Abstract
Objective
To assess the impact of mild-moderate systemic lupus erythematosus (SLE) disease activity during a 12-month period on the risk of death or subsequent organ system damage.
Methods
1168 patients with ≥24 months of follow-up from the Hopkins Lupus Cohort were included. Disease activity in a 12-month observation period was calculated using adjusted mean Safety of Estrogens in Lupus Erythematosus National Assessment (SELENA) version of the SLE Disease Activity Index (SLEDAI), defined as the area under the curve divided by the time interval. Damage accrual in the follow-up period was defined as change in Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SDI) score ≥1 among patients without prior damage. Patients visited the clinic quarterly and had SELENA-SLEDAI and SDI assessed at every visit.
Results
During follow-up (median 7 years), 39% of patients accrued new damage in any organ system (7% cardiovascular and 3% renal) and 8% died. In adjusted models, an increased SELENA-SLEDAI score increased the risk of death (HR=1.22, 95% CI 1.13 to 1.32, p<0.001), renal damage (HR=1.24, 95% CI 1.08 to 1.42, p=0.003) and cardiovascular damage (HR=1.17, 95% CI 1.07 to 1.29, p<0.001). Hydroxychloroquine use reduced the risk of death (HR=0.46, 95% CI 0.29 to 0.72, p<0.05) and renal damage (HR=0.30, 95% CI 0.13 to 0.68, p<0.05). Non-steroidal anti-inflammatory drug use increased the risk of cardiovascular damage (HR=1.66, 95% CI 1.04 to 2.63, p<0.05). Without prior damage, an increased adjusted mean SELENA-SLEDAI score increased the risk of overall damage accrual (HR=1.09, 95% CI 1.04 to 1.15, p<0.001).
Conclusions
Each one-unit increase in adjusted mean SELENA-SLEDAI during a 12-month observation period was associated with an increased risk of death and developing cardiovascular and renal damage.
Keywords: lupus erythematosus, systemic, anti-inflammatory agents, non-steroidal, antirheumatic agents
Key messages.
What is already known about this subject?
Previous studies have demonstrated that systemic lupus erythematosus (SLE) disease activity and damage accrual are strong predictors of SLE prognosis and survival.
What does this study add?
This study involved patients with SLE from the large and racially diverse Hopkins Lupus Cohort, composed of 55% white patients and 39% patients of Black African Ancestry. This prospective analysis demonstrated that increases in adjusted mean Safety of Estrogens in Lupus Erythematosus National Assessment-SLE Disease Activity Index (SELENA-SLEDAI) score during a 12-month observational period were associated with an increased risk of death or developing renal or cardiovascular damage.
Chronic non-steroidal anti-inflammatory drug (NSAID) use resulted in significantly increased risk of developing cardiovascular damage.
SLE may progress to irreversible damage in selected organ systems among patients with stable, mild-to-moderate SLE disease activity over 7 years.
How might this impact on clinical practice or future developments?
This analysis demonstrates that, even in patients with mild-to-moderate disease activity, an increase in adjusted mean SELENA-SLEDAI score and NSAID use during a 12-month period increases the risk of death and organ specific damage, highlighting the need for more active measures to manage SLE disease activity over time and to limit NSAID use.
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease characterised by a complex and multifactorial aetiology. It affects multiple organ systems, which contributes to the complicated patient burden of SLE. Some patients with SLE experience a waxing and waning pattern of disease activity during the course of disease, whereas others have continuous activity.1 Cumulative SLE disease activity2 and SLE therapies3 4 impact the risk of developing organ system damage and survival.5–7 The 5-year survival among individuals with SLE has increased dramatically in the last four decades to 95%,7 with similar trends observed for 10-year survival.7 8 The impact of irreversible organ system damage in the prognosis of SLE remains a major concern because patients who develop damage are more likely to accrue additional damage and die.9 Published research from prospective cohorts has demonstrated that SLE disease activity is a strong predictor of SLE prognosis and survival.10 11
The objective of this analysis was to investigate whether the pattern of SLE disease activity (as measured by the Safety of Estrogens in Lupus Erythematosus National Assessment (SELENA) version of the SLE Disease Activity Index (SLEDAI)) during a 12-month period after enrolment in a racially diverse cohort of patients prospectively followed quarterly by protocol impacted the risk of developing damage (overall or by specific organ system) and death over time.
Methods
Patient population
Demographic, clinical and laboratory data were collected prospectively in enrolled patients in the Hopkins Lupus Cohort from 1987 to 2010.12–15 Cohort patients with a minimum of 24-month follow-up were included in this analysis. Patients were seen by a single rheumatologist in the Hopkins Lupus Center (MAP) and had a clinical diagnosis of SLE according to the revised American College of Rheumatology (ACR) or Systemic Lupus International Collaborating Clinics (SLICC) criteria.16–18 The study was approved by the Johns Hopkins University School of Medicine Institutional Review Board. All patients provided informed written consent. Patients with a SLICC/ACR Damage Index (SDI) score ≥3, end-stage kidney disease, history of major organ transplant or malignant neoplasm at cohort entry were excluded from this analysis.
Patient follow-up
Follow-up time in the cohort was then divided into three discrete periods: (a) background (background period: first 12 months after cohort entry), (b) observation (observation period: second 12 months after cohort entry) and (c) follow-up (follow-up period: remainder of time under observation until damage occurred, death or end of available data). SLE disease duration was defined as the time interval from SLE clinical diagnosis (revised ACR or SLICC criteria) until the end of the follow-up period or death.
Study variables
Key variables included in the observation period were demographic characteristics, SLE clinical characteristics, laboratory parameters, medication use and comorbid medical conditions (hypertension, diabetes mellitus, obesity, etc.). Hypertension was defined as systolic blood pressure greater than 140 mm Hg or diastolic blood pressure greater than 90 mm Hg or requiring antihypertensive medication. Diabetes mellitus was defined as requiring an oral hypoglycaemic agent or insulin therapy. Immunosuppressive therapies excluded steroids and included the following: leflunomide, mycophenolate, cyclophosphamide, tacrolimus, azathioprine, methotrexate or rituximab (biologic). Antimalarial therapies were almost exclusively comprised of hydroxychloroquine and non-steroidal anti-inflammatory drug (NSAID) therapy included several drugs (prescription and over-the-counter) in this class, but almost exclusively naproxen. For the aforementioned SLE therapies, use was defined as ever prescribed during the observation period (yes/no). Oral steroid (prednisone) use in the observational period was stratified into the following groups: ever prescribed any dose (yes) or never prescribed (no). Among those prescribed oral steroids, daily dose during the observation period was stratified into two categories: (a) prescribed >7.5 mg/day at least once during the observation period or (b) only prescribed ≤7.5 mg/day during the observation period. Antinuclear antibody positivity (ANA+) was defined as a titre ≥1:80 and antidouble stranded DNA positivity (anti-dsDNA+) was defined as a titre ≥1:10 on the Crithidia luciliae indirect immunofluorescence test.
Disease activity was measured at every clinic visit (quarterly by protocol or more often if clinically warranted) using the SELENA-SLEDAI.19 To describe disease activity over time in this analysis, adjusted mean SELENA-SLEDAI20 21 was calculated from the cumulative area under the curve for each period divided by the time interval under evaluation and reported in months. At least one clinic visit per period was required to estimate adjusted mean SELENA-SLEDAI for each period. Damage accrual was assessed at each visit by the SDI.22 23
Data analysis
All statistical analyses were completed in SAS V.9.13 (SAS Institute, Cary, North Carolina, USA).
Continuous variables were reported as median values unless otherwise noted. Categorical variables were described as frequency counts. SLE outcomes of interest included (a) development of overall organ system damage, (b) development of specific organ system damage or (c) death during the follow-up period. The outcome of death was defined as all-cause mortality that may or may not have been clinically attributable to SLE. Damage accrual analyses were restricted to patients without prior damage in the organ system of interest through the end of the observation period. Accrued damage was defined as at least a one (≥1) unit increase in SDI score between the observation period and last recorded visit in the follow-up period.
In this analysis, organ specific system damage was restricted to renal, cardiovascular, peripheral vascular, neuropsychiatric, pulmonary, musculoskeletal, seizure or stroke. Damage due to malignancy was not explored as it was an exclusion criterion. Damage accrual in organ systems not commonly associated with death (ocular, gastrointestinal, skin, or gonadal failure) was not evaluated in this analysis.
Adjusted Mean SELENA-SLEDAI was our key explanatory variable. Laboratory parameters were not considered in models evaluating the impact of adjusted mean SELENA-SLEDAI on the outcomes of interest since these variables are components of the SELENA-SLEDAI score. Time-varying variables that were measured during the observation period and SLE clinical history variables were individually evaluated in univariate, unadjusted models for the outcomes of death or damage accrual (overall or specific organ systems). When more than one variable was available to evaluate comorbidity status, the variable that was most relevant to the time period of interest was used. If variables were comparable, then both were retained in the univariate analyses and the results from the univariate analysis determined which variable was considered in the multivariable model (when applicable). Variables with a p<0.10 in the univariate analysis were considered as potential covariates in the multivariable models for the outcomes of death or damage accrual. Cox proportional hazard models were used to estimate the impact of adjusted mean SELENA-SLEDAI as a time-dependent variable on the risk of death or developing any new organ damage over time. Kaplan–Meier survival curves were also generated to explore the impact of adjusted mean SELENA-SLEDAI in the observation period on the risk of death over time during the follow-up period.
For each outcome, a multivariable model was created to adjust for well-established potential confounding factors that included age, gender, race, SLE duration, overall SDI score at the start of the follow-up period and ever prescribed oral prednisone >7.5 mg/day in the observation period. With these factors forced in the model, other covariates identified in the univariate analyses were then evaluated for inclusion in the model using backward elimination methods with the criteria of p<0.10 for entry into the model and p<0.05 for retention. To evaluate racial and gender differences in the association of adjusted mean SELENA-SLEDAI and organ system damage, models were stratified by race and gender (data not shown).
Patient and public involvement
We did not involve patients and/or the public in this work.
Results
Characteristics
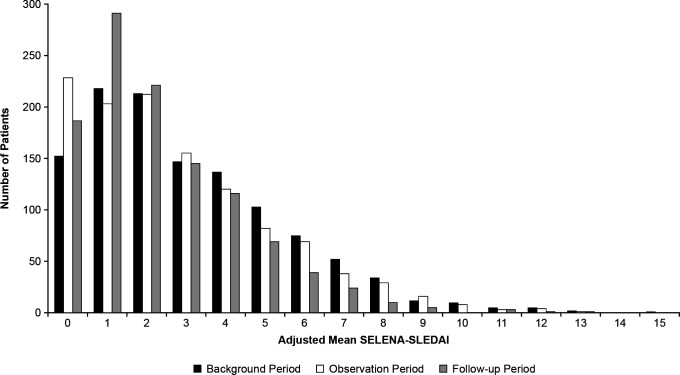
Overall, 1168 adult patients with SLE were included in this study. Patients were predominantly women (93%), 55% were white and 39% were of Black African Ancestry (table 1). During the background period, the median (range) adjusted mean SELENA-SLEDAI was 3 (0–16) and 55% of patients had mild-to-moderate disease activity, defined by an adjusted mean SELENA-SLEDAI <3 (table 1). Median (range) adjusted mean SELENA-SLEDAI in the observation period was 2 (0–13) and remained the same during the follow-up period. The distribution of adjusted mean SELENA-SLEDAI, stratified by period, is shown in figure 1.
Table 1.
Demographic and clinical characteristics of the Hopkins Lupus Cohort analytical cohort (N=1168)
| N (%) | |
| Women | 1085 (92.9) |
| Race | |
| White | 643 (55.1) |
| Black African Ancestry | 460 (39.4) |
| Other | 65 (5.6) |
| History of seropositive status | |
| ANA+ | 1075 (92.0) |
| Anti-dsDNA+ | 591 (50.6) |
| ANA+ or anti-dsDNA+ | 1093 (93.6) |
| Current smoker at cohort entry | 202 (17.3) |
| Past smoker at cohort entry | 465 (39.8) |
| Adjusted mean SELENA-SLEDAI <3 in background period | 641 (54.9) |
| SLE therapies* ever prescribed in observation period | |
| Oral prednisone (any dose) | 702 (60.1) |
| Oral prednisone >7.5 mg/day | 428 (36.6) |
| HCQ | 759 (65.0) |
| NSAID | 447 (38.3) |
| Immunosuppressants† | 262 (22.4) |
| Obesity in observation period (BMI >27.8 for men and BMI >27.3 for women) | 604 (51.7) |
| Hypertension in observation period (SBP ≥140 or DBP ≥90 mm Hg) | 652 (55.8) |
| Diabetes therapy with oral hypoglycaemic agent or insulin | 118 (10.1) |
| Median (range) | |
| Age at cohort entry‡, years | 36 (11–77) |
| Age at SLE diagnosis§, years | 31(5–75) |
| Age at last assessment in follow-up period, years | 46 (20–85) |
| Adjusted mean SELENA-SLEDAI in background period | 3 (0–16) |
| Disease duration from SLE diagnosis to cohort entry, years | 2 (0–39) |
| Disease duration from SLE diagnosis to last assessment, years | 11 (2–48) |
| Follow-up time from cohort entry to last assessment, years | 7 (2–23) |
*Categories not mutually exclusive.
†Leflunomide, mycophenolate, cyclophosphamide, tacrolimus, azathioprine, methotrexate or rituximab (biologic).
‡Less than 2% of cohort enrolled <18 years of age.
§Less than 5% of cohort diagnosed with SLE <18 years of age.
ANA, antinuclear antibodies; BMI, body mass index in kg/m2; DBP, diastolic blood pressure; dsDNA, double-stranded DNA; HCQ, hydroxychloroquine; NSAID, non-steroidal anti-inflammatory drug; SBP, systolic blood pressure; SELENA-SLEDAI, Safety of Estrogens in Lupus Erythematosus National Assessment-SLE Disease Activity Index; SLE, systemic lupus erythematosus.
Figure 1.
Distribution of adjusted mean SELENA-SLEDAI stratified by period (N=1168). SELENA-SLEDAI, Safety of Estrogens in Lupus Erythematosus National Assessment-Systemic Lupus Erythematosus Disease Activity Index.
Death
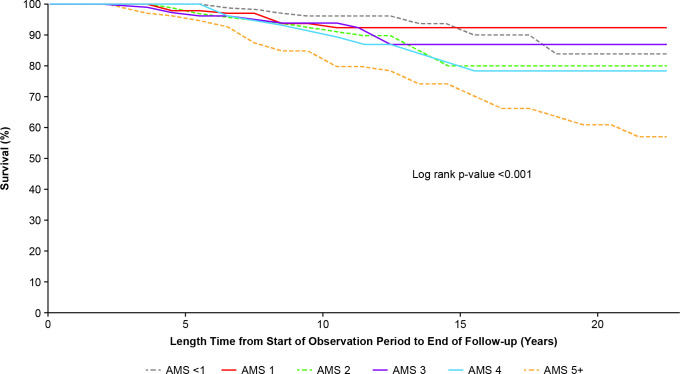
Ninety-two of 1168 patients (8%) died during the follow-up period (table 2). When exploring the association between adjusted mean SELENA-SLEDAI in the observation period and risk of death in the follow-up period, an adjusted mean SELENA-SLEDAI ≥5 significantly increased the risk of death after 7 years of follow-up (Log rank p<0.001; figure 2). In the multivariable model, each one-unit adjusted mean SELENA-SLEDAI increase in the observation period was significantly associated with a 22% increased risk of death in the follow-up period (HR=1.22, 95% CI 1.13 to 1.32, p<0.001; table 2). Use of hydroxychloroquine in the observation period was significantly associated with a 54% lower risk of death in the follow-up period (HR=0.46, 95% CI 0.29 to 0.72, p<0.05; table 2). The following variables were considered but not retained in the model: have a history of smoking at cohort entry, hypertension and diabetes.
Table 2.
Final models for adjusted mean SELENA-SLEDAI in the observation period as predictors of death/organ system damage in the follow-up period
| Death (n=1168) |
Overall damage* (n=888) |
Renal damage* (n=1147) |
Cardiovascular damage*(n=1135) | |
| Patients with outcome, % | 7.9 | 38.5 | 2.7 | 6.5 |
| Adjusted mean SELENA-SLEDAI in observation period, adjusted HR (95% CI)† |
1.22 (1.13 to 1.32)‡ | 1.09 (1.04 to 1.15) | 1.24 (1.08 to 1.42)§ | 1.17 (1.07 to 1.29)¶ |
| P value | <0.001 | <0.001 | 0.003 | <0.001 |
| Variables**, adjusted HR (95% CI) | ||||
| Age at cohort entry (years) | 1.05 (1.04 to 1.07)†† | 1.04 (1.03 to 1.05)†† | 1.02 (0.98 to 1.05) | 1.04 (1.02 to 1.06)†† |
| Men | 1.53 (0.81 to 2.89) | 0.94 (0.62 to 1.41) | 1.97 (0.52 to 7.46) | 1.86 (0.90 to 3.85) |
| Non-white | 1.24 (0.79 to 1.97) | 1.05 (0.84 to 1.31) | 2.28 (1.00 to 5.18) | 1.01 (0.62 to 1.63) |
| Duration of SLE at cohort entry (years) | 1.02 (0.99 to 1.05) | 1.01 (1.00 to 1.03) | 0.98 (0.92 to 1.04) | 1.00 (0.96 to 1.04) |
| SDI score at start of follow-up period | 1.41 (1.02 to 1.94)†† | N/A | 6.77 (4.32 to 10.61)†† | 2.40 (1.78 to 3.23)†† |
| Oral prednisone use in observation period | ||||
| None | Ref | Ref | Ref | Ref |
| ≤7.5 mg/day | 1.47 (0.77 to 2.80) | 1.29 (0.97 to 1.71) | 0.98 (0.28 to 3.45) | 0.98 (0.50 to 1.94) |
| >7.5 mg/day | 1.54 (0.88 to 2.71) | 1.85 (1.40 to 2.44)†† | 0.82 (0.32 to 2.10) | 1.05 (0.56 to 1.95) |
| HCQ ever use in observation period | 0.46 (0.29 to 0.72)†† | NS | 0.30 (0.13 to 0.68)†† | NS |
| Current smoker at cohort entry | 1.74 (1.09 to 2.76)†† | NS | NS | NS |
| NSAIDs use in observation period | NS | NS | NS | 1.66 (1.04 to 2.63)†† |
*In patients without a prior history of damage in the organ system of interest at the start of the follow-up period.
†HR for a one-unit increase in adjusted mean SELENA-SLEDAI during the observation periods. Models are adjusted for age at cohort entry (years), gender, non-white, duration of SLE at cohort entry (years), damage accrued (SDI score) in any other organ system but the outcome of interest through the end of the observation period (excluding the outcome of overall damage in any organ system), oral prednisone use in observation period (>7.5 mg/day).
‡Additional model adjustments include ever use of hydroxychloroquine during the observation period and current smoker at cohort entry.
§Additional model adjustments include ever use of hydroxychloroquine during the observation period.
¶Additional model adjustments include NSAID use during the observation period.
**SLE therapies defined as ever use during observation period.
††P<0.05.
CI, confidence interval; HCQ, hydroxychloroquine; HR, Cox proportional hazards ratio; NS, not a statistically significant predictor in the final Cox model; NSAID, non-steroidal anti-inflammatory drug; Ref, reference group; SDI, SLICC/ACR damage index; SELENA-SLEDAI, Safety of Estrogens in Lupus Erythematosus National Assessment-SLE Disease Activity Index; SLE, systemic lupus erythematosus.
Figure 2.
Kaplan–Meier curve: time to death by adjusted mean SELENA-SLEDAI score (N=1168). AMS, adjusted mean SELENA-SLEDAI; SELENA-SLEDAI, Safety of Estrogens in Lupus Erythematosus National Assessment-Systemic Lupus Erythematosus Disease Activity Index.
Overall damage accrual
After restricting the analysis (univariate and multivariable) to patients without a prior history of overall damage (SDI=0; n=888) at the start of the follow-up period, more than one-third (39%) of patients developed damage (SDI≥1) in any organ system by the end of the follow-up period (table 2). In multivariable models restricted to patients without prior damage in the organ system at the start of the follow-up period, adjusted for the effects of age, race, gender, SLE duration and ever prescribed >7.5 mg/day oral prednisone during the observation period, a one-unit increase in adjusted mean SELENA-SLEDAI during the observation period was significantly associated with an increased risk of accruing subsequent overall damage (HR=1.09, 95% CI 1.04 to 1.15, p<0.001; table 2). Among women without prior damage at the start of the follow-up period, adjusted mean SELENA-SLEDAI during the observation period was associated with an increased risk of accruing subsequent overall damage (HR=1.08, 95% CI 1.03 to 1.13, p=0.002; data not shown). No significant differences were observed for the outcomes of interest when stratified by race (HR=1.07, 95% CI 0.99 to 1.16, p=0.089 in white patients and HR=1.04, 95% CI 0.96 to 1.12, p=0.343 in patients of Black African Ancestry; data not shown).
Renal damage accrual
Approximately 3% of patients without renal damage in the observational period developed renal damage (SDI≥1; n=1147) in the follow-up period (table 2). Each one-unit increase in adjusted mean SELENA-SLEDAI during the observation period significantly increased the risk of renal damage by 24% (HR=1.24, 95% CI 1.08 to 1.42, p=0.003; table 2) in the follow-up period. Ever use of hydroxychloroquine in the observation period was significantly associated with lowering the risk of renal damage in the follow-up period by 70% (HR=0.30, 95% CI 0.13 to 0.68, p<0.05; table 2). When we restricted the analysis to women, the results did not change (HR=1.23, 95% CI 1.06 to 1.42, p=0.005). It was not possible to evaluate renal damage among men due to only one man having developed renal damage.
Cardiovascular damage accrual
Subsequent cardiovascular damage (SDI≥1; n=1135) was observed in approximately 7% of patients during the follow-up period (table 2). A one-unit increase in adjusted mean SELENA-SLEDAI during the observation period was associated with a 17% (HR=1.17, 95% CI 1.07 to 1.29, p<0.001; table 2) significant increased risk of cardiovascular damage accrual in the adjusted model. Patients who had been prescribed NSAID therapies in the observation period had a 66% (HR=1.66, 95% CI 1.04 to 2.63; table 2) increased risk of cardiovascular damage accrual. On further exploration, patients with any antihypertensive use in the observation period had an 81% (HR=1.81, 95% CI 1.09 to 3.02, p<0.05) significant increased risk of cardiovascular damage accrual.
Other organ system or subsystem damage accrual
Adjusted mean SELENA-SLEDAI during the observation period did not impact the risk of developing damage in the following organ systems or subsystems in our analysis: peripheral vascular (HR=1.12, 95% CI 0.96 to 1.32, p=0.154), pulmonary (HR=0.96, 95% CI 0.87 to 1.07, p=0.484), neuropsychiatric (HR=1.01, 95% CI 0.92 to 1.10, p=0.880), musculoskeletal (HR=1.05, 95% CI 0.98 to 1.12, p=0.194), stroke or seizure (data not shown).
Discussion
This analysis described the impact of disease activity during a 12-month period on the subsequent risk of death or organ system damage in a large, prospective cohort of racially diverse patients with SLE. This study included a large proportion of patients of Black African Ancestry (39.4%), which contrasts with previous Spanish studies (eg, RELESSER cohort)24–26 where the majority of patients were white (>90%), and Latin American studies (eg, GLADEL),27–29 which had lower percentages of African-Latin American patients (<13%). In this cohort of patients with SLE and mild-to-moderate disease activity at cohort entry, adjusted mean SELENA-SLEDAI measured during a prior 12-month period (corresponding to the length of a typical Phase III trial) significantly impacted the risk of renal and cardiovascular damage accrual (SDI≥1) and risk of death in the follow-up period. In previous studies, patients of Black African Ancestry were reported to experience a higher prevalence of SLE, greater disease severity, higher risk of cardiovascular events, greater organ damage and higher mortality rates, compared with white patients.30–37 In the current study, although non-white (Black African Ancestry and other race) patients had higher disease activity in the follow-up period compared with white patients, no significant racial differences were observed for the outcomes of interest. Therefore, results from this cohort provide an important contribution in characterising non-white patient populations. In adjusted Cox regression models, a one-unit increase in adjusted mean SELENA-SLEDAI during the 12-month observation period was associated with a 22% increased risk of death and a 17% and 24% increased risk of subsequent cardiovascular and renal damage accrual, respectively. Adjusted mean SELENA-SLEDAI in the observation period was associated with overall damage accrual after adjustment for age, gender, race, SLE duration, ever prescribed oral prednisone therapy >7.5 mg/day in observation period and SDI at the start of the follow-up period. Hydroxychloroquine use in the observation period was associated with a 54% and 70% decreased risk of death and renal damage, respectively, and NSAID use in the observation period increased the risk for cardiovascular damage accrual by approximately 70%.
Adjusted mean SELENA-SLEDAI in a 12-month prior period increased the risk of developing new renal and cardiovascular damage accrual (SDI≥1) and risk of death, which corroborates findings from another published Hopkins Lupus Cohort analysis with different analytical methods.38 Other studies have demonstrated that high disease activity increased the risk of poor SLE outcomes including accrual of overall organ damage20 39–44 and risk of death.7 40 42 45 The findings in this analysis corroborate the influence of disease activity for renal and cardiovascular damage accrual and death and also extend the findings to patients with SLE and mild-to-moderate disease activity.
Although 61.5% of the cohort remained free of any organ system damage through the end of the follow-up period, a reasonable proportion of patients with SLE with mild-to-moderate disease activity at cohort entry and through the start of the follow-up period accrued damage (renal and cardiovascular) (9.2%) in a relatively short time (median 7 years). Similarly, 8% of patients died during follow-up despite overall mild-to-moderate disease activity in the 24 months after cohort entry.
Detailed methods from real-world, large, prospective SLE cohorts have been described elsewhere10–13 46 47 and have contributed to SLE disease understanding over the past four decades. It is well recognised that damage accrual, due to active inflammation, comorbidities, previous SLE disease activity and/or exposure to SLE therapies (particularly corticosteroids), is an important prognostic factor for death and has a clear impact on burden of disease for patients,15 20 40 48 49 but the time for interval for damage to manifest has varied somewhat between different studies. Rahman et al found that patients with SLE exhibiting damage within the first year of admission to the clinic had a higher mortality rate after 10 years as compared with patients with no early damage,50 whereas results from Gladman et al suggested that damage accrued gradually over the 15 years of follow-up.2 In a study by Becker-Merok and Nossent, damage accrual occurred in 54% of patients with SLE in a linear fashion over the first 10 years of the disease.42 Similarly, a Swedish study also observed that 54% of patients with SLE had damage accrual in the first 5 years after diagnosis.51 Our findings demonstrate that SLE may progress to accrual of irreversible damage in selected organ systems among patients with stable, mild-to-moderate disease activity over 7 years. Our study confirms the results from a Norwegian SLE cohort that showed disease activity at baseline was a predictor of accrued organ damage that occurred over 2 years of follow-up in a small cohort of patients with relatively stable disease activity52 but extends them to specific organ damage subtypes. Unlike the findings from the SLICC inception cohort, we did not observe a pattern of decreasing disease activity over the first 5 years in conjunction with organ damage accrual.10 This may be in part due to the combination of inception and prevalent patients with SLE at the start of the observation period in this Hopkins Lupus Cohort analysis.
Subsequent accrual of organ system damage attributed to active SLE disease has been observed in several SLE prospective cohorts of patients with SLE receiving care from rheumatology specialty centres.5 10 11 20 48 53–55 One strength of this analysis is that Hopkins Lupus Cohort patients had clinic visits on average every 3 months per registry protocol. Ibañez et al recently demonstrated that adjusted mean SELENA-SLEDAI derived from quarterly clinic visits was more reliable than adjusted mean SELENA-SLEDAI based on more infrequent clinic visits.56
We recognise the limitations of this analysis and similar evaluations in prospective SLE cohorts. One limitation is that all patients in this analysis received care at a single tertiary medical centre, under the care of a single provider, and their SLE clinical characteristics, and hence our findings, may not extend to all patients with SLE. Furthermore, although we attempted to adjust for the effects of known risk factors and potential confounders, unmeasured or residual confounding factors may have influenced our findings.
We observed that adjusted mean SELENA-SLEDAI during a prior 12-month period impacted the risk of death and developing damage in a racially diverse cohort of patients with SLE, who had on average mild-to-moderate disease activity during a median follow-up of 7 years, after adjusting for potential confounders. Exposure to hydroxychloroquine during a prior 12-month period decreased the risk of death and developing renal damage. The London University College Hospital Lupus Cohort reported that more than half of patients with SLE were prescribed hydroxychloroquine during a 12-month observation period and this exposure was also associated with a decreased risk of death (50% reduction) and renal damage accrual (47% reduction) in adjusted models.11 A new finding in our analysis was the 66% increased risk of cardiovascular organ damage accrual associated with NSAID use in the previous 12 months, after controlling for the effects of other covariates in the model. It has been reported that NSAIDs may negatively affect the cardiovascular system, yet they also decrease inflammation, which is an independent risk factor in cardiovascular pathology.57 In our analysis, we observed that chronic NSAID use, commonly taken by patients with SLE to alleviate musculoskeletal pain, resulted in a significantly increased risk of cardiovascular damage accrual. NSAID use is also correlated with an increase in blood pressure, which may have an effect on cardiovascular damage.58 Indeed, in our study, we observed a significant increase in cardiovascular damage accrual with antihypertensive use. This may suggest that the known cardiovascular risk of NSAIDs in the general population is also applicable to patients with SLE59–62 and highlights the importance of assessing cardiovascular risk in this patient population.
In summary, our findings corroborate other published data that demonstrated that cumulative SLE disease activity over time impacted risk of developing organ damage (ie, SLE prognosis) and was associated with an increased risk of death. Furthermore, the findings suggest routine clinical care and SLE disease management, characterised by minimal fluctuations in disease activity, may not prevent development of organ damage or risk of premature death. An increase in adjusted mean SELENA-SLEDAI score during a 12-month period increased the risk of death and developing renal and cardiovascular organ system damage, even in patients with mild-to-moderate disease severity, which underscores the need for active measures to manage SLE disease activity over time.
Acknowledgments
Medical writing support was provided by Helen Taylor, of Fishawack Indicia Ltd, UK, part of Fishawack Health, and was funded by GSK.
Footnotes
Contributors: Conception or design: DDH, PJE, QF, MAP and AME. Acquisition of data: MAP. Data analysis or interpretation: DDH, PJE, QF, MAP and AME.
Funding: The Hopkins Lupus Cohort is supported by grants R01AR043727 and R01AR069572 from the National Institutes of Health (NIH). This work was funded by GSK Study Number: WEUKBRE4566. Johns Hopkins University School of Medicine received a research grant to support this study. AME is currently supported by NIH NCATS Award Number 1KL2TR002554.
Competing interests: DDH, PJE and QF were paid employees of GlaxoSmithKline (GSK) with stock options and conducted the study as part of their employment using GSK resources. AME was a paid contractor for GSK. MAP was a paid consultant to GSK.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Author note: DDH current affiliation is Center for Observational and Real-World Evidence, Pharmacoepidemiology, Merck & Co, Inc, Kenilworth, New Jersey, USA.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study was approved by the Johns Hopkins University School of Medicine Institutional Review Board (Study number NA_00039294).
References
- 1.Barr SG, Zonana-Nacach A, Magder LS, et al. Patterns of disease activity in systemic lupus erythematosus. Arthritis Rheum 1999;42:2682–8. [DOI] [PubMed] [Google Scholar]
- 2.Gladman DD, Urowitz MB, Rahman P, et al. Accrual of organ damage over time in patients with systemic lupus erythematosus. J Rheumatol 2003;30:1955–9. [PubMed] [Google Scholar]
- 3.Thamer M, Hernán MA, Zhang Y, et al. Prednisone, lupus activity, and permanent organ damage. J Rheumatol 2009;36:560–4. 10.3899/jrheum.080828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zonana-Nacach A, Barr SG, Magder LS, et al. Damage in systemic lupus erythematosus and its association with corticosteroids. Arthritis Rheum 2000;43:1801–8. [DOI] [PubMed] [Google Scholar]
- 5.Nossent J, Cikes N, Kiss E, et al. Current causes of death in systemic lupus erythematosus in Europe, 2000--2004: relation to disease activity and damage accrual. Lupus 2007;16:309–17. 10.1177/0961203307077987 [DOI] [PubMed] [Google Scholar]
- 6.Urowitz MB, Gladman DD, Tom BDM, et al. Changing patterns in mortality and disease outcomes for patients with systemic lupus erythematosus. J Rheumatol 2008;35:2152–8. 10.3899/jrheum.080214 [DOI] [PubMed] [Google Scholar]
- 7.Kasitanon N, Magder LS, Petri M. Predictors of survival in systemic lupus erythematosus. Medicine 2006;85:147–56. 10.1097/01.md.0000224709.70133.f7 [DOI] [PubMed] [Google Scholar]
- 8.Cervera R, Khamashta MA, Font J, et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine 2003;82:299–308. 10.1097/01.md.0000091181.93122.55 [DOI] [PubMed] [Google Scholar]
- 9.Alarcón GS, Roseman JM, McGwin G, et al. Systemic lupus erythematosus in three ethnic groups. XX. damage as a predictor of further damage. Rheumatology 2004;43:202–5. 10.1093/rheumatology/keg481 [DOI] [PubMed] [Google Scholar]
- 10.Urowitz MB, Gladman DD, Ibañez D, et al. Evolution of disease burden over five years in a multicenter inception systemic lupus erythematosus cohort. Arthritis Care Res 2012;64:132–7. 10.1002/acr.20648 [DOI] [PubMed] [Google Scholar]
- 11.Lopez R, Davidson JE, Beeby MD, et al. Lupus disease activity and the risk of subsequent organ damage and mortality in a large lupus cohort. Rheumatology 2012;51:491–8. 10.1093/rheumatology/ker368 [DOI] [PubMed] [Google Scholar]
- 12.Petri M. Hopkins lupus cohort. 1999 update. Rheum Dis Clin North Am 2000;26:199–213. v. 10.1016/s0889-857x(05)70135-6 [DOI] [PubMed] [Google Scholar]
- 13.Petri M. Lupus in Baltimore: evidence-based 'clinical pearls' from the Hopkins Lupus Cohort. Lupus 2005;14:970–3. 10.1191/0961203305lu2230xx [DOI] [PubMed] [Google Scholar]
- 14.Petri M. Monitoring systemic lupus erythematosus in standard clinical care. Best Pract Res Clin Rheumatol 2007;21:687–97. 10.1016/j.berh.2007.01.003 [DOI] [PubMed] [Google Scholar]
- 15.Al Sawah S, Zhang X, Zhu B, et al. Effect of corticosteroid use by dose on the risk of developing organ damage over time in systemic lupus erythematosus-the Hopkins lupus cohort. Lupus Sci Med 2015;2:e000066. 10.1136/lupus-2014-000066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hochberg MC. Updating the American College of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:40:1725. 10.1002/art.1780400928 [DOI] [PubMed] [Google Scholar]
- 17.Petri M, Orbai A-M, Alarcón GS, et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. 10.1002/art.34473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271–7. 10.1002/art.1780251101 [DOI] [PubMed] [Google Scholar]
- 19.Petri M, Kim MY, Kalunian KC, et al. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med 2005;353:2550–8. 10.1056/NEJMoa051135 [DOI] [PubMed] [Google Scholar]
- 20.Ibañez D, Gladman DD, Urowitz MB. Adjusted mean systemic lupus erythematosus disease activity Index-2K is a predictor of outcome in SLE. J Rheumatol 2005;32:824–7. [PubMed] [Google Scholar]
- 21.Ibañez D, Urowitz MB, Gladman DD. Summarizing disease features over time: I. adjusted mean SLEDAI derivation and application to an index of disease activity in lupus. J Rheumatol 2003;30:1977–82. [PubMed] [Google Scholar]
- 22.Gladman DD, Urowitz MB. The SLICC/ACR damage index: progress report and experience in the field. Lupus 1999;8:632–7. 10.1191/096120399680411335 [DOI] [PubMed] [Google Scholar]
- 23.Gladman D, Ginzler E, Goldsmith C, et al. Systemic lupus international collaborative clinics: development of a damage index in systemic lupus erythematosus. J Rheumatol 1992;19:1820–1. [PubMed] [Google Scholar]
- 24.Rúa-Figueroa Íñigo, Richi P, López-Longo FJ, et al. Comprehensive description of clinical characteristics of a large systemic lupus erythematosus cohort from the Spanish rheumatology Society lupus registry (RELESSER) with emphasis on complete versus incomplete lupus differences. Medicine 2015;94:e267. 10.1097/MD.0000000000000267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pego-Reigosa JM, Lois-Iglesias A, Rúa-Figueroa Íñigo, et al. Relationship between damage clustering and mortality in systemic lupus erythematosus in early and late stages of the disease: cluster analyses in a large cohort from the Spanish Society of rheumatology lupus registry. Rheumatology 2016;55:1243–50. 10.1093/rheumatology/kew049 [DOI] [PubMed] [Google Scholar]
- 26.Riveros Frutos A, Holgado S, Sanvisens Bergé A, et al. Late-onset versus early-onset systemic lupus: characteristics and outcome in a national multicentre register (RELESSER). Rheumatology 2020. 10.1093/rheumatology/keaa477. [Epub ahead of print: 27 Oct 2020]. [DOI] [PubMed] [Google Scholar]
- 27.Pons-Estel BA, Catoggio LJ, Cardiel MH, et al. The GLADEL multinational Latin American prospective inception cohort of 1,214 patients with systemic lupus erythematosus: ethnic and disease heterogeneity among "Hispanics". Medicine 2004;83:1–17. 10.1097/01.md.0000104742.42401.e2 [DOI] [PubMed] [Google Scholar]
- 28.Ugarte-Gil MF, Pons-Estel GJ, Molineros J, et al. Disease features and outcomes in United States lupus patients of Hispanic origin and their Mestizo counterparts in Latin America: a commentary. Rheumatology 2016;55:436–40. 10.1093/rheumatology/kev280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ugarte-Gil MF, Wojdyla D, Pons-Estel GJ, et al. Remission and low disease activity status (LDAS) protect lupus patients from damage occurrence: data from a multiethnic, multinational Latin American lupus cohort (GLADEL). Ann Rheum Dis 2017;76:2071–4. 10.1136/annrheumdis-2017-211814 [DOI] [PubMed] [Google Scholar]
- 30.Stojan G, Petri M. Epidemiology of systemic lupus erythematosus: an update. Curr Opin Rheumatol 2018;30:144–50. 10.1097/BOR.0000000000000480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barbhaiya M, Feldman CH, Guan H, et al. Race/ethnicity and cardiovascular events among patients with systemic lupus erythematosus. Arthritis Rheumatol 2017;69:1823–31. 10.1002/art.40174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis MJ, Jawad AS. The effect of ethnicity and genetic ancestry on the epidemiology, clinical features and outcome of systemic lupus erythematosus. Rheumatology 2017;56:i67–77. 10.1093/rheumatology/kew399 [DOI] [PubMed] [Google Scholar]
- 33.Munroe ME, Vista ES, Merrill JT, et al. Pathways of impending disease flare in African-American systemic lupus erythematosus patients. J Autoimmun 2017;78:70–8. 10.1016/j.jaut.2016.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rees F, Doherty M, Grainge MJ, et al. The worldwide incidence and prevalence of systemic lupus erythematosus: a systematic review of epidemiological studies. Rheumatology 2017;56:1945–61. 10.1093/rheumatology/kex260 [DOI] [PubMed] [Google Scholar]
- 35.Pons-Estel GJ, Alarcón GS, Scofield L, et al. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin Arthritis Rheum 2010;39:257–68. 10.1016/j.semarthrit.2008.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uribe AG, Alarcón GS. Ethnic disparities in patients with systemic lupus erythematosus. Curr Rheumatol Rep 2003;5:364–9. 10.1007/s11926-003-0022-8 [DOI] [PubMed] [Google Scholar]
- 37.González LA, Toloza SMA, McGwin G, et al. Ethnicity in systemic lupus erythematosus (SLE): its influence on susceptibility and outcomes. Lupus 2013;22:1214–24. 10.1177/0961203313502571 [DOI] [PubMed] [Google Scholar]
- 38.Watson P, Brennan A, Birch H, et al. An integrated extrapolation of long-term outcomes in systemic lupus erythematosus: analysis and simulation of the Hopkins lupus cohort. Rheumatology 2015;54:623–32. 10.1093/rheumatology/keu375 [DOI] [PubMed] [Google Scholar]
- 39.Tsang-A-Sjoe MWP, Bultink IEM, Heslinga M, et al. Both prolonged remission and lupus low disease activity state are associated with reduced damage accrual in systemic lupus erythematosus. Rheumatology 2017;56:121–8. 10.1093/rheumatology/kew377 [DOI] [PubMed] [Google Scholar]
- 40.Stoll T, Sutcliffe N, Mach J, et al. Analysis of the relationship between disease activity and damage in patients with systemic lupus erythematosus--a 5-yr prospective study. Rheumatology 2004;43:1039–44. 10.1093/rheumatology/keh238 [DOI] [PubMed] [Google Scholar]
- 41.Toloza SMA, Roseman JM, Alarcón GS, et al. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA): XXII. predictors of time to the occurrence of initial damage. Arthritis Rheum 2004;50:3177–86. 10.1002/art.20578 [DOI] [PubMed] [Google Scholar]
- 42.Becker-Merok A, Nossent HC. Damage accumulation in systemic lupus erythematosus and its relation to disease activity and mortality. J Rheumatol 2006;33:1570–7. [PubMed] [Google Scholar]
- 43.Peschken CA, Katz SJ, Silverman E, et al. The 1000 Canadian faces of lupus: determinants of disease outcome in a large multiethnic cohort. J Rheumatol 2009;36:1200–8. 10.3899/jrheum.080912 [DOI] [PubMed] [Google Scholar]
- 44.Taraborelli M, Cavazzana I, Martinazzi N, et al. Organ damage accrual and distribution in systemic lupus erythematosus patients followed-up for more than 10 years. Lupus 2017;26:1197–204. 10.1177/0961203317693096 [DOI] [PubMed] [Google Scholar]
- 45.Cardoso CRL, Signorelli FV, Papi JAS, et al. Initial and accrued damage as predictors of mortality in Brazilian patients with systemic lupus erythematosus: a cohort study. Lupus 2008;17:1042–8. 10.1177/0961203308093829 [DOI] [PubMed] [Google Scholar]
- 46.Alarcón GS. Lessons from LUMINA: a multiethnic US cohort. Lupus 2008;17:971–6. 10.1177/0961203308094359 [DOI] [PubMed] [Google Scholar]
- 47.Urowitz MB, Gladman DD. Contributions of observational cohort studies in systemic lupus erythematosus: the University of Toronto lupus clinic experience. Rheum Dis Clin North Am 2005;31:211–21. 10.1016/j.rdc.2005.01.008 [DOI] [PubMed] [Google Scholar]
- 48.Alarcón GS, McGwin G, Bartolucci AA, et al. Systemic lupus erythematosus in three ethnic groups. IX. Differences in damage accrual. Arthritis Rheum 2001;44:2797–806. [DOI] [PubMed] [Google Scholar]
- 49.Bruce IN, O'Keeffe AG, Farewell V, et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the systemic lupus international collaborating clinics (SLICC) inception cohort. Ann Rheum Dis 2015;74:1706–13. 10.1136/annrheumdis-2013-205171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rahman P, Gladman DD, Urowitz MB, et al. Early damage as measured by the SLICC/ACR damage index is a predictor of mortality in systemic lupus erythematosus. Lupus 2001;10:93–6. 10.1191/096120301670679959 [DOI] [PubMed] [Google Scholar]
- 51.Nived O, Jönsen A, Bengtsson AA, et al. High predictive value of the systemic lupus international collaborating Clinics/American College of rheumatology damage index for survival in systemic lupus erythematosus. J Rheumatol 2002;29:1398–400. [PubMed] [Google Scholar]
- 52.Gilboe IM, Kvien TK, Husby G. Disease course in systemic lupus erythematosus: changes in health status, disease activity, and organ damage after 2 years. J Rheumatol 2001;28:266–74. [PubMed] [Google Scholar]
- 53.Mimica M, Barra I, Ormeño R, et al. Predictors of damage accrual in systemic lupus erythematosus: a longitudinal observational study with focus on neuropsychological factors and anti-neuronal antibodies. Clin Rheumatol 2019;38:3129–37. 10.1007/s10067-019-04707-x [DOI] [PubMed] [Google Scholar]
- 54.Petri M, Purvey S, Fang H, et al. Predictors of organ damage in systemic lupus erythematosus: the Hopkins lupus cohort. Arthritis Rheum 2012;64:4021–8. 10.1002/art.34672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Segura BT, Bernstein BS, McDonnell T, et al. Damage accrual and mortality over long-term follow-up in 300 patients with systemic lupus erythematosus in a multi-ethnic British cohort. Rheumatology 2020;59:524–33. 10.1093/rheumatology/kez292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ibañez D, Gladman DD, Touma Z, et al. Optimal frequency of visits for patients with systemic lupus erythematosus to measure disease activity over time. J Rheumatol 2011;38:60–3. 10.3899/jrheum.100575 [DOI] [PubMed] [Google Scholar]
- 57.Zavodovsky BV, Sivordova LE. Cardiovascular safety of non-steroidal anti-inflammatory drugs in chronic inflammatory rheumatic diseases. Ter Arkh 2018;90:101–6. 10.26442/terarkh2018908101-106 [DOI] [PubMed] [Google Scholar]
- 58.Munguia-Realpozo P, Mendoza-Pinto C, Sierra Benito C, et al. Systemic lupus erythematosus and hypertension. Autoimmun Rev 2019;18:102371. 10.1016/j.autrev.2019.102371 [DOI] [PubMed] [Google Scholar]
- 59.Chinthapalli K. High dose NSAIDs may double the risk of heart attacks and heart failure, says new study. BMJ 2013;346:f3533. 10.1136/bmj.f3533 [DOI] [PubMed] [Google Scholar]
- 60.Marsico F, Paolillo S, Filardi PP. NSAIDS and cardiovascular risk. J Cardiovasc Med 2017;18:e40–3. 10.2459/JCM.0000000000000443 [DOI] [PubMed] [Google Scholar]
- 61.Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA 2001;286:954–9. 10.1001/jama.286.8.954 [DOI] [PubMed] [Google Scholar]
- 62.Simon LS. Non-steroidal anti-inflammatory drugs and their benefits and harms: the challenge of interpreting meta-analyses and observational data sets when balanced data are not analyzed and reported. Arthritis Res Ther 2015;17:130. 10.1186/s13075-015-0650-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request.