
The mechanisms underlying the differences in COVID-19 susceptibility and disease presentation are currently unknown, although viral and host genetic variants are probable factors influencing both disease severity and immune response outcomes. Host genetic variation may result in different susceptibility to SARS-CoV-2. Although this may account for the broad spectrum of the symptoms and disease severity associated with COVID-19, it cannot explain the observed improved fatality rates in the population, as the interval required for human genome mutations to occur is incomparably high (10−8 per site per generation).29
Alterations in the viral genome are another possible explanation for the apparent reduced pathogenicity. The single-stranded RNA viruses accumulate mutations at a rate of 10−6–10−4 per replication cycle and might result in enhanced abilities to escape the host immune system or cause increased virulence.30 The mutation rate in the SARS-CoV-1 genome was estimated to be 0.80–2.38×10–3 nucleotides/genome/year, which is in the same order of magnitude as of other RNA viruses.31 For SARS-CoV-2, the mutation rate has been found to be approximately 6×10−4 nucleotides/genome/year.32 The frequency at which the mutations are found in a viral population is different from the mutation rate and depends on several other processes such as natural selection, random genetic drift, host immune responses and recombination among others.30 Natural selection acts on individual alleles based on their mutational fitness effect (MFE). A positive MFE results in fixation of beneficial alleles, whereas deleterious and lethal alleles are removed from the population by negative selection.30 The zoonotic origin of the SARS-CoV-2 implies the filtering of a multitude of viral strains of different strengths during its transition to a human host, allowing for the least lethal to efficiently replicate. The rate at which the environment of a virus population changes has been found to be closely related with the dynamics of the RNA evolution.33 Thus, a faster changing environment would prompt rapid evolutionary changes, such as the case of influenza. A recent mutation in the spike protein appears to have significantly increased the transmissibility of SARS-CoV-2, and the strains containing this mutation are spreading fast through Europe and the USA.29 Therefore, continued surveillance for mutations and understanding their impact on the biology of the virus remain crucial.
SARS-CoV-2 as well as SARS-CoV-1 and MERS-CoV display increased pathogenicity when compared with the seasonal coronaviruses. A proposed theory that has been investigated for dengue virus, HIV, Ebola and other respiratory viruses is the antibody dependent enhancement of the infection, where poorly neutralising antibodies elicited by a previous contact with the virus facilitate the viral entry resulting in severe forms of disease.34 Other studies dispute the cross-reactivity with other coronaviruses and suggest the increased pathogenicity as a result of humans’ serologically naivety to SARS-CoV-2.34 35 Nonetheless, when compared with recent novel virus outbreaks, such as SARS and MERS, the mortality rate is significantly lower with COVID-19. SARS accounted for 8098 laboratory confirmed cases between 2002 and 2004 and 774 deaths, while MERS led to 2494 confirmed cases and 858 associated deaths in 2012.36 Similarly, a total of 28 616 Ebola cases were reported between 2014 and 2016 with 11 310 deaths.37 As of 7 September, there were 26 763 217 SARS-CoV-2 cases and 876 616 deaths reported worldwide. The overall lower fatality potential of COVID-19 compared with these other novel viruses combined with its rapid spread across the world since March may have provided further evolutionary opportunity in favour of a less virulent but more infectious virus, manifesting in reduced fatality rates over time.
Limitations
One of the main limitations of our study is related to the variations in the testing technology both between different countries and also over time in the same country. Furthermore, the way test results were reported was not always consistent, especially when multiple tests were performed in the same individuals. By using data from multiple sources in each country, we aimed to minimise the effect of these confounding factors.
Conclusions
COVID-19 is a novel virus and there is much to learn about its biology and behaviour. Since early 2020, the virus has spread fast with catastrophic loss of life and impact on the society. Nonetheless, our data show a gradual but significant reduction in the virus-related mortality over time which is difficult to be wholly explained by public health measures. Understanding the basic biology of the virus and how it interacts with host’s immune system and leveraging that knowledge might ultimately hold the key to defeating this disease. Till then our results show the hospital and ICU admission rates to be useful predictors of patient outcomes and could be used as early warning signs for escalation of public health measures.
Supplementary Material
Footnotes
Contributors: MAV organised, executed, wrote and reviewed the study. LF organised, wrote and reviewed the study. JJ organised, executed and reviewed the study. KA conceived, organised, supported, wrote and reviewed the study. All authors have seen and approved the final version of manuscript being submitted. The article is the authors’ original work, has not received prior publications and is not under consideration for publication elsewhere.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Disclaimer: The views of the authors do not necessarily reflect those of the National Health System, National Institute for Health Research or the Department of Health.
Reporting statement: The manuscript was written following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. Bsg.ox.ac.uk . World’S first COVID-19 government response tracker launched Today, 2020. Available: https://www.bsg.ox.ac.uk/news/worlds-first-covid-19-government-response-tracker-launched-today
- 2. Who.int . Coronavirus disease (COVID-19) situation reports, 2020. Available: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
- 3. Our World in Data . Coronavirus pandemic (COVID-19), 2021. Available: https://ourworldindata.org/coronavirus
- 4. Mscbs.gob.es . Ministerio de Sanidad, Consumo y Bienestar Social - Profesionales - Situación actual Coronavirus, 2021. Available: https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/situacionActual.htm
- 5. Isciii.es . Guía COVID-19, 2021. Available: https://www.isciii.es/QueHacemos/Servicios/Biblioteca/Paginas/Guia-COVID.aspx
- 6. Salute M. Ministero DELLA salute, 2021. Available: http://www.salute.gov.it/portale/home.html
- 7. Daticovid.it . Dati COVID-19 Italia, 2021. Available: https://daticovid.it/
- 8. Rki.de . RKI - Startseite, 2021. Available: https://www.rki.de/DE/Home/homepage_node.html
- 9. Santepubliquefrance.fr . Coronavirus (COVID-19), 2021. Available: http://www.santepubliquefrance.fr/dossiers/coronavirus-covid-19
- 10. GOV.UK . Coronavirus (COVID-19): guidance and support, 2021. Available: https://www.gov.uk/coronavirus
- 11. Who.int . Laboratory testing of 2019 novel coronavirus (2019-Ncov) in suspected human cases: interim guidance, 17 January 2020, 2020. Available: https://apps.who.int/iris/handle/10665/330676
- 12. Who.int . Laboratory testing for coronavirus disease (COVID-19) in suspected human cases: interim guidance, 19 March 2020, 2020. Available: https://apps.who.int/iris/handle/10665/331501
- 13. Healthdata.gov . COVID-19 diagnostic laboratory testing (PCR testing) time series, 2020. Available: https://healthdata.gov/dataset/covid-19-diagnostic-laboratory-testing-pcr-testing-time-series
- 14. Geodes.santepubliquefrance.fr . Nombre de patients testés - Hebdomadaire - tous âges2020-S38, 2020. Available: https://geodes.santepubliquefrance.fr/#c=indicator&f=0&i=sp_pos_heb.t&s=2020-S38&t=a01&view=map1
- 15. Johns Hopkins Coronavirus Resource Center . Testing - Johns Hopkins Coronavirus resource center, 2020. Available: https://coronavirus.jhu.edu/testing
- 16. Rki.de . RKI - Coronavirus SARS-Cov-2 - Hinweise Zur Testung Von Patienten Auf Infektion Mit Dem Neuartigen Coronavirus SARS-Cov-2, 2020. Available: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Vorl_Testung_nCoV.html
- 17. Who.int . Estimating mortality from COVID-19, 2020. Available: https://www.who.int/news-room/commentaries/detail/estimating-mortality-from-covid-19
- 18. Meyerowitz-Katz G, Merone L. A systematic review and meta-analysis of published research data on COVID-19 infection fatality rates. Int J Infect Dis 2020;101 pp.:138–48. 10.1016/j.ijid.2020.09.1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang J-F, Zhang T-L, Fu B-J. A measure of spatial stratified heterogeneity. Ecol Indic 2016;67 pp.:250–6. 10.1016/j.ecolind.2016.02.052 [DOI] [Google Scholar]
- 20. Who.int . WHO director-general’s opening remarks at the media briefing on COVID-19 - 15 April 2020, 2020. Available: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-15-april-2020
- 21. Who.int . Antigen-detection in the diagnosis of SARS-Cov-2 infection using rapid immunoassays, 2020. Available: https://www.who.int/publications/i/item/antigen-detection-in-the-diagnosis-of-sars-cov-2infection-using-rapid-immunoassays
- 22. Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet 2020;396 pp.:535–44. 10.1016/S0140-6736(20)31483-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rki.de . RKI - Coronavirus SARS-Cov-2 - Serologische Untersuchungen Von Blutspenden Auf Antikörper Gegen SARS-Cov-2 (Sebluco-Studie), 2020. Available: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Projekte_RKI/SeBluCo_Zwischenbericht.html
- 24. Centers for Disease Control and Prevention . Coronavirus disease 2019 (COVID-19) in the U.S, 2020. Available: https://covid.cdc.gov/covid-data-tracker/#national-lab
- 25. Kronbichler A, Kresse D, Yoon S, et al. Asymptomatic patients as a source of COVID-19 infections: a systematic review and meta-analysis. Int J Infect Dis 2020;98 pp.:180–6. 10.1016/j.ijid.2020.06.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nussbaumer-Streit B, Mayr V, Dobrescu A. Quarantine alone or in combination with other public health measures to control COVID-19: a rapid review. Cochrane Database of Systematic Reviews 2020;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wellinghausen N, Plonné D, Voss M, et al. SARS-CoV-2-IgG response is different in COVID-19 outpatients and asymptomatic contact persons. J Clin Virol 2020;130:104542. 10.1016/j.jcv.2020.104542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou R, Li F, Chen F, et al. Viral dynamics in asymptomatic patients with COVID-19. Int J Infect Dis 2020;96 pp.:288–90. 10.1016/j.ijid.2020.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ovsyannikova IG, Haralambieva IH, Crooke SN, et al. The role of host genetics in the immune response to SARS-CoV-2 and COVID-19 susceptibility and severity. Immunol Rev 2020;296 pp.:205–19. 10.1111/imr.12897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sanjuán R, Domingo-Calap P. Mechanisms of viral mutation. Cell Mol Life Sci 2016;73 pp.:4433–48. 10.1007/s00018-016-2299-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao Z, Li H, Wu X, et al. Moderate mutation rate in the SARS coronavirus genome and its implications. BMC Evol Biol 2004;4:21. 10.1186/1471-2148-4-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Dorp L, Acman M, Richard D, et al. Emergence of genomic diversity and recurrent mutations in SARS-CoV-2. Infect Genet Evol 2020;83:104351. 10.1016/j.meegid.2020.104351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dolan PT, Whitfield ZJ, Andino R. Mapping the evolutionary potential of RNA viruses. Cell Host Microbe 2018;23 pp.:435–46. 10.1016/j.chom.2018.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee WS, Wheatley AK, Kent SJ, et al. Antibody-Dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat Microbiol 2020;5 pp.:1185–91. 10.1038/s41564-020-00789-5 [DOI] [PubMed] [Google Scholar]
- 35. Siracusano G, Pastori C, Lopalco L. Humoral immune responses in COVID-19 patients: a window on the state of the art. Front Immunol 2020;11:1049. 10.3389/fimmu.2020.01049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hu T, Liu Y, Zhao M, et al. A comparison of COVID-19, SARS and MERS. PeerJ 2020;8:e9725. 10.7717/peerj.9725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Who.int . Ebola virus disease, 2020. Available: https://www.who.int/news-room/fact-sheets/detail/ebola-virus-disease
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.