Highlights
-
•
Two lentil protein isolates (LPIs) and a lentil flour (LF) were prepared in pilot-scale.
-
•
Nutritional and anti-nutritional properties of LPIs were examined in comparison to LF.
-
•
Total galacto-oligosaccharides (GOS) contents of LPIs were reduced by 58–91%.
-
•
Trypsin inhibitor activity (TIA) levels of LPIs were reduced by 81–87%.
-
•
In vitro protein digestibility (IVPD) values of LPIs were improved by 35–53%.
Abbreviations: AA, amino acids; ANC(s), anti-nutritional compound(s); ANOVA, analysis of variance; DH, degree of hydrolysis; DM, dry matter; E:S ratios, enzyme:substrate ratios; FODMAPs, fermentable oligo-, di- and monosaccharides, and polyols; FOS, Fructans and fructo-oligosaccharides; GOS, galacto-oligosaccharides; HPAEC-PAD, high performance anion exchange chromatography coupled with pulsed amperometric detection; IBS, irritable bowel syndrome; IEP, isoelectric precipitation; IVPD %, in vitro protein digestibility; IVPD P %, pepsin digestibility; IVPD PT % 1+1 h, short-term protein digestibility; IVPD PT % 1+3 h, medium-term protein digestibility; IVPD PT % 1+24 h, long-term protein digestibility; l–BAPA, N–α–benzoyl–l–arginine–4–nitroanilide; LF(s), lentil flour(s); LP, lentil protein(s); LPC(s), lentil protein concentrate(s); LPI(s), lentil protein isolate(s); LPI–IEP, lentil protein isolate prepared by IEP; LPI–UF, lentil protein isolate prepared by UF; MW(s), molecular weight(s); PD, protein digestibility; OPA, o-phthaldialdehyde; RFO, raffinose family oligosaccharides; TCA, trichloroacetic acid; TIA, trypsin inhibitor activity; TU, trypsin activity unit; TIU, trypsin inhibitor unit; TNBS, trinitrobenzenesulfonic acid; UF, ultrafiltration
Keywords: Lens culinaris, Lentil flour, Lentil protein isolates, Alternative protein sources, Pilot-scale processing, Trypsin inhibitor activity, In vitro protein digestibility, FODMAPs
Abstract
Lentil (Lens culinaris) is a high-protein crop with a promising potential as a plant-based protein source for human nutrition. This study investigated nutritional and anti-nutritional properties of whole seed lentil flour (LF) compared to lentil protein isolates (LPIs) prepared in pilot-scale by isoelectric precipitation (LPI–IEP) and ultrafiltration (LPI–UF). Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) profiles showed significant reductions in total galacto-oligosaccharides (GOS) contents by 58% and 91% in LPI–IEP and LPI–UF, respectively, compared to LF. Trypsin inhibitor activity (TIA) levels based on dry protein mass were lowered by 81% in LPI–IEP and 87% in LPI–UF relative to LF. Depending on the stage of digestion, the in vitro protein digestibility (IVPD) of LPIs was improved by 35–53% compared to LF, with both products showing a similar long-term protein digestibility to that of bovine serum albumin (BSA). This work supports the use of purified LPI products as a novel source of high quality protein for food applications.
1. Introduction
The global demand for protein is growing rapidly and has been projected to be more than doubled by 2050 (Westhoek et al., 2011). In the food industry there is an increased interest in replacing animal proteins with new alternative protein sources of high nutritional quality. Plant-based protein sources can be a cost-effective way to meet the future demand for protein and improve the overall dietary quality at all levels of income (Aggarwal & Drewnowski, 2019). Plant proteins may also offer an improved environmental and sustainability profile, as well as other health benefits such as a reduced risk of mortality in certain individuals (Song et al., 2016).
Lentil (Lens culinaris) is a low-fat, high-protein, and high-fibre pulse crop belonging to the legume family (Jarpa-Parra, 2018). Similar to most legumes, lentil provides an excellent source of dietary protein for human nutrition, containing 20.6–31.4% protein on dry weight basis (Urbano, Porres, Frías, & Vidal-Valverde, 2007). Lentil proteins (LP) mostly consist of storage proteins classified according to their solubility behavior as albumins (water-soluble), globulins (salt-soluble), glutelins (dilute acid/base soluble), and prolamins (alcohol-soluble). In regard to protein composition, LP is usually composed of around 16% albumins, 70% globulins, 11% glutelins, and 3% prolamins (Boye et al., 2010, Jarpa-Parra, 2018). The globulins consist of two types of proteins, vicilin and legumin, which are traditionally known as 7S and 11S proteins based on their sedimentation coefficients. Vicilin is composed of trimers of glycosylated subunits, each with a molecular weight (MW) of 40–70 kDa connected without disulphide bridges (López-Torrejón et al., 2003). Legumin is a hexameric protein with a MW of 320–380 kDa. The six subunits of legumin are non-covalently linked, and each subunit is composed of an acidic (~40 kDa) and a basic (~20 kDa) polypeptide chain linked by one disulphide bond (Shewry, Napier, & Tatham, 1995). The albumin, glutelin, and prolamin fractions have MWs of about 20 kDa, 17–46 kDa, and 16–64 kDa, respectively, consisting of approximately 13, 4, and 10 polypeptides (Boye, Zare, et al., 2010). The isoelectric points of proteins in lentils have been reported to range from pH 4.5–5.9 (Aydemir & Yemenicioğlu, 2013). All protein fractions in lentils (albumins, legumins, vicilins, glutelins, and prolamins) are glycosylated, especially the vicilins that contain about 2.8% carbohydrate (Boye, Zare, et al., 2010).
Lentil proteins generally have a high nutritional quality and an acceptable amino acid (AA) composition with relatively good leucine/isoleucine and leucine/lysine ratios (Boye, Zare, et al., 2010). These proteins are especially rich in lysine, but are also limited in sulphur-containing AA, and thus require mixing with other plant protein sources such as cereal grains to obtain an adequately well-balanced AA profile (Jarpa-Parra, 2018). The nutritional potential of proteins derived from lentils and other pulses may be limited by the presence of allergens and anti-nutritional compounds (ANCs) such as phytic acid, protease inhibitors, tannins, and lectins (Nosworthy and House, 2017, Urbano et al., 2007). Protease inhibitors like trypsin and chymotrypsin inhibitors may significantly lower the protein digestibility if not properly removed or inactivated during processing (Boye, Zare, et al., 2010). However, the negative effects of ANCs on the nutritional value and digestibility of LP can be greatly diminished by different processing methods (Nosworthy et al., 2018). Lentil protein concentrates (LPCs) or isolates (LPIs) have mostly been prepared by a combination of aqueous alkaline extraction and isoelectric precipitation (IEP) methods (Aydemir and Yemenicioğlu, 2013, Johnston et al., 2015). Alkaline extraction coupled with IEP has been shown to reduce the trypsin inhibitor activity (TIA) levels of LPCs by about 66–82%, respectively, compared to whole seed lentil flours (LF) (Barbana & Boye, 2013). Membrane filtration processing methods like ultrafiltration (UF) have been demonstrated to yield purified LP products with a slightly higher protein content (82.7–88.6%) compared to the IEP method (78.2–79.1%) (Boye, Aksay, et al., 2010). Depending on the applied processing method and the lentil variety, the in vitro protein digestibility (IVPD) of LFs and LPCs/LPIs have been reported to range from about 66–88% and 76–85%, respectively (Carbonaro et al., 2012, Barbana and Boye, 2013, Aryee and Boye, 2016, Nosworthy et al., 2018). Highly purified LPCs/LPIs exhibit IVPD values (75–77%) between those of similar protein products produced from faba bean (74–75%) and pea (~78%), while all these products show lower IVPD than both animal-based foods (e.g. milk, cheese, and meat) and their isolated proteins (e.g. caseins, ~84%) (Carbonaro et al., 2012, Nosworthy and House, 2017).
Lentils and other pulses reveal in their native composition a high content of fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs). The main saccharides found in pulses considered as FODMAPs are galacto-oligosaccharides (GOS), which are also referred to as raffinose family oligosaccharides (RFO). These are α-galactose derivates (1 → 6 linked) from sucrose (α-glucose 1 → 2 linked to ß-fructose), with raffinose (trisaccharide), stachyose (tetrasaccharide) and verbascose (pentasaccharide) being the most abundant representatives (Ispiryan et al., 2019, Martínez-Villaluenga et al., 2008). Since the human gut lacks the enzyme α-galactosidase, these carbohydrates are not digested and fermented by the colonic microflora. Thereby, short-chain fatty acids and gases are produced (Tahir, Lindeboom, Båga, Vandenberg, & Chibbar, 2011). Although the colonic fermentation of non-digestible dietary carbohydrates is known to be beneficial for the human digestive health, only a few studies have investigated prebiotic properties from GOS derived from pulses, since bacterial growth on these substrates is less specific in comparison to the structurally similar β-GOS. These consist of a terminal β-linked glucose and are commercially produced by enzymatic β-galactose transfer on lactose; β-GOS represent a major group of well investigated prebiotics (Wilson and Whelan, 2017). On the contrary, α-GOS from pulses have been largely in focus due to their flatulence-inducing properties. In particular, for individuals with functional gastrointestinal disorders such as irritable bowel syndrome (IBS), the ingestion of those carbohydrates may be more problematic, causing gastrointestinal discomfort and various symptoms. Thus, α-GOS belong to the often described group of FODMAPs associated with gastrointestinal symptoms of IBS. In addition to GOS, FODMAPs comprise the most abundant dietary fermentable, small and osmotically active carbohydrates, being fructans and fructo–oligosaccharides (FOS), lactose, fructose in excess of glucose and polyols. A reduction of dietary FODMAPs (a low FODMAPs diet) has shown significant improvement of symptoms and the patients’ wellness (Halmos et al., 2014, Staudacher et al., 2011).
The hypothesis of this study was that the nutritional and anti-nutritional properties of LPIs would be changed compared to whole seed LF. Therefore, the aim was to investigate if LPIs produced by pilot–scale processing methods involving IEP (LPI–IEP) and UF (LPI–UF) differed compared to LF in relation to nutritional and anti-nutritional properties such as IVPD, FODMAP content, and TIA level.
2. Materials and methods
2.1. Chemicals and reagents
Analytical grade chemicals were supplied by Sigma–Aldrich Denmark A/S (Copenhagen, DK), unless otherwise stated. Sodium hydroxide solution (50% w/w) was purchased from Thermo Fisher Scientific (ACROS OrganicsTM; Dublin, IE). Electrochemical-grade sodium acetate was purchased from Thermo Fisher Scientific (DionexTM AAA-Direct Reagents; Dublin, IE). HPLC-grade methanol, potassium hexacyanoferrate (II) trihydrate (Carrez I) and zinc acetate dihydrate (Carrez II) were purchased from Sigma-Aldrich (Darmstadt, DE) and glacial acetic acid from Thermo Fisher Scientific (J.T. BakerTM; Loughborough, UK). Sodium azide was obtained from Thermo Fisher Scientific (Alfa Aesar; Lancashire, UK). All carbohydrate reference standards and analytical enzyme preparations of high purity were purchased from Megazyme (Bray, IE), Carbosynth (Compton, UK), or Sigma-Aldrich (Darmstadt, DE). Pepsin (P7000, porcine gastric mucosa, 920 U/mg protein), pancreatin (P1750, 4 × USP, porcine pancreas), trypsin (T0134, porcine pancreas, Type IX, 16,300 U/mg protein), and bovine serum albumin (BSA; heat shock fraction, pH 5.2, purity ≥ 96%) were purchased from Sigma–Aldrich Denmark A/S (Copenhagen, DK). Ultrapure water obtained from either a Milli–Q Plus system (Millipore Corporation, Milford, MA, USA) or a DionexTM IC PureTM Water Purification System (Thermo Fisher Scientific, Sunnyvale, CA, USA) was used for all buffers, reagents, and sample preparations. All experimental analyses were performed in triplicates, unless otherwise stated.
2.2. Raw material and protein isolation
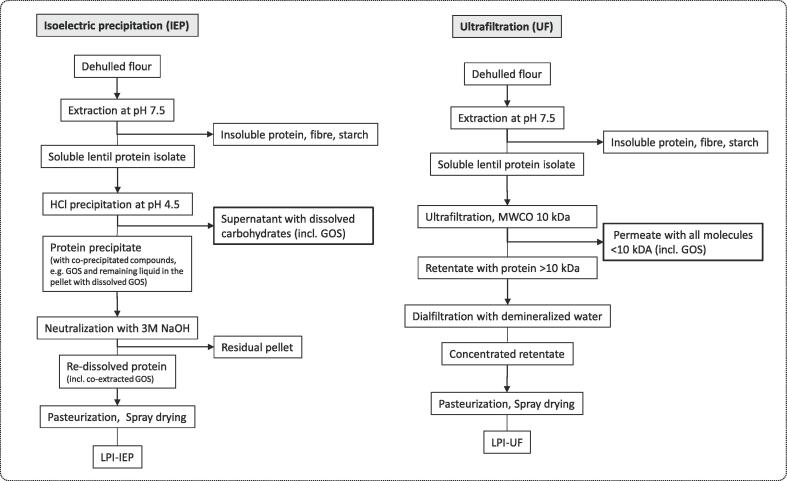
Brown lentils (Lens culinaris, cv. Itaca) of commercial quality were provided by Agroservice S.P.A. (San Severino Marche, Italy), and used as raw material for preparation of whole seed LF. LPI products were recovered by a single-batch, pilot-scale processing procedure involving either isoelectric precipitation (LPI-IEP) or ultrafiltration (LPI-UF), according to a previously described procedure (Alonso-Miravalles et al., 2019), as shown in Fig. 1. Each batch of the protein extraction trials was carried out with an input of 150 kg of lentil flour and an extraction volume of 1050 L (1.05 m3) of water. The protein contents (N × 6.25) of LF, LPI-IEP, and LPI-UF based on dry matter (DM) were 30.9, 85.9, and 93.4 g/100 g DM, respectively.
Fig. 1.
LPI-IEP and LPI-UF preparation processes according to Alonso-Miravalles et al. (2019) with indicated impact on FODMAP (GOS) extraction and isolation.
2.3. Molecular weight (MW) analysis
Molecular weight (MW) of proteins was analyzed under non-reducing and reducing conditions by electrophoresis using the Bioanalyzer 2100 (Agilent Technologies, Palo Alto, CA, USA), according to the instructions given in the manual for the Protein 80 + chip. The analysis was carried out in a MW range of 4.5–95 kDa.
2.4. Quantification of fermentable oligo-, di- and monosaccharides, and polyols (FODMAPs)
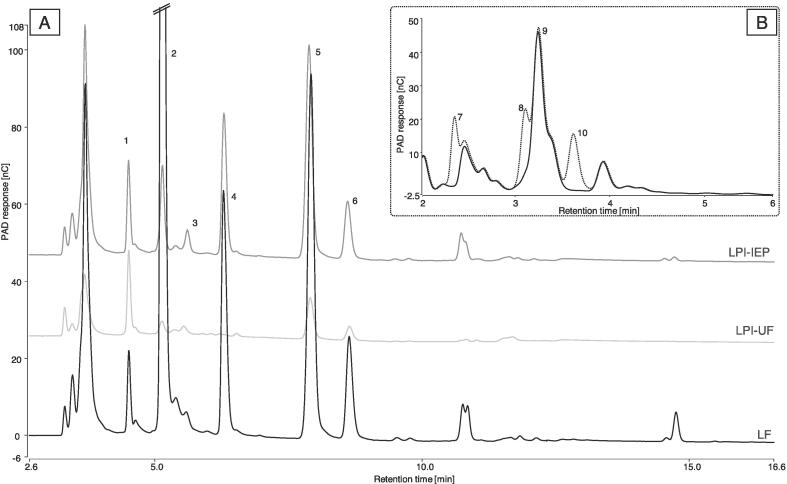
Quantification of monosaccharides, disaccharides, oligosaccharides (including fructans), and polyols was performed using high performance anion exchange chromatography coupled with pulsed amperometric detection (HPAEC-PAD), using a DionexTM ICS-5000+ system (Sunnyvale, CA, USA) mounted with DionexTM CarboPacTM PA1 and PA200 columns (Thermo Fisher Scientific, Sunnyvale, CA, USA), as previously described (Ispiryan, Heitmann, Hoehnel, Zannini, & Arendt, 2019). Briefly, 400 mg of the samples were mixed with 1 mL MeOH, 100 μL internal standard rhamnose (9 mg/mL), and 20 mL 80 °C H2O (containing 50 mg/L NaN3). The mixture was subjected to the first extraction step, using a Sonoplus homogenizer for 2 × 15 s. After centrifugation at 1520 g for 5 min, the supernatant was transferred into a 100 mL volumetric flask and the extraction was repeated with 20 mL 80 °C H2O (containing 50 mg/L NaN3). Two hundred microliters of Carrez I and Carrez II were added to the supernatants, respectively. The extracts were centrifuged at 3000 g for 10 min, filtered through 0.2 µm polyamide syringe filters (Chromafil AO-20/25; Machery Nagel, Düren, DE), and further diluted for HPAEC-PAD analysis. The calculation of total GOS content was based on the amount of raffinose, stachyose, and verbascose. All results are presented in g analyte per 100 g sample on a dry weight basis (g/ 100 g DM). For further verification and identification of unknown or suspected peaks, sample extracts were spiked with a mixture of reference standards, as shown in Fig. 3.
Fig. 3.
HPAEC-PAD chromatograms. (1) rhamnose (internal standard), (2) glucose, (3) fructose, (4) sucrose, (5) raffinose/stachyose, (6) verbascose, (7) xylitol, (8) sorbitol, (9) unknown (predicted cyclitol-derivate), (10) mannitol. (A) CarboPac PA200 profile of LF overlaid with LPI–IEP and LPI–UF, (B) CarboPac PA1 profiles of LF overlaid with LF spiked with standard mixture.
The total fructan content was determined after enzymatic hydrolysis. Two aliquots of 500 μL from the extracts, obtained as described above, were mixed with 150 μL of the enzyme mixture A (1:1:1 mixture of α-galactosidase, amyloglucosidase, and 100 mM sodiumacetate-buffer) and enzyme mixture B (1:1:1 mixture of α-galactosidase, amyloglucosidase, and inulinase), respectively. The mixtures were incubated 30 min at 60 °C, heated to 100 °C for 40 min, diluted to a total volume of 1 mL, and analyzed by HPAEC-PAD. The calculation of total fructan content was based on the amount of glucose and fructose released from fructans. All results are presented in g analyte per 100 g sample on a dry weight basis (g/ 100 g DM).
2.5. Trypsin inhibitor activity (TIA)
Trypsin inhibitors were extracted by solubilization of the samples in sodium acetate buffer (0.1 M, pH 4.9) at 14% (w/v) powder concentrations, followed by Ultra-Turrax homogenization for 2 min, and centrifugation at 13,500 RPM for 5 min (ScanSpeed mini; LaboGene, Lynge, DK). Supernatants were transferred to new tubes prior to another centrifugation step. Purified supernatants were stored overnight at 5 °C and centrifuged again for 5 min prior to the TIA assay.
TIA levels of lentil protein sources were determined using a previously described assay method (Joehnke et al., 2018). In this assay, one trypsin inhibitor unit (TIU) represents the amount of inhibitor required to reduce the enzyme activity by one trypsin activity unit (TU). TU is defined as the amount of enzyme that catalyzes hydrolysis of 1 µmol l–BAPA substrate into the product (4–nitroaniline) in 1 min at pH 8.2 and 37 °C. All samples were corrected by subtraction of background absorbance in blank samples containing only buffer and substrate. TIA levels were determined against purified trypsin enzyme and expressed as either TIU/mg sample or TIU/mg protein on a dry weight basis.
2.6. In vitro protein digestibility (IVPD)
Simulated gastrointestinal digestion was performed, with a few modifications, according to a previously published static IVPD method (Joehnke et al., 2018, Joehnke et al., 2019). Briefly, all protein samples were weighed to contain 50 mg protein based on DM and solubilized in protein concentrations of 0.5% (w/v). Equivalent amounts of BSA (reference protein) and free alanine samples, as well as blank samples containing only buffer were run in tandem. The enzymatic digestion consisted of hydrolysis by pepsin (1 h at 37 °C) followed by pancreatin (+1 h, +3 h, or + 24 h at 37 °C) at constant enzyme:substrate ratios (E:S ratios) of approximately 1:50 and 1:10 (w/w) enzyme to substrate protein, respectively. Aliquots of digestion products were withdrawn progressively from the untreated, pepsin digested, and pepsin + pancreatin digested samples. IVPD of samples was quantified using an in–house developed trinitrobenzenesulfonic acid (TNBS)–based assay method (Joehnke et al., 2018). IVPD (%) at each stage of digestion was calculated based on the relative concentration of free α–amino groups in samples and an alanine standard solution used in the TNBS–based assay. The results were subsequently expressed relative to the regular alanine samples representing 100% protein digestibility (PD). The starting level of hydrolysis in untreated samples was subtracted to obtain corrected values for pepsin digestibility (IVPD P %, 1 h) and pepsin + pancreatin protein digestibility (IVPD PT %; 1 + 1 h, short-term protein digestibility; 1 + 3 h, medium-term protein digestibility; 1 + 24 h, long-term protein digestibility). This correction also accounted for enzymatic autolysis by deducting the value of the blank samples.
2.7. Statistical analysis
Statistical analysis was performed using GraphPad Prism 7 (GraphPad Software Inc., San Diego, CA, USA) and SPSS Statistic 24 (IBM Corp., Armonk, NY, USA). The results are presented as mean ± standard deviation (mean ± SD, n = 3). One–way analysis of variance (ANOVA) followed by Tukey’s post hoc test for pairwise multiple comparisons was used to assess statistically significant differences amongst several lentil protein sources. Multiplicity adjusted P values were calculated and differences were considered statistically significant at a base level of P < 0.05. However, in the discussion of results, the significance is stated as either P < 0.05, P < 0.01, or P < 0.001 to further indicate the magnitude of the statistical difference.
3. Results and discussion
3.1. Molecular weight
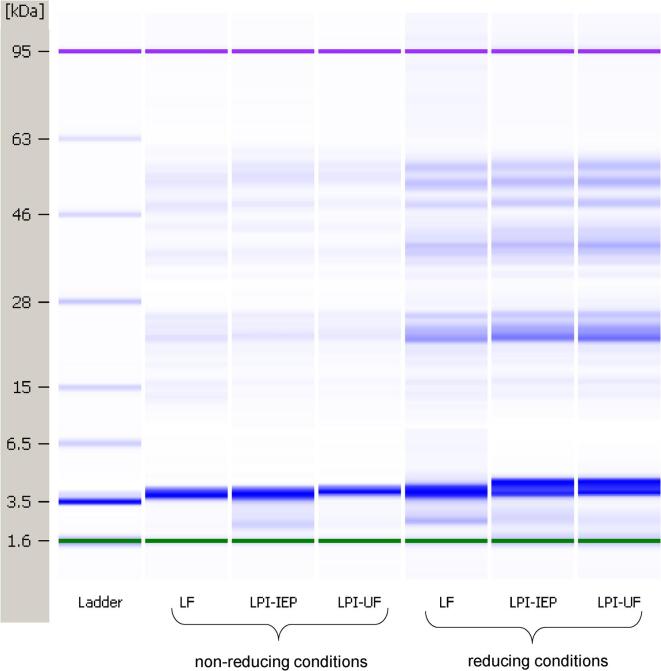
LF and the two LPIs were analyzed for their protein profile using the Agilent Bioanalyzer and the results are presented in Fig. 2. Typical protein profiles corresponding to the subunits of the main storage proteins legumin 11S and vicilin 7S were found. Several common bands corresponding to the subunits of vicilin were detected under non-reducing and reducing conditions between 40 and 70 kDa. The band at MW ~60 kDa detected under non-reducing conditions may correspond to intact legumin subunits. Under reducing conditions, the band at ~60 kDa vanished, whilst bands corresponding to the acidic (~40 kDa) and basic subunits (~20 kDa) of legumin showed a more pronounced intensity. In general, all lentil samples showed similar protein profiles with several common bands between LF, LPI–IEP, and LPI–UF. Thus, the different isolation techniques did not seem to have a major impact on the MW distribution of proteins in the LPIs compared to LF. These results are similar to those previously reported for LF, LPCs, and LPIs, where only subtle differences in band patterns were shown by SDS-PAGE under both non–reducing and reducing conditions (Barbana and Boye, 2013, Alonso-Miravalles et al., 2019).
Fig. 2.
Gel-like images of the lentil protein sources under non-reducing and reducing conditions obtained from Bioanalyzer using an Agilent 80 + protein chip.
3.2. FODMAP contents
FODMAP contents in LF and LPIs are presented in Table 1. In accordance with other studies, LF contained a total GOS content of ~4 g/ 100 g DM, with the most abundant GOS being the tetrasaccharide stachyose (Dilis and Trichopoulou, 2009, Johnson et al., 2013). In this study, the sum of raffinose and stachyose in LF resulted in 3.41 g/ 100 g DM. The concentration of the pentasaccharide verbascose in lentils varies depending on variety and origin, ranging between 0.6 and 3.1 g/ 100 g DM (Martínez-Villaluenga et al., 2008). Thus, the amount of verbascose determined in the LF (0.75 g/ 100 g DM) is within the range reported in other studies. In contrast to Johnson et al. (2013), no sorbitol was determined in the LF. Spiking of the LF-extract (Fig. 3) with the reference standard of sorbitol resulted in a separated elution of sorbitol from the unknown peak, originating from the sample extract. The compound eluting very closely to sorbitol is suspected to be a metabolite (cyclitol) of the biosynthesis of the GOS (Fig. 3) (Martínez-Villaluenga et al., 2008, Sengupta et al., 2015). However, this compound could not be identified and hence, no reference standard could be acquired. The approximate amount was estimated by determination of the unknown compound as sorbitol, with ~0.9 g/ 100 g DM. No fructose in excess of glucose, no lactose, and only traces of fructans were determined. Hence, the predominant FODMAPs in lentils are GOS. The analysis revealed that only 9% of the GOS from the LF remained in LPI–UF, whereas a higher GOS content of 42% was recovered in LPI–IEP. Both protein isolation processes involved steps for the removal of soluble carbohydrates such as FODMAPs (GOS). Thereby, ultrafiltration resulted in an effective removal of compounds with a molecular weight below 10 kDa, including GOS. Within the acid precipitation step of the IEP process, GOS were only partially removed, dissolved in the supernatant, while a proportion of the GOS presumably remained in the residual liquid of the pellet or was co-precipitated in the protein-matrix (Fig. 1). Ispiryan et al. (2020) also reported highly variable GOS contents in different, partly commercial pulse-protein ingredients, expected as a result from different preparation processes. Faba bean and lupin protein isolates obtained by IEP contained only traces of GOS (Ispiryan, Zannini, & Arendt, 2020). Hence, modifications of the IEP processing steps, such as an additional acid precipitation step as reported in a recent study (Vogelsang-O’Dwyer et al., 2020), may result in lower GOS levels in the LPI-IEP. The clinically relevant cut-off level for oligosaccharides (i.e. the sum of GOS and fructans) triggering symptoms in IBS patients is 0.3 g per serve, when referring to typical serving sizes of food products (Muir et al., 2009, Varney et al., 2017). Hence, a level of ~2 g/ 100 g DM total GOS in LPI–IEP may result in a high FODMAP product, depending on proportion of protein isolate in a food recipe. Contrariwise, the low amount of GOS in LPI–UF (0.37 g/100 g DM) enables its application as a high nutritional value ingredient in low FODMAPs formulations.
Table 1.
FODMAP content in lentil protein sources.1
|
FODMAP content (g/100 g DM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Mono-/Disaccharides |
Polyols |
Oligosaccharides |
|||||||
| Glucose | Fructose2 | Excess fructose3 | Lactose2 | Estimation unknown4 | Raffinose / Stachyose | Verbascose | ∑GOS5 | Total fructan6 | |
| LF | 0.20 ± 0.04b | 0.03 ± 0.00b | – | n.d. | 0.87 ± 0.02a | 3.41 ± 0.15a | 0.75 ± 0.03a | 4.17 ± 0.15a | n.d. |
| LPI-IEP | 0.29 ± 0.01a | 0.10 ± 0.00a | – | n.d. | 0.38 ± 0.01b | 1.43 ± 0.04b | 0.35 ± 0.01b | 1.77 ± 0.04b | n.d. |
| LPI-UF | 0.05 ± 0.00c | n.d. | – | n.d. | 0.08 ± 0.00c | 0.29 ± 0.01c | 0.08 ± 0.00c | 0.37 ± 0.01c | n.d. |
The results are presented as mean ± SD (n = 3). Values within one column with different letter superscript are significantly different (P < 0.05).
Fructose and lactose n.d. = not detected (below 0.005 g/100 g DM).
Excess fructose = fructose – glucose.
Unknown polyol, i.e. presumed to be α-galactose linked cyclitol, estimated as sorbitol.
Total galacto-oligosaccharides (GOS) = Raffinose / Stachyose + Verbascose.
Total fructan n.d. = not detected (below 0.1 g/100 g DM).
3.3. Trypsin inhibitor activity (TIA)
TIA levels of the lentil protein sources determined against purified trypsin enzyme are presented in Table 2. Based on dry sample mass, LF showed a significantly higher TIA than both LPI–IEP and LPI–UF (P < 0.05). These products had TIA levels based on dry sample mass that were reduced by 44% and 59%, respectively, compared to LF. TIA levels standardized according to dry protein mass were also significantly higher for LF compared to both LPIs (P < 0.001). LPI–IEP and LPI–UF exhibited reductions in TIA levels based on dry protein mass of 81% and 87%, respectively, relative to LF. These results indicate that a major proportion of trypsin inhibitors were removed from LF during the pilot-scale protein extraction and processing procedure applied for preparation of the LPIs. However, TIA values obtained in this study were generally higher than those reported in previous studies (Barbana and Boye, 2013, Aryee and Boye, 2017). Minor variations in absolute values may be attributed to differences in the TIA determination, including the definitions provided for trypsin activity units (TU) and trypsin inhibitor units (TIU). Barbana and Boye (2013) reported TIA values for LFs and LPCs ranging from 0.94 to 1.94 and 0.17–0.66 TIU/mg protein, respectively, depending on the lentil variety. These authors found reductions in the TIA levels of green and red LPCs produced by alkaline extraction and IEP ranging from about 66–82%, respectively, relative to LFs (Barbana & Boye, 2013). In another study, the TIA level of an IEP protein isolate prepared by a similar processing method was found to be reduced by 69% (based on sample mass) or 91% (based on protein mass) compared to LF (Aryee & Boye, 2017). Therefore, although the absolute TIA values were higher in this study, the relative reductions for LPIs compared to LF correspond well with those reported elsewhere.
Table 2.
Trypsin inhibitor activity (TIA) of lentil protein sources.1
| Based on dry sample mass |
Based on dry protein mass |
|||
|---|---|---|---|---|
| TIU/mg sample | Reduction (%)2 | TIU/mg protein | Reduction (%)2 | |
| LF | 1.75 ± 0.27a | 6.17 ± 0.96a | ||
| LPI–IEP | 0.98 ± 0.18b | 44 | 1.19 ± 0.22b | 81 |
| LPI–UF | 0.72 ± 0.19b | 59 | 0.81 ± 0.22b | 87 |
TIA levels based on either dry sample mass or dry protein mass were determined against purified trypsin enzyme and expressed as TIU/mg sample or TIU/mg protein, respectively. The results are presented as mean ± SD (n = 3). Values within one column with different letter superscript are significantly different (P < 0.05).
Reduction (%) in TIA levels of LPIs compared to LF.
Lentil seeds generally have a lower TIA content per sample mass (3–8 TIU/mg) compared to other legume seed cultivars such as soybean (43–84 TIU/mg), chickpea (15–19 TIU/mg), pea (6–15 TIU/mg), and faba bean (5–10 TIU/mg) (Guillamón et al., 2008). TIA levels of lentil seeds may be effectively decreased by dehulling or application of different thermal treatments such as boiling, autoclaving, extrusion cooking, and microwave cooking (Aryee and Boye, 2017, Rathod and Annapure, 2016, Wang et al., 2009). Seeds of lentil are known to contain a variety of different protease inhibitors and isoinhibitors that primarily belong to the Bowman–Birk trypsin inhibitor family (Weder & Kahley, 1998). Bowman–Birk inhibitors present in legume seeds are usually small proteins composed of a single polypeptide chain, with MWs of 6–10 kDa, high cystine contents (five to seven disulphide bonds), and two reactive sites capable of binding to trypsin and chymotrypsin enzymes (Qi, Song, & Chi, 2005). Therefore, Bowman–Birk inhibitors are usually considered to be ANCs due to their ability to inhibit the activity of digestive enzymes, potentially leading to a reduced digestibility of dietary proteins.
3.4. In vitro protein digestibility (IVPD)
In this study, the IVPD procedure consisted of pepsin digestion for 1 h followed by pancreatin digestion for 1 h (short-), 3 h (medium-), or 24 h (long-term protein digestibility, respectively). The sequential enzymatic digestion by pepsin and pancreatin was performed with constant E:S ratios of approximately 1:50 and 1:10 (w/w) enzyme to substrate protein, respectively. These levels of added enzymes were initially optimized to gain a maximal short-term digestibility of BSA (1 + 1 h). BSA served as a positive control in this assay, since it constitutes a highly digestible protein (Joehnke et al., 2018).
IVPD of the different protein sources according to stage of digestion are presented in Table 3. The pepsin digestibility (IVPD P %) values obtained for the lentil protein sources ranged from 4.3–6.4%. LPI–IEP and LPI–UF possessed a similar (P = 0.09) and significantly improved pepsin digestibility compared to LF (~35% and ~49%, respectively, P < 0.05). However, all lentil protein sources showed significantly lower pepsin digestibility values than obtained for the BSA control (P < 0.01). The increased pepsin digestibility of LPIs is indicative of a higher susceptibility of peptide bonds towards enzymatic hydrolysis by pepsin compared to the proteins found in LF. The pepsin hydrolysis time of 1 h used in this study was relatively short, which may explain the relatively low IVPD values obtained compared to other studies (A. Suliema et al., 2008, Bamdad et al., 2009, Barbana et al., 2011, Aryee and Boye, 2016).
Table 3.
IVPD of lentil protein sources according to the stage of digestion.1
|
IVPD P (%) |
IVPD PT (%) |
|||
|---|---|---|---|---|
| 1 h | 1 + 1 h | 1 + 3 h | 1 + 24 h | |
| LF | 4.3 ± 0.4c | 17.0 ± 0.9c | 21.3 ± 1.2d | 28.3 ± 1.0b |
| LPI–IEP | 5.8 ± 0.4b | 25.5 ± 0.5b | 29.8 ± 0.3c | 42.9 ± 2.1a |
| LPI–UF | 6.4 ± 0.1b | 26.0 ± 0.5b | 32.3 ± 0.9b | 42.1 ± 1.9a |
| BSA (control) | 7.6 ± 0.2a | 31.7 ± 0.7a | 36.6 ± 0.1a | 42.8 ± 0.6a |
Pepsin digestibility (IVPD P %, 1 h) and pepsin + pancreatin protein digestibility in the short-term (IVPD PT %, 1 + 1 h), medium-term (IVPD PT %, 1 + 3 h), and long-term (IVPD PT %, 1 + 24 h). The results are presented as mean ± SD (n = 3). One-way ANOVA was performed within each stage of digestion and values within one column with different letter superscript are significantly different (P < 0.05).
Short-term protein digestibility (IVPD PT % 1 + 1 h) of the lentil proteins ranged from 17.0–26.0%. The results showed a significantly higher short-term protein digestibility of BSA compared to all lentil protein sources (P < 0.001). LPIs exhibited significantly higher short-term protein digestibility compared to the LF (~50–53%, P < 0.05), but similar values were obtained for the two LPIs (P = 0.84). These short-term protein digestibility values correspond to average chain lengths of peptides released from LF and LPIs of approximately six and four AAs (e.g. 100%/17.0% ≈ 6 AAs), respectively, indicating as expected an incomplete digestion of the lentil proteins within the initial 2 h of sequential digestion with pepsin and pancreatin. A similar incremental increase in the absolute digestibility values (+ ~20%) were observed for the LPIs from the pepsin digestion to the short-term protein digestion (corresponding to the pancreatin digestibility within 1 h), whilst this was considerably lower for LF (+ ~13%). These results indicate a major removal and/or inactivation of ANCs (e.g. trypsin inhibitors) from the LPIs during processing, which can otherwise adversely affect the protein digestibility. Indeed, a reduction in the content of ANCs (e.g. trypsin inhibitors, phytic acid, and tannins) by appropriate processing techniques is an efficient strategy to lower the degree of interactions and complexations amongst molecules, thereby facilitating an improved protein digestibility (Sarwar Gilani, Wu Xiao, & Cockell, 2012). Furthermore, differences in the short-term protein digestibility could also be facilitated by structural modifications of the proteins occurring during extraction and processing steps (e.g. denaturation or unfolding) or within the IVPD procedure (e.g. acid- or pepsin-induced changes) (Aryee and Boye, 2016, Aryee and Boye, 2017). Carbonaro et al. (2012) studied the relationship between structure and digestibility of legume proteins, showing a strong inverse correlation between the β–sheet content and IVPD. These authors also demonstrated that structural modifications induced by thermal treatments (e.g. dry heating or autoclaving) can lead to changes in the β–sheet arrangements and digestibility of legume proteins (Carbonaro et al., 2012). In this study, the pilot-scale preparation processes of LPI–IEP and LPI–UF both included thermal treatments by pasteurization (65 °C, 30 min) and spray-drying (Tin: 180 °C, Tout: 75 °C), as described elsewhere (Alonso-Miravalles et al., 2019). However, Alonso–Miravalles et al. (2019) reported only limited secondary structure conformational modifications induced by extraction and processing of the LPIs. Similarly, in another study, only slight differences were observed in the secondary structures of two lentil flours and a LPI prepared by IEP (Aryee & Boye, 2017). Hence, the variation in the protein digestibility of LF and LPIs found in this study is most likely not due to structural modifications induced by the extraction and processing steps. Instead, as previously mentioned, these differences may largely result from the lower content of ANCs like trypsin inhibitors in the LPIs compared to LF.
In general, the IVPD values obtained in this study were relatively low compared to those reported for lentil protein sources in other studies, whilst markedly higher improvements were shown here for the IVPD of LPIs compared to LF (Bamdad et al., 2009, Barbana and Boye, 2013, Aryee and Boye, 2016). This relationship is to be expected, considering the ability to attain a higher percentage improvement in the IVPD when comparing similar absolute differences between two relatively low values (e.g. 20% vs. 25% = 25% increase) compared to higher IVPD values (e.g. 50% vs. 55% = 10% increase). Furthermore, differences in the reported results may be attributed to variations in the applied processing methods, IVPD model systems, and IVPD quantification methods. Previous in vitro studies have reported IVPD and degree of hydrolysis (DH) values for LFs and LPCs/LPIs ranging from about 66–88% and 76–85%, respectively, depending on the lentil variety and the applied processing procedure (Carbonaro et al., 2012, Barbana and Boye, 2013, Aryee and Boye, 2016, Nosworthy et al., 2018). Barbana and Boye (2013) demonstrated using the 10 min pH–drop method that the IVPD of LPCs was improved by 8–10% compared to the LF. Aryee and Boye (2016) showed using the same method that the IVPD of a LPI obtained by IEP was improved by 26% relative to the LF. Whole seed LF possess a similar short-term IVPD (79%) as soybean (80%), as well as a higher digestibility than other legume sources such as common bean (74%) and chickpea (77%) (Carbonaro et al., 2012). Furthermore, in a recent study, LFs prepared by different thermal processing methods (extrusion, baking, or cooking) were reported to show improved IVPD values ranging from 79–88%, respectively (Nosworthy et al., 2018). Amongst pulse cultivars, LPCs and LPIs exhibit intermediate IVPD values (75–77%) compared to those of faba bean (74–75%) and pea (78%), while having lower digestibility than casein (84%) (Nosworthy & House, 2017). To sum up, in agreement with our findings, legume protein sources have generally been reported to possess lower short-term IVPD values compared to both animal-based foods (e.g. milk, cheese, and meat) and individual animal proteins (e.g. caseins) (Carbonaro et al., 2012, Nosworthy and House, 2017).
Medium-term protein digestibility (IVPD PT % 1 + 3 h) of the lentil protein sources varied from 21.3–32.3%. In parallel to the short-term protein digestibility, the highest medium-term protein digestibility was obtained for BSA compared to the LPIs (P < 0.001). Amongst the lentil protein sources, LPI–UF exhibited a significantly higher medium-term protein digestibility compared to both LF (P < 0.001) and LPI–IEP (P < 0.05). For LPI–UF, the rate of proteolysis remained relatively constant from the short- to medium-term protein digestion, whilst it was temporarily lowered for LPI–IEP. However, LPI–IEP and LPI–UF both exhibited a significantly higher medium-term protein digestibility compared to LF (~40% and ~52%, respectively, P < 0.001). Barbana et al. (2011) applied a sequential pepsin–trypsin–α–chymotrypsin digestion combined with a TNBS method to assess the DH of two LPCs produced by alkaline extraction and IEP. Similar to the results obtained in this study, these authors reported medium-term IVPD values for red and green LPCs ranging from 27–29% (Barbana et al., 2011). With respect to the differences in IVPD between lentil protein sources and BSA, a previous study found both the peptic and medium-term protein digestibility of a LPI to be augmented more than a whey protein concentrate, which presumably contained a mixture of proteins such as α–lactalbumin, β–lactoglobulin, and BSA (Bamdad et al., 2009). These authors applied a two–step sequential in vitro digestion method involving pepsin hydrolysis for 2 h and trypsin/chymotrypsin hydrolysis for 2.5 h, followed by an OPA quantification method for determining DH. Therefore, these contrary results may be attributed to differences in the purity levels of the protein references and application of various quantification methods for determining DH or IVPD. Besides these differences, the broad variations in IVPD values and improvements reported for lentil protein sources may also be related to the selectivity, specificity, order, and combination of enzymes added in the in vitro model system. In this study, a sequential digestion procedure was applied involving pepsin hydrolysis for 1 h followed by a variable time of pancreatin digestion. The addition of pepsin prior to the sequential digestion by pancreatin may lead to hydrolysis of more peptide bonds and exposure of new sites not naturally available to enzymes in pancreatin (e.g. trypsin, chymotrypsin, and exopeptidases). In this study, relatively high E:S ratios were used compared to some other studies, which would be expected to facilitate a higher protein digestibility (Bamdad et al., 2009, Barbana and Boye, 2013, Aryee and Boye, 2016). However, the high enzymatic levels were partially counterbalanced by a relatively short hydrolysis time, especially within the pepsin digestion phase, as previously mentioned. This may have resulted in a lower degree of predigestion by pepsin and less exposure of new proteolytic sites, thereby leading to a reduction in the protein digestibility. Nevertheless, it is extremely difficult to determine the relative contribution of such methodological differences on variations in DH and IVPD values.
Long-term protein digestibility (IVPD PT % 1 + 24 h) of the lentil protein sources ranged from 28.3–42.9%. Relative to LF, the long-term protein digestibility of LPI–IEP and LPI–UF was improved by approximately 52% (P < 0.001) and 49% (P < 0.001). Interestingly, in contrast to the short- and medium-term protein digestibility, the LPIs showed long–term digestibility values that were statistically equivalent to purified BSA (P > 0.05). These results may be attributed to an increased unfolding of the protein structures and improved accessibility towards enzymatic activities with a prolonged incubation time, possibly leading to a higher protein digestibility. Monsoor and Yusuf (2002) studied the long-term IVPD of legume protein concentrates extracted from lentil, pea, and chickpea using the TCA method after sequential hydrolysis with pepsin for 3 h and pancreatin for 24 h. These authors reported a higher long-term IVPD of lentil compared to pea and chickpea, being elevated to a level statistically indifferent from a casein protein reference (Monsoor & Yusuf, 2002). In a similar study, Sulieman et al. (2008) found both the pepsin and pepsin–pancreatin digestibility of raw lentil seeds to be dependent on the specific cultivar type, with long-term protein digestibility values ranging from about 45–52% and 82–100%, respectively. As previously discussed, these differences in the reported results may be related to the application of different processing procedures and IVPD quantification methods, but literature and results of this study generally agree with an increased long-term IVPD.
4. Conclusion
The nutritional and anti–nutritional properties of LPIs produced by pilot-scale processing involving IEP and UF were found to be markedly altered compared to LF. Both protein isolation procedures led to preparation of LPIs with majorly increased protein contents, reduced TIA (ANCs) levels, and improved IVPD. TIA levels based on dry protein mass were reduced by 81–87% compared to LF. Furthermore, a better tolerability of the LPIs in comparison to LF for IBS patients is expected, since the main FODMAPs in lentils (GOS) were reduced by 58–91% in LPIs compared to LF. IVPD of LPIs was markedly improved relative to LF, depending on the stage of digestion. Pepsin digestibility of LPIs was increased by 35–49% compared to LF. Short-, medium-, and long-term protein digestibility of LPIs was improved by 50–53%, 40–52%, and 49–52%, respectively, relative to LF. Long-term protein digestibility of LPIs reached a level similar to that of the highly digestible protein BSA, indicating a high protein digestibility of purified LPIs under the tested conditions. Further studies under physiological conditions are needed to confirm the high nutritional value of LPIs per se and as part of a mixed diet. The results of this work indicate that the highest purity product, LPI-UF, may be the most promising ingredient for human nutrition due to its high nutritional quality with a relatively low FODMAP content, low TIA level, and high protein digestibility. However, a similar nutritional quality was obtained with the less purified LPI-IEP product, which may be used in food applications where a low FODMAP content is not required.
Funding
The present work has been conducted as part of the PROTEIN2FOOD project (2015–2019), which has received funding from the European Union’s Horizon 2020 Framework Programme for Research and Innovation (grant no. 635727).
CRediT authorship contribution statement
Marcel Skejovic Joehnke: Conceptualization, Methodology, Validation, Formal analysis, Resources, Investigation, Writing - original draft, Visualization. Stephanie Jeske: Conceptualization, Methodology, Validation, Formal analysis, Resources, Investigation, Writing - original draft, Visualization. Lilit Ispiryan: Conceptualization, Methodology, Validation, Formal analysis, Resources, Investigation, Writing - original draft, Visualization. Emanuele Zannini: Conceptualization, Supervision, Project administration, Funding acquisition. Elke K. Arendt: Conceptualization, Methodology, Validation, Formal analysis, Resources, Writing - original draft, Supervision, Project administration, Funding acquisition. Jürgen Bez: Conceptualization, Methodology, Validation, Formal analysis, Resources, Writing - original draft, Supervision, Project administration, Funding acquisition. Jens Christian Sørensen: Conceptualization, Methodology, Validation, Formal analysis, Resources, Supervision, Project administration, Funding acquisition. Iben Lykke Petersen: Conceptualization, Methodology, Validation, Formal analysis, Resources, Writing - original draft, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Marcel Skejovic Joehnke, Email: marcel@food.ku.dk.
Stephanie Jeske, Email: stephanie.jeske@web.de.
Lilit Ispiryan, Email: lilit.ispiryan@umail.ucc.ie.
Emanuele Zannini, Email: e.zannini@ucc.ie.
Elke K. Arendt, Email: e.arendt@ucc.ie.
Jürgen Bez, Email: juergen.bez@ivv.fraunhofer.de.
Jens Christian Sørensen, Email: jens.sorensen@siccadania.com.
Iben Lykke Petersen, Email: ilp@food.ku.dk.
References
- Aggarwal, A., & Drewnowski, A. (2019). Plant- and animal-protein diets in relation to sociodemographic drivers, quality, and cost: Findings from the Seattle Obesity Study. The American Journal of Clinical Nutrition, 110(2), 451–460. https://doi.org/10.1093/ajcn/nqz064. [DOI] [PMC free article] [PubMed]
- Alonso-Miravalles L., Jeske S., Bez J., Detzel A., Busch M., Krueger M., Wriessnegger C.L., O’Mahony J.A., Zannini E., Arendt E.K. Membrane filtration and isoelectric precipitation technological approaches for the preparation of novel, functional and sustainable protein isolate from lentils. European Food Research and Technology. 2019;245(9):1855–1869. doi: 10.1007/s00217-019-03296-y. [DOI] [Google Scholar]
- Aryee A.N.A., Boye J.I. Improving the Digestibility of Lentil Flours and Protein Isolate and Characterization of Their Enzymatically Prepared Hydrolysates. International Journal of Food Properties. 2016;19(12):2649–2665. doi: 10.1080/10942912.2015.1123269. [DOI] [Google Scholar]
- Aryee A.N.A., Boye J.I. Comparative Study of the Effects of Processing on the Nutritional, Physicochemical and Functional Properties of Lentil: Comparative Study. Journal of Food Processing and Preservation. 2017;41(1):e12824. doi: 10.1111/jfpp.12824. [DOI] [Google Scholar]
- Aydemir L.Y., Yemenicioğlu A. Potential of Turkish Kabuli type chickpea and green and red lentil cultivars as source of soy and animal origin functional protein alternatives. LWT - Food Science and Technology. 2013;50(2):686–694. doi: 10.1016/j.lwt.2012.07.023. [DOI] [Google Scholar]
- Bamdad F., Dokhani S., Keramat J., Zareie R. The Impact of Germination and In Vitro Digestion on the Formation of Angiotensin Converting Enzyme (ACE) Inhibitory Peptides from Lentil Proteins Compared to Whey Proteins. Engineering and Technology. 2009;49:36–46. [Google Scholar]
- Barbana C., Boucher A.C., Boye J.I. In vitro binding of bile salts by lentil flours, lentil protein concentrates and lentil protein hydrolysates. Food Research International. 2011;44(1):174–180. doi: 10.1016/j.foodres.2010.10.045. [DOI] [Google Scholar]
- Barbana C., Boye J.I. In vitro protein digestibility and physico-chemical properties of flours and protein concentrates from two varieties of lentil (Lens culinaris) Food & Function. 2013;4(2):310–321. doi: 10.1039/C2FO30204G. [DOI] [PubMed] [Google Scholar]
- Boye J.I., Aksay S., Roufik S., Ribéreau S., Mondor M., Farnworth E., Rajamohamed S.H. Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Research International. 2010;43(2):537–546. doi: 10.1016/j.foodres.2009.07.021. [DOI] [Google Scholar]
- Boye J., Zare F., Pletch A. Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Research International. 2010;43(2):414–431. doi: 10.1016/j.foodres.2009.09.003. [DOI] [Google Scholar]
- Carbonaro M., Maselli P., Nucara A. Relationship between digestibility and secondary structure of raw and thermally treated legume proteins: A Fourier transform infrared (FT-IR) spectroscopic study. Amino Acids. 2012;43(2):911–921. doi: 10.1007/s00726-011-1151-4. [DOI] [PubMed] [Google Scholar]
- Dilis V., Trichopoulou A. Nutritional and health properties of pulses. Mediterranean Journal of Nutrition and Metabolism. 2009;1(3):149–157. doi: 10.1007/s12349-008-0023-2. [DOI] [Google Scholar]
- Guillamón E., Pedrosa M.M., Burbano C., Cuadrado C., Sánchez M.d.C., Muzquiz M. The trypsin inhibitors present in seed of different grain legume species and cultivar. Food Chemistry. 2008;107(1):68–74. doi: 10.1016/j.foodchem.2007.07.029. [DOI] [Google Scholar]
- Halmos E.P., Power V.A., Shepherd S.J., Gibson P.R., Muir J.G. A Diet Low in FODMAPs Reduces Symptoms of Irritable Bowel Syndrome. Gastroenterology. 2014;146(1):67–75.e5. doi: 10.1053/j.gastro.2013.09.046. [DOI] [PubMed] [Google Scholar]
- Ispiryan L., Heitmann M., Hoehnel A., Zannini E., Arendt E.K. Optimization and Validation of an HPAEC-PAD Method for the Quantification of FODMAPs in Cereals and Cereal-Based Products. Journal of Agriculture and Food Chemistry. 2019;67(15):4384–4392. doi: 10.1021/acs.jafc.9b00382.s001. [DOI] [PubMed] [Google Scholar]
- Ispiryan L., Zannini E., Arendt E.K. Characterization of the FODMAP-profile in cereal-product ingredients. Journal of Cereal Science. 2020;92:102916. doi: 10.1016/j.jcs.2020.102916. [DOI] [Google Scholar]
- Jarpa-Parra M. Lentil protein: A review of functional properties and food application. An overview of lentil protein functionality. International Journal of Food Science & Technology. 2018;53(4):892–903. doi: 10.1111/ijfs.13685. [DOI] [Google Scholar]
- Joehnke M.S., Lametsch R., Sørensen J.C. Improved in vitro digestibility of rapeseed napin proteins in mixtures with bovine beta-lactoglobulin. Food Research International. 2019;123:346–354. doi: 10.1016/j.foodres.2019.05.004. [DOI] [PubMed] [Google Scholar]
- Joehnke M.S., Rehder A., Sørensen S., Bjergegaard C., Sørensen J.C., Markedal K.E. In Vitro Digestibility of Rapeseed and Bovine Whey Protein Mixtures. Journal of Agriculture and Food Chemistry. 2018;66(3):711–719. doi: 10.1021/acs.jafc.7b04681. [DOI] [PubMed] [Google Scholar]
- Johnson C.R., Thavarajah D., Combs G.F., Jr., Thavarajah P. Lentil (Lens culinaris L.): A prebiotic-rich whole food legume. Food Research International. 2013;51(1):107–113. doi: 10.1016/j.foodres.2012.11.025. [DOI] [Google Scholar]
- Johnston S.P., Nickerson M.T., Low N.H. The physicochemical properties of legume protein isolates and their ability to stabilize oil-in-water emulsions with and without genipin. Journal of Food Science and Technology. 2015;52(7):4135–4145. doi: 10.1007/s13197-014-1523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Torrejón G., Salcedo G., Martín-Esteban M., Díaz-Perales A., Pascual C.Y., Sánchez-Monge R. Len c 1, a major allergen and vicilin from lentil seeds. Journal of Allergy and Clinical Immunology. 2003;112(6):1208–1215. doi: 10.1016/j.jaci.2003.08.035. [DOI] [PubMed] [Google Scholar]
- Martínez-Villaluenga C., Frias J., Vidal-Valverde C. Alpha-Galactosides: Antinutritional Factors or Functional Ingredients? Critical Reviews in Food Science and Nutrition. 2008;48(4):301–316. doi: 10.1080/10408390701326243. [DOI] [PubMed] [Google Scholar]
- Monsoor M.A., Yusuf H.K.M. In vitro protein digestibility of lathyrus pea (Lathyrus sativus), lentil (Lens culinaris), and chickpea (Cicer arietinum) Int J Food Sci Tech. 2002;37(1):97–99. doi: 10.1046/j.1365-2621.2002.00539.x. [DOI] [Google Scholar]
- Muir J.G., Rose R., Rosella O., Liels K., Barrett J.S., Shepherd S.J., Gibson P.R. Measurement of Short-Chain Carbohydrates in Common Australian Vegetables and Fruits by High-Performance Liquid Chromatography (HPLC) Journal of Agriculture and Food Chemistry. 2009;57(2):554–565. doi: 10.1021/jf802700e. [DOI] [PubMed] [Google Scholar]
- Nosworthy M.G., House J.D. Factors Influencing the Quality of Dietary Proteins: Implications for Pulses. Cereal Chemistry Journal. 2017;94(1):49–57. doi: 10.1094/CCHEM-04-16-0104-FI. [DOI] [Google Scholar]
- Nosworthy M.G., Medina G., Franczyk A.J., Neufeld J., Appah P., Utioh A., Frohlich P., House J.D. Effect of processing on the in vitro and in vivo protein quality of red and green lentils (Lens culinaris) Food Chemistry. 2018;240:588–593. doi: 10.1016/j.foodchem.2017.07.129. [DOI] [PubMed] [Google Scholar]
- QI R.-F., SONG Z.-W., CHI C.-W. Structural Features and Molecular Evolution of Bowman-Birk Protease Inhibitors and Their Potential Application. Acta Biochim Biophys Sinica. 2005;37(5):283–292. doi: 10.1111/j.1745-7270.2005.00048.x. [DOI] [PubMed] [Google Scholar]
- Rathod R.P., Annapure U.S. Effect of extrusion process on antinutritional factors and protein and starch digestibility of lentil splits. LWT - Food Science and Technology. 2016;66:114–123. doi: 10.1016/j.lwt.2015.10.028. [DOI] [Google Scholar]
- Sarwar Gilani G., Wu Xiao C., Cockell K.A. Impact of Antinutritional Factors in Food Proteins on the Digestibility of Protein and the Bioavailability of Amino Acids and on Protein Quality. British Journal of Nutrition. 2012;108(S2):S315–S332. doi: 10.1017/S0007114512002371. [DOI] [PubMed] [Google Scholar]
- Sengupta S., Mukherjee S., Basak P., Majumder A.L. Significance of galactinol and raffinose family oligosaccharide synthesis in plants. Frontiers in Plant Science. 2015;6 doi: 10.3389/fpls.2015.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewry P.R., Napier J.A., Tatham A.S. Seed storage proteins: Structures and biosynthesis. The Plant Cell. 1995;7(7):945–956. doi: 10.1105/tpc.7.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M., Fung T.T., Hu F.B., Willett W.C., Longo V., Chan A.T., Giovannucci E.L. Animal and plant protein intake and all-cause and cause-specific mortality: Results from two prospective US cohort studies. JAMA Internal Medicine. 2016;176(10):1453–1463. doi: 10.1001/jamainternmed.2016.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudacher, H. M., Whelan, K., Irving, P. M., & Lomer, M. C. E. (2011). Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) versus standard dietary advice in patients with irritable bowel syndrome. Journal of Human Nutrition and Dietetics, 24(5), 487–495. https://doi.org/10.1111/j.1365-277X.2011.01162.x. [DOI] [PubMed]
- A. Suliema M., B. Hassan A., A. Osman G., M. El Tyeb M., A.I. El Kh E., H. El Tina A., E. Babiker E. Changes in Total Protein Digestibility, Fractions Content and Structure During Cooking of Lentil Cultivars. Pakistan J. of Nutrition. 2008;7(6):801–805. doi: 10.3923/pjn.2008.801.805. [DOI] [Google Scholar]
- Tahir M., Lindeboom N., Båga M., Vandenberg A., Chibbar R. Composition and correlation between major seed constituents in selected lentil (Lens culinaris. Medik) genotypes. Canadian Journal of Plant Science. 2011;91(5):825–835. doi: 10.4141/cjps2011-010. [DOI] [Google Scholar]
- Urbano, G., Porres, J. M., Frías, J., & Vidal-Valverde, C. (2007). Nutritional Value. In S. S. Yadav, D. L. McNeil, & P. C. Stevenson (Eds.), Lentil: An Ancient Crop for Modern Times (pp. 47–93). Dordrecht: Springer Netherlands. https://doi.org/10.1007/978-1-4020-6313-8_5.
- Varney J., Barrett J., Scarlata K., Catsos P., Gibson P.R., Muir J.G. FODMAPs: Food composition, defining cutoff values and international application. Journal of Gastroenterology and Hepatology. 2017;32(S1):53–61. doi: 10.1111/jgh.13698. [DOI] [PubMed] [Google Scholar]
- Vogelsang-O’Dwyer, M., Bez, J., Petersen, I. L., Joehnke, M. S., Detzel, A., Busch, M., … Zannini, E. (2020). Techno-Functional, Nutritional and Environmental Performance of Protein Isolates from Blue Lupin and White Lupin. Foods, 9(2). https://doi.org/10.3390/foods9020230. [DOI] [PMC free article] [PubMed]
- Wang N., Hatcher D.W., Toews R., Gawalko E.J. Influence of cooking and dehulling on nutritional composition of several varieties of lentils (Lens culinaris) LWT - Food Science and Technology. 2009;42(4):842–848. doi: 10.1016/j.lwt.2008.10.007. [DOI] [Google Scholar]
- Weder, J. K. P., & Kahley, R. (1998). Isolation and characterisation of four trypsin-chymotrypsin inhibitors from lentil seeds. Journal of the Science of Food and Agriculture, 78(3), 429–434.
- Westhoek, H., Rood, T., van den Berg, M., Janse, J., Nijdam, D., Reudink, M., & Stehfest, E. (2011). The protein puzzle: The consumption and production of meat, dairy and fish in the European Union (p. 221). The Hague: PBL Netherlands Environmental Assessment Agency. Retrieved from The Hague: PBL Netherlands Environmental Assessment Agency website: http://www.pbl.nl/en/publications/2011/meat-dairy-and-fish-options-for-changes-in-production-and-consumption.
- Wilson B., Whelan K. Prebiotic inulin-type fructans and galacto-oligosaccharides: Definition, specificity, function, and application in gastrointestinal disorders. Journal of Gastroenterology and Hepatology. 2017;32(S1):64–68. doi: 10.1111/jgh.13700. [DOI] [PubMed] [Google Scholar]