Abstract
Non Alcoholic Steatohepatitis (NASH) is the most severe histological form of non-alcoholic fatty liver disease (NAFLD). It progress to cirrhosis in 20% population and 40% will have death due to liver pathology. Still consensus on pharmacotherapy is yet to be evolved and till date there is no US FDA approved drug for NASH. Ayurveda formulation Katukyadi churna is explored in the possible management of NASH. Study is a single arm with pre and post test design. Sonologically diagnosed patients of fatty liver (n = 30) were screened. 11 patients meeting elastoghraphic criteria (6.4–11.7 kPa) were enrolled in the study. K. churna was administered in the dose of 6 g twice a day with water at the middle of the meal for a period of 6 months. Subjective parameters were Aruchi (Anorexia), Agnimandhya (loss of appetite), Ajeerna (indigestion), Gouravata. Follow up assessments were done on every 30th day. Study showed that K. churna produced significant improvement in various parameters. Significant decrease in weight, (p < 0.001), BMI (p < 0.001), Elastography (p = 0.001), total bilirubin (p = 0.02), Alanine Aminotransferase (ALT) (p < 0.001), Aspartate Aminotransferase (AST) (p = 0.001), Albumin (p = 0.04), Triglycerides (p = 0.005) were observed. Subjective symptoms like Ajeerna (p = 0.002), Agnimandhya (p = 0.004), Arochaka (p = 0.001), Gouvravata (p = 0.002) showed significant improvement. K. churna showed clinical significance in terms of improvement from pathological ranges to normative ranges in elastography, total bilirubin, AST, Albumin. K. churna reduced weight, BMI, hardness and stiffness of the liver, liver function derangements, triglycerides and improved other subjective clinical parameters. Drug has promising results in NASH and warrants further studies.
Keywords: Non Alcoholic Steatohepatitis (NASH), Elastography, Ayurveda, Katukyadi churna
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is wide spectrum of disease comprising from adipose infiltration in liver to steatosis, steatohepatitis, and cirrhosis. NAFLD is defined as the presence of more than 5% hepatic steatosis without evidence of hepatocyte ballooning. Non-alcoholic steatohepatitis (NASH) is a commonly occurring silent liver and forms a component of non-alcoholic fatty liver disease (NAFLD). NASH is a metabolic liver disease characterized by the presence of more than 5% hepatic steatosis along with hepatocyte inflammation with or without hepatic fibrosis [1]. NAFLD comprises of a simple steatosis, NASH, fibrosis and cirrhosis.
Prevalence of NAFLD in Indian population based on Ultra sound was 17% [2]. MRI based study in US reported prevalence of 31% [3]. About 90% patients of NAFLD have simple steatosis [4], 10–30% will progress to NASH and 25–40% progress to liver fibrosis and to cirrhosis in 20–30% [5,6]. NASH is frequently seen in the age group of 35–55 years and in females. 6% of all chronic hepatitis patients do suffer from NASH [7], 20–25% of patients with NASH will progress to cirrhosis [8]. NASH cirrhosis have increased risk of development of hepato cellular carcinoma [9], liver mortality and cardiovascular death [10]. Indian population study showed that the prevalence of NAFLD and steatohepatitis ranges from 15 to 39% and 1.2–4.8% respectively in the patients undergoing liver biopsy. Imaging studies have shown the ranges of steatosis and steatohepatitis as 9.7–23% and 1.2–4.8% respectively [11].
Aetiology for NASH is not clearly known. Predisposing factors are insulin resistance, release of toxic inflammatory cytokines by fat cells and oxidative stress. Post orthotopic liver transplantation, recurrence of NASH suggests the role of extra hepatic metabolic factors like genetic and acquired abnormalities of fatty acid turnover and oxidative stress. The alcohol consumption should not exceed 30 g/day for men and 20 g/day for women according to the second NIH Consensus Conference statement [12]. Steatohepatitis is the inflammation in the hepatocytes caused due to various factors like alcohol, drug induced and dietary factors. Various drugs like antiarrhythmic drugs, methotrexate, tamoxifen, valproic acid, nucleoside reverse transcriptase inhibitors, and chemotherapy have a possible role in induction of NASH [13]. In NASH histologically changes are macrovesicular steatosis, balloon degeneration of hepatocytes, lobular inflammation with neutrophilic cells infiltrate, and zone-3 pericellular fibrosis [14].
NASH has associations with Type 2 Diabetes mellitus, family history of Type 2 diabetes mellitus, insulin resistance with or without glucose intolerance, central obesity, hypertriglyceridaemia, rapid and massive weight loss in overweight subjects. Hence prevalence of NASH may rise with the global raise in obesity and T2DM. NASH manifestations include asymptomatic phase to fatigue, weight loss, weakness in the advanced stages and to cirrhosis.
Liver biopsy is the gold standard for diagnosis of NASH and for disease progression risk prediction. NASH and fatty liver can be only differentiated through biopsy. However biopsy has various limitations like being an invasive technique has potential mortality, morbidity [15] and sampling error. Hence NASH remains to be under diagnosed in clinical practise [16]. Non invasive laboratory tests like serum hyaluronic acid levels, aspartate aminotransferase (ASAT) to platelet ratio index, Fibrotest, assess the extent of liver fibrosis. Fibrometer have their own advantages and limitations. Ultra sonography and computerized tomography are sensitive to hepatic steatosis but cannot differentiate NASH from other variants of NAFLD.
Ultrasound based Transient Elastography [Fibroscan] (TE) has promising results in assessing liver fibrosis by evaluating liver stiffness [17]. EASL-ALEH Clinical Practice Guidelines [18] recommends, Transient elastography assessment as the first line procedure for identification of patient with low risk to sever fibrosis or cirrhosis. It evaluates the stiffness, hardness and is the diagnostic and prognostic marker for NAFLD [19]. Liver stiffness occurs before the onset of fibrosis and this could be due to changes in cellular matrix characters, micro environment leading to transformation of hepatic stellate cells into a fibrogenic phenotype [20] Liver stiffness assessments can be affected by space occupying liver tissue abnormalities like edema, inflammation, extra hepatic cholestasis. Transient elastography results range from 1.5 to 75 kPa. Indian population based study [20] showed cut off ranges for liver stiffness measures in healthy individuals was 5.4 kPa and in NAFLD was 5.9 kPa. In NAFLD with no fibrosis/minimal fibrosis 7.79, moderate fibrosis 11.73, advanced fibrosis 24.88 ± 13.0 kP. In NASH score was 6.4 kPa [21]. NASH with no fibrosis or minimal fibrosis with TE values of 6.4–11.7 kPa were included in the study.
NASH can be compared with Kaphaja yakrut roga in Ayurveda as there is similarity in their manifestations. Manifestations of kaphaja yakrut roga [22] are Mandha vyatha (mild aches), Kathinyata (increase in hardness), Gouvravata (increase in heaviness), Arochaka (Deranged taste), Agnimandhya (decrease in digestion). NASH has similar presentations like pain in right upper quadrant, enlarged, hard liver, feeling of heaviness and loss of appetite.
Effective management of NASH can prevent the possible risks of cirrhosis, terminal liver failure and hepatocellular carcinoma. Current treatment of NASH is only through dietary changes and life style modifications. No pharmacological agent has received FDA or EMEA approval [23]. However studies have shown weight reduction [24], poly-unsaturated fatty acid (PUFA) therapy [25] and exercise [26] have beneficial role. Ayurveda herbal drugs have shown beneficial effect on liver. Many of the Ayurveda drugs have hepatotrophic activity. Drugs like Katuki (Picrorhiza kurroa Royle Benth.), Nimba (Azadirachta indica A. Juss.), Amritha (Tinospora cordifolia Miers.), Bhringraj (Eclipta alba Hassk.), Bhumyamalaki (Phyllanthus niruri Linn.) having Tikta predominant rasa have action on Yakrit (Hepatotrophic action). Katukyadi churna has shown beneficial effect in liver disorders in our clinical settings. Hence Katukyadi churna formulation is explored for it’s possible role in management of NASH.
2. Materials and methods
The objective of the study was to evaluate the effect of Katukyadi churna in patients of Non-alcoholic steatohepatitis.
Patients attending OPD and IPD of the KAHER’s Shri B.M.K. Ayurveda Mahavidyalaya & Hospital were selected for the study.
2.1. Patients
Thirty patients sonologically diagnosed as fatty liver were subjected to Elastographic assessments. 11 patients meeting the diagnostic criteria of stetohepatitis (sonological and elastographic) were recruited from patients visiting outpatient department of KAHER’s Shri. BMK Ayurveda Hospital Belagavi, Karnataka, India.
2.1.1. Inclusion criteria
Patient of either sex, between the age group of 20–60 yrs, Sonologically diagnosed cases of fatty liver, elastographic reading between 6.4 and 11.7 kPa.
2.1.2. Exclusion criteria
Alcoholic stetohepatitis, cirrhosis of liver, pregnant and lactating women.
2.1.3. Screening methods
All patients included in this study were examined thoroughly and data was recorded systematically. Various laboratory and Ayurveda variables were assessed. Laboratory investigations were carried out at clinical laboratory, KAHER’s Shri BMK Ayurveda Mahavidyalaya, Belagavi in all patients at baseline and on 180th day of intervention. Elastography evaluation were done at Dr Varadaraj Gokak’s Liver & Gastroenterology clinic. Belagavi. Clinical trial was conducted from October 2016 to December 2017.
2.2. Study design
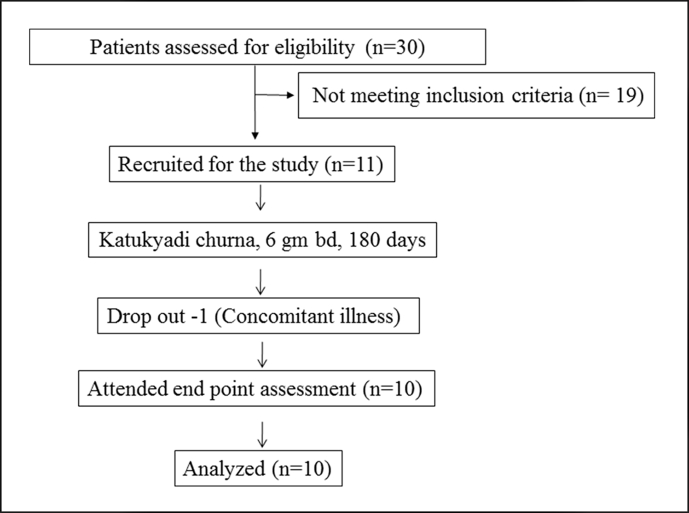
The study was an open label clinical study. Study has single arm with pre and post test design. The scholars involved in distribution and administration of study articles were different from the investigators.The flow chart of the study is depicted in Fig. 1.
Fig. 1.
Study flow chart.
2.2.1. Intervention
Raw drugs of the Katukyadi churna were procured from authentic source. Identification, raw material assessment, quality assessment of final product (analytical and microbial) was done at AYUSH Approved drug testing laboratory for ASU Drugs, Govt. of India, KLE Shri B M Kankanawadi Ayurveda Mahavidyalaya & Research Centre, Belagavi. (Table 1). Katukyadi churna was prepared in GMP certified KLEU’s pharmacy Belagavi as per the standard procedures for Churna (Powder) preparations.
Table 1.
Katukyadi churna- Ingredients, part used, chemical constituents and proportion.
| S.no | Name of the drug | Botanical name and Family | Part Used | Important chemical constituents | Proportion |
|---|---|---|---|---|---|
| 1 | Katuki | Picrorhiza kurroa Royle Benth. | Roots | Phenolic glycoside, Iridoid glycoside, Cucurbitacins glycoside, Aliphatic homomonocyclic glycosides. Sesquiterpene | 1 part |
| 2 | Nimba | Azadirachta indica A.Juss. | Stem bark | Tannins, Saponins, Phlobatanins, Flavanoids, Cardiac glycosides, alkaloids | 1 part |
| 3 | Amrita | Tinospora cordifolia Miers. | Stem | Phenols, Flavanoids, Alkaloids, Saponins, cardiac glycosides, steroids | 1 part |
| 4 | Bhringaraj | Eclipta alba Hassk. | Whole plant | Alkaloids, Flavanoids, glycosides, triterpenoids, polyacetylenes | 1 part |
| 5 | Bhumyamalki | Phyllanthus niruri Linn. | Whole plant | Alkaloids, Tannins, Saponin, Phlobatannins, Sterols, glycosides, Resins. | 1 part |
K. churna was administered in the dose of 6 gm (Sachets) twice a day with water. For 180 days. Administration was in the middle of the food intake. Observations were made on 30th, 60th, 90th, 120th, 150th& 180th day of intervention.
Study obtained permission from the Institutional Ethics Committee of KAHER’s Shri B.M.K. Ayurveda Mahavidyalaya & Hospital (BMK/15/PG/KC/02. Dated 19/02/2016). CTRI Number (CTRI/2018/01/011490). Patients were asked to adhere to the treatment protocol and report any adverse event to the investigators at the earliest. All such manifestations were screened for the possible adverse events.
2.3. Criteria for assessment criteria-
Patients screening was done for viral markers for Hepatitis B & C.
2.3.1. Primary outcome
Elastography (Model 402, Echosens Company, medium probe).
Elastography is a novel, non invasive technique which measures elastic properties of soft tissue like liver through quantitative or qualitative methods. Mechanism includes transmission of vibration to the tissue by a vibrator. Elastic shear wave induced by vibrator propagates through the tissue. Concurrently pulse-echo ultrasonic acquisitions are done to follow shear wave propagation and its velocity. Harder the tissue faster is the propagation. It is expressed in kilopascal (kPa) [27].
2.3.2. Secondary outcome
Subjective symptoms like aruchi (Anorexia), agnimandhya (decrease in digestion), ajeerna (indigestion), gouravata (Subjective feeling of heaviness in whole body). Random blood sugar, liver function test (serum bilirubin total, serum bilirubin direct, alanine aminotransferase (ALT), aspertate aminotransferase (AST), serum total protein, serum albumin, albumin/globulin (A/G) ratio, serum alkaline phosphatase). Normal reference ranges of liver function tests used for this study are: Total bilirubin: 0.0–1.5 mg/dL, AST: 5–34 U/L, ALT: 0–55 U/L, Alkaline phosphatase: 50–125 U/L, albumin is 3.5–5.0 g/dL [28]. Lipid profile (Cholesterol total, HDL, LDL, Triglyceride) and were assessed. Normal reference ranges of lipid profile used for this study are LDL<100, TC < 200, HDL<40, Triglyceride<150 [29].
2.4. Statistical methods
Statistical analysis was carried out using SPSS Version 20. Comparison at different time points was carried out by Student’s ‘t’ test for parametric test and for non-parametric McNemar’s test was applied. Values are reported as mean ± standard deviation. All tests were considered statistically significant at p < 0.05.
3. Results
A total of 11 patients participated in the study. Two patients suffered from loose stools 2–3 times/day for the first 8 days. However patients did not suffer from dehydration and had no disturbance to their daily functioning. And no remedial measures were done. One patient dropped out of our study due to concomitant illness of Nephrolithiasis (Fig. 1).
3.1. Patient characteristics
Mean age of patients was 43.19 yrs, mean duration of the illness was 1.9 yrs, maximum number of patients were female (54.5%), vegetarians (63.63%), secondary level education (63.63%), sedentary activity (81.1%), Vata pradhana pitta prakurti (45.45%) (see Table 1). Mean weight of the participants was 74.3 Kgs and mean BMI was 29.5. Manifestations profile in the patient showed decrease in abhyavarana (63.63%) and jarana shakti (90.9%). They had Ajeerna (100%), Agnimandya (100%), Arochaka (100%), Gouravata (90.9%), sour eructation (63.63%), mild pain in right hypochondriac abdominal region (63.63%). Other manifestations were increase in BMI (63.63%), Total bilirubin (45.45%), direct bilirubin (54.54%), AST (63.63%), ALT (27.27%), Total Cholesterol (27.27%) and Triglycerides (63.63%). Derangement in LDL (72.72%) and decrease in HDL (18.18%) were observed (Table 2, Table 3).
Table 2.
Patient profile: Expressed in Mean, standard deviations (S.D.) and percentage.
| S.No | Clinical Profile | Number | Percentage (%) | |
|---|---|---|---|---|
| 1 | Age (yrs) | 43.09 ± 10.70 | ||
| 2 | Sex | Male | 5 | 45.45 |
| Female | 6 | 54.5 | ||
| 3 | Socio Economic Status | Poor | 2 | 18.18 |
| Middle class | 9 | 81.1 | ||
| 4 | Food | Vegetarian | 7 | 63.63 |
| Mixed | 4 | 36.36 | ||
| 5 | Educational Status | Primary | 1 | 9 |
| Secondary | 7 | 63.63 | ||
| Graduate | 3 | 27.27 | ||
| 6 | Lifestyle | Sedentary activity | 9 | 81.1 |
| Moderate activity | 1 | 9 | ||
| Heavy activity | 1 | 9 | ||
| 7 | Prakurti (Body constitution) | Vatapradhana Pitta | 5 | 45.45 |
| Pitta pradhanaKapha | 1 | 9 | ||
| VatapradhanaKapha | 1 | 9 | ||
| Pitta pradhanaVata | 2 | 18.18 | ||
| kaphapradhanaVata | 2 | 18.18 | ||
| 8 | Agni | Abhyavaharan Shakti – Avara | 7 | 63.63 |
| Madhyama | 4 | 36.36 | ||
| Jarana shakti – Avara | 10 | 90.9 | ||
| Madhyama | 1 | 9 | ||
| 9 | Duration of illness (In Years) | 1.92 ± 1.33 | ||
| 10 | Weight (Kgs) | 74.30 ± 12.51 | ||
| 11 | BMI | 29.59 ± 7.20 | ||
| 12 | Drop outs | 1 | 9 | |
| 13 | Study completed | 10 | 90.9 | |
| 14 | Total | 11 | ||
Table 3.
Clinical Manifestations in the patients.
| S.No | Manifestation | Number | Percentage % | |
|---|---|---|---|---|
| 1 | Ajeerna (Indigestion) | 11 | 100 | |
| 2 | Agnimandya (loss of appetite) | 11 | 100 | |
| 3 | Gouravata (heaviness of body) | 10 | 90.9 | |
| 4 | Arochaka (Anorexia) | 11 | 100 | |
| 5 | Mild abdominal Pain (Right hypochondriac Region) | 7 | 63.63 | |
| 6 | Amlapitta (Sour erectations) | 7 | 63.63 | |
| 7 | BMI | Normal (18.5–22.9) | 2 | 18.18 |
| 8 | Over weight (23–24.9) | 1 | 9 | |
| 9 | Pre Obese (25–29.9) | 4 | 36.36 | |
| 10 | Obese- Type 1 (30–40) | 3 | 27.27 | |
| 11 | Obese- Type 2 (40–50) | 1 | 9 | |
| 12 | Increased Total Bilirubin (>1.2 mg/dL) | 5 | 45.45 | |
| 13 | Increased Direct Bilirubin (>0.3 mg/dL) | 6 | 54.54 | |
| 14 | Increased AST (>40 IU/mL) | 7 | 63.63 | |
| 15 | Increased ALT (>45 IU/mL) | 3 | 27.27 | |
| 16 | Hypoalbuminemia (<3 mg/dL) | 1 | 9 | |
| 17 | Increased Total Cholesterol (<200 mg/dL) | 3 | 27.27 | |
| 18 | Decreased HDL (40–59 mg/dL) | 2 | 18.18 | |
| 19 | Deranged LDL (100–129 mg/dL) | 8 | 72.72 | |
3.2. Primary outcome
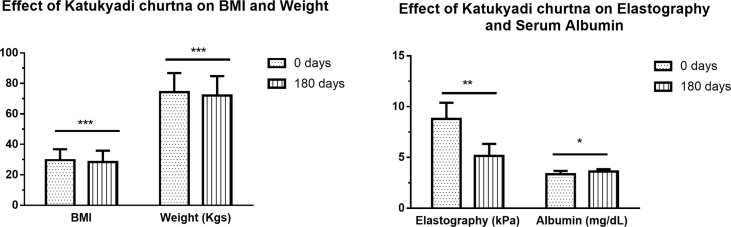
Study showed significant improvement in Elastography readings (p = 0.001). Intervention of K. churna produced significant reduction in elastographic reading to normalcy at 180th day of intervention (Fig. 2) (Table 5).
Fig. 2.
Effect of Katukyadi churna on BMI Weight, Elastography and serum Albumin.
Table 5.
Effect of interventions on parameters of liver and lipid.
| S.No | Parameters | 0TH DAY | 180TH DAY | P value |
|---|---|---|---|---|
| 1 | Elastography (kPa) | 8.78 ± 1.60 | 5.14 ± 1.19 | 0.001 |
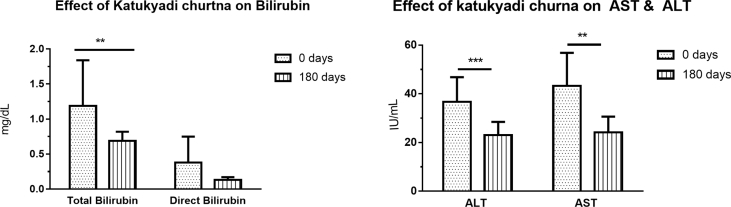
| 2 | Total Bilirubin (mg/dL) | 1.19 ± 0.65 | 0.69 ± 0.13 | 0.020 |
| 3 | Direct Bilirubin (mg/dL) | 0.38 ± 0.37 | 0.13 ± 0.04 | 0.068 |
| 4 | ALT (IU/mL) | 36.7 ± 10.13 | 23.00 ± 5.49 | <0.001 |
| 5 | AST (IU/mL) | 43.3 ± 13.53 | 24.20 ± 6.44 | 0.001 |
| 6 | Total Protein (mg/dL) | 6.69 ± 0.16 | 6.53 ± 0.37 | 0.27 |
| 7 | Albumin (mg/dL) | 3.33 ± 0.34 | 3.62 ± 0.22 | 0.04 |
| 8 | A/G ratio | 0.96 ± 0.18 | 1.06 ± 0.22 | 0.322 |
| 9 | Alkaline Phosphatase (IU/mL) | 73.5 ± 13.08 | 77.10 ± 7.51 | 0.309 |
| 10 | Total Cholesterol (mg/dL) | 191.1 ± 46.41 | 169.7 ± 27.93 | 0.12 |
| 11 | HDL (mg/dL) | 45.8 ± 5.67 | 43.30 ± 5.53 | 0.069 |
| 12 | LDL (mg/dL) | 85.1 ± 30.14 | 88.4 ± 24.15 | 0.645 |
| 13 | Triglyceride (mg/dL) | 276.9 ± 116.44 | 186.8 ± 51.08 | 0.005 |
| 14 | Random Blood Sugar (mg/dL) | 102.7 ± 48.07 | 106.9 ± 49.14 | 0.490 |
3.3. Secondary outcomes
K. churna intervention showed improvement in LFT parameter like total bilirubin (p = 0.02), ALT (p < 0.001), AST (p = 0.001), albumin (p = 0.04). Significant improvement in triglyceride (p = 0.005) was observed among the lipid profile assessments. Reduction in Ajeerna (p = 0.002), Agnimandhya (p = 0.004), Arochaka (p = 0.001), Gouvravata (p = 0.002), Weight (p < 0.001) and BMI (p < 0.001) were observed (Fig. 2, Fig. 3, Fig. 4) (Table 4, Table 5).
Fig. 3.
Effect of Katukyadi churna on bilirubin, AST and ALT
Fig. 4.
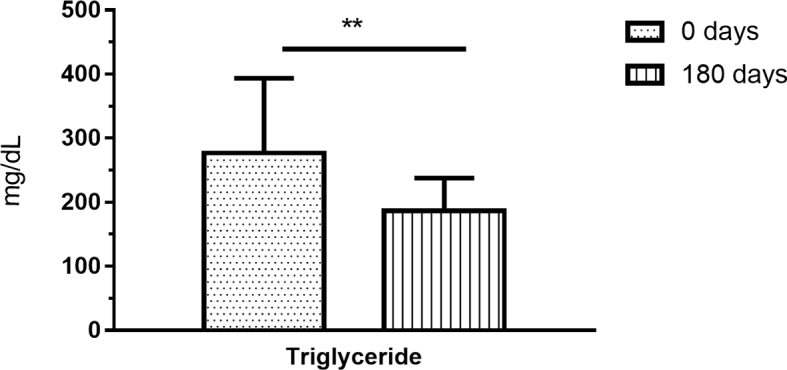
Effect of Katukyadi churna on triglycerides.
Table 4.
Effect of interventions on parameters- Ajeerna, Agnimandhya, Arochaka, Gouvravata.
| S.No | Parameters | 0TH DAY | 30TH DAY | 60TH DAY | 90TH DAY | 120TH DAY | 150TH DAY | 180TH DAY | 0–30 | 0–60 | 0–90 | 0–120 | 0–150 | 0–180 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ajeerna | 1.00 ± 0.00 | 1.00 ± 0.00 | 0.82 ± 0.40 | 0.80 ± 0.42 | 0.60 ± 0.51 | 0.10 ± 0.31 | 0.00 ± 0.00 | 0.50 | 0.50 | 0.50 | 0.125 | 0.004 | 0.002 |
| 2 | Agnimandhya | 1.00 ± 0.00 | 1.00 ± 0.00 | 0.82 ± 0.40 | 0.90 ± 0.31 | 0.70 ± 0.48 | 0.60 ± 0.51 | 0.10 ± 0.31 | 0.50 | 0.50 | 1.00 | 0.250 | 0.125 | 0.004 |
| 3 | Arochaka | 1.00 ± 0.00 | 1.00 ± 0.00 | 0.73 ± 0.46 | 0.64 ± 0.50 | 0.36 ± 0.50 | 0.09 ± 0.30 | 0.00 ± 0.00 | 0.25 | 0.25 | 0.125 | 0.016 | 0.002 | 0.001 |
| 4 | Gouvravata | 0.91 ± 0.30 | 0.91 ± 0.30 | 0.82 ± 0.40 | 0.45 ± 0.52 | 0.45 ± 0.52 | 0.27 ± 0.46 | 0.00 ± 0.00 | 1.00 | 1.00 | 0.063 | 0.063 | 0.016 | 0.002 |
| 5 | Weight (Kgs) | 74.30 ± 12.51 | 74.13 ± 12.63 | 73.90 ± 12.58 | 73.29 ± 13.07 | 73.03 ± 12.93 | 72.64 ± 13.15 | 72.02 ± 12.79 | 0.083 | 0.019 | 0.002 | 0.003 | <0.001 | <0.001 |
| 6 | BMI | 29.59 ± 7.20 | 29.39 ± 7.30 | 29.23 ± 7.44 | 29.06 ± 7.79 | 28.91 ± 7.66 | 28.69 ± 7.69 | 28.42 ± 7.48 | 0.171 | 0.036 | 0.012 | 0.003 | <0.001 | <0.001 |
4. Discussion
The study showed that K. churna is effective in the management of NASH. K. churna showed not only significant reduction in the elastographic values but also in reducing to the normative liver values. K. churna also showed significant reduction in Ajeerna, Agnimandya, Arochaka, Gouravata, weight, BMI, total bilirubin, ALT, AST, albumin values, triglycerides. Increased AST and hypo albuminemia changed to normative levels post intervention. Intervention did not produce any major adverse effects and had good compliance. Loose motions in two patients could be due to the bhedana (piercing quality producing purgative action) [30] effect of the drug Katuki.
Majority of the patients were suffering from grade II fatty liver (72.7%) and few were with grade I fatty liver (27.3%) in sonography findings. Patients noticed symptoms which could be related to NASH since 2–3 years. All the patients were diagnosed with NASH for the first time. Majority of the patients were in middle age (43 yrs) similar to the findings (35–55yrs) in other study [31]. Our study showed predominant female gender involvement (55%) which was similar to the observation of other study [32], where biopsy proven NASH among female and male was in the 2:1 ratio. This could be due to gender specific biology. Prakurti assessment showed predominant vata pittaja prakurti (46%). Patients had irregularity in diet and dietary patterns. Diet was more of Abhishyandhi ahara [picchila (thick), guru (heavy), dravam (liquid), sroto avarodhaka (obstructing the channels) which causes more of Kapha properties], vidahi (causes burning sensation and increase in pitta dosha), madhura (sweet), guru (heavy) properties. Dietary patterns were vishamashana (deranged eating practises), and adhyashana (excess food consumption). Digestion was impaired with decreased (64%) abhyavarana shakti (quantity of food intake) and jarana shakti (time required for food digestion) in 91% patients. Sedentary activity (91%) was prevalent in patients. Hence probable pathogenesis in NASH or Kaphaja Yakruta Roga based on our observation is predominant intake of vidahi, abhishyandi, guru, madhur foods. Derangement in dietary patterns are vishamashana and adhyashana along with sedentary activity. These etiological factors can cause agnimandhya (decrease in abhyavaharana shakti, and jarana shakti) and increase in ama (product of deranged metabolism) production. Kapha and Pitta dosha gets increase in annavaha srotas (Gastro intestinal tract) and Yakruta. Increase of kapha dosha and ama in location of pitta like liver and pachamanashaya (digital part of stomach) causes excess fat accumulation in hepatic cells (kapha vruddhi) and Sroroavrodha (obstruction to channels). Manifestations includes metabolic derangements like ajeerna (indigestion), yakruta guruta and kathinata leading to Kaphaja Yakruta roga. However in the current patients kathinata of liver or abdomen was not observed and it might manifest in the advanced stages of NASH. However subclinical presentations of hardness and stiffness was demonstrated through elastographic evaluations.
Katukyadi churna contains 5 drugs like {Katuki (P. kurroa Royle Benth.), Nimba (A. indica A. Juss.), Amritha (T. cordifolia Miers.), Bhringraj (E. alba Hassk.), Bhumyamalaki (P. niruri Linn.)}. (Table 1) This formulation was conceptualised considering the tikta pradhana rasa, hepatotropic action and rasayana (regenerative) effect of these drugs. Katukyadi choorna interventions in patients of liver disorders with predominant pitta and kapha dosha showed beneficial effect in our hospital settings (unpublished). Hence the current study was planned. Administration of the drug was in the middle of food as it is the time administered for samana vayu and agni derangements. The study showed that intervention produced significant improvement in gastro intestinal parameters like agnimandhya, ajeerna, aruchi, gouravata. Hardness and stiffness of the liver are the indicators of NASH. These could be due to kapha increase in the locations of pitta like liver. Intervention for 180 days showed reverting of deranged elastrographic values (p = 0.001) to the normative levels. This effect could be due to properties of the Katukyadi churna ingredients like ushna virya (hot potency), tikta rasa, ruksha, laghu guna, srotoshodhaka and kapha pitta shamana.
Liver function evaluation showed significant improvement and restoration to normative ranges post intervention. Significant reduction was noted in total bilirubin (p = 0.02), AST (p = 0.001) and ALT (p < 0.001) and improvement in Albumin (p = 0.04). Derangement in synthesis and elimination of triglycerides cause lipid accumulation in hepatocytes. This can cause progressive inflammation and derangements in AST and ALT leading to steatohepatitis. ALT specifically indicates hepatic necrosis and AST reflects cholestatic damage. Normalization of AST and ALT values were noted with the intervention. These effects could be due to pharmacological activities of ingredients of Katukyadi churna. They have properties like tikta (bitter) rasa, deepana, pachana, grahi, yakrit uttejaka, kleda shoshana, srotoshodhana, Kapha pittahara, medahara effects. Studies have shown that all ingredients have properties like hepatoprotective, Anti-inflammatory, Anti oxidant, immunomodulatory effects and anti NAFLD effects. Katuki (P. kurroa Royle Benth.) [33] through contents of picroside I showed hepatoprotective effect and decrease in triglycerides, ALT, AST, fatty infiltration of liver, anti NAFLD effect in NAFLD animal model. Nimba (A. indica A.Juss.) leaf extract [34] has hepatoprotective effect may be through phytochemicals like Azadirachtin, Nimbidin, Nimbin, Nimbinin. Guduchi (T. cordifolia Miers.) [35] has anti-inflammatory, immunomodulatory, hepatoprotective, antioxidant and antidiabetic effects. E. alba Hassk. [36] has also hepato protective effect. P. niruri Linn. (Bhumyamalaki) [37] in animal model showed decrease in hepatomegaly, visceral fat weight, serum total cholesterol, low-density lipoprotein, free fatty acids (FFAs), ALT, ALP, insulin concentration, homeostatic model assessment of insulin resistance (HOMA-IR), hepatic content of cholesterol, triglyceride, malondialdehyde, prevented fibrosis, and has anti NAFLD effect. Katukyadi churna produced significant reduction in BMI (p < 0.001), weight (p < 0.001) and Triglyceride (p = 0.005) values. These could be due to decrease in kapha and meda, apatarpana, lekhaniya and langhana effects. Drugs reducing insulin resistance like metformin and thiazolidinediones [38,39] have shown beneficial effect. Cochrane analysis of probiotic treatment in NAFLD and NASH did not show beneficial outcome.
Alternative and complimentary medication have shown beneficial effect in NAFLD and NASH. Clinical studies have shown that Polyherbal formulation [40], Yiqi Sanju Formula (YQSJF) (traditional Chinese herbal medicine) [41], extracts of grape seed [42] and Gynostemma pentaphyllum [43] have demonstrated beneficial outcome in patients with NAFLD/NASH.
Significance of the study is that a 6 months intervention of a polyherbal Ayurveda formulation showed significant improvement in subjective and objective parameters. Parameters of gastrointestinal manifestations, liver functions and elastographic values reverted to normal values. Assessment was through non invasive method of elastography. Attempt was made to understand the pathophysiology as per Ayurveda through the observations made during the study.
Study has limitations like small sample size, open clinical trial, single arm with no comparator. Small sample was selected as available patient pool was less and also due to difficulty in access to elastography instrument. Comparator arm would have been helpful in giving clarity of clinical improvement. Clinical improvement may not be due to placebo, spontaneous or natural history improvement. As NASH is a silent and progressive disorder. Non Liver biopsy based NASH diagnosis is a limitation of this study.
5. Conclusion
K. churna showed promising results in the management of NASH. Improvement were observed in both subjective and objective parameters. Change from deranged levels to normative levels were observed in liver function and elastography parameters. Hence further studies through randomized controlled trial design are needed. Assessment of biological effect of Katukyadi churna on liver histopathological changes of NASH will be beneficial.
Source(s) of funding
None.
Conflict of interest
None.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 2.Amarapurkar D., Kamani P., Patel N., Gupte P., Kumar P., Agal S. Prevalence of non-alcoholic fatty liver disease: population based study. Ann Hepatol. 2007;6:161–163. [PubMed] [Google Scholar]
- 3.Williams C.D., Stengel J., Asike M.I., Torres D.M., Shaw J., Contreras M. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 4.Teli M.R., James O.F., Burt A.D., Bennett M.K., Day C.P. The natural history of non alcoholic fatty liver: a follow up study. Hepatology. 1995;22:1714–1719. [PubMed] [Google Scholar]
- 5.Matteoni C.A., Younossi Z.M., Gramlich T., Boparai N., Liu Y.C., McCullough A.J. Non alcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 6.Dyson J.K., Anstee Q.M., McPherson S. Non-alcoholic fatty liver disease: a practical approach to diagnosis and staging. Frontline Gastroenterol. 2014;5(3):211–218. doi: 10.1136/flgastro-2013-100403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byron D., Minuk G.Y. Profile of an urban hospital-based practice. Hepatology. 1996;24:813–815. doi: 10.1002/hep.510240410. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal S.R., Malhotra V., Sakhuja P., Sarin S.K. Clinical, biochemical and histological profile of nonalcoholic steatohepatitis. Indian J Gastroenterol. 2001;20:183–186. [PubMed] [Google Scholar]
- 9.Ascha M.S., Hanouneh I.A., Lopez R., Tamimi T.A., Feldstein A.F., Zein N.N. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 10.Ekstedt M., Franzen L.E., Mathiesen U.L., Thorelius L., Holmqvist M., Bodemar G. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 11.Singh S.P., Nayak S., Swain M., Rout N., Mallik R.N., Agrawal O. Prevalence of non-alcoholic fatty liver disease in coastal eastern India : a preliminary ultrasonographic survey. Trop Gastroenterol. 2004;25:76–79. [PubMed] [Google Scholar]
- 12.National Institutes of Health consensus development conference statement. Management of hepatitis C. Hepatology. 2002;36(Suppl. 1):S3–S21. [Google Scholar]
- 13.Rabinowich L., Shibolet O. Drug induced steatohepatitis: an uncommon culprit of a common disease. Biomed Res Int. 2015;2015:168905. doi: 10.1155/2015/168905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunt E.M. Pathology of nonalcoholic steatohepatitis. Hepatol Res. 2005;33:68–71. doi: 10.1016/j.hepres.2005.09.006. [PubMed:16214395] [DOI] [PubMed] [Google Scholar]
- 15.Cadranel J.F., Rufat P., Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the group of epidemiology of the French association for the study of the liver (AFEF) Hepatology. 2000;32:477–481. doi: 10.1053/jhep.2000.16602. [DOI] [PubMed] [Google Scholar]
- 16.Graudal N., Leth P., Marbjerg L., Galloe A.M. Characteristics of cirrhosis undiagnosed during life: a comparative analysis of 73 undiagnosed cases and 149 diagnosed cases of cirrhosis, detected in 4929 consecutive autopsies. J Intern Med. 1991;230:165–171. doi: 10.1111/j.1365-2796.1991.tb00425.x. [DOI] [PubMed] [Google Scholar]
- 17.Sandrin L., Fourquet B., Hasquenoph J.M., Yon S., Fournier C., Mal F. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–1713. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 18.European Association for the Study of the Liver, Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63(1):237–264. doi: 10.1016/j.jhep.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Boursier J., Vergniol J., Guillet A., Hiriart J.B., Lannes A., Le Bail B. Diagnostic accuracy and prognostic significance of blood fibrosis tests and liver stiffness measurement by FibroScan in non-alcoholic fatty liver disease. J Hepatol. 2016;65(3):570–578. doi: 10.1016/j.jhep.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 20.Das K., Sarkar R., Mahiuddin Ahmed S.K., Mridha A.R., Mukherjee P.S. Kshaunish Das et al. ‘“Normal”’ Liver Stiffness Measure (LSM) Values Are Higher in Both Lean and Obese Individuals: a Population-Based Study From a Developing Country. Hepatology. 2012;55(2):584–593. doi: 10.1002/hep.24694. [DOI] [PubMed] [Google Scholar]
- 21.Cocciolillo S., Parruti G., Marzio L. CEUS and Fibroscan in non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. World J Hepatol. 2014;6(7):496–503. doi: 10.4254/wjh.v6.i7.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mishra B.S. Bhavaprakasha of Bhavamishra. 9th ed. choukambha Sanskrit Samsthana; Varanasi: 2005. p. 348. (Madhya khanda; Pliha yakruta adhikara, chapter 33/ 6.). [Google Scholar]
- 23.Thounaojam M.C., Jadeja R.N., Devkar R.V., Ramachandran A.V. Non-alcoholic steatohepatitis: an overview including treatments with herbals as alternative therapeutics. J Appl Biomed. 2012;10:119–136. [Google Scholar]
- 24.Promrat K., Kleiner D.E., Niemeier H.M., Jackvony E., Kearns M., Wands J.R. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51(1):121–129. doi: 10.1002/hep.23276. PMID: 19827166; PMCID: PMC2799538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y.H., Yang L.H., Sha K.H., Liu T.G., Zhang L.G., Liu X.X. Efficacy of poly-unsaturated fatty acid therapy on patients with nonalcoholic steatohepatitis. World J Gastroenterol. 2015;21(22):7008–7013. doi: 10.3748/wjg.v21.i22.7008. PMID: 26078579; PMCID: PMC4462743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Houghton D., Thoma C., Hallsworth K., Cassidy S., Hardy T., Burt A.D. Exercise reduces liver lipids and visceral adiposity in patients with nonalcoholic steatohepatitis in a randomized controlled trial. Clin Gastroenterol Hepatol. 2017;15(1):96–102. doi: 10.1016/j.cgh.2016.07.031. Epub 2016 Aug 10. PMID: 27521509; PMCID: PMC5196006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li G.Y., Cao Y. Mechanics of ultrasound elastography. Proc Math Phys Eng Sci. 2017;473(2199):20160841. doi: 10.1098/rspa.2016.0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed Z., Ahmed U., Walayat S., Ren J., Martin D.K., Moole H. Liver function tests in identifying patients with liver disease. Clin Exp Gastroenterol. 2018;11:301–307. doi: 10.2147/CEG.S160537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Cholesterol Education Program(NCEP) Expert panel on detection, evaluation,and treatment of high blood cholesterol in adults (adult treatment panel III).Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 30.Srivastava S. Sharangadhara Samhita of Acharaya Sharangadhara. 3rd ed. Choukamba Orientlia; Varanasi: 2003. p. 31. (Purva khanda, Deepapachanadi adhikara,Chapter 4/6). [Google Scholar]
- 31.Byron D., Minuk G.Y. Profile of an urban hospital-basedpractice. Hepatology. 1996;24:813–815. doi: 10.1002/hep.510240410. [DOI] [PubMed] [Google Scholar]
- 32.Neuschwander-Tetri B.A., Clark J.M., Bass N.M., Van Natta M.L., Unalp-Arida A., Tonascia J. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology. 2010;52:913–924. doi: 10.1002/hep.23784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shetty S.N., Mengi S., Vaidya Rama, Vaidya Ashok D.B. A study of standardized extracts of Picrorhiza kurroa Royle ex Benth in experimental nonalcoholic fatty liver disease. J Ayurveda Integr Med. 2010;3(1):203–210. doi: 10.4103/0975-9476.72622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chattopadhyay R.R. Possible mechanism of hepatoprotective activity of Azadirachtaindica leaf extract: Part II. J Ethnopharmacol. 2003;89(2–3):217–219. doi: 10.1016/j.jep.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Upadhyay A.K., Kumar K., Kumar A., Mishra H.S. Tinospora cordifolia (willd.) Hook. F. and Thoms. (Guduchi) – validation of the ayurvedic pharmacology through experimental and clinical studies. Int J Ayurveda Res. 2010;1(2):112–121. doi: 10.4103/0974-7788.64405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh B., Saxena A.K., Chandan B.K., Agarwal S.G., Anand K.K. In vivo hepatoprotective activity of active fraction from ethanolic extract of Eclipta alba leaves. Indian J Physiol Pharmacol. 2001;45:435–441. [PubMed] [Google Scholar]
- 37.Zarzour R.H.A., Ahmad M., Zaini Asmawi Mohd, Kaur G., Saeed M.A., Al-Mansoub M.A. Phyllanthus niruri standardized extract alleviates the progression of non-alcoholic fatty liver disease and decreases atherosclerotic risk in sprague–dawley rats. Nutrients. 2017;9:766. doi: 10.3390/nu9070766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tahan V., Eren F., Avsar E., Yavuz D., Yuksel M., Emekli E. Rosiglitazone Attenuates liver inflammation in a rat model of non-alcoholic steatohepatitis. Dig Dis Sci. 2007;52:3465–3472. doi: 10.1007/s10620-007-9756-x. [DOI] [PubMed] [Google Scholar]
- 39.Sanyal A.J., Chalasani N., Kowdley K.V., McCullough A., Diehl A.M., Bass N.M. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chande N., Laidlaw M., Adams P., Marotta P. Yo Jyo Hen Shi Ko (YHK) improves transaminases in nonalcoholic steatohepatitis (NASH): a randomized pilot study. Dig Dis Sci. 2006;51:1183–1189. doi: 10.1007/s10620-006-8030-y. [DOI] [PubMed] [Google Scholar]
- 41.Lou S.Y., Liu Y., Ma Y.Y., Chen H.Y., Chen W.H., Ying J. Effects of Yiqi Sanju Formula on non-alcoholic fatty liver disease: a randomized controlled trial. Zhong Xi Yi Jie He Xue Bao. 2008;6:793–798. doi: 10.3736/jcim20080805. [DOI] [PubMed] [Google Scholar]
- 42.Khoshbaten M., Aliasgarzadeh A., Masnadi K., Farhang S., Tarzamani M.K., Babaei H. Grape seed extract to improve liver function in patients with nonalcoholic fatty liver change. Saudi J Gastroenterol. 2010;16:194–197. doi: 10.4103/1319-3767.65197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chou S.C., Chen K.W., Hwang J.S., Lu W.T., Chu Y.Y., Lin J.D. The add-on effects of Gynostemma pentaphyllum on nonalcoholic fatty liver disease. Alternative Ther Health Med. 2006;12(3):34–39. [PubMed] [Google Scholar]