Abstract
Background
The mortality and morbidity rate of diabetes patients is increasing worldwide which requires an ideal treatment to prevent the disease worsening. Traditional medicine is gaining more attention in diabetes due to its efficacy and safety. We, therefore performed a systematic review study of clinical trials to assess the comparative effect of polyherbal formulations in type 2 Diabetes mellitus.
Objectives
To find the effectiveness of polyherbal formulations in blood sugar and lipid level for type 2 Diabetes mellitus.
Material and methods
PubMed, Scopus and CINAHL databases for clinical trials investigating the effect of polyherbal formulations in Type 2 Diabetes mellitus patients were searched. Meta-analysis of eligible trials was conducted employing Revman 5.2 software.
Results
Fourteen randomized controlled trials were found eligible for meta-analysis. Meta-analysis of findings showed a significant effect of polyherbal formulations on blood sugar level compared to control group. The estimated standard mean changes at 95% confidence interval, following polyherbal formulations treatment were −0.59, (−0.91 to – 0.27) mg/dL; for fasting blood sugar(p < 0.001), −0.69, (−1.18 to −0.21) mg/dL; for postprandial blood sugar (p = 0.005) and −0.46, (−0.88 to −0.04) gm%; for glycated haemoglobin (p = 0.03). The reduction in postprandial sugar and glycated haemoglobin was statistically significant with polyherbal formulations compared to metformin treatment but not for fasting sugar. Similarly in lipid profile the reduction for total cholesterol and triglycerides was statistically significant with polyherbal formulations compared to control group but was not significant for HDL and LDL whereas in other group of polyherbal formulations and metformin only HDL was favouring polyherbal formulations.
Conclusion
Polyherbal formulations occurred to be effective in lowering blood sugar level in Type 2 diabetes but their further efficacy in managing diabetes needs to be validated. Therefore, a qualitative, long term, randomized placebo-controlled trials of adequate sample size are necessary to determine the efficacy of polyherbal formulation in managing diabetes.
Keywords: Type 2 diabetes, Polyherbal formulation, Ayurvedic formulation
1. Introduction
Type 2 Diabetes mellitus is the most threatening disease in many countries and the growing prevalence of diabetic macrovascular and microvascular complications has led to remarkable concern [[1], [2], [3], [4], [5]]. According to the report of the International Diabetes Federation, there were about 451 million people with diabetes worldwide in 2017 [6]. India stands at second place in diabetes in the world and accounts for about 72 million cases of diabetes as on 2017. The occurrence of Diabetes mellitus in India is showing a sharp rise and the most worrying problem is that the switch in the onset age of diabetes from adult to adolescent [7]. This could have a burden on a nation’s health and economy. It is estimated in India, 67% of deaths will occur due to diabetes in the year 2020 [8,9]. Despite of recent progression in glycaemic control from various drugs, Diabetes mellitus remains to be the main health problem due to its notable growth in mortality and morbidity rate [10]. The quality of life is adversely affected by severe hyperglycemia, hyperlipedemia and related complications. There is a constant necessity for the best possible treatment for the management of this disease [11]. The therapeutic approach of an anti-diabetic medicine should not only focus on glycaemic control but also be able to prevent the progression of diabetic complications. The standard allopathic management proved to be effective in managing Diabetes mellitus, but the success of such therapy is sometimes limited [12]. Recently alternative therapies for diabetes have become increasingly popular because of their effectiveness in lowering blood glucose levels and the least side effects [[13], [14], [15]]. The phytochemical component in the herbs such as alkaloids, flavonoids, saponins, led to the desired healing effect in Diabetes mellitus [16]. A single plant may even contain more than one component of phytochemical and thus the combination of several such plants or herbs work symbiotically with each other giving out effective pharmacological action [17]. This holistic approach, if shown effective, could prove safer and better tolerated. Additionally, the lack of supporting studies would focus on the break-in our understanding of the importance of Ayurvedic medicine and may lead to the initiation to conduct more qualitative randomized controlled trials.
This review mainly summarizes the importance of poly herbalism as a potential treatment to control blood sugar, fasting insulin and lipid level in type 2 diabetes mellitus patients.
2. Methods
2.1. Search strategy
We searched PubMed, Scopus, and CINAHL complete up to December 2019 using Mesh terms “polyherbal formulation”, “Ayurvedic formulation” “herbal formulation” and “type 2 diabetes mellitus”. We also manually searched references of key articles and google scholar. All articles obtained were scanned on the title and possibly on abstract. The full report was obtained on the confirmed eligible abstract to decide whether the trial met the inclusion criteria.
2.2. Study selection
The clinical trials irrespective of blinding were considered eligible for this review. Patients with pre-existing or newly diagnosed Type 2 diabetes aged between 18 and 70 years and treatment duration more than 8 weeks were included. The polyherbal formulations used as intervention included three or more than three herbs in combination.
2.3. Data retrieval and quality assessment
The data was retrieved from each article using a standardized data extraction form. Reviewers abstracted characteristics of each trial and appraised methodological characteristics of trials such as study design, outcomes and follow up procedures but did not use a scoring system to rate study quality. We did not contact the authors to request any additional information about the study. The main outcomes recorded were fasting blood sugar (FBS), postprandial blood sugar (PPBS), glycated haemoglobin (HbA1c), fasting insulin, and lipid profile such as total cholesterol (TC), triglycerides (TG), High density lipoprotein (HDL), and low density lipoprotein (LDL).
2.4. Statistical analysis
Review Manager 5.2 software was used to conduct meta-analysis and to calculate the standard mean difference (SMD) and 95% confidence (CI). A random-effect model and inverse variance were adapted to evaluate the heterogeneity of the study. The meta-analysis was done on biochemical parameters such as fasting blood sugar, postprandial blood sugar, glycated haemoglobin, fasting insulin, total cholesterol, triglycerides, high density lipoprotein, and low density lipoprotein. Forest plots were used to illustrate the study findings. A ‘P value’ of 0.05 or less than 0.05 indicates statistical significance.
3. Result
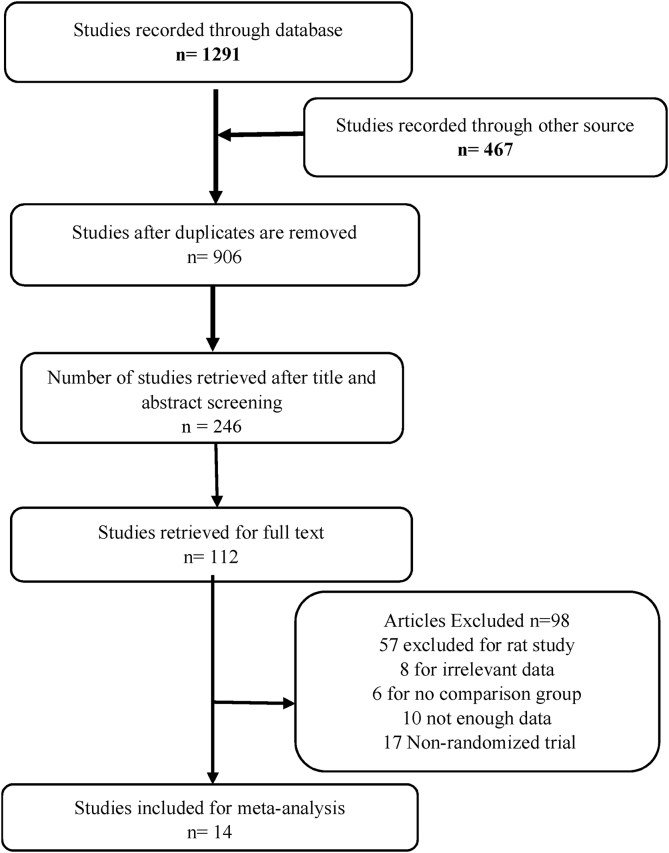
Fig. 1 represents the study selection procedure for this review. Fourteen out of twenty studies involving polyherbal formulations and Type 2 Diabetes mellitus in humans were found to be eligible for this review.
Fig. 1.
Literature search strategy flow diagram.
We excluded six studies Kohli et al. [18], Sudha et al. [19], Kurian et al. [20], Kushwaha et al. [21] for irrelevant data and Sangeetha et al. [22], Kanwar et al. [23] for no satisfactory comparison group. The meta-analysis involved fourteen trials which comprised 1436 participants.
3.1. Study description
All the trials included in this review have been carried out worldwide. The studies were published between 2000 and 2019. The characteristic of selected trials is depicted in Table 1. Seven trials were conducted in India [2,[24], [25], [26], [27], [28], [29]], two from Iran [31,33] and five from China [30,32,[34], [35], [36]]. Three trials [24,25,36] described for diet control while the diet was not controlled in four trials [27,28,31,33] and not mentioned in the other five trials [2,26,30,32,34]. The interventions in all the trials were in either tablet or capsule or powdered form. Additionally, the participants in six trials [24,25,[31], [32], [33], [34]] were given intervention along with existing oral hypoglycaemic agents. The angiotensin receptor blockers were the existing medicine in diabetic nephropathy in one study [30].
Table 1.
Characteristics of the studies included.
| Study | Design | Partici-pants | Age | Control | Intervention | Herbal combinations | Duration | Outcome |
|---|---|---|---|---|---|---|---|---|
| Mohan et al., 2001 [24] | Randomized double blind | 30 | 54 ± 12 | placebo | DCBT 2345 | Gymnema sylvestre, Syzygium jambolinium, Cephalandra indica. | 6 month | FBS, PPBS, HbA1c, Insulin |
| Poongothai et al., 2002 [25] | Randomized double blind | 40 | 45 ± 15 | placebo | Hyponidd | GymnemaSylvestre,Syzigiumcumine, Pterocarpus marsupium, Gurcuma Longa, Emblicaofficianale, Meliaazadirachta, Trivang Bhasma and Shilajit. | 3 month | FBS, PPBS, HbA1c, TG, TC, HDL, LDL, Insulin |
| Guptha et al., 2018 [26] | Randomized parallel group | 64 | 42.5 ± 17.5 | placebo | BGR-34 | Tinospora cordifolia, Berberis aristata, Pterocarpus marsupium, Rubia cordifoila, Gymnema sylvestre, and Trigonella foenum-graecum. | 16 week | FBS, PPBS, HbA1c. |
| Sharma et al., 2010 [27] | Randomized double blind | 50 | 47.5 ± 17.5 | placebo | GlucoCare capsules | Gymnema sylvestre, Commiphora wightii, Pterocarpus marsupium, Shilajeet, Glycyrrhiza glabra, Syzygium cumini, Casearia esculenta, Sphaeranthus indicus, Boerhaavia diffusa, Tinospora cordifolia, Phyllanthus amarus, Gossypium herbaceum, Gmelina arborea, Triphala, Momordica charantia, Abutilon indicum, Asparagus racemosus, Tribulus terrestris, Aloe vera, Curcuma longa, Piper nigrum, Ocimum santum, Rumex maritimus, Trikatu | 3 month | FBS, PPBS, HbA1c. |
| Awasthia et al., 2015 [28] | Open label randomized active control | 93 | 40 ± 20 | metformin | Poly herbal formulation | Cyperus rotundus, Emblica officinalis, Berberis aristata, Terminalia chebula Cedrus deodara, and Terminalia bellirica. | 24 weeks | FBS, PPBS, HbA1c, TG,TC, HDL, LDL |
| Deshpande et al., 2018 [29] | Open label randomized active control | 61 | 42.5 ± 17.5 | Metformin | Vidangadi Yoga | Embelia ribes, Shorea robusta, Terminalia arjuna, Myrica esculeuta, Anthocephalus indicus, Symplocos racemosa,Pterocarpus marsupium, Holarrhena antidysenterica. | 90 days | FBS, PPBS, HbA1c, TG,TC, HDL, LDL |
| Xiang L et al., 2016 [30] | Randomized, Parallel-Controlled Trial | 91 | 57.21 ± 13.2 | Placebo | Qidan Dihuang grain | Radixastragali, Radix Salviae Miltiorrhizae, Radixrehmanniae,Rhizoma Diosscoreae, Radixglycyrrhizae | 12 weeks | FBS, PPBS, HbA1c, TG, TC, HDL, LDL |
| Nakanekar A et al., 2019 [2] | randomized double blind trial | 77 | 49 ± 15.1 | Placebo | PDBT | Gymnema sylvestre, Momordica charantia, Pterocarpus marsupium, Zingiber officinale | 6 month | FBS, PPBS, HbA1c, Insulin |
| Khalili N et al., 2017 [31] | Randomized double blind trial | 60 | 56.1 ± 10.3 | Placebo | Herbal formulation | Urtica dioica, Silybum marianumGaertn, Olibanum gum (olibanum) | 90 days | FBS, PPBS, HbA1c, TG, TC, HDL, LDL |
| Yuan H et al., 2015 [32] | Randomized double blind trial | 111 | 55.6 ± 10.7 | Placebo | JYTK | A.senticosus,E. alatus, and Rhizoma Anemarrhenae | 26 weeks | FBS, PPBS, HbA1c, TG, TC, HDL, LDL |
| Shokoohi R et al., 2017 [33] | Randomized double blind trial | 86 | 49.7 ± 6.4 | Placebo | Polyherbal formulation | C mukul, C myrrh, and T chebula | 3 month | FBS, HbA1c, TG, TC, HDL, LDL |
| Lian F et al., 2015 [34] | Randomized double blind trial | 186 | 55.5 ± 9.8 | Placebo | Jinlida | Coptis chinensis, Ginseng, flavescentis, salvia, Puerariae, Ophiopogon japonicus, Polygonommultiflori, dogwood, Rehmanniae,Poria, epimedium, Semen litchi, Cortex lycii radices, Perrin, anemarrhena | 12 weeks | FBS, PPBS, HbA1c |
| Zhu J et al., 2019 [35] | Randomized double blind trial | 88 | 63.3 ± 7.88 | Placebo | Polyherbal formulation | Gymnema, Trigonella foenum-graecum, Momordica charantia, Tinospora cordifolia, Azadirachta indica, Cinnamon, Kino tree, Bael tree, Ficus carica | 12 weeks | FBS, PPBS, HbA1c |
| Tong XL et al., 2013 [36] | Randomized double blind trial | 399 | 54.4 ± 7.7 | Placebo | TM81 | Rhizomacoptidis, Pericarpiumcirtrireticulatae, Rhizoma Rhei, Radix Paeoniae Alba, Radix Scutellariae | 12 weeks | FBS |
Note: FBS: Fasting blood sugar, PPBS: Postprandial blood sugar, HbA1c: Glycated haemoglobin, TG: Triglycerides, TC: Total cholesterol, HDL: High density lipoprotein, LDL: Low density lipoprotein.
3.2. Quality of study
Few of the included studies in the meta-analysis were poor in quality because some did not specify randomization, blinding and allocation concealment process. Nine trials reported randomization and blinding process [2,24,25,[31], [32], [33], [34], [35], [36]] and rest of the trials reported randomization only. The high heterogeneity in the study may be due to the duration of intervention, different comparison group and diversity of results.
3.3. Meta-analysis
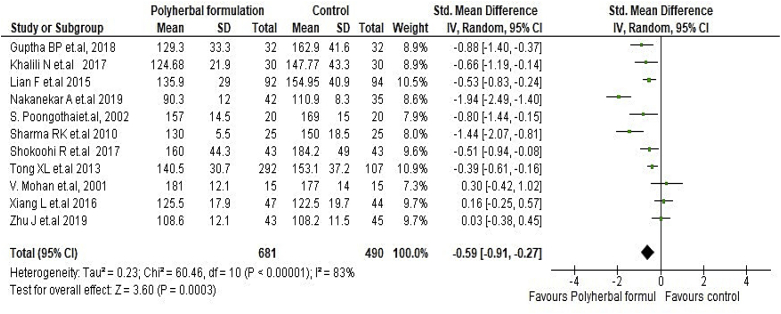
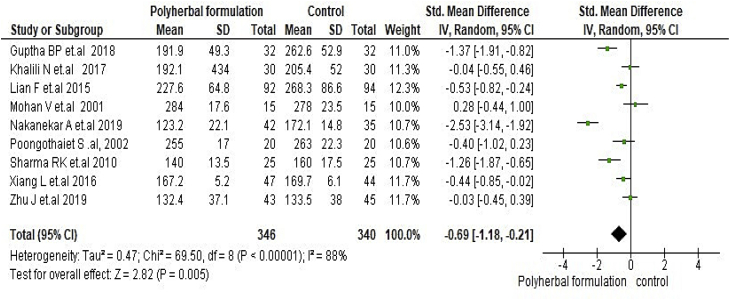
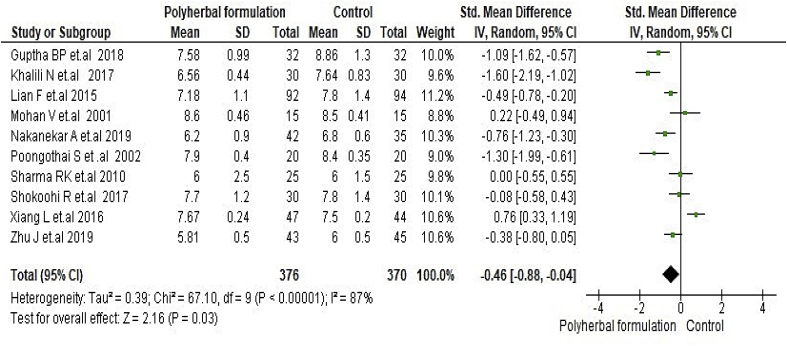
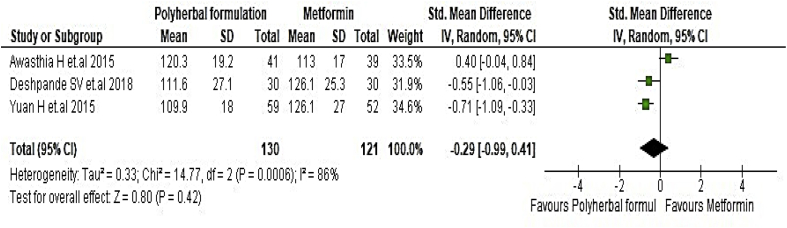
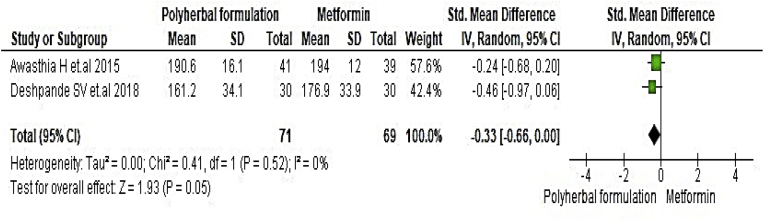
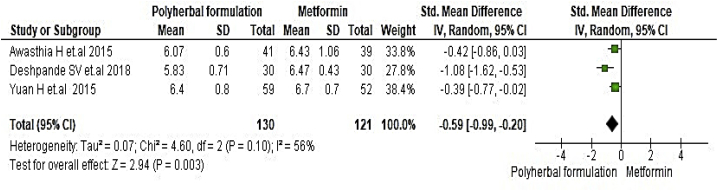
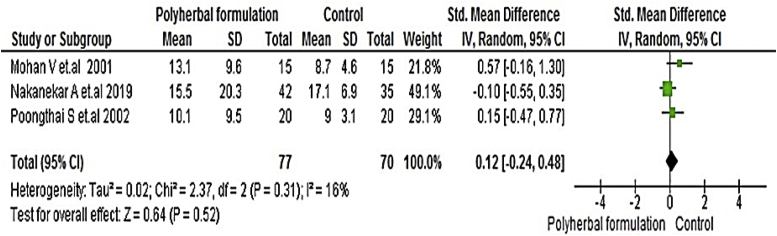
All included studies were evaluated to assess the antidiabetic effect of polyherbal formulations. The parameters were analysed based on two groups for comparator; polyherbal formulations and control group, and polyherbal formulation and metformin group. As shown in Fig. 2 the result for fasting blood glucose level manifested a notable difference between polyherbal formulations treated and control group [−0.59, 95% CI (−0.91 to –0.27) mg/dL; p = 0.0003]. In same manner postprandial blood sugar in Fig. 3 showed to be significant [-0.69, 95% CI (−1.18 to −0.21) mg/dL; p = 0.005] favouring polyherbal formulations treatment. HbA1c as shown in Fig. 4 proved to be statistically significant [-0.46, 95% CI (−0.88 to −0.04) gm%; p = 0.03]. The result of polyherbal formulations and metformin group for postprandial blood sugar [-0.33, 95% CI (−0.66 to −0.00)) mg/dL; p = 0.05] in Fig. 5 and HbA1c [ −0.59, 95% CI (−0.99 to −0.20) gm%; p = 0.003] in Fig. 6 showed to be statistically significant whereas fasting blood sugar [−0.29, 95% CI (−0.99 to 0.41)) mg/dL; p = 0.42] as shown in Fig. 7 was not favouring the polyherbal formulations. The result for fasting insulin [0.12, 95% CI (−0.24 to 0.48) mIU/ml; p = 0.52] in Fig. 8 showed to be favouring towards control group.
Fig. 2.
The effect of polyherbal formulations on fasting blood sugar (mg/dL) compared with control treatment (SMD and 95% CI).
Fig. 3.
The effect of polyherbal formulations on postprandial blood sugar (mg/dL) compared with control treatment (SMD and 95% CI).
Fig. 4.
The effect of polyherbal formulations on glycated haemoglobin (gm %) compared with control group (SMD and 95% CI).
Fig. 5.
The effect of polyherbal formulations on fasting blood sugar (mg/dL) compared with Metformin treatment (SMD and 95% CI).
Fig. 6.
The effect of polyherbal formulations on postprandial blood sugar (mg/dL) compared with Metformin treatment (SMD and 95% CI).
Fig. 7.
The effect of polyherbal formulations on glycated haemoglobin (gm %) compared with Metformin group (SMD and 95% CI).
Fig. 8.
- The effect of polyherbal formulations on fasting insulin (mIU/ml) compared with control group (SMD and 95% CI).
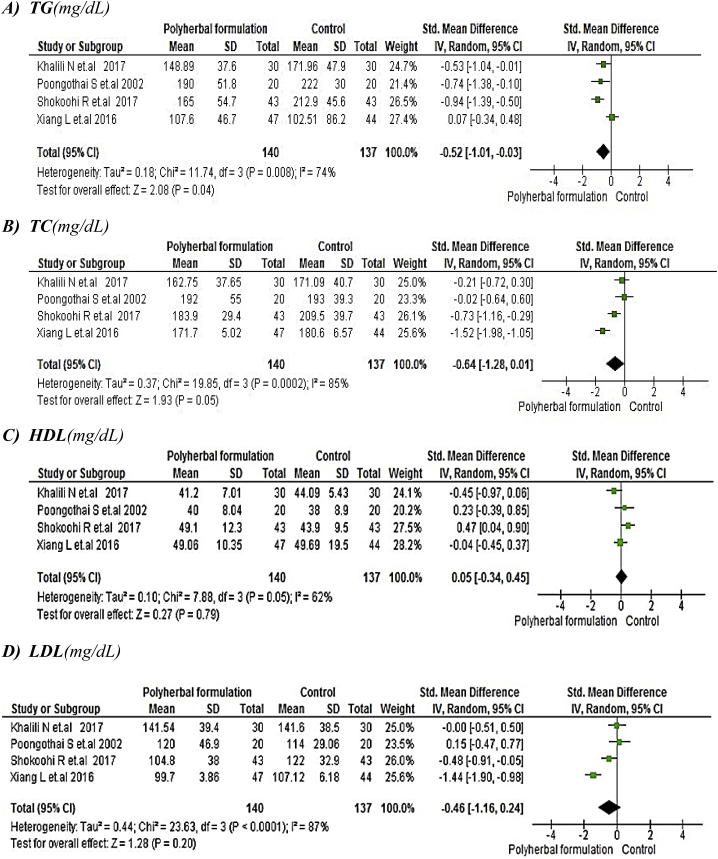
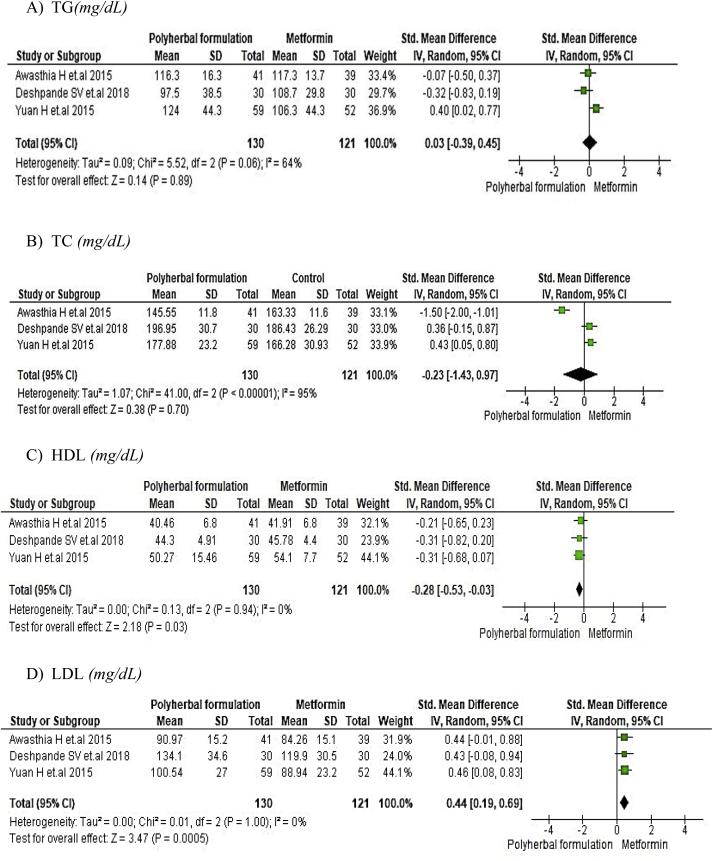
The meta-analysis for polyherbal formulations and control group as shown in Fig. 9 for lipid profile such as triglycerides [-0.52, 95% CI (−1.01 to −0.03) mg/dL; p = 0.04] and total cholesterol [-0.64, 95% CI (−1.28 to 0.01) mg/dL; p = 0.05] showed to be statistically significant whereas HDL [0.05, 95% CI (−0.34 to 0.45) mg/dL; p = 0.79] and LDL [-0.46, 95% CI (−1.16 to 0.24) mg/dL; p = 0.20] was not favouring polyherbal formulations. In polyherbal formulations and metformin group as shown in Fig. 10 triglycerides [0.03, 95% CI (−0.39 to −0.45) mg/dL; p = 0.89], total cholesterol [-0.23, 95% CI (−1.43 to 0.97) mg/dL; p = 0.70] and LDL [0.44, 95% CI (0.19–0.69) mg/dL; p = 0.0005] favoured metformin treatment whereas HDL [-0.28, 95% CI (−0.53 to 0.03) mg/dL; p = 0.03] proved to be statistically significant.
Fig. 9.
The effect of polyherbal formulations on lipid profile compared with Control group (SMD and 95% CI).
Fig. 10.
The effect of polyherbal formulations on lipid profile compared with Metformin group (SMD and 95% CI).
3.4. Adverse effect
Adverse effect was reported in twelve trials [[25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36]] whereas it is unknown in two trial [2,24]. Haematological test, liver function test, and renal function test were performed in eight studies [25,[27], [28], [29],[32], [33], [34],36] which did not show any significant changes. In two trials [26,30] the haematological and biochemical test were not reported but described to have no side effects. There was mild side effects like diarrhoea in 3 subjects [30], 1 subject [35] and 1 subject [36].
4. Discussion
The result of meta-analysis for polyherbal formulations and control group indicates that polyherbal formulation is effective in lowering fasting blood sugar levels. The review also suggests that polyherbal formulations is also effective in reducing postprandial blood sugar and glycated haemoglobin. The polyherbal formulations was effective in lowering fasting blood sugar and glycated haemoglobin in comparison with metformin group. These outcomes suggest that polyherbal formulations have a potential hypoglycaemic effect as good as other oral hypoglycaemic agents. But the effect of polyherbal formulations on lipid profile was not much satisfactory. Total cholesterol and triglycerides were reduced in polyherbal formulations compared to placebo group but lipoproteins did not find any beneficiary effect. Similarly in polyherbal and metformin group HDL was found to be reduced in polyherbal formulations treatment whereas total cholesterol, triglycerides and LDL was reduced in metformin treatment. Also the effect of polyherbal formulations on fasting insulin was not statistically significant in polyherbal formulations and control group. Besides, herbal formulations evaluated in this study is generally considered to be safe. Even though these studies proved the efficacy of herbal formulations treatment on glycaemic control to be significant, their effect on lipid profile needs to be proved more.
The abnormality in lipid profile is most common factor in type 2 diabetes due to insufficient insulin secretion and insulin resistance that directly affects the enzymatic pathway of glucose and lipid metabolism [37]. Since carbohydrate and lipid pathways are interlinked, any irregularity in carbohydrate metabolism leads to lipid malformation [38]. The increased level of lipids in diabetic patients is a risk factors for cardiovascular disease [39]. The study proved that high triglyceride level is associated with poor glycaemic control of diabetes [40]. Lifestyle management with diet changes and exercise are keystones for management of diabetes along with anti-diabetic medicine that helps to control blood sugar along with lipid management [41]. Therefore, improving glycaemic control can substantially reduce the lipids in diabetic patients. For this the qualitative randomized placebo controlled study should be conducted with long follow-up and larger sample size to find better efficacy of polyherbal formulation in blood sugar and lipid control in diabetic patient. The diet management and physical activity along will also mark important that has to be added in the trial.
Additionally, Systematic reviews perform an important role in connecting the scientific gap between traditional and western medical practices that require better research output and thorough meta-analysis [42].This review is different from a previous systematic review [11], as this study involves the clinical trial which included only polyherbal formulation rather than single herb as a treatment in diabetic patients.This systematic review also has several limitations; few studies were of poor quality, the allocation concealment was not clear in some studies and the smaller sample size in many trials which might affect the significance of the result.
To validate the potential benefit of the polyherbal formulation more, an in-depth research is needed. Since many studies performed in India are low in quality this has to be resolved by taking corrective steps for research trials. Standardization and quality control of herbal medicine is feasible but difficult to perform. Herbal formulations for diabetes have different characteristics when compared to synthetic drugs. Apart from this, the regulations of herbal formulations differ across the country [43]. The development of herbal formulations requires a comprehensive understanding of the whole plant system characteristics [44]. The steps for the development of herbal formulations starting from the collection of raw material to isolation of active ingredients of plants are to be followed according to the general guidelines of Ayush and WHO as per the countries requirement [45,46]. In Ayurveda, it is well known that the “Prakriti” determines the efficacy of various herbal medicines that holds a response to drugs and also a significant factor to be regarded in clinical trials as an inclusion/exclusion criterion. The use of the same in clinical trials would yield a better outcome of Ayurvedic treatments that can assist in the preparation of a successful trial protocol [47]. It is important to calculate the dose of the formulation based on whether the crude drug or extract is used and to calculate the dose of the extract on the basis of the extractive value [48]. In general, Ayurvedic practitioners prescribe the dose of medication based on the features of the patient’s body, disease diagnosis, and treatment prognosis. The patients should be prescribed and given the exact dose of medicine according to study protocol in clinical trials which should be checked by patient compliance regularly [49]. In the case of safety and efficacy studies of herbal medicine, the placebo-controlled, double-blind randomized trial may be considered as a gold standard. Besides, the findings of herbal medicine in randomized clinical trials may go a long way to uncovering new knowledge that will lead to better health for everyone [50]. The placebo group or other comparator with conventional treatment is ideal to select in trials involving Ayurvedic capsules or tablets with perfect matching of 1:1 sample size in diabetic patients. The basic requirement for placebo design is, it should be physically identical and pharmacologically inert [51]. Presently, it is hard to make a perfectly matching placebo of herbal formulation because of its special taste, colour, and smell. Therefore, a standardized methodological protocol should be established for designing herbal placebos. In the case of herbal powder or decoction where the placebo has to be considered, it’s ideal to select a placebo group that contains no or very low concentration (e.g. 5–10%) of the intervention [52]. Blinding is an important element of RCT as it helps to isolate the placebo effect and observer bias. The patient and investigators will not know who receives the interventions and also the placebo and Ayurvedic medicine looks similar in appearance [49,53]. The suggested approach to ensure blinding in Ayurvedic interventions is that the Investigator does not assess the treatment outcome. The assessment is carried out by a third person who is unaware of the actual treatment [54]. The study duration of a minimum of six months towards long follow-up up to a year can be considered to yield a reliable effect of anti-diabetic herbal drugs [53].
Based on ethnobotanical knowledge, more than 800 plants are used as traditional remedies in some form or another for the treatment of diabetes. However, only a few herbs have been scientifically proved. Few of these herbs, such as Momordica charantia, Tinospora cordifolia, Pterocarpus marsupium, and Trigonella foenum-greacum, have been proved to be beneficial in treating type 2 diabetes [55]. The selection of appropriate herbs would be useful in proper planning of polyherbal preparation and building a standard protocol. The selection of patients is a key requirement that should have stringent inclusion and exclusion criteria so that the indication and treatment can be assessed reliably [55,56]. Many studies prefer to select newly diagnosed diabetes patients which are ideal to assess the drug response efficiently. However many studies have been performed that involved diabetic complications. In this case, it depends on the herbal formulation that plays an effective role in different diabetic complications like nephropathy, retinopathy, for which the formulations can be chosen efficiently. It is quite difficult to study long term outcomes such as nephropathy, retinopathy, neuropathy, cardiovascular or peripheral vascular or Cerebrovascular outcomes or mortality in the case of herbal treatments as this requires long term follow up and huge funding [57,58]. Few anti-diabetic herbs may have an adverse effect such as hypoglycemia and ketosis, so it makes it important to analyze these outcomes. Assessment of safety is the most important method to be identified during the trial of Ayurvedic interventions which should report safety laboratory parameters (complete blood count, liver function test, renal function test, and urine protein test), clinical signs and symptoms, methods for safety assessment and reporting of adverse effect. This will provide clear knowledge and a better understanding of herbal formulations [53].
The Ayurvedic sector should understand and recognize all these needs for scientific evidence. Ayurvedic clinical trials of high standard of quality with suitable methodology and systematic documentation reporting good clinical science will generate creditable move towards Evidence-based Ayurveda [58,59].
5. Conclusion
This systematic review and meta-analysis show that polyherbal formulations has a blood sugar lowering effect in type 2 Diabetes mellitus patients. The efficacy of polyherbal formulations remains to be validated due to the lack of good quality randomized trial. However, advanced well designed randomized controlled clinical trials with a larger sample size are required to determine the better therapeutic efficacy of polyherbal formulations in managing blood sugar and lipid level in diabetic patients. This review should also help develop evidence-based research in Ayurveda particularly in India.
Source(s) of funding
None.
Conflict of interest
None.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Zheng Y., Ley S.H., Hu F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 2.Nakanekar A., Kohli K., Tatke P. Ayurvedic polyherbal combination (PDBT) for prediabetes: a randomized double blind placebo controlled study. J Ayurveda Integr Med. 2019;10(4):284–289. doi: 10.1016/j.jaim.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krug E.G. Trends in diabetes: sounding the alarm. Lancet. 2016;387:1485–1486. doi: 10.1016/S0140-6736(16)30163-5. [DOI] [PubMed] [Google Scholar]
- 4.Tancredi M., Rosengren A., Svensson A.M., Kosiborod M., Pivodic A., Gudbjornsdottir S. Excess mortality among persons with type 2 diabetes. N Engl J Med. 2015;373:1720–1732. doi: 10.1056/NEJMoa1504347. [DOI] [PubMed] [Google Scholar]
- 5.Upadhyay J., Polyzos S.A., Perakakis N., Thakkar B., Paschou S.A., Katsiki N. Pharmacotherapy of type 2 diabetes: an update. Metab Clin Exp. 2018;78:13–42. doi: 10.1016/j.metabol.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Cho N., Shaw J.E., Karuranga S., Huang Y., da Rocha Fernandes J.D., Ohlrogge A.W. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 7.Mohan V., Sandeep S., Deepa R., Shah B., Varghese C. Epidemiology of type 2 diabetes: Indian scenario. Indian J Med Res. 2007;125:217–230. http://www.icmr.nic.in/ijmr/2007/march/0302.pdf [PubMed] [Google Scholar]
- 8.Reddy K.S., Shah B., Varghese C., Ramadoss A. Responding to the threat of chronic diseases in India. Lancet. 2005;366:1744–1749. doi: 10.1016/S0140-6736(05)67343-6. [DOI] [PubMed] [Google Scholar]
- 9.Bhojani U., Devedasan N., Mishra A., De Henauw S., Kolsteren P., Criel B. Health system challenges in organizing quality diabetes care for urban poor in South India. PLoS One. 2014;9 doi: 10.1371/journal.pone.0106522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedewald W.T., Buse J.B., Bigger J.T., Gerstein H.C., Miller M.E., Byington R.P. Effects of intensive glucose lowering in type 2 diabetes. Nav Eng J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sridharan K., Mohan R., Ramaratnam S., Panneerselvam D. Ayurvedic treatments for diabetes mellitus. Cochrane Database Syst Rev. 2011;12 doi: 10.1002/14651858.CD008288.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandey A., Tripathi P., Pandey R., Srivatava R., Goswami S. Alternative therapies useful in the management of diabetes: a systematic review. J Pharm Bio Allied Sci. 2011;3:504–512. doi: 10.4103/0975-7406.90103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang E.S., Brown S.E., Ewigman B.G., Foley E.C., Meltzer D.O. Patient perceptions of quality of life with diabetes-related complications and treatments. Diabetes Care. 2007;30:2478–2483. doi: 10.2337/dc07-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manya K., Champion B., Dunning T. The use of complementary and alternative medicine among people living with diabetes in Sydney. BMC Complement Altern Med. 2012;12(1):2. doi: 10.1186/1472-6882-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeh G.Y., Eisenberg D.M., Davis R.B., Phillips R.S. Use of complementary and alternative medicine among persons with diabetes mellitus: results of a national survey. Am J Pub Health. 2002;92:1648–1652. doi: 10.2105/ajph.92.10.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh R., Kaur N., Kishore L., Gupta G.K. Management of diabetic complications: a chemical constituents based approach. J Ethnopharmacol. 2013;150:51–70. doi: 10.1016/j.jep.2013.08.051. [DOI] [PubMed] [Google Scholar]
- 17.Meena A.K., Bansal P., Kumar S. Plants-herbal wealth as a potential source of Ayurvedic drugs. Asian J Tradit Med. 2009;4:152–170. http://asianjtm.syphu.edu.cn/EN/Y2009/V4/I4/152 [Google Scholar]
- 18.Kohli K.R., Shilin G., Kolhapure S.A. Evaluation of the clinical efficacy and safety of Diabecon in NIDDM. Antiseptic. 2004;101:487–494. [Google Scholar]
- 19.Shalini S. Efficacy and tolerability of Dianex in Type 2 diabetes mellitus: a non randomized, open label non-comparative study. Med J Malaysia. 2005;60:204–211. [PubMed] [Google Scholar]
- 20.Kurian G.A., Manjusha V., Nair S.S., Varghese T., Padikkala J. Short-term effect of G-400, polyherbal formulation in the management of hyperglycemia and hyperlipidemia conditions in patients with type 2 diabetes mellitus. Nutrition. 2014;30:1158–1164. doi: 10.1016/j.nut.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 21.Kushwaha V., Kar A. Clinical study on ayurvedic herbal drug (mustadi kwatha ghanavti) therapy in patients with type 2 diabetes. Indian Journal of Traditional Knowledge. 2017;16:40–46. [Google Scholar]
- 22.Banerji S., Banerjee S. A formulation of grape seed, Indian gooseberry, turmeric and fenugreek helps controlling type 2 diabetes mellitus in advanced-stage patients. Eur J Integr Med. 2016;8:645–653. doi: 10.1016/j.eujim.2016.06.012. [DOI] [Google Scholar]
- 23.Singh K.S., Chandola H., Kaur M., Ravishankar B. Evaluation of Saptarangyadi Ghanavati in the management of Apathyanimittaja Prameha wsr to type-2 diabetes mellitus. Ayu. 2012;33:368–373. doi: 10.4103/0974-8520.108825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohan V., Poongothai S., Deepa R., Lakshmi S. Efficacy of DCBT 2345–an Ayurvedic compound in treatment of type 2 diabetic patients with secondary failure to oral drugs-randomized double blind placebo control study. Int J Diabetes Dev Ctries. 2001;21:176–183. http://ijddc.com/article.asp?issn=0973-3930;year=2 [Google Scholar]
- 25.Poongothai S., Karkuzhali K., Sharadha J., Deepa R., Mohan V. Evaluation of safety and efficacy of Hyponidd, an Ayurvedic compound: a double blind, placebo controlled study in type 2 diabetic patients with secondary failure to oral drugs. Int J Diabetes Dev Ctries. 2002;22:19–27. [Google Scholar]
- 26.Gupta B.P., Sharma I., Kohli N., Sharma S., Rathi A., Sharma A.K. Preliminary clinical assessment and non-toxicity evaluation of an ayurvedic formulation BGR-34 in NIDDM. J Tradit Complement Med. 2018;8:506–514. doi: 10.1016/j.jtcme.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma R.K., Patki P.S. Double-blind, placebo-controlled clinical evaluation of an Ayurvedic formulation (GlucoCare capsules) in non-insulin dependent diabetes mellitus. J Ayurveda Integr Med. 2010;1:45–51. doi: 10.4103/0975-9476.59827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Awasthi H., Nath R., Usman K., Mani D., Khattri S., Nischal A. Effects of a standardized Ayurvedic formulation on diabetes control in newly diagnosed Type-2 diabetics; a randomized active controlled clinical study. Complement Ther Med. 2015;23:555–561. doi: 10.1016/j.ctim.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Deshpande S.V., Jadhav K.S. Efficacy and safety of Vidangadi Yoga (ayurvedic polyherbal medicine) in type 2 diabetes mellitus: a randomized controlled clinical study. Clin Trials Degener Dis. 2018;3:123–129. doi: 10.4103/2542-3975.248011. [DOI] [Google Scholar]
- 30.Xiang L., Jiang P., Zhou L., Sun X., Bi J., Cui L. 2016. Additive effect of qidan dihuang grain, a traditional Chinese medicine, and angiotensin receptor blockers on albuminuria levels in patients with diabetic nephropathy: a randomized, parallel-controlled trial. Evidence-based Complementary and Alternative Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khalili N., Fereydoonzadeh R., Mohtashami R., Mehrzadi S., Heydari M., Huseini H.F. Silymarin, olibanum, and nettle, a mixed herbal formulation in the treatment of type II diabetes: a randomized, double-blind, placebo-controlled, clinical trial. J Evid Based Complementary Altern Med. 2017;22(4):603–608. doi: 10.1177/2156587217696929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu Y., Zhou X., Liu P., Wang B., Duan D.M., Guo D.H. A comparison study of metformin only therapy and metformin combined with Chinese medicine jianyutangkang therapy in patients with type 2 diabetes: a randomized placebo-controlled double-blind study. Compl Ther Med. 2016;24:13–18. doi: 10.1016/j.ctim.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Shokoohi R., Kianbakht S., Faramarzi M., Rahmanian M., Nabati F., Mehrzadi S. Effects of an herbal combination on glycemic control and lipid profile in diabetic women: a randomized, double-blind, placebo-controlled clinical trial. J Evid Based Complementary Altern Med. 2017;22(4):798–804. doi: 10.1177/2156587217737683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lian F., Tian J., Chen X., Li Z., Piao C., Guo J. The efficacy and safety of Chinese herbal medicine Jinlida as add-on medication in type 2 diabetes patients ineffectively managed by metformin monotherapy: a double-blind, randomized, placebo-controlled, multicenter trial. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0130550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu J., Xing G., Shen T., Xu G., Peng Y., Rao J. Postprandial glucose levels are better associated with the risk factors for diabetes compared to fasting glucose and glycosylated hemoglobin (HbA1c) levels in elderly prediabetics: beneficial effects of polyherbal supplements—a randomized, double-blind, placebo controlled trial. Evid Based Complement Alternat Med. 2019;(5):1–13. doi: 10.1155/2019/7923732. Article ID 7923732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tong X.L., Wu S.T., Lian F.M., Zhao M., Zhou S.P., Chen X.Y. The safety and effectiveness of TM81, a Chinese herbal medicine, in the treatment of type 2 diabetes: a randomized double-blind placebo-controlled trial. Diabetes Obes Metabol. 2013;15(5):448–454. doi: 10.1111/dom.12051. [DOI] [PubMed] [Google Scholar]
- 37.Dixit A.K., Dey R., Suresh A., Chaudhuri S., Panda A.K., Mitra A. The prevalence of dyslipidemia in patients with diabetes mellitus of ayurveda Hospital. J Diabetes Metab Disord. 2014;13(1):58. doi: 10.1186/2251-6581-13-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chatterjee M.N., Shinde R. Metabolism of carbohydrates. 6th ed. Jaypee Brothers Medical publisher; Delhi-India: 2005. Text book of medical laboratory technology; pp. 266–330. [Google Scholar]
- 39.Kannel W.B. Lipids, diabetes, and coronary heart disease: insights from the Framingham Study. Am Heart J. 1985;110(5):1100–1107. doi: 10.1016/0002-8703(85)90224-8. [DOI] [PubMed] [Google Scholar]
- 40.Nau D.P., Mallya U. Sex disparity in the management of dyslipidemia among patients with type 2 diabetes mellitus in a managed care organization. Am J Manag Care. 2005;11(2):69–73. [PubMed] [Google Scholar]
- 41.Al-Habori M., Al-Mamari M., Al-Meeri A. Type II Diabetes Mellitus and impaired glucose tolerance in Yemen: prevalence, associated metabolic changes and risk factors. Diabetes Res Clin Pract. 2004;65(3):275–281. doi: 10.1016/j.diabres.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Gopalakrishnan S., Ganeshkumar P. Systematic reviews and meta-analysis: understanding the best evidence in primary healthcare. J Fam Med Prim Care. 2013;2:9–14. doi: 10.4103/2249-4863.109934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sahoo N., Manchikanti P., Dey S. Herbal drugs: standards and regulation. Fitoterapia. 2010;81(6):462–471. doi: 10.1016/j.fitote.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Calixto J.B. Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents) Braz J Med Biol Res. 2000;33(2):179–189. doi: 10.1590/S0100-879X2000000200004. [DOI] [PubMed] [Google Scholar]
- 45.General guidelines for drug development of Ayurvedic formulations- Volume – I. Central council for research in Ayurvedic Sciences. Ministry of AYUSH; 2018. [Google Scholar]
- 46.General guidelines for methodologies on research and evaluation of traditional medicine. World Health Organization; Geneva: 2000. WHO/EDM/TRM. [Google Scholar]
- 47.Dahanukar S.A., Thatte U.M. Current status of Ayurveda in phytomedicine. Phytomedicine. 1997;4(4):359–368. doi: 10.1016/S0944-7113(97)80048-7. [DOI] [PubMed] [Google Scholar]
- 48.Sharma A.K., Kumar R., Mishra A., Gupta R. Problems associated with clinical trials of Ayurvedic medicines. Revista Brasileira de Farmacognosia. 2010 May;20(2):276–281. doi: 10.1590/S0102-695X2010000200023. [DOI] [Google Scholar]
- 49.Bansal D., Hota D., Chakrabarti A. Research methodological issues in evaluating herbal interventions. Open Access J Clin Trials. 2010;2:15–21. doi: 10.2147/OAJCT.S9029. [DOI] [Google Scholar]
- 50.Parveen A., Parveen B., Parveen R., Ahmad S. Challenges and guidelines for clinical trial of herbal drugs. J Pharm BioAllied Sci. 2015;7(4):329. doi: 10.4103/0975-7406.168035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dube A., Manthata L.N., Syce J.A. The design and evaluation of placebo material for crude herbals: artemisia afra herb as a model. Phytother Res. 2007;21(5):448–451. doi: 10.1002/ptr.2084. [DOI] [PubMed] [Google Scholar]
- 52.Zhang X., Tian R., Zhao C., Tang X., Lu A., Bian Z. Placebo design in WHO-registered trials of Chinese herbal medicine need improvements. BMC Compl Alternative Med. 2019;19(1):299. doi: 10.1186/s12906-019-2722-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Srikanth N. Ministry of AYUSH; 2018. General guidelines for clinical evaluation of ayurvedic interventions-guidelines seris:III. Central council for research in ayurvedic scviences. [Google Scholar]
- 54.Ministry of Health & Family Welfare Government of India . 2013. Good clinical practice guidelines for Clinical Trails in Aryuveda, Siddha and Unani Medicine (GPC-ASU) pp. 1–114. [Google Scholar]
- 55.Saxena A., Vikram N.K. Role of selected Indian plants in management of type 2 diabetes: a review. J Alternative Compl Med. 2004;10(2):369–378. doi: 10.1089/107555304323062365. [DOI] [PubMed] [Google Scholar]
- 56.Narahari S.R., Ryan T.J., Aggithaya M.G., Bose K.S., Prasanna K.S. Evidence-based approaches for the ayurvedic traditional herbal formulations: toward an ayurvedic CONSORT model. J Alternative Compl Med. 2008;14:769–776. doi: 10.1089/acm.2007.0818. [DOI] [PubMed] [Google Scholar]
- 57.Gupta D., Agrahari R., Sachdev K., Garg R. Ayurvedic management of madhumeha (type 2 diabetes mellitus) and its complications – a review article. Wjpmr. 2018;4(1):67–70. [Google Scholar]
- 58.Mathur A., Sankar V. Standards of reporting Ayurvedic clinical trials—is there a need? J Ayurveda Integr Med. 2010;1(1):52. doi: 10.4103/0975-9476.59828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patwardhan B. Bridging Ayurveda with evidence-based scientific approaches in medicine. EPMA J. 2014;5(1):19. doi: 10.1186/1878-5085-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]