Abstract
Background
Traditional medicine intervention has been used in rheumatoid arthritis (RA) treatment due to limitations of conventional drugs.
Objective
This study aimed at evaluating the anti-arthritic potentials of ethanol and aqueous extracts of stem bark of Cleistopholis patens (SBCP) in complete Freund's adjuvant (CFA) induced rheumatoid arthritis in rats.
Materials and methods
Rheumatoid arthritis was induced in groups 2 to 9 by intradermal injection of 0.1 mlkg−1 chicken type II collagen in CFA into the left hind paw of the rats. Group 1 served as normal control. Group 2 (negative control) received 5 mlkg−1 body weight normal saline while group 3 (positive control) received 10 mg/kg body weight standard drug (indomethacin). Groups 4 to 9 received varied doses of the extracts. After 10 days of RA induction, rats were treated with ethanol and aqueous extracts of SBCP orally at a dose of 400, 600 and 800 mgkg−1 for 21 days. The paw size, body weight changes, inflammatory parameters, lipid peroxidation maker and malondialdehyde (MDA) were assessed.
Results
Rheumatoid arthritis induction caused marked (p < 0.05) increase in paw size, inflammatory makers and MDA while significant (p < 0.05) reduction was observed in body weight relative to normal control. Treatment with extracts analogous to indomethacin markedly (p < 0.05) decreased the paw size and caused weight gain while the altered inflammatory parameters and MDA were reversed relative to negative control.
Conclusion
The findings suggest that the extracts of SBCP have good antiarthritic potentials comparable to indomethacin and hence could be used in rheumatoid arthritis management.
Keywords: Adjuvant, Rheumatoid arthritis, Inflammatory parameters, Malondialdehyde
1. Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by joint swelling and immobility, destruction of cartilage and bone all over the joints [1]. It is associated with MHC genes, T-cell dependent and more severe in females than males [2]. Kaur et al. reported that RA is not a genetic disorder but some researchers believed that some people have genes that make them vulnerable to the disease and these people will not always develop RA except if the genes are activated by “trigger” such as an infection or environmental factors [1]. There are inappropriate reactions by the body's immune system to these stimuli which culminate in the production of substances that attack the joint instead of protecting it, resulting in development of rheumatoid arthritis [1]. The generations of oxygen free radicals from metabolism of oxygen and an increase in reactive oxygen species (ROS) have been reported to have important roles in the pathogenesis of RA [3], [4]. Oxygen-free radicals have been linked as the cause of joint tissue damage in RA [5] and in experimentally induced arthritis [6]. Besides, anti-oxidants can mop up free radicals and limit damage. The imbalance between pro-oxidants and anti-oxidants in RA and arthritis model has been suggested to be due to acceleration of some cellular reactions or perturbed antioxidant defense mechanism [7].
Rheumatoid arthritis is commonly managed using two groups of drugs viz: nonsteroidal anti-inflammatory drugs (NSAIDS) and disease modifying anti-rheumatic drugs (DMARDS) [8]. NSAIDS are use in short term management of RA. Indomethacin, ibuprofen, aspirin, naproxen are members of this category of drugs. The cellular mechanism of antiarthritic and anti-inflammatory potentials of indomethacin is attributed to its inhibition of cyclooxygenase 1 and 2 (COX-1 and COX-2), thus halting the synthesis of prostaglandins implicated in inflammatory arthritis. It has both analgesic and anti-inflammatory potentials but do not prevent the progression of RA. Some of the negative effects of NSAIDS are gastrointestinal distress and ulcer [9]. However, adverse effects, potential toxicity and high cost of NSAIDS and DMARDS limit their use [10]. Consequently, the field of arthritis research has progressed rapidly towards herbal treatment that is regarded to be safe and effective in ameliorating the pain associated with the disease.
Medicinal plants have been proven to be potent and efficacious in the management of RA [11]. The use of medicinal plants provides another alternative approach for the management of RA and currently a number of medicinal plants are under exploration for development of novel drugs [12]. The anti-arthritic potentials of several medicinal plants such as Zingiber officinale, Aloe barbadensis, Withania somnifera Linn have been reported [13]. Thus, it is expedient to exploit the curative efficacy and deleterious actions, if any, of these herbal plants for providing novel and better treatment alternatives with minimal side effects [12]. In this study, we have tried to investigate the therapeutic potentials of Cleistopholis patens in the management of RA.
C. patens (C- patens) Benth is a tree of the Family Annonaceae mostly seen in Sierra Leone eastwards into Uganda and Zaire and grows up to 30 m high [14]. It has been reported that alkaloids (eupolaurine) and 3-methoxy champangine from the extract of C. patens are potent anti-fungal agents [15]. Nigerians and other African countries use the root, bark and leaves of this plant for the treatment of typhoid fever and urogenital infections. Traditional healers from South East, Nigeria use the extracts from the leaves of this plant in the treatment of coronary diseases [15]. In Nigeria, it is called “ojo” by the Igbos while the Yorubas call it “apako” [14]. Analysis of the phytochemical composition of C. patens leaves showed the presence of glycosides, alkaloids, steroids, saponins, terpenoids, favonoids and carbohydrates [15]. Its anti-arthritic potentials are yet to be explored but the people of Ugwulangwu claimed its use in the treatment of rheumatoid arthritis. However, from my personal interviews of different traditional herbalists from Ugwulangwu, they affirmed that they use the ethanol and aqueous extracts of SBCP in the management of rheumatoid arthritic conditions.
2. Materials and methods
2.1. Chemicals and reagents
Chicken type 11 collagen, CFA and other reagents used were of standard grade for analysis and were bought from Sigma Aldrich U.S.A.
2.2. Collection and identification of plant material
The SBCP were collected in a farmland at Umuigboke Ugwulangwu in Ohaozara Local Government of Ebonyi State, Nigeria. They were identified and authenticated by Mr. Alfred Ozioko, a plant taxonomist at the International Centre for Ethnomedicine and Drug Development Nsukka, Enugu State with voucher specimen no: Interced/505-cleitopholis.
2.3. Preparation of the plants extracts
The ethanol and aqueous extracts of SBCP were prepared by method described by Oluduro and Aderiye [16].
2.4. Experimental animals
Albino rats weighing 155–185 g were obtained from the Animal House of Faculty of Veterinary Medicine, University of Nigeria, Nsukka. The rats were acclimatized for a period of two weeks in the animal house of Department of Biochemistry Ebonyi State University, Abakaliki under standard laboratory conditions and fed with growers mash and water ad libitum. The Faculty of Medicine Pre-Clinical Research and Ethics Committee of Ebonyi Stated University, Abakaliki, Nigeria granted ethical approval for this animal study in November, 2017 with ethical code no: EBSU/REC/FBMS/1808/02/002. Animals were given humane care according to NIH Publication on Guide for Care and Use of Laboratory Animals.
2.5. Acute toxicity
Acute toxicity of SBCP ethanol and aqueous extracts was carried out by the modified method described by Lork [17]. Sixty eight (68) rats were used for the acute toxicity test. Prior to the acute toxicity study, the rats were weighed and fasted overnight and were assigned into three experimental groups A, B and C. Group A which had four rats served as the control group and was administered normal saline. The B group received ethanol extract of SBCP while group C received aqueous extract of SBCP. Groups B and C were further sub-divided into eight groups with each group having four rats. The sub-groups were orally given ethanol and aqueous extracts of SBCP at 200, 400, 800, 1200, 1800, 2000, 3000 and 5000 mgkg−1 body weight respectively and the test animals were observed for 24 h. Further, they were continuously observed for the first 2 h for morbidity and up to 24 h for mortality.
2.6. Experimental design
2.6.1. Induction of arthritis
RA was induced according to the method previously described by Pearson [18]. Arthritis was induced intradermally by injection of 0.1 mL chicken type II collagen in CFA [heat killed Mycobacterium tuberculosis and sterile paraffin oil (10 mgmL−1)] into the left hind paws of rats groups 2 to 9 according to their body weights. Treatment with ethanol and aqueous extracts of SBCP started from the 10th day after arthritis induction and continued for 21 days. The treatment lasted for three weeks after which the animals were humanely immobilized by cervical dislocation. Thereafter, blood samples were collected by cardiac puncture into sterile bottles. The blood samples were centrifuged (3000× g for 15 min) and serum separated for biochemical analyses.
2.6.2. Experimental groups
A total number of one hundred and thirty five (135) Wistar albino rats divided into nine groups of 15 rats each were used.
Group 1: Normal control received normal saline 5 mLkg−1
Group 2: Negative control (untreated arthritic rats) received 5 mLkg−1 normal saline
Group 3: Positive control (arthritic rats) treated with indomethacin (10 mgkg−1) standard drug
Group 4: Arthritic rats + 400 mg/kg stem bark C. patens ethanol extract (SBCPEE)
Group 5: Arthritic rats + 600 mgkg−1SBCPEE
Group 6: Arthritic rats + 800 mgkg−1 SBCPEE
Group 7: Arthritic rats + 400 mgkg−1 stem bark C. patens aqueous extract (SBCPAE)
Group 8: Arthritic rats + 600 mgkg−1 SBCPAE
Group 9: Arthritic rats + 800 mgkg−1 SBCPAE
2.7. Evaluation of physical parameters
The changes in paw size and body weight were measured on 10th, 17th, 24th and 31st day of the study using digital vernier caliper and weighing balance.
2.8. Inflammatory parameters
C-reactive protein (CRP) was determined by the method described by Voila [19]. Rheumatoid factor (RF) was determined according to method described by Johnson and Faulk [20]. Adenosine deaminase (ADA) was assayed by the method described by Bergmeyer [21]. Erythrocyte sedimentation rate (ESR) was determined according to method described by Westergreen [22].
2.9. Determination of lipid peroxidation maker
Malondialdehyde (MDA) in serum was determined spectrophotometrically by measuring thiobarbituric acid reactive substance (TBARS) as described by Gavrilov et al. [23].
2.10. Histopathological study of joint
The interphalangeal joints were fixed in formol acetic acid solution and were later removed and cleaned with distilled water twice. The samples were soaked in graded 30, 50, 70, and 95%, ethanol each for 2 h and finally in absolute ethanol overnight for dehydration. The dehydrated samples were cleared by placing them in turn through 3:2, 1:1, and 1:3 volume/volume of absolute ethanol/xylene series and then through pure xylene for 3 h. Each of them was kept in molten wax at 50–60 °C for 48 h for infiltration of the wax into the tissues. The specimens were placed in the embedding mould and molten wax poured on them, and set aside to cool. Plastic block was then attached to the wax block containing the sample and to a rocking microtome for sectioning. The cut sections were laid on slides daubed with albumin of an egg, cleared with graded ethanol – xylene solutions and dyed with hematoxylin and eosin. The slides were allowed to dry in an oven, thereafter microscopic examinations was done using light microscope and photomicrograph.
2.11. Statistical analysis
All the data were expressed as mean ± standard deviation. Continuous data were assessed for significant differences between means using the one way ANOVA test. A significance threshold of p < 0.05 was adopted for the analyses. Data were analyzed using the IBM-SPSS (version 20) statistical software (IBM, Corp., Atlanta, GA). Value of (p < .05) was viewed to be statistically significant.
3. Results
3.1. Acute toxicity
The ethanol and aqueous extracts of SBCP were subjected to acute toxicity testing in Wistar albino rats and the animals were monitored for 24 h. Both ethanol and aqueous extracts of SBCP did not cause any death up to 5000 mgkg−1, and hence 400, 600 and 800 mgkg−1 were selected for the present study.
3.2. Effect of ethanol and aqueous extracts of SBCP on paw size
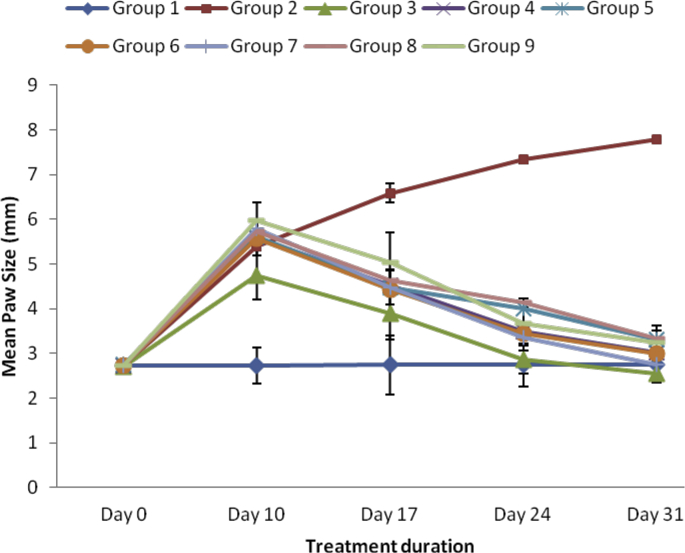
The results showed significant (p < 0.05) increase in paw size on induction of arthritis (Fig. 1). However, ethanol and aqueous extracts of SBCP (400, 600 and 800 mgkg−1) significantly (p < 0.05) reduced paw swelling of treated arthritic groups relative to negative control. The SBCPAE was more effective; it showed peak effect at 400 mgkg−1 body weight on day 31.
Fig. 1.
Effect of ethanol and aqueous extracts of SBCP on paw size of adjuvant-induced arthritic rats. Group 1 = normal control, Group 2 = negative control, Group 3 = positive control, Group 4 = ethanol extract 400 mgkg−1, Group 5 = ethanol extract 600 kg−1, Group 6 = ethanol extract 800 mgkg−1, Group 7 = aqueous extract 400 mgkg−1, Group 8 = aqueous extract 600 mgkg−1, Group 9 = aqueous extract 800 mgkg−1.
3.3. Effect of ethanol and aqueous extract of SBCP on weight of adjuvant-induced arthritic rats
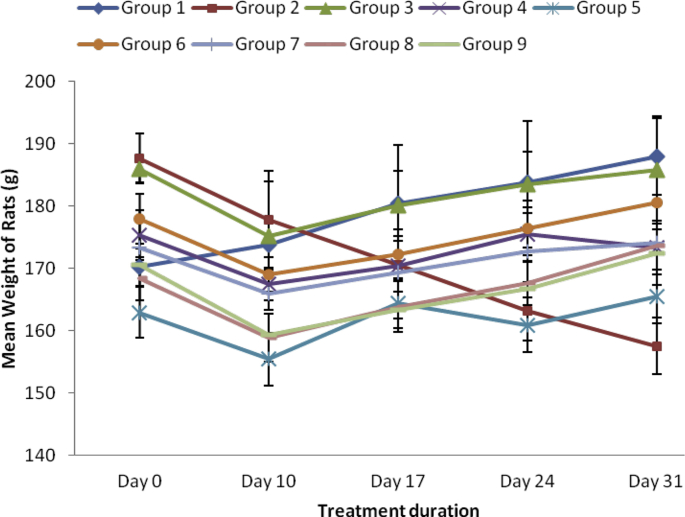
As shown in Fig. 2, the ethanol and aqueous extracts of SBCP (400, 600 and 800 mgkg−1) inhibited loss of weight in treated arthritic rats relative to negative control. The 600 mg/kg SBCPEE was more effective and peaked on day 31. Likewise treatment with the reference drug, indomethacin considerably increased the body weight of the rats in the same manner like the extracts.
Fig. 2.
Effect of ethanol and aqueous extract of SBCP weight (g) of adjuvant-induced arthritic rats. Group 1 = normal control, Group 2 = negative control, Group 3 = positive control, Group 4 = ethanol extract 400 mg/kg, Group 5 = ethanol extract 600 mg/kg, Group 6 = ethanol extract 800 mg/kg, Group 7 = aqueous extract 400 mg/kg, Group 8 = aqueous extract 600 mg/kg, Group 9 = aqueous extract 800 mg/kg.
3.4. Effect of ethanol and aqueous extract of SBCP on inflammatory parameters
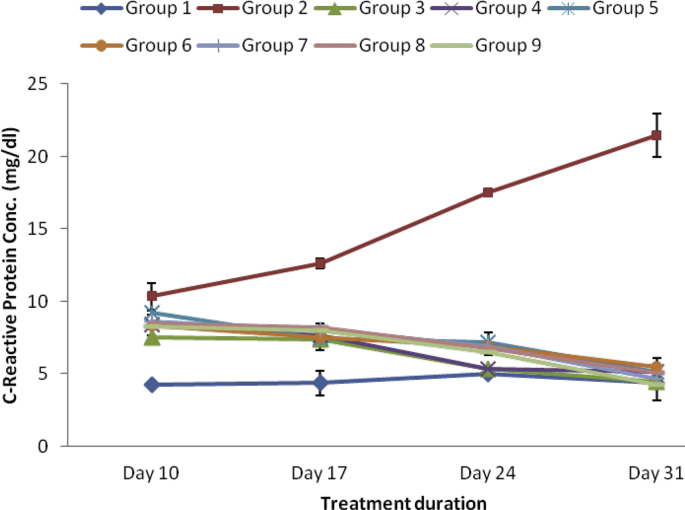
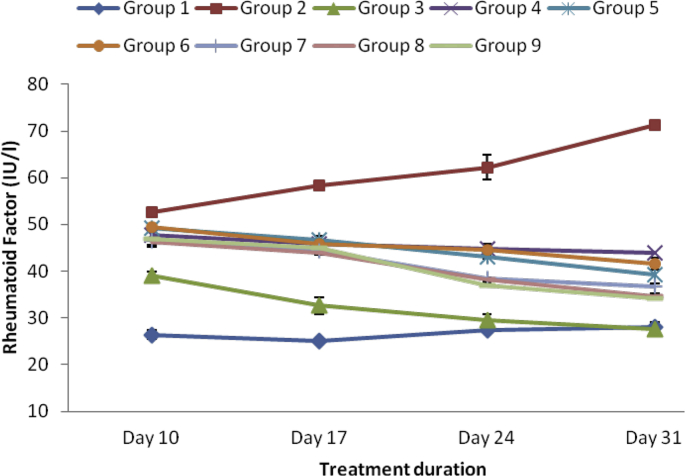
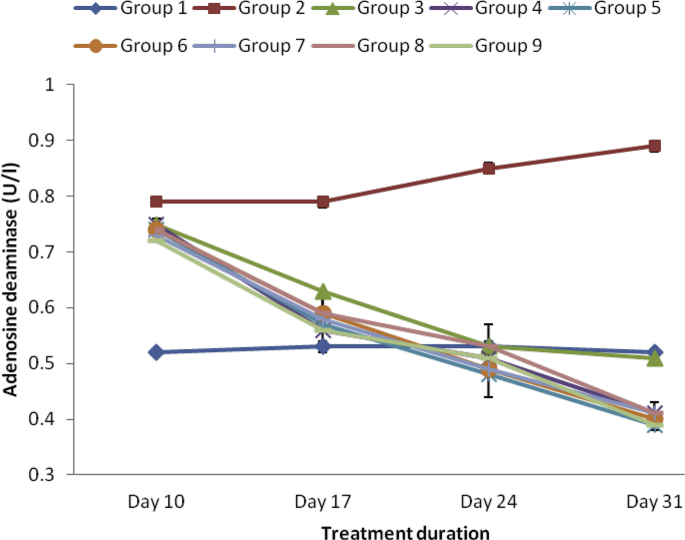
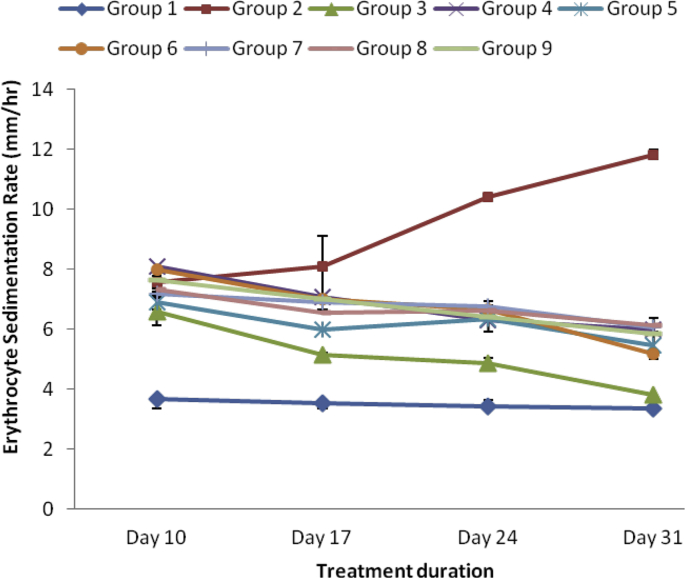
As revealed in Fig. 3, Fig. 4, Fig. 5, Fig. 6 CRP, RF, ADA and ESR respectively were markedly elevated in the serum of arthritic rats relative to the normal control group. However, treatment with varied doses of the ethanol and aqueous extracts of SBCP significantly (p < 0.05) reduced serum levels of CRP, RF and likewise the activities of ADA. The effects of the extracts were analogous to that of indomethacin (Group 3).
Fig. 3.
Effect of ethanol and aqueous extract of SBCP on CRP of adjuvant-induced arthritic rats. Group 1 = normal control, Group 2 = negative control, Group 3 = positive control, Group 4 = ethanol extract 400 mgkg−1, Group 5 = ethanol extract 600 mgkg−1, Group 6 = ethanol extract 800 mgkg−1, Group 7 = aqueous extract 400 mgkg−1, Group 8 = aqueous extract 600 mgkg−1, Group 9 = aqueous extract 800 mgkg−1.
Fig. 4.
Effect on ethanol and aqueous extract of SBCP on RF of adjuvant-induced arthritic rats. Group 1 = normal control, Group 2 = negative control, Group 3 = positive control, Group 4 = ethanol extract 400 mgkg−1, Group 5 = ethanol extract 600 mgkg−1, Group 6 = ethanol extract 800 mgkg1, Group 7 = aqueous extract 400 mgkg−1, Group 8 = aqueous extract 600 mgkg−1, Group 9 = aqueous extract 800 mgkg−1.
Fig. 5.
Effect of ethanol and aqueous extract of SBCP on ADA activity of adjuvant-induced arthritic rats. Group 1 = normal control, Group 2 = negative control, Group 3 = positive control, Group 4 = ethanol extract 400 mgkg−1, Group 5 = ethanol extract 600 mgkg−1, Group 6 = ethanol extract 800 mgkg−1, Group 7 = aqueous extract 400 mgkg−1, Group 8 = aqueous extract 600 mgkg−1, Group 9 = aqueous extract 800 mgkg−1.
Fig. 6.
Effect of ethanol and aqueous extract of SBCP on ESR of adjuvant induced arthritic rats. Group 1 = normal control, Group 2 = negative control, Group 3 = positive control, Group 4 = ethanol extract 400 mgkg−1, Group 5 = ethanol extract 600 mgkg−1, Group 6 = ethanol extract 800 mgkg−1, Group 7 = aqueous extract 400 mgkg−1, Group 8 = aqueous extract 600 mgkg−1, Group 9 = aqueous extract 800 mgkg−1.
3.5. Effect of ethanol and aqueous extract of SBCP on lipid peroxidation maker
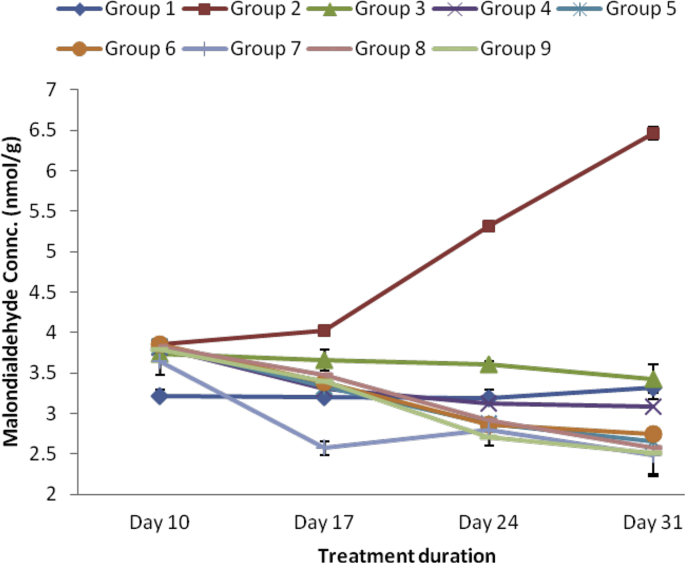
The results for serum concentrations of MDA in complete Freund's adjuvant induced arthritic rats were shown in Fig. 7. Intradermal injection of 0.1 mLkg−1 of Chicken Type II collagen in complete Freund's adjuvant (CFA) into the left hind paw of the rats caused significant rise in the concentrations of MDA relative to the normal control. However, treatment with both SBCPEE and SBCPAE at 400, 600 and 800 mgkg−1 produced significant (p < 0.05) reduction in the levels of MDA. This was more pronounced at 400 mgkg−1 of the aqueous extract of SBCP.
Fig. 7.
Effect of ethanol and aqueous extract of SBCP on lipid peroxidation maker (MDA). Group 1 = normal control, Group 2 = negative control, Group 3 = positive control, Group 4 = ethanol extract 400 mgkg−1, Group 5 = ethanol extract 600 mgkg−1, Group 6 = ethanol extract 800 mgkg−1, Group 7 = aqueous extract 400 mgkg−1, Group 8 = aqueous extract 600 mgkg−1, Group 9 = aqueous extract 800 mgkg−1.
3.6. Effect of ethanol and aqueous extract of SBCP on histopathological changes of the joint
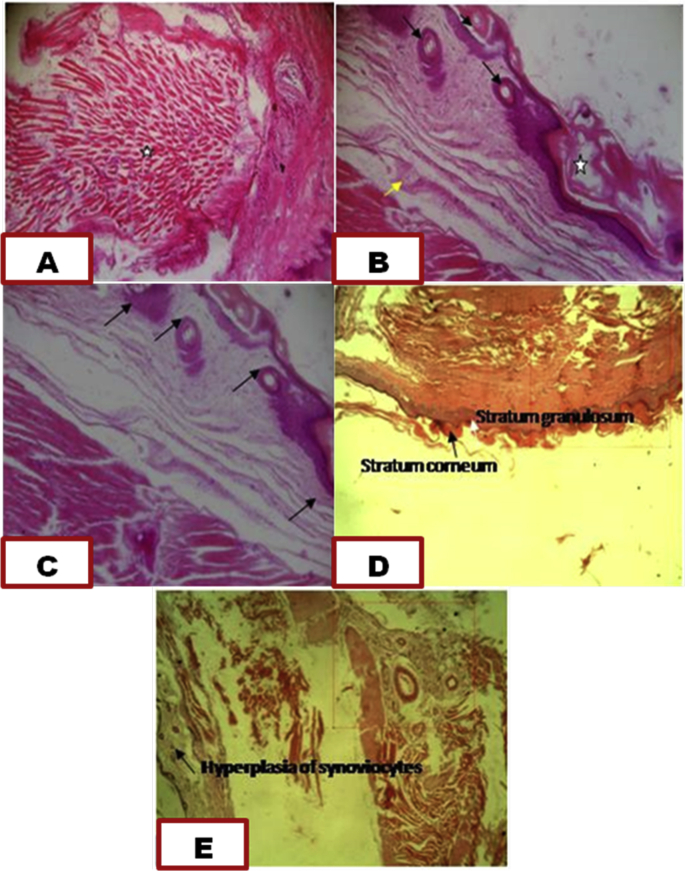
The result of the histopathological changes of the paw joints is shown in Fig. 8. The results indicate that joint of normal control was intack with thick collagen fiber (white star), destruction of epidermal layers, hyperplasia of synoviocytes (black arrow) with the presence of synoviocytes (yellow arrow) in the negative control, regeneration of damaged stratum corneum and stratum granulosum and reduction of inflammation (black arrow] in the positive control (treated with indomethacin, regeneration and increase in thickness of stratum corneum and stratum granulosum in SBCPEE treated group, and hyperplasia of synoviocytes and reduction of inflammation in SBCPAE treated group.
Fig. 8.
A = normal control, B = negative control, C = Positive control, D = Treated with SBCPEE, E = Treated with SBCPAE.
4. Discussion
Complete Freund's adjuvant (CFA) has previously been used in subclinical trials for exploration of novel drugs for the cure of rheumatoid arthritis [24] Wistar albino rats were used for the study due to close resemblance between rat and human rheumatoid disease [25].
In this study, the LD50 for SBCP was found to be greater than 5000 mgkg−1. Thus, acute toxicity testing shows no sign of toxicity or fatality in any of the animal groups and this indicated that SBCP used is safe for the treatment of the conditions linked with experimental arthritis.
Rat paw swelling used in assessing the degree of inflammation and the therapeutic effects of SBCP in this study reduced in volume. This may be attributed to immunological protection provided by the plants extracts, preventing systemic spread and ultimately reducing joint destruction in the rats. Reports have shown that prolonged inflammation is associated with the provocation of number of substances which initiate and moderate inflammatory response such as cytokines, granulocyte macrophage colony stimulating factor (GM-CSF), interferons and prostaglandins F (PGDF) which are connected with pain and degeneration of cartilage and bone that can culminate in severe impairment [26].
The study indicated loss in weight of the arthritic rats relative to the normal control. This could be attributed to increment in production of leptin (a cytokine-hormone) on administration of complete Freund's adjuvant which may also lead to reduced feed intake and hence weight loss [27]. Similar observations were reported by Winder et al [28]. Reduction in weight often accompanies prolonged arthritis due to the systemic or local action of inflammatory cytokines such as TNF-α and IL-1β produced primarily by monocytes and macrophages [29] which trigger muscle degeneration. The study showed that treatment of the RA rats with SBCP extracts induced weight gain. Thus, SBCP may be capable of reversing muscle degeneration caused by RA.
The level of CRP serves as indices of inflammation. CRP induces the release of inflammatory cytokines such as IL-1β, IL-6 and TNF-α by monocyte and may also directly function as proinflammatory boost to phagocytic cells [30]. In this study, treatment of the arthritic rats with SBCP reduced serum CRP as compared with the untreated arthritic rats. Similar reports have shown that the level of CRP in the blood had direct relationship with RA development and gravity of the disease akin to RF [31].
Serum RF correlates with the quantity of serum IgM [32] and the quantity of RF in the serum have direct link with the development of inflammation [33], [34]. In this study, the marked increase in the concentration of rheumatoid factor in the arthritic rats significantly reduced upon treatment with SBCP. This showed that the extracts exhibited anti-arthritic activity and the mechanism could be attributed to the making of autoantibodies towards the Fc fragment thereby shielding the breakdown of cartilage. Similar reports with other anti-arthritic agents are documented [35], [36].
The study indicated that the marked elevations in the activity of ADA in arthritic rats were reduced to near normal (comparable to controls) upon treatment with SBCP. Reports have proposed that the potentiality of ADA (and other ailments) in serum is due to the action of monocyte/macrophage or their rapid growth [37], [38]. The elevation in the concentration of monocyte/macrophages in the synovium heightens their effector functions in rheumatoid disease [39].
The study indicated increase in the concentration of ESR in the arthritic rats relative to the normal control rats. This probably might be due to rise in the rate of making of tissue proteins such as such as fibrinogen and α, β-globulin, and this elevation in the level of ESR is suggestive of serious but vague disease process. The acute phase protein (APP) in ESR exhibit similar features of presenting rise in the level in reaction to stress or inflammation such as injection, surgery, wound and death of tissues [40]. From the study, treatment of the arthritic rats with SBCP showed marked reduction of ESR. The ability of SBCP to reduce the ESR may be attributed to its phytochemical content such as flavonoid because such phytochemical has been implicated in modulation of proinflammatory gene expression, and as such may cause diminution of swollen joints [41].
The increase in lipid peroxidation maker, MDA in arthritic rats recorded in this study is suggestive of compromise in the antioxidant capacity and increased oxidative stress in RA [42]. MDA is biomarker that could furnish information on the overall lipid peroxidation level of a cell [43]. Reports have shown that oxidative damage engendered by ROS is a crucial mechanism that underlies destructive and proliferative synovitis and articular degradation [44], and a substantial rise in ROS and H2O2 in arthritic rats is observed [45]. Also, reduced antioxidant level is a risk factor of RA that aggravates the seriousness of the condition [46]. Treatment with various doses of ethanol and aqueous extracts of SBCP significantly decreased serum MDA in the arthritic rats relative to the negative control suggesting that the curative potential of the extracts could be ascribed to their antioxidant potentials probably due to their phytochemical constituents which help to mop up free radicals.
The results of the histopathological observations substantiated biochemical findings as there was regeneration of the damaged epidermal layers as result of exposure to the adjuvant by treatment with the extracts. The effect of the extracts was comparable to that of indomethacin.
5. Conclusion
From the present study, it is evident that both ethanol and aqueous extracts of SBCP is safe even at a higher dose of 5000 mgkg−1. The findings suggest that the extracts of SBCP have good anti-arthritic potentials comparable to indomethacin and hence could be used in RA management. The anti-arthritic effect might be attributed to the phytochemical components; hence the SBCP extracts could be used as herbal intervention in the treatment of rheumatoid arthritis.
Source(s) of funding
None.
Conflict of interest
None.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Castro-Santos P., Díaz-Peña R. Genetics of rheumatoid arthritis: a new boost is needed in Latin American populations. Rev Bras Reumatol. 2016;56(2) doi: 10.1016/j.rbre.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Vingsbo C., Sahlstrand P., Johan G.B., Jonsson R., Saxne T., Holmdahl R. Pristane –Induced arthritis in rats: a new model for rheumatoid arthritis with a chronic disease across influenced by both major histocompatibility complex and non major histocompatibility complex genes. Am J Pathol. 1996;149(5):1675–1683. [PMC free article] [PubMed] [Google Scholar]
- 3.Flora S.J. Role of free radicals and antioxidants in health and disease. Cell Mol Biol (noisy-le-grand) 2007;53:1–2. [PubMed] [Google Scholar]
- 4.Mirshafiey A., Mohsenzadegan M. The role of reactive oxygen species in immunopathogenesis of rheumatoid arthritis. Iran J Allergy Asthma Immunol. 2008;7:195–202. [PubMed] [Google Scholar]
- 5.Hitchon C.A., El-Gabalawy H.S. Oxidation in rheumatoid arthritis. Arthritis Res Ther. 2004;6:265–278. doi: 10.1186/ar1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hemshekhar M., Sebastin S.M., Sunitha K., Thushara R.M., Kemparaju K., Rangappa K.S. A dietary colorant crocin mitigates arthritis and associated secondary complications by modulating cartilage deteriorating enzymes, inflammatory mediators and antioxidant status. Biochimie. 2010;94:2723–2733. doi: 10.1016/j.biochi.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Minuz P., Fava C., Cominacini L. Oxidative stress, antioxidants, and vascular damage. Br J Clin Pharmacol. 2006;61:774–777. doi: 10.1111/j.1365-2125.2006.02681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y.Z., Sun S.Q., Zhou Y.B. Extract of the dried heartwood of Caesalpinia sappan L.attenuates collagen induced arthritis. J Ethnopharmacol. 2011;136(1):271–278. doi: 10.1016/j.jep.2011.04.061. [DOI] [PubMed] [Google Scholar]
- 9.Ofman J.J., Badamgarav E., Henning J.M. Utilization of nonsteroidal anti-inflammatory drugs and antisecretory agents: a managed care claims analysis. Am J Med. 2004;116:835–842. doi: 10.1016/j.amjmed.2004.02.028. [DOI] [PubMed] [Google Scholar]
- 10.Shen X., Li C., Zaho H., Li S., Li J., Chen Y. Inhibitory effects of a traditional Chinese herbal formula TBL-II on type II collagen-induced arthritis in mice. J Ethnopharmacol. 2011;134:399–405. doi: 10.1016/j.jep.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 11.Cameron M., Gagnia J.J., Chrubasik S. Herbal therapy for treating rheumatoid arthritis. Cochrane Database Syst Rev. 2011;16(2):CD002948. doi: 10.1002/14651858.CD002948.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Rathore B., Mahdi A.A., Paul B.N., Saxena P.N., Das S.K. Indian herbal medicines: possible potent therapeutic agents for rheumatoid arthritis. J Clin Biochem Nutr. 2007;41:12–17. doi: 10.3164/jcbn.2007002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kadhim M.J., Kaizal A.F., Hameed I.H. Medicinal plants used for treatment of rheumatoid arthritis: a review. Int J Pharm Clin Res. 2016;8(12):1685–1694. [Google Scholar]
- 14.Burkill H.M. vol. 4. Royal Botanic Gardens; Kew: 1997. (The useful plants of west tropical Africa. 2). [PubMed] [Google Scholar]
- 15.Liu S.C., Oguntimein B., Hufford C.D., Clark A.M. 3-Methoxysampangine, a novel antifungal copyrine alkaloid from Cleistopholis patens. Antimicrob Agents Chemother. 1990;34(4):529–533. doi: 10.1128/aac.34.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oluduro A.O., Aderiye B.I. Effect of Moringa oleifera seed extract on vital organs and tissue enzymes activities of male albino rats. Afr J Microbiol Res. 2009;3(9):537–540. [Google Scholar]
- 17.Lork D. A new approach to practical acute toxicity tests. Arch Toxicol. 1983;54(2):275–287. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 18.Pearson C.M. Development of arthritis, periarthritis and periostitis in rats given adjuvants. Proc Soc Exp Bio Med. 1956;91(1):95–101. doi: 10.3181/00379727-91-22179. [DOI] [PubMed] [Google Scholar]
- 19.Voila M., Ruoslanti L., Enguall E. Immunology methods. J Immunol Methods. 1981;42:11–115. [Google Scholar]
- 20.Johnson P.M., Faulk W.P. Rheumatoid factor: its nature, specificity, and production in rheumatoid arthritis. Clin Immunol Immunopathol. 1976;6(3):414–430. doi: 10.1016/0090-1229(76)90094-5. [DOI] [PubMed] [Google Scholar]
- 21.Bergmeyer H.U. 3rd ed. VerlagChemie; Deerfield Beach: 1983. Methods of enzymatic analysis; pp. 135–136. [Google Scholar]
- 22.Westergren A. Diagnostic tests: the erythrocyte sedimentation rate range an limitations of the technique. Triangle. 1957;3(1):20–25. [PubMed] [Google Scholar]
- 23.Gavrilov V.B., Gavrilova A.R., Mazhul L.M. Methods of determining lipid peroxidation products in the serum using a thiobarbituric acid test. Vopr Med Khim. 1987;33:118–122. [PubMed] [Google Scholar]
- 24.Kalaiselvan S., Rasool M.K. The anti-inflammatory effect of triphala in arthritic-induced rats. Pharm Biol. 2015;53(1):51–60. doi: 10.3109/13880209.2014.910237. [DOI] [PubMed] [Google Scholar]
- 25.Singh S., Majumdar D.K. Effect of fixed oils of Ocimum sanctum against experimentally induced arthritis and joint edema in laboratory animals. Int J Pharmacogn. 1996;34(3):218–222. [Google Scholar]
- 26.Eric G.B., Lawrence J.L. 6th ed. Williams and Wilkins Company; Baltimore: 1996. Rheumatoid arthritis and its therapy, the Textbook of therapeutic drugs and disease management; pp. 579–595. [Google Scholar]
- 27.Mariam A.H.K. Anti-arthritic activity of ethanolic extract of Lawsonia inermis in Freund's adjuvant induced arthritic rats. J Agric Vet Sci. 2016;9(6):2319–2372. [Google Scholar]
- 28.Winder C.V., Lembke L.A., Stephens M.D. Comparative bioassay of drugs in adjuvant induced arthritis in rats: flufenamic acid, mefenamic acid and phenylbutazone. Arthritis Rheum. 2005;12(5):472–482. doi: 10.1002/art.1780120503. [DOI] [PubMed] [Google Scholar]
- 29.Choy E.H., Panayi G.S. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 30.Bharadway D., Stein M.P., Vozer M., Mold C., Duclos T.W. The major receptor for C reactive protein on leukocytes is FC -ã receptor II. J Exp Med. 1999;190:585–590. doi: 10.1084/jem.190.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung H.J., Nam J.H., Choi J., Lee K.T., Park H.J. Antiinflammatory effects of Chiisanoside and Chiisanogenin obtained from the leaves of Acanthopanax chiisanensis in the carrageenan and Freund's complete adjuvant-induced rats. J Ethnopharmacol. 2005;97:359–367. doi: 10.1016/j.jep.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 32.Veys E.M., Gabriel P.A., Coigne E., Mielants H. Rheumatoid factor and serum IgG, IgM and IgA levels in rheumatoid arthritis with vasculitis. Scand J Rheumatol. 1976;5(1):1–6. [PubMed] [Google Scholar]
- 33.Viswanatha G., Akinapally N., Shylaja H., Nandakumar K., Srinath R., Janardhanan S. Analgesic, anti-inflammatory and antiarthritic activity of newly synthesized bicyclothieno 1, 2, 3-triazines. Maced J Med Sci. 2011;4(2):131–138. [Google Scholar]
- 34.Kshirsagar A.D., Panchal P.V., Harle U.N., Nanda R.K., Shaikh H.M. Anti-inflammatory and antiarthritic activity of anthraquinone derivatives in rodents. Int J Inflamm. 2014;2014:12. doi: 10.1155/2014/690596. Article ID 690596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeh E.T.H. CRP as a mediator of disease. Circulation. 2004;109(21):11–14. doi: 10.1161/01.CIR.0000129507.12719.80. [DOI] [PubMed] [Google Scholar]
- 36.Nielen M.M.J., van Schaardenburg D., Reesink H.W., Twisk J.W.R., van de Stadt R.J., van der Horst-Bruinsma I.E. Simultaneous development of acute phase response and autoantibodies in preclinical rheumatoid arthritis. Ann Rheum Dis. 2006;65(4):535–537. doi: 10.1136/ard.2005.040659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gakis G., Calia G.M., Naitana A.G., Pirino D., Serru G. Serum adenosine deaminase activity in HIV positive subjects. A hypothesis on the significance of ADA-2. Panminerva Med. 1989;31:107–113. [PubMed] [Google Scholar]
- 38.Ungerer J.P., Oosthuizen H.M., Bisbort S.H., Vermaah W.J. Serum adenosine deaminase: isoenzymes and diagnostic applications. Clin Chem. 1992;38:1322–1326. [PubMed] [Google Scholar]
- 39.Stamp L.K., James M.J., Cleland L.O. Interleukin -17: the missing link between T-cell accumulation and effector cell actions in rheumatoid arthritis? Immunol Cell Biol. 2004;82(1):1–9. doi: 10.1111/j.1440-1711.2004.01212.x. [DOI] [PubMed] [Google Scholar]
- 40.Petchi R.R., Vijaya C., Parasuraman S. Anti-arthritic activity of ethanolic extract of Tridax procumbens (Linn.) in Sprague Dawley rats. Pharmacognosy Res. 2013;5:113–117. doi: 10.4103/0974-8490.110541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.García-Lafuente A., Guillamón E., Villares A., Mauricio A.R., Martínez J.A. Flavonoids as anti-inflammatory agents: implications in cancer and cardiovascular disease. Inflamm Res. 2009;58(9):537–552. doi: 10.1007/s00011-009-0037-3. [DOI] [PubMed] [Google Scholar]
- 42.Suresh P., Kavitha C.H.N., Babu S.M., Reddy V.P., Latha A.K. Effect of ethanol extract of Trigonella foenum graecum (Fenugreek) seeds on Freund's adjuvant-induced arthritis in albino rats. Inflammation. 2012 doi: 10.1007/s10753-012-9444-7. [DOI] [PubMed] [Google Scholar]
- 43.Nielsen F., Mikkelsen B.B., Nielsen J.P., Andersen H.R., Grandjean P. Plasma malondialdehide as biomarker of oxidative stress: reference interval and effects of lifestyle factors. Clin Chem. 1997;43:1209–1214. [PubMed] [Google Scholar]
- 44.Hemshekhar M., Sebastin Santhosh M., Sunitha K., Thushara R.M., Kemparaju K., Rangappa K.S. A dietary colorant crocin mitigates arthritis and associated secondary complications by modulating cartilage deteriorating enzymes, inflammatory mediators and antioxidant status. Biochimie. 2010;94:2723–2733. doi: 10.1016/j.biochi.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 45.Walker A.F., Bundy R., Hicks S.M., Middleton R.W. Bromelain reduces mild acute knee pain and improves well-being in a dose-dependent fashion in an open study of otherwise healthy adults. Phytomedicine. 2002;9:681–686. doi: 10.1078/094471102321621269. [DOI] [PubMed] [Google Scholar]
- 46.Balbir-Gurman A., Fuhrman B., Braun-Moscovici Y., Markovits D., Aviram M. Consumption of pomegranate decreases serum oxidative stress and reduces disease activity in patients with active rheumatoid arthritis: a pilot study. IMAJ. 2011;13:474–479. [PubMed] [Google Scholar]