Abstract
Background
Guggulutiktaka ghritam is an ayurvedic medicine which has been traditionally used to treat various chronic inflammatory conditions. However, the mechanism of action of the Ayurvedic medication in control of inflammatory conditions has not been clearly evaluated.
Objective
In the current study, the effect of the Guggulutiktaka ghritam extract (GTG) on the lipoxygenase pathway and in the production of proinflammatory cytokines involved in the pathogenesis of chronic inflammation was studied.
Materials and methods
The effect of GTG in the production of leukotriene was determined by enzyme inhibition studies on 12- lipoxygenase. The assay was carried out by ferrous oxidation of xylenol orange (FOX assay) and was compared to a positive control nordihydroguaiaretic acid. The effect of GTG on the production of proinflammatory cytokines TNF-α and IL-1β in monocytes were studied. For this, the monocytes were pretreated with various concentrations of GTG and subsequently stimulated with lipopolysaccharide. The cytokines TNF-α and IL-1β produced were quantified by ELISA and the results were compared to positive controls Rolipram and Dexamethasone respectively. The gene expression studies were carried out using qRT-PCR. The IC50 values were calculated and evaluated statistically.
Results
The result indicates that GTG in comparison to the positive control Nordihydroguaiaretic acid significantly reduced the activity of 12- lipoxygenase. Also, there was significant inhibition in the production of proinflammatory cytokines in LPS stimulated monocytes pretreated with GTG as compared to positive control Rolipram and Dexamethasone. There was significant downregulation of IL-1β gene in LPS stimulated monocytes pretreated with GTG as compared to control. These changes are further supported by Raman spectra obtained for GTG treated and untreated cells.
Conclusion
The study revealed that GTG is a leukotriene and cytokine inhibitor. The inhibition in the production of cytokines may be due to the down-regulation of genes for TNF-α and IL-1β. The study provides a scientific validation on the possible anti-inflammatory mechanism of action of this traditionally used medicine. Identification of bioactive molecules would aid in developing newer therapeutics for control of chronic inflammation.
Keywords: Ayurveda, Guggulutiktaka ghritam, Lipoxygenase, Monocytes, TNF-α, IL-1β, Raman spectroscopy
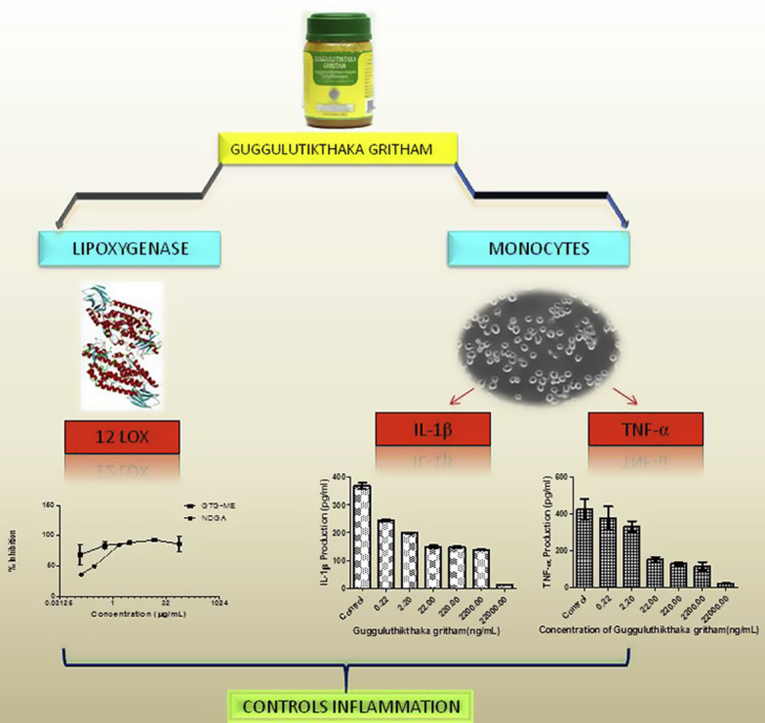
Graphical abstract
Highlights
-
•
Guggulutiktaka ghritham (GTG) has multiple targeted actions in controlling inflammation.
-
•
GTG treatment decreases the production of proinflammatory cytokines TNF-α and IL-1β and inhibits lipoxygenase activity.
-
•
Studies indicate a simple and easy protocol to evaluate efficacy of drugs or natural products.
1. Introduction
Complex interactions between various immunomodulators comprising of cytokines and different signaling pathways are the hallmark of chronic inflammation [1]. It has been reported that the leukotriene product of 12-lipoxygenase (12–LOX) has an important role in the progression of inflammatory conditions [2], [3]. Monocytes and macrophages contribute to chronic inflammatory conditions by producing higher amounts of Prostagladins-2 (PGE2), superoxide anions and lysosomal enzymes leading to tissue damage [4]. 12–LOX and its products are involved in the adhesion of monocytes to the endothelial cells, which is an important step in inflammatory pathways [5]. In addition, they are also involved in the production of TNF-α and interleukins which further complicate the chronic inflammatory conditions.
Cytokines are key mediators of the inflammatory process. They orchestrate inflammatory responses and are major determinants of cellular activation and systemic response to inflammation. Cytokines have a multifunctional role in having both autocrine and paracrine action as well as in having a synergistic and antagonistic inflammatory activity [6]. Several cytokines play an important role in the progression of inflammation. Among these TNF-α and IL-1β are potent inflammatory mediators. TNF-α and IL-1β are involved in the synthesis of prostaglandins and cellular adhesion molecules, B and T cell maturation, the release of lysosomal enzymes in case of arthritis, diabetes and obesity [7]. IL-1 is localized in synovial pannus produced by cells lining bones and joints and by chondrocytes and is more potent in the induction of tissue-damaging matrix metalloproteinases (MMPs) in rheumatoid arthritis [8]. Cytokine therapies, which include anti-TNF-α and IL-1β, have been the recent trend in the treatment of inflammation [9], [10]. Anti IL-1β therapy before the onset of arthritis has proven to be beneficial [11]. Studies have shown inhibition of both TNF-α and IL-1 decreases inflammation due to arthritis [12]. But various side effects like nausea, gastrointestinal disturbances, the occurrence of opportunistic infections, lymphomas and cancer are associated with such therapies [7]. The use of herbal medicines with comparatively few to no side effects may be beneficial in such circumstances.
Ayurveda is the traditional platform of medicine, prevalent in India, Sri Lanka and other South Asian countries, having a history which dates back to 5000 B.C [13], [14]. Clinical studies of ayurvedic treatment were found to be as effective as conventional treatment [15], [16]. Gugguglutiktaka ghritam, (GTG) also known as Pancatikta guggulu ghrita/nimbadi ghrita, is an ayurvedic lipid formulation which, according to the classical ayurvedic text Astanga hridayam, is used for the treatment of bone and associated disorders [17] and has been indexed in Ayurveda pharmacopoeia of India (A.F.I. Part-I, 6:27) [18] as a drug used in the treatment of chronic inflammatory conditions as Sandhigata vata, Vatarakta, Asthigata vata, (Osteoarthritis, Gout, Osteoporosis). Ghritas are ayurvedic formulations prepared in ghee (clarified butter) along with polyherbal decoctions. The lipid base helps in better absorption and delivery of the active constituents [19]. Efficacy of Guggulutiktaka ghritam in the management of arthritic conditions like rheumatoid arthritis and osteoarthritis have been studied [17], [20]. The dosage and method of administration of drug have been previously reported [17]. However, scientific evidence on the mechanism of action of the drug in controlling inflammation is not available. Since most of the Ayurvedic drugs are poly herbal in nature it may influence multiple metabolic pathways. Not much work has been carried out in determining the cytokine and lipoxygenase modulatory activity of Ayurvedic medicines as compared to traditional Chinese medicines [21].
The present study aims to elucidate the possible mechanism of action of GTG in the control of proinflammatory cytokines production by monocytes and also on lipoxygenase enzymes which are involved in the progression of chronic inflammation associated with various disorders.
2. Materials and methods
2.1. Reagents
Pseudomonas aeruginosa LPS (LPS), Dexamethasone and Rolipram from Sigma–Aldrich (USA), Nordihydroguaiaretic acid (NDGA) from Cayman Chemicals (USA), ELISAMAX™ DELUX HUMAN TNF-α and IL-1β ELISA kits from Biolegend (USA), Thermo Scientific Detoxi-Gel Endotoxin Removing Columns from Thermoscientific (USA), Methanol, EDTA from Merck (USA) and Antibiotic solution, EZ™ MTT cell assay kit, Ferrous sulfate, Fetal bovine serum (FBS), HISEP™, Linoleic acid, RPMI 1640, Trypan blue, Tris base and Tween 20 from Himedia Laboratories (India) were purchased.
2.2. Preparation of Guggulutiktaka ghritam and validation of the drug by HPLC
Guggulutiktaka ghritam was obtained from Arya Vaidyasala Kottakkal, India (Batch No: 145426, Mfg.Lic.No: 1/25D/76, QC report provided in supplementary data). Industrially Guggulutiktaka ghritam is prepared by boiling ghee with the decoctions of ingredients 2–6 and paste of ingredients 7–31 (Supplementary Table.1), stirred well and attained to medium consistency, filtered and packed. The Guggulutiktaka ghritam extract (GTG) was prepared by maceration in HPLC grade Methanol. 20 gm of Guggulutiktaka ghritam yielded 1.998 gm of extract. The extract obtained was lyophilized, weighed and redissolved in 0.1% DMSO for cell culture studies and in HPLC grade methanol for enzyme inhibition studies and for further analysis. Prior to analysis, the extracts were passed through Thermo Scientific Detoxi-Gel Endotoxin Removing Columns for the removal of endotoxins.
The drug validation was performed as per the guidelines of WHO [22] for herbal medicines and as described earlier [23]. In order to validate the commercially obtained drug, two different batches were used. Reports on the use of bioactive constituents present in different plant components of polyherbal formulations as marker compounds for standardization is available [23]. HPLC fingerprinting was done and compared with bioactive constituents/standards present in the herbal preparation. Bioactive molecules identified and used for fingerprinting chromatography are lupeol of C. paniculatus, H. antidysentrica and P. rosea, piperine of P. longum and P. nigrum, piperlongumine of P. longum, rutin of A. indica, stigmasterol of C. longa, ferulic acid of C. mukul and A. indica and gallic acid of A. indica. For HPLC analysis 20 μl of methanolic extract of Guggulutiktaka ghritam was used (Fig. 1). HPLC analysis was performed on Shimadzu LC20AP, Japan with Enable C18 250 × 4.6 mm column having a particle size 5 μm and pore size 120 A0. An isocratic system consisting of 60% Acetonitrile (solvent B) in 0.1% TFA in water (solvent A) was run for 60 min with a flow rate of 1.0 mL/min for both the GTG extract and the standards. The UV detector was set at 254 nm. The data obtained were analyzed by LC solution software by Shimadzu, Japan.
Fig. 1.
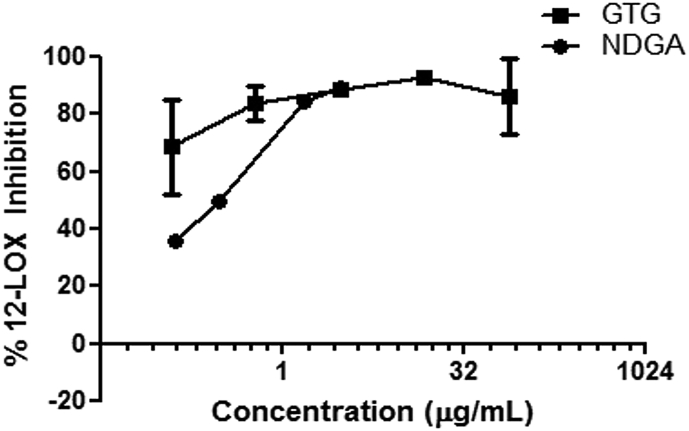
Figure showing percentage inhibition of 12-LOX by GTG as compared to that of NDGA. Values from three independent experiments are plotted as mean ± S.D.
2.3. Platelet 12-lipoxygenase isolation
Platelet 12-LOX was isolated from human platelets (Institutional human ethical clearance No: IEC/IRB No: 4/IHEC20082015) as per the protocols described earlier [24] with slight modifications. Briefly, human platelets isolated from platelet-rich plasma (PRP) obtained from blood bank was initially centrifuged at 230 g for 5 min followed by centrifugation of supernatant at 500 g for 20 min. The pellet obtained was resuspended in phosphate buffer saline (pH 7.4, 2 mM EDTA) and again centrifuged at 230 g for 5 min. The supernatant was discarded and the pellets were suspended in fresh calcium-free tyrodes buffer (pH 7.4) and kept at room temperature for 30 min. The pellets were lysed by adding Triton X 100 to 0.1% of pellet suspension and followed by freeze-thawing. The cell debris was removed by centrifugation and the enzyme was stored at −80° C until use. The total protein was estimated by Bradford assay [25].
2.4. Effect of GTG on platelet 12-lipoxygenase
Inhibition of platelet 12 lipoxygenases by GTG extract was carried out by FOX assay as described earlier [26]. FOX reagent was prepared by dissolving Xylenol orange (100 μM) in methanol: water (9:1) containing sulphuric acid (30 μM) and ferrous sulfate (100 μM). For FOX assay, 20 μL of different concentrations of GTG and positive control Nordihydroguaiaretic acid (NDGA) separately were incubated with 20 μL of platelet 12- LOX (100 ng of protein) for 20 min at room temperature. 50 μL of the linoleic acid substrate (140 μM) was added, mixed and incubated for 20 min 130 μL of FOX reagent was added and kept in dark for 30 min at room temperature. Fox reagent and methanol was used as negative control. Bio-Rad microplate reader was used to record the absorbance at 590 nm. Percentage inhibition was further calculated using the formula
where absorbance of control represents uninhibited enzyme activity whereas that of the test represents the enzyme activity either in the presence of GTG or positive control. From the percentage inhibition, IC50 value was calculated by using Graph pad prism 5 TM software. The values obtained were compared to that obtained for positive control Nordihydroguaiaretic acid (NDGA).
2.5. Isolation and culture of monocytes and cytotoxicity assay
Ethical clearance from institutional human ethical committee (IEC/IRB No: 4/IHEC20082015) and consent from healthy volunteers were obtained for drawing blood. Fresh blood was collected by venipuncture and monocytes were isolated by density gradient centrifugation using HISEP™ according to manufacturer's instructions. The cells isolated were suspended in RPMI1640. The viability of cells was determined by Trypan blue exclusion assay. MTT (3-(4, 5-Dimethylthiazol-2-yl)-2, 5-Diphenyltetrazolium Bromide) assay using EZ™ MTT cell assay kit was performed to check the cytotoxicity of the GTG extract. Briefly, from a stock cell suspension of 1 × 106 cells/mL of PBMC, 100 μL of cell suspension were seeded onto 96 well plates and incubated for 2 h at 37 °C for monocytes cell adhesion. Nonadherent cells were removed and to the remaining adherent cells, varying concentrations of GTG extract (0.0044–4.4 μg/mL) was added and incubated for 2 h, after which 10 μL of MTT reagent was added and the plates were kept for 4 h for the formation of formazan crystals. The crystals formed were dissolved in solubilizing solution (DMSO) and absorbance was read at 570 nm. Concentrations, which proved to be non-cytotoxic, were used for further cell culture studies.
2.6. Antigenic stimulation, cytokine quantification and gene expression
For cytokine production by monocytes and gene expression studies P. aeruginosa LPS was used as the antigen. Monocytes were seeded at a cell density of 1 × 106 cells/mL in RPMI1640 containing 10% heat-inactivated fetal bovine serum, 4.5 g glucose/L, 100 U/mL of penicillin and 0.1 mg/mL of streptomycin at 37 °C at 5% CO2. The cells were pretreated with varying concentration of GTG and positive standards Rolipram and Dexamethasone for 2 h before LPS stimulation. LPS (at a concentration of 100 ng/mL/well) dissolved in 0.1% DMSO was added to the media and further incubated for 24 h. Monocytes with solvent but without LPS and GTG treatment was kept as negative control. A control group consisting of cells without GTG and positive control instead with solvents and LPS was also kept for comparison.
For cytokine quantification, the cell suspension was centrifuged and the supernatant was taken. The cytokines were estimated by ELISAMAX™ DELUX HUMAN TNFα and IL1 β kits as per the protocols provided along with the kits. The absorbance was recorded with Thermo Scientific Varioskan multimode reader and the data were analyzed for concentration by four parameter logistic curve equation. The value of blank was subtracted from the control and test. The percentage inhibition values were calculated as ((Value of control- Value of test)/Value of control) ×100. The IC50 values for the GTG and positive controls were obtained from the percentage inhibition curve drawn using GraphPad Prism 5™ software.
For gene expression studies, total cellular RNA was isolated from the cells obtained after centrifugation using RNAiso Plus reagent (Takara, Japan) according to the kit protocol. The extracted RNA was dissolved in nuclease-free water. DNase I (1U/μL) was added to the RNA solution to remove residual DNA if any. DNase was denatured by heating at 75 °C for 10 min 100 ng of the extracted RNA was converted to cDNA using Bio-Rad iScript cDNA synthesis kit. Real-time qPCR was carried out using Roche SYBR Green I master mix using 2 μL of synthesized cDNA along with primers of various inflammatory markers:TNF-α, forward: 5′-GTTCTATGGCCCAGACCTCACA-3′, reverse: 3′-TACCAGGGTTTGAGCTCAGC-5’; IL-1β, forward: 5′-GATGAAGTGCTCCTTCCAGG-3′, reverse: 3′-GGAGAACACCACTTGTTGCT-5′, GAPDH, forward: 5′-GACCACAGTCCATGCCATCAC- 3′, reverse:3′-TCCACCACCCTGTTGCTGTAG-5’; β-actin, forward: 5′-ATCTGGCACCACACCTTCTACAATGAGCTGCG-3′,reverse: 3′CGTCATACTCCTGCTTGCTGATCCACATCTG-5′.
Real-Time PCR was performed by Roche Light cycler 96™ PCR instrument. The reaction involves an initial denaturation at 95 °C for 5 min, amplification and quantification at 95 °C for 10 s, followed by that at 72 °C for 30 s. Melting curve program was set at 65–97 °C for 1 min with a ramp rate of 0.1 °C per second and a continuous fluorescence measurement and cooling at 40 °C for 10 s. The data obtained were analyzed using Light Cycler 96 SW 1.0 software. The results were normalized with respect to the reference gene GAPDH and β-actin and the fold expressions were determined.
2.7. Raman spectroscopy on cultured monocytes
For Raman spectroscopic studies, the monocytes were cultured on sterile glass coverslips kept in 24-well cell culture plates at a cell density of 1 × 106 cells/mL in RPMI1640 containing 10% heat-inactivated fetal bovine serum, 4.5 g glucose/L, 100 U/mL of penicillin and 0.1 mg/mL of streptomycin at 37 °C at 5% CO2. The cells were pretreated with GTG (0.22 μg/mL) for 2 h before LPS stimulation. LPS at a concentration of 100 ng/mL was added to the media and further incubated for 24 h. For control, media containing 0.1% DMSO without GTG extract was taken.
After 24 h, the cell culture media was removed and the cells were washed thrice with PBS. The cells were then fixed with 4% formaldehyde solution for 15 min and excess formaldehyde was washed off with sterile distilled water. Raman spectra of the air-dried fixed cells were taken. Raman spectra were acquired by WItec Alpha 300RA (WITec GmbH, Ulm, Germany). Spectra were obtained using a 532 nm DPSS laser (with a power after single mode fiber of around 60 mW) which is coupled into an optical microscope (Modified Zeiss Axio Scope with an objective of 100X Zeiss with 0.9 NA). The signal is collected into a multimode optical fiber connected to UHTS300 spectrograph with a back-illuminated CCD. The spectra were measured in the range of 500-3500 cm−1 with a spectral resolution of 4 cm−1 and were averaged over 10 scans with an exposure time of 10 s. The results were normalized with the intensity of the peak at 1745 cm−1.
2.8. Statistical analysis
The experiments were done in triplicate. The results were expressed as a mean ± standard error. Statistical analysis was done by one-way ANOVA with Tukey's multiple comparison post-test having a significance level of 95% confidence interval using GraphPad Prism 5™. A p-value ≤ 0.05 was considered as statistically significant.
3. Results
3.1. Standardization of GTG extract
Chromatographic fingerprinting by means of HPLC revealed a similar pattern of chromatogram for two different batches of the drug and also bioactive compounds as indicated by retention time (Supplementary Figure 1). This proves consistency in composition and manufacturing process. HPLC chromatogram shows major peaks at retention time 3.4, 4.1, 5.2, 5.9, 6.8, 8.6, 9.3, 11.2, 12.3, 13.6, 14.9, 17.9, 19.6, 21.3, 23.8, 25.0, 26.7, 28.0, 35.2, 38.8, 40.1 and 45.2 min for both batches.
3.2. Effect of GTG on inhibition of 12-LOX
The effect of GTG on leukotriene production by 12 LOX was carried out as described in methods. The percentage inhibition and IC50 values were calculated for the extracts and compared with positive control NDGA (Fig. 1). In case of 12-LOX, the IC50 value was 3.883 ± 0.8719 ng/mL for GTG and 270.9 ± 1.0414 ng/mL for NDGA (Table 1).
Table 1.
IC50 values of GTG and NDGA on 12-LOX.
| 12-LOX IC50 (ng/mL) | |
|---|---|
| GTG | ∗∗∗3.883 ± 0.8719 |
| NDGA | 270.9 ± 1.0414 |
∗∗∗p-value <0.001 as compared to positive control NDGA.
Values are expressed as mean ± S.E of three independent experiments.
3.3. Effect of GTG on production of TNF-α and IL-1β
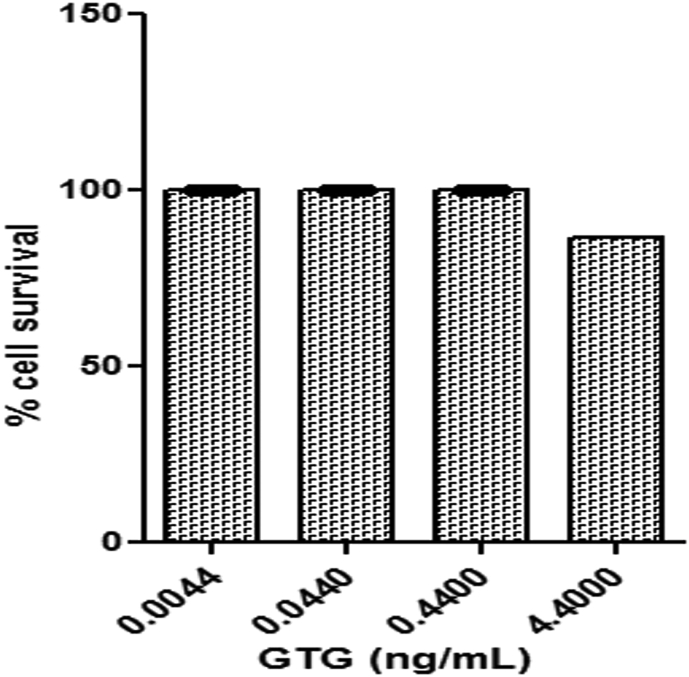
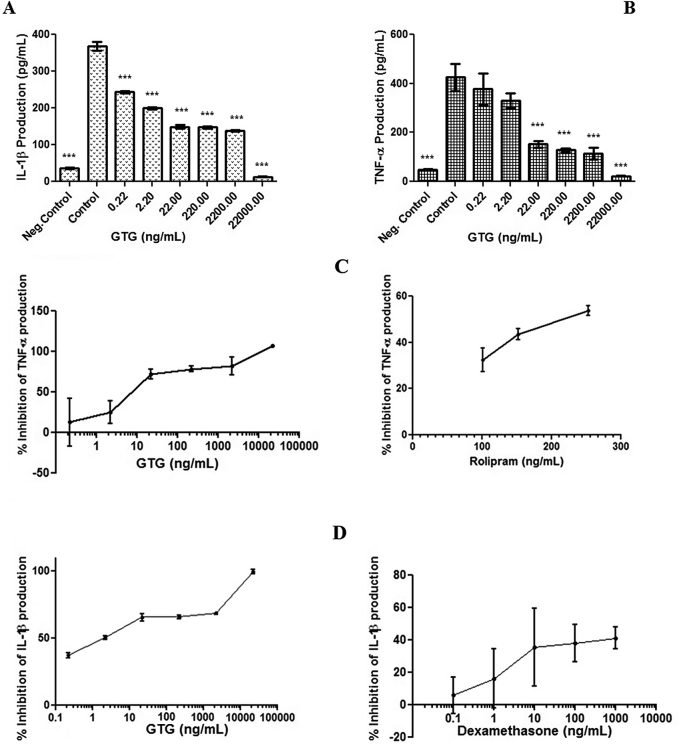
MTT assay showed the absence of cytotoxicity of GTG at concentrations (0.0044–4.4 μg/mL) towards monocytes (Fig. 2). Cells pretreated with either GTG or positive controls Rolipram for TNF-α and Dexamethasone for IL-1β were incubated with LPS for 24 h and the amount of cytokine produced by the cells was quantified (Fig. 3) The IC50 values for GTG in inhibiting TNF-α and IL-1β were obtained and compared to the IC50 values of positive controls as shown in Table 2. An IC50 of 9.402 ± 0.1993 ng/mL and 2.482 ± 0.2673 ng/mL was obtained for the inhibition in production of TNF-α and IL-1β respectively for GTG whereas the positive control for TNF-α inhibition Rolipram was 211.2 ± 0.0011 ng/mL and for Dexamethasone the positive control for IL-1β the value was 2039 ± 0.0095 ng/mL.
Fig. 2.
Figure showing the percentage cell survival of monocytes on incubation with different concentration of GTG as determined by MTT assay. Values plotted are from three independent experiments as mean ± S.D.
Fig. 3.
Effect of GTG on the production of (A) IL-1β and (B) TNF-α in LPS activated monocytes after 24 h of incubation as compared to the control group. Graph showing the percentage inhibition of TNF-α production by GTG and Rolipram (C) and IL-1β production by GTG and Dexamethasone (D). (Neg.Control-negative control; Control contains LPS treated monocytes whereas GTG contains LPS + GTG treated monocytes). *** p-value < 0.001, as compared to control. Values plotted are from three independent experiments as mean ± SD.
Table 2.
IC50 values of GTG and NDGA on TNF-α and IL-1β.
| IC50 (ng/mL) |
||
|---|---|---|
| TNF-α | IL-1β | |
| GTG | ∗∗∗9.402 ± 0.1993 | ∗∗∗2.482 ± 0.2673 |
| Rolipram | 211.2 ± 0.0011 | – |
| Dexamethasone | – | 2039 ± 0.0095 |
∗∗∗p-value <0.001, as compared to respective positive controls.
Values are expressed as mean ± S.E of three independent experiments.
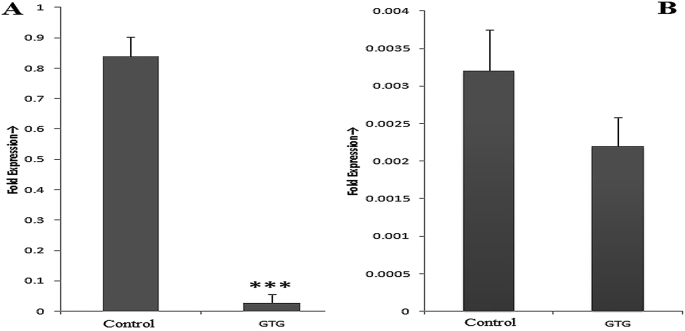
Quantitative real-time PCR revealed that in the control group consisting of only LPS stimulated monocytes, gene expression of both proinflammatory cytokines are increased. Upon pretreatment with GTG, there was decreased gene expression compared to that of control. Gene expression of IL-1β was down-regulated by 96.63% and TNF-α by 31.25% as compared to that of control (Fig. 4).
Fig. 4.
Quantitative Real-Time PCR: Expressions of (A) IL1-β and (B) TNF-α in LPS treated monocytes. (Control contains LPS treated monocytes whereas GTG contains LPS + GTG (0.22 μg/mL) treated monocytes). Values plotted are from three independent experiments as mean ± SD. *** p-value < 0.001 as compared to control.
3.4. Effect of GTG on total cellular changes
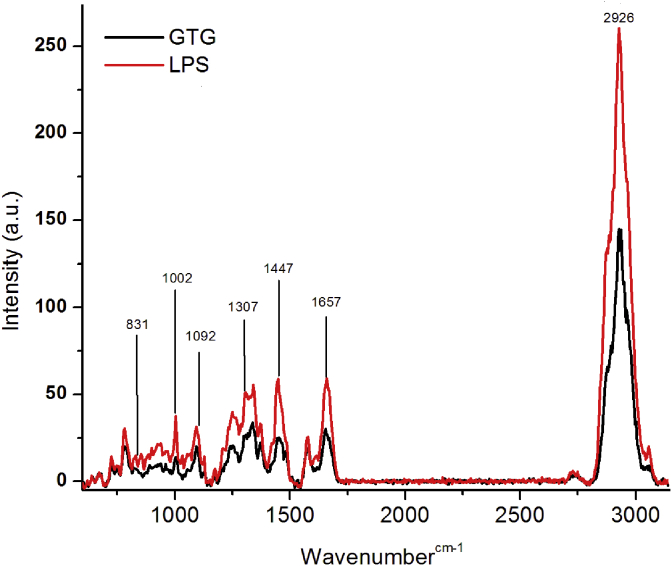
The total cellular changes brought about by LPS stimulation and by GTG on LPS treated monocytes were visualized by Raman spectroscopy. Raman spectra obtained in LPS stimulated monocytes (control) and cells that were pretreated with GTG, showed a clear difference. The changes were noticeable in case of peak intensities and in presence or absence of certain peaks. Major peaks that differed in Raman intensities between pretreated and control cells are shown in Fig. 5.
Fig. 5.
Raman spectra of LPS treated monocytes. (Control contains LPS treated monocytes whereas GTG contains LPS + GTG treated monocytes).
4. Discussion
Inflammation is a complex process involving enzymes of prostaglandin synthesis and various cytokine pathways. Though reports on the inhibitory activity of lipoxygenase by various plants used in herbal medicine are available, the effects of polyherbal formulations used in Ayurveda are relatively unknown. GTG is a polyherbal formulation, mentioned in ancient Ayurvedic text Astanga hridayam and in Ayurvedic pharmacopeia of India, used for the treatment of chronic inflammatory conditions like rheumatism and related disorders. Clinical trials have shown that GTG reduces pain, swelling, tenderness and aids in enhanced joint movements in osteoarthritis patients [17]. Results presented in Fig. 1 indicate the potential of the polyherbal formulation GTG in inhibiting 12 LOX. The inhibition of 12-LOX by GTG is compared with that of positive control Nordihydroguaiaretic acid (NDGA), a selective lipoxygenase inhibitor [27]. It has been reported that 12-LOX products mediate monocyte-endothelial interactions in inflammatory conditions [5], [28] and leukotriene-lipoxygenase pathways are associated with the pathophysiology of acute and chronic inflammation and hence, drugs that can interfere with the pathway, are of great potential to control inflammation [29].
Lipopolysaccharide-stimulated production of pro-inflammatory cytokines by monocytes and macrophages and their inhibition is a widely chosen in vitro model for studies on screening for anti-inflammatory molecules [7], [30]. LPS is a bacterial endotoxin which stimulate immune response in monocytes/macrophages through TLR4 resulting in activation of various inflammatory mediators including TNF-α [31]. Results presented in Table 2 indicate that IC50 values shown by GTG in inhibition of production of proinflammatory cytokines, TNF-α and IL-1β, in LPS stimulated monocytes was statistically lower compared to that treated with positive controls Rolipram and Dexamethasone. Besides comparing the IC50 values, a statistical comparison of various concentrations of GTG with different concentrations of Rolipram and Dexamethasone was done (supplementary table 2–3). The results presented in supplementary Table 2–3 show that the inhibition of TNF-α and IL-1β by GTG is at par with Rolipram and Dexamethasone and at a certain concentration, the inhibition was much better. The IC50 values (Table 2) show that GTG is much more potent than the respective positive control used in the inhibition of pro-inflammatory cytokines TNF-α and IL-1β. Inhibition of pro-inflammatory cytokines production by extracts of GTG is in line with the new approach in the treatment of chronic inflammatory diseases [32].
Further studies were carried out to investigate the efficacy of GTG extract at the level of TNF-α and IL-1β gene expression in LPS stimulated monocytes. Several pathological conditions like Rheumatoid arthritis, Ankylosing spondylitis, Diabetes, Obesity, Parkinson's Disease, Spondyloarthropathies, Crohn's disease, Ulcerative colitis, Gastritis etc. are manifested by overexpression of cytokines like TNF-α and IL-1β [7]. The gene expression study (Fig. 4) shows that GTG has reduced the expression of both pro-inflammatory cytokines. The effect was more pronounced in IL-1β gene than TNF-α. Both TNF-α and IL-1β are primary mediators in the pathogenesis of chronic inflammation in rheumatoid arthritis and are potent stimulators of synovial fibroblasts, osteoclasts and chondrocytes [33]. As TNF-α is involved in joint swelling and IL-1β in cell invasion and erosive cartilage damage [34], the inhibition, of both these proinflammatory cytokine production, plays a pivotal role in controlling inflammatory arthritis. Further studies have to be carried out to elucidate the role of GTG in controlling inflammation in arthritis, as the mechanism by which LPS elicit inflammation (through TLR4) is different from the inflammatory response in Rheumatoid arthritis, which involves a wide array of mechanism and cells other than monocytes. However, the data obtained from this work is promising, as inhibition of production of TNF-α and IL-1β are identified as potential therapeutic targets for pathophysiological conditions associated with chronic inflammation.
Data observed at gene expression level are further confirmed by results of Raman spectroscopy. The cells which were treated with LPS with and without pretreatment of GTG (control) were subjected to Raman spectroscopic analysis. The spectra obtained for monocytes are in line with the previous reports [35]. Raman spectra obtained in LPS stimulated monocytes (control) and cells that were pretreated with GTG before LPS stimulation, showed a clear difference in Raman spectra (Fig. 5). Increased production of proinflammatory cytokines by monocytes occurs during inflammatory conditions and also during LPS stimulation [7]. The peak at 1447 cm−1 which corresponds to the total cellular proteins has been found to be increased in control cells, whereas in GTG treated cells the intensity was less. The increase in Raman peak intensity at 1447 cm−1, which denotes the CH2 bending, is a measure of total cellular protein concentration and is not affected by protein secondary structure or various protein interactions [36], [37]. This increased concentration of proteins in cells is probably a reflection of increased production of proinflammatory molecules TNF-α and IL-1β which are proteinaceous in nature. Similarly, the peak at 1002 cm−1 assigned to the C-C symmetric ring breathing of phenylalanine was found to be more in LPS control cells and had decreased with GTG pretreatment. The production of cytokines TNF-α and IL-1β in LPS stimulated cells and decreases on pretreatment of cells with GTG, as confirmed by ELISA. This observation is further supported by the changes in Raman spectral peaks corresponding to proteins. During inflammatory conditions, the expression of various genes including TNF-α and IL-1β are found to be increased in monocytes [38]. Raman shift near 1092 cm−1 reflecting stretching vibration of the DNA–phosphate backbone was observed in LPS stimulated monocytes. This stretching of DNA backbone can be possibly due to binding of transcription factors to DNA. This observation is further confirmed by an increase in the intensity of RNA peak at 831 cm−1, indicating an increased concentration of RNA leading to an increase in expression of TNF-α and IL-1β in LPS stimulated cells. This increase in expression of proinflammatory genes, specifically that of TNF-α and IL-1β is more as has been observed by qRT-PCR and confirmed by the results of ELISA quantification of TNF-α and IL-1β in LPS stimulated cells. Besides, the protein and nucleic acid peaks, peaks at 1307 and 1657 cm−1 (CH3/CH2 twisting or bending) corresponding to the presence of lipids were also found [36]. The intensities of these peaks increased in case of LPS treated control and was less in GTG pretreated cells. This increase in lipid content can be explained in terms of increased production of lipid moieties like eicosanoids during inflammation due to increased activity of pro-inflammatory enzymes like cyclooxygenase and lipoxygenases and also from the fact that there could be an increased release of fatty acids like arachidonic acids from cell membrane during inflammatory conditions [39], [40], [41], [42]. A broad peak at 2926 cm−1, which denotes overall changes in lipids and proteins, was decreased in GTG pretreated cells. Further, the study also indicated that pretreatment of monocytes with GTG protected the cells by decrease gene expression of TNF-α and IL-1β.
In the present study, GTG has been shown to exert its effect on multiple targets i.e. inhibition of leukotriene production by inhibiting 12- LOX activity, inhibiting production and secretion of TNF-α and IL-1β as well as decreasing the gene expressions of TNF-α and IL-1β, all of which are processes which could control chronic inflammation. Further studies to identify bioactive molecules that interact with multiple targets would help in developing newer therapeutic molecules for control of chronic inflammation. The result obtained from this study supports the clinical finding and effectiveness of GTG and also validates its ethnopharmacological uses.
5. Conclusion
Ayurvedic medications have been used for many decades for the treatment of various ailments, but the underlying mechanism of action has not been clearly elucidated. Reports are available on the interactions of pure plant bioactive molecules with various inflammatory pathways but such studies in case of Ayurvedic polyherbal formulations have seldom been reported. This study brings an insight into the mechanism by which a polyherbal Ayurvedic formulation inhibits the inflammatory pathways of cytokines and the activity of lipoxygenase enzymes. It is possible that GTG exerts its anti-inflammatory property by inhibiting the pro-inflammatory cytokine production and the lipoxygenase enzymatic pathway. The study showed that GTG is more efficient in inhibiting IL-1β, which in turn maybe responsible in controlling various immunological conditions. It is quite possible that a disease is caused due to multiple factors, cure of which would requires treatment of multiple targets. Hence, a single drug with single target fails to completely cure a disease condition. Ayurveda, an alternative form of therapy uses polyherbal decoctions and ghritam with a large number of bioactive molecules which act synergistically at different targets to control a disease.
Source(s) of funding
None declared.
Conflict of interest
None.
Acknowledgment
The authors thank DBT MSUB project (BT/PR4800/INF/22/152/2012 dated 22/03/2012) and Inter-University Instrumentation Center for instrument facility. Authors also thank Vaidya Ratnam P.S. Variers Arya Vaidya Sala, Kottakal, Kerala, India for their constant support.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jaim.2018.05.007.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Choy E. Understanding the dynamics: pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology. 2012;51(Suppl. 5):v3–v11. doi: 10.1093/rheumatology/kes113. [DOI] [PubMed] [Google Scholar]
- 2.Liagre B., Vergne P., Rigaud M., Beneytout J.L. Expression of arachidonate platelet-type 12-lipoxygenase in human rheumatoid arthritis typeB synoviocytes. FEBS Lett. 1997;414:159–164. doi: 10.1016/s0014-5793(97)00904-6. [DOI] [PubMed] [Google Scholar]
- 3.Wu M.-Y., Lin T.-H., Chiu Y.-C., Liou H.-C., Yang R.-S., Fu W.-M. Involvement of 15-lipoxygenase in the inflammatory arthritis. J Cell Biochem. 2012;113:2279–2289. doi: 10.1002/jcb.24098. [DOI] [PubMed] [Google Scholar]
- 4.Fujii I., Shingu M., Nobunaga M. Monocyte activation in early onset rheumatoid arthritis. Ann Rheum Dis. 1990;49:497–503. doi: 10.1136/ard.49.7.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reilly K.B., Srinivasan S., Hatley M.E., Patricia M.K., Lannigan J., Bolick D.T. 12/15-Lipoxygenase activity mediates inflammatory monocyte/endothelial interactions and atherosclerosis in vivo. J Biol Chem. 2004;279:9440–9450. doi: 10.1074/jbc.M303857200. [DOI] [PubMed] [Google Scholar]
- 6.Feghali C.A., Wright T.M. Cytokines in acute and chronic inflammation. Front Biosci. 1997;2:d12–d26. doi: 10.2741/a171. [DOI] [PubMed] [Google Scholar]
- 7.Abhimannue A.P., Mohan M.C., Kumar P.B. Inhibition of tumor necrosis factor-α and Interleukin-1β production in lipopolysaccharide-stimulated monocytes by methanolic extract of elephantopus scaber linn and identification of bioactive components. Appl Biochem Biotechnol. 2016;179:427–443. doi: 10.1007/s12010-016-2004-0. [DOI] [PubMed] [Google Scholar]
- 8.Dayer J.M. The pivotal role of interleukin-1 in the clinical manifestations of rheumatoid arthritis. Rheumatology (Oxford) 2003;42(Suppl. 2):ii3–ii10. doi: 10.1093/rheumatology/keg326. [DOI] [PubMed] [Google Scholar]
- 9.Federici S., Martini A., Gattorno M. The central role of anti-IL-1 blockade in the treatment of monogenic and multi-factorial autoinflammatory diseases. Front Immunol. 2013;4 doi: 10.3389/fimmu.2013.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seymour H.E., Worsley a., Smith J.M., Thomas S.H.L. Anti-TNF agents for rheumatoid arthritis. Br J Clin Pharmacol. 2001;51:201–208. doi: 10.1046/j.1365-2125.2001.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van den Berg W.B., Joosten L.A., Helsen M., van de Loo F.A. Amelioration of established murine collagen-induced arthritis with anti-IL-1 treatment. Clin Exp Immunol. 1994;95:237–243. doi: 10.1111/j.1365-2249.1994.tb06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joosten L.A., Helsen M.M., Saxne T., van De Loo F.A., Heinegard D., van Den Berg W.B. IL-1 alpha beta blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas TNF-alpha blockade only ameliorates joint inflammation. J Immunol. 1999;163:5049–5055. [PubMed] [Google Scholar]
- 13.Mukherjee P.K., Wahile A. Integrated approaches towards drug development from Ayurveda and other Indian system of medicines. J Ethnopharmacol. 2006;103:25–35. doi: 10.1016/j.jep.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 14.Patwardhan B., Vaidya A.D.B., Chorghade M. Ayurveda and natural products drug discovery. Curr Sci. 2004;86:789–799. [Google Scholar]
- 15.Witt C.M., Michalsen A., Roll S., Morandi A., Gupta S., Rosenberg M. Comparative effectiveness of a complex Ayurvedic treatment and conventional standard care in osteoarthritis of the knee--study protocol for a randomized controlled trial. Trials. 2013;14:149. doi: 10.1186/1745-6215-14-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krishna Kumar P. The efficacy of Ayurvedic treatment for rheumatoid arthritis: cross-sectional experiential profile of a longitudinal study. Int J Ayurveda Res. 2011;2:8. doi: 10.4103/0974-7788.83177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patil B.D., Sonaje M.L. Role of guggulutiktaka ghrita in the management of osteoarthritis W.S.R. to knee joint. Int J Res Ayurveda Pharm. 2013;4:71–73. [Google Scholar]
- 18.Anonymous . The ayurvedic pharmacopoeia of India, First. Ministry of Health and Family Welfare Government of India Department of Ayurveda, Yoga – Naturopathy, Unani, Siddha & Homeopathy (AYUSH); 2008. The ayurvedic pharmacopoeia of India, Part II, vol.II; pp. 170–174. [Google Scholar]
- 19.Duraipandi S., Selvakumar V., Er N.Y. Reverse engineering of Ayurvedic lipid based formulation, ghrita by combined column chromatography, normal and reverse phase HPTLC analysis. BMC Compl Altern Med. 2015;15:1–6. doi: 10.1186/s12906-015-0568-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Survase M.R., Sonawane V.G. A clinical study of Kokilaksha Ksheervasti in rheumatoid arthritis w . r . t . Vatashonita. Int J Ayurvedic Med. 2014;5:321–328. [Google Scholar]
- 21.Burns J.J., Zhao L., Taylor E.W., Spelman K. The influence of traditional herbal formulas on cytokine activity. Toxicology. 2010;278:140–159. doi: 10.1016/j.tox.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Anonymous . World Health Organization (WHO); 2000. General guidelines for methodologies on research and evaluation of traditional medicine. doi:WHO/EDM/TRM/2000.1. [Google Scholar]
- 23.Pandit S., Kanjilal S., Awasthi A., Chaudhary A., Banerjee D., Bhatt B.N. Evaluation of herb-drug interaction of a polyherbal Ayurvedic formulation through high throughput cytochrome P450 enzyme inhibition assay. J Ethnopharmacol. 2017;197:165–172. doi: 10.1016/j.jep.2016.07.061. [DOI] [PubMed] [Google Scholar]
- 24.Waslidge N.B., Hayes D.J. A colorimetric method for the determination of lipoxygenase activity suitable for use in a high throughput assay format. Anal Biochem. 1995;231:354–358. doi: 10.1006/abio.1995.0063. [DOI] [PubMed] [Google Scholar]
- 25.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 26.Mohan M.C., Abhimannue A.P., Kumar B.P. Identification and characterization of Berberine in tinospora cordifolia by liquid chromatography Quadrupole time of flight mass spectrometry (LC MS/MS q-tof) and evaluation of its anti inflammatory potential. Pharmacogn J. 2017;9:350–355. [Google Scholar]
- 27.Mikuni M., Yoshida M., Hellberg P., Peterson C.A., Edwin S.S., Brännström M. The lipoxygenase inhibitor, nordihydroguaiaretic acid, inhibits ovulation and reduces leukotriene and prostaglandin levels in the rat ovary. Biol Reprod. 1998;58:1211–1216. doi: 10.1095/biolreprod58.5.1211. [DOI] [PubMed] [Google Scholar]
- 28.Hedrick C.C., Kim M.D., Natarajan R.D., Nadler J.L. 12-Lipoxygenase products increase monocyte:endothelial interactions. Adv Exp Med Biol. 1999;469:455–460. doi: 10.1007/978-1-4615-4793-8_67. [DOI] [PubMed] [Google Scholar]
- 29.Abe M., Yoshimoto T. Leukotriene-lipoxygenase pathway and drug discovery. Nihon Yakurigaku Zasshi. 2004;124:415–425. doi: 10.1254/fpj.124.415. [DOI] [PubMed] [Google Scholar]
- 30.Jacob J., Babu B.M., Mohan M.C., Abhimannue A.P., Kumar B.P. Inhibition of proinflammatory pathways by bioactive fraction of Tinospora cordifolia. Inflammopharmacology. 2018;26:531–538. doi: 10.1007/s10787-017-0319-2. [DOI] [PubMed] [Google Scholar]
- 31.Fang H., Pengal R.A., Cao X., Ganesan L.P., Wewers M.D., Marsh C.B. Lipopolysaccharide-Induced macrophage inflammatory response is regulated by SHIP. J Immunol. 2004;173:360–366. doi: 10.4049/jimmunol.173.1.360. [DOI] [PubMed] [Google Scholar]
- 32.Venkatesha S., Dudics S., Acharya B., Moudgil K. Cytokine-modulating strategies and newer cytokine targets for arthritis therapy. Int J Mol Sci. 2014;16:887–906. doi: 10.3390/ijms16010887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choy E.H.S., Panayi G.S. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 34.van den Berg W.B., Joosten L.A., van de Loo F.A. TNF alpha and IL-1 beta are separate targets in chronic arthritis. Clin Exp Rheumatol. 1999;17:S105–S114. [PubMed] [Google Scholar]
- 35.Zinin P.V., Misra A., Kamemoto L., Yu Q., Hu N., Sharma S.K. Visible, near-infrared, and ultraviolet laser-excited Raman spectroscopy of the monocytes/macrophages (U937) cells. J Raman Spectrosc. 2010;41:268–274. [Google Scholar]
- 36.Talari A.C.S., Movasaghi Z., Rehman S., ur Rehman I. Raman spectroscopy of biological tissues. Appl Spectrosc Rev. 2015;50:46–111. [Google Scholar]
- 37.Puppels G.J., Garritsen H.S., Segers-Nolten G.M., de Mul F.F., Greve J. Raman microspectroscopic approach to the study of human granulocytes. Biophys J. 1991;60:1046–1056. doi: 10.1016/S0006-3495(91)82142-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki T., Hashimoto S., Toyoda N., Nagai S., Yamazaki N., Dong H.Y. Comprehensive gene expression profile of LPS-stimulated human monocytes by SAGE. Blood. 2000;96:2584–2591. [PubMed] [Google Scholar]
- 39.Garrelds I.M., Van Hal P.T.W., Haakmat R.C., Hoogsteden H.C., Saxena P.R., Zijlstra F.J. Time dependent production of cytokines and eicosanoids by human monocytic leukaemia U937 cells; effects of glucocorticosteroids. Mediators Inflamm. 1999;8:229–235. doi: 10.1080/09629359990397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar B.P., Abhimannue A.P., Mohan M.C., Jacob J., Babu B.M. Inhibition of lipoxygenase by elephantopus scaber extract and determination of its inhibition pattern. J Adv Sci Res. 2015;6:1–5. [Google Scholar]
- 41.Jacob J., Prakash K.B., Abhimannue A.P., Mohind M., Babu B.M. Inhibition of lipoxygenase enzymes by extracts of Tinospora cordifolia: a study of enzyme kinetics. J Nat Prod. 2014;7:203–209. [Google Scholar]
- 42.Sokolowska M., Chen L.Y., Eberlein M., Martinez-Anton A., Liu Y., Alsaaty S. Low molecular weight hyaluronan activates cytosolic phospholipase A 2?? and eicosanoid production in monocytes and macrophages. J Biol Chem. 2014;289:4470–4488. doi: 10.1074/jbc.M113.515106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.