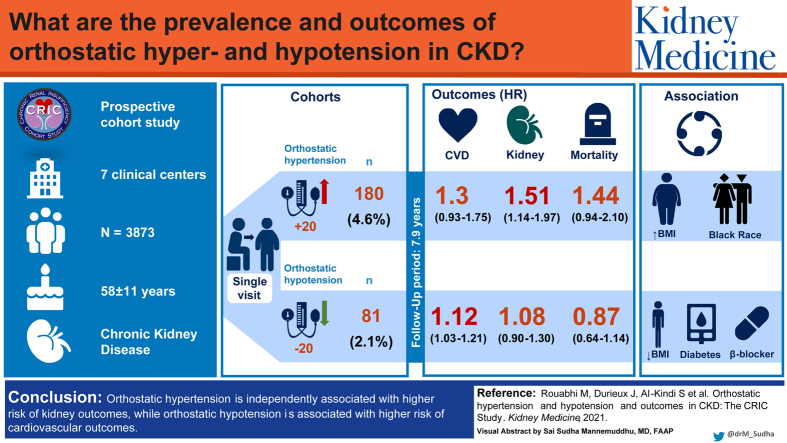
Abstract
Rationale & Objective
There are limited data about the prevalence and prognostic significance of orthostatic hypo- and hypertension in patients with chronic kidney disease. The objective of this study is to determine the prevalence of orthostatic hypo- and hypertension in a cohort of patients with chronic kidney disease and examine their association with clinical outcomes.
Study Design
Prospective cohort study: Chronic Renal Insufficiency Cohort (CRIC) Study.
Setting & Population
7 clinical centers, participants with chronic kidney disease.
Exposures
Orthostatic hypotension (decline in systolic blood pressure [BP] > 20 mm Hg) and orthostatic hypertension (increase in systolic BP > 20 mm Hg) from seated to standing position.
Outcomes
Cardiovascular and kidney outcomes and mortality.
Analytical Approach
Logistic regression was used to determine factors associated with orthostatic hypo- and hypertension; Cox regression was used to examine associations with clinical outcomes.
Results
Mean age of study population (n = 3,873) was 58.1 ± 11.0 years. There was a wide distribution of change in systolic BP from seated to standing (from −73.3 to +60.0 mm Hg); 180 participants (4.6%) had orthostatic hypotension and 81 (2.1%) had orthostatic hypertension. Diabetes, reduced body mass index, and β-blocker use were independently associated with orthostatic hypotension. Black race and higher body mass index were independently associated with orthostatic hypertension. After a median follow-up of 7.9 years, orthostatic hypotension was independently associated with high risk for cardiovascular (HR, 1.12; 95% CI, 1.03-1.21) but not kidney outcomes or mortality. Orthostatic hypertension was independently associated with high risk for kidney (HR, 1.51; 96% CI, 1.14-1.97) but not cardiovascular outcomes or mortality.
Limitations
Orthostatic change in BP was ascertained at a single visit.
Conclusions
Orthostatic hypotension was independently associated with higher risk for cardiovascular outcomes, whereas orthostatic hypertension was associated with higher risk for kidney outcomes. These findings highlight the importance of orthostatic BP measurement in practice and the need for future investigation to understand the mechanisms and potential interventions to minimize the risk associated with orthostatic changes in BP.
Index Words: chronic kidney disease, orthostatic hypotension, orthostatic hypertension, cardiovascular outcomes, renal outcomes, chronic renal insufficiency cohort
Graphical abstract
Plain-Language Summary.
When patients stand from a seated position, some patients have a significant decrease in blood pressure (orthostatic hypotension) and others may have a significant increase in blood pressure (orthostatic hypertension). These blood pressure profiles have not been well studied in the chronic kidney disease population. Our study sought to identify the prevalence of orthostatic hypo- and hypertension and factors associated with these conditions in this population. Additionally, we wanted to characterize cardiovascular and kidney outcomes for individuals with these blood pressure profiles. We used a multicenter cohort study to analyze blood pressure data alongside demographics, comorbid conditions, medications, and laboratory studies. We found that diabetes, body mass index, race, and β-blocker use were associated with blood pressure changes on standing. Participants with orthostatic hypertension were at risk for developing kidney outcomes, whereas those with orthostatic hypotension were at high risk for cardiovascular outcomes.
Orthostatic changes in blood pressure (BP) reflect alterations in the homeostatic response to change in body position; appropriate compensatory mechanisms to maintain BP on standing are integral for continued perfusion to vital organs. With standing, blood pools in the lower extremities and abdominal vasculature, setting off a cascade of decreased venous return to the heart and decreased cardiac output and in turn reduced firing of carotid artery and aortic arch baroreceptors.1 In most individuals, autonomic regulation increases cardiac output and vasoconstriction to return BP to seated/supine levels.1
Autonomic neuropathy, neuronal damage, and arterial stiffness, among other factors, can lead to suboptimal BP response, resulting in orthostatic changes in BP.2 Orthostatic hypotension, a significant decrease in BP on standing, has been shown to be a risk factor for adverse cardiovascular and neurologic events, including stroke, coronary artery disease, heart failure, atrial fibrillation, cognitive dysfunction, and all-cause mortality.3, 4, 5, 6, 7, 8, 9, 10 However, though many physiologic and homeostatic changes that are associated with orthostatic hypotension, such as neuropathy and vascular stiffness, are common in patients with chronic kidney disease (CKD), less is known about orthostatic hypotension in these patients. Cross-sectional studies show that orthostatic hypotension is more common in patients with lower estimated glomerular filtration rates (eGFRs), but whether orthostatic hypotension is associated with increased risk for kidney or cardiovascular events over time is not known.11, 12, 13, 14
Although research has focused mainly on the effects associated with abnormal reductions in BP with standing, evidence has shown that increases in BP with standing are also common.11,15,16 This likely reflects overcompensation of the neuronal and vascular pathway involved in BP maintenance.17 Previous studies evaluating the long-term prognostic significance of orthostatic hypertension have demonstrated inconsistent results; to our knowledge, no previous studies have examined this in patients with CKD.
To our knowledge, our study represents the largest evaluation of orthostatic changes in BP in patients with CKD. The objectives of this work were to examine the prevalence of orthostatic hypo- and hypertension in patients with mild to moderate kidney disease enrolled in the Chronic Renal Insufficiency Cohort (CRIC) Study, identify characteristics associated with higher prevalence of orthostatic hypo- and hypertension in CRIC participants, and evaluate whether the presence of orthostatic hypo- or hypertension is associated with risk for kidney and cardiovascular outcomes.
Methods
Study Population
Data from the CRIC Study were used to conduct analyses reported in the article. This data set was publicly available through the National Institute for Diabetes and Digestive and Kidney Diseases repository.
CRIC is a prospective multicenter cohort study and included 3,939 participants with mild to moderate CKD (eGFR, 20-70 mL/min/1.73 m2) aged 21 to 74 years at baseline. The design and baseline characteristics of this group have been previously published.18 Exclusion criteria included a diagnosis of polycystic kidney disease and active immunosuppression for glomerulonephritis, as well as cirrhosis, class III or IV heart failure, HIV infection, cancer, and pregnancy. The study protocol was approved by the institutional review board of each participating site, and written informed consent was obtained from all participants. This analysis was limited to deidentified data and was considered exempt by the local institutional review board. All research was conducted in adherence with the Declaration of Helsinki.
At study visits, demographic and physical measurements, medical history, medication use, and serum and urine for laboratory assessments were collected. Participants were followed up annually with in-person clinic visits and also contacted by telephone approximately 6 months apart. Diabetes was determined as at least 1 of the following: self-reported insulin or oral hypoglycemic medication use, fasting blood glucose level ≥ 126 mg/dL or a nonfasting level ≥ 200 mg/dL, or glycated hemoglobin level ≥ 6.5%. GFR was estimated using the CRIC Study equation.19
BP was measured during the baseline clinic visit by trained study staff following standardized protocols as recommended by the American Heart Association using aneroid sphygmomanometers. Three seated measurements were obtained after 5 minutes of rest; subsequently, a standing BP measurement was obtained following 2 minutes of standing. Change in BP on standing was calculated as the standing measurement minus the average of 3 seated measurements.
Orthostatic hypotension was defined as a reduction in systolic BP ≥ 20 mm Hg on standing.1 Orthostatic hypertension was defined as an increase in systolic BP ≥ 20 mm Hg on standing.2 We also conducted sensitivity analyses with alternate thresholds for defining orthostatic hypo- and hypertension.
Cardiovascular events were ascertained during the course of the study by asking participants about hospitalizations during clinic or telephone visits. Hospital records were then obtained and were adjudicated using event-specific guidelines by 2 clinicians. Cardiovascular events evaluated during follow-up included myocardial infarction, stroke, heart failure, and peripheral arterial disease. In addition to participant report, end-stage kidney disease was also ascertained through the US Renal Data System. Mortality was determined through report from next of kin, retrieval of death certificates or obituaries, review of hospital records, and linkage with the Social Security Mortality Master File. A composite cardiovascular outcome of the first occurrence of heart failure, myocardial infarction, stroke, or peripheral arterial disease was considered as the cardiovascular outcome; the development of end-stage kidney disease or a 50% decline in eGFR was defined as the kidney outcome.
Statistical Methods
Baseline characteristics were summarized as mean ± standard deviation for continuous variables and frequency (number) and percentage for discrete measures by orthostatic hypo- and hypertension. Distribution of the change in BP with orthostasis was examined as a continuous variable and values were plotted as a frequency histogram.
Associations between orthostatic hypo-/hypertension and covariates, selected based on univariate associations and/or scientific feasibility of the variable as a possible contributor to orthostatic change in BP, were first modeled separately to provide unadjusted odds ratios. The independent associations between each outcome variable and covariates (clinical center, age, sex, race, diabetes status, cardiovascular disease history, smoking status, body mass index [BMI], ankle-brachial index, β-blocker use, angiotensin-converting enzyme [ACE] inhibitor or angiotensin receptor blocker [ARB] use, eGFR, total cholesterol level, hemoglobin level, calcium level, phosphate level, and urinary albumin-creatinine ratio) were then modeled by way of multivariable logistic regression to provide the adjusted odds ratios. Estimates were computed using Firth’s penalized maximum likelihood method of estimation, and 95% profile likelihood CIs were calculated to describe the sampling distribution.20
To evaluate the relationship between orthostatic hypo-/hypertension and the occurrence of cardiovascular and kidney outcomes, the survival probability was estimated using the Kaplan-Meier method. Log-rank test was used to determine whether survival curves were statistically different from each other. To compare the relative survival experiences between groups, unadjusted and adjusted hazard ratios (HRs) were computed using semi-parametric Cox proportional hazards models. Schoenfeld residuals were used to evaluate whether the covariate’s effects on the hazard rate were constant over time. If the proportional hazard assumption was violated, interactions were included to address nonproportionality. The exact method of approximation was used for tied event times. We evaluated the association between orthostatic changes in BP as a continuous variable and clinical outcomes using unadjusted penalized spline curves with Cox regression.
We performed sensitivity analysis to determine the impact of alternative threshold values for orthostatic hypo- and hypertension and their effect on kidney and cardiovascular outcomes. To evaluate the impact of each criterion on survival outcomes, separate adjusted Cox proportional hazards models were fit to the data.
The distribution of changes in BP histogram was created using SPSS, version 25 (IBM Corp). All other analyses were conducted using SAS, version 9.4 (SAS Inc).
Results
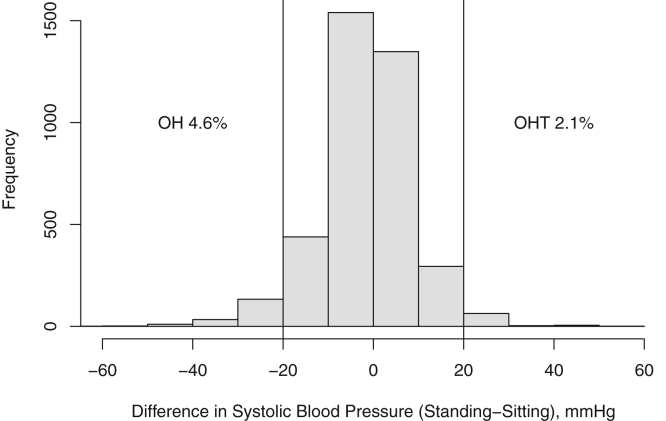
Of the 3,939 participants enrolled in the CRIC Study, baseline BP measurements were missing in 66 participants; therefore, data from 3,873 participants are the basis for this report. The mean age of the study population was 58 ± 11 years, 55% were men, 48% had diabetes, and 42% were African American. Mean eGFR was 45 ± 17 mL/min/1.73 m2. Figure 1 demonstrates the distribution of changes in systolic BP; 180 individuals (4.6%) had orthostatic hypotension and 81 (2.1%) had systolic orthostatic hypertension, as defined. There was a wide range of changes in systolic BP on standing (−73.3 to 60.0 mm Hg).
Figure 1.
Distribution of changes in blood pressure on standing in Chronic Renal Insufficiency Cohort (CRIC) Study participants. Abbreviations: OH, orthostatic hypotension; OHT, orthostatic hypertension.
Orthostatic Hypotension
Baseline characteristics of patients stratified by the presence of orthostatic hypotension are reported in Table 1. Patients in the orthostatic hypotension group were more likely to be of Hispanic ethnicity, have diabetes at baseline, and have a history of cardiovascular disease. In addition, there were several differences in clinical, demographic, and laboratory characteristics in participants with orthostatic hypotension compared with those without orthostatic hypotension. Patients with orthostatic hypotension were more likely to be prescribed α-blockers or β-blockers (Table 1). In a cross-sectional multivariable binary logistic regression model, diabetes at baseline, reduced BMI, and β-blocker use were independently associated with the presence of orthostatic hypotension (Table 2).
Table 1.
Baseline Characteristics of Patients by Presence or Absence of Orthostatic Hypotension
| No Orthostatic Hypotension (n = 3,693) | Orthostatic Hypotension (n = 180) | Total (N = 3,873) | |
|---|---|---|---|
| Demographics | |||
| Age, y | 58±11 | 59±11 | 58±11 |
| Male sex | 2,025 (54.8%) | 96 (53.3%) | 2,121 (54.8%) |
| Black race | 1,567 (42.4%) | 66 (36.7%) | 1,633 (42.2%) |
| Hispanic ethnicity | 419 (11.3%) | 50 (27.7%) | 469 (12.1%) |
| Comorbid conditions (history of) | |||
| Diabetes mellitus | 1,736 (47.0%) | 128 (71.1%) | 1,864 (48.1%) |
| Myocardial infarction/revascularization | 793 (21.5%) | 46 (25.6%) | 839 (21.7%) |
| Peripheral vascular disease | 232 (6.3%) | 20 (11.1%) | 252 (6.5%) |
| Congestive heart failure | 349 (9.5%) | 21 (11.7%) | 370 (9.6%) |
| Stroke | 353 (9.6%) | 28 (15.6%) | 381 (9.8%) |
| Cardiovascular disease [n (%)] | 1,200 (32.5%) | 80 (44.4%) | 1,280 (33.0%) |
| Hypertension [n (%)] | 3,157 (85.5%) | 173 (96.1%) | 3,330 (86.0%) |
| Ever smoker [n (%)] | 2,021 (54.7%) | 97 (53.9%) | 2,118 (54.7%) |
| Current smoker [n (%)] | 485 (13.1%) | 25 (13.9%) | 510 (13.2%) |
| BP data | |||
| Seated pulse, beats/min | 67.8±11.3 | 67.0±12.0 | 67.8±11.4 |
| Standing pulse, beats/min | 72.3±12.4 | 71.8±13.3 | 72.2±12.5 |
| Seated systolic BP, mm Hg | 127.5±21.5 | 147.5±27.0 | 128.5±22.2 |
| Seated diastolic BP, mm Hg | 71.3±12.9 | 75.2±14.8 | 71.5±13.0 |
| Pulse pressure, mm Hg | 56.2±18.8 | 72.3±20.8 | 56.95±19.2 |
| Standing systolic BP, mm Hg | 127.5±22.0 | 120.6±27.1 | 127.1±22.3 |
| Standing diastolic BP, mm Hg | 73.5±13.4 | 69.7±15.5 | 73.4±13.5 |
| Laboratory data | |||
| Triglycerides, mg/dL | 156.6±116.2 | 161.6±118.6 | 156.9±116.3 |
| Total cholesterol, mg/dL | 183.3±44.4 | 196.5±64.9 | 183.9±45.6 |
| HDL cholesterol, mg/dL | 47.5±15.5 | 48.9±15.2 | 47.5±15.5 |
| LDL cholesterol, mg/dL | 102.6±35.2 | 109.8±42.7 | 102.9±35.6 |
| VLDL cholesterol, mg/dL | 33.4±26.0 | 37.9±30.3 | 33.6±26.2 |
| BMI, kg/m2 | 32.2±7.8 | 29.3±5.9 | 32.1±7.8 |
| Ankle-brachial index | 1.05±.20 | 1.01±.23 | 1.04±0.20 |
| Hemoglobin, g/dL | 12.7±1.8 | 11.8±1.9 | 12.6±1.8 |
| Hemoglobin A1c, % | 6.6±1.5 | 7.1±1.9 | 6.6±1.6 |
| Serum albumin, g/dL | 4.0±.46 | 3.7±.57 | 3.9±0.47 |
| Calcium, mg/dL | 9.2±.50 | 9.1±.58 | 9.2±0.51 |
| Phosphate, mg/dL | 3.7±.67 | 4.0±.60 | 3.7±0.67 |
| Urinary albumin-creatinine ratio, μg/mg | 48.0 [8.2-434.3] | 238.0 [23.1-1740.5] | 51.9 [8.7-458.8] |
| eGFR,a mL/min/1.73 m2 | 45±17 | 38±13 | 45±17 |
| Medications | |||
| ACE inhibitor use | 1,807 (49.2%) | 80 (45.5%) | 1,887 (49.1%) |
| Angiotensin receptor blocker use | 923 (25.1%) | 55 (31.3%) | 978 (25.4%) |
| α2-agonist use | 314 (8.6%) | 24 (13.6%) | 338 (8.8%) |
| α-Blocker use | 517 (14.1%) | 38 (21.6%) | 555 (14.4) |
| β-Blocker use | 1,781 (48.5%) | 108 (61.4%) | 1,889 (49.1%) |
Note: Values expressed as mean ± standard deviation, number (percent), or median [interquartile range].
Abbreviations: ACE, angiotensin-converting enzyme; BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very low-density lipoprotein.
eGFR calculated using the Chronic Renal Insufficiency Cohort Study equation.
Table 2.
Factors Independently Associated With Orthostatic Hypotension
| Reference Category (for categorical variables) | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI) | |
|---|---|---|---|
| Age, y | 1.01 (0.99-1.03) | 1.02 (0.99-1.04) | |
| Female sex | Male | 0.93 (0.69-1.25) | 0.94 (0.64-1.39) |
| Black race | Non-Black | 0.80 (0.59-1.09) | 0.72 (0.47-1.11) |
| Hispanic ethnicity | Non-Hispanic | 2.99 (2.12-4.19) | 1.32 (0.65-2.55) |
| Diabetes mellitus | Nondiabetic | 2.78 (2.02-3.89) | 1.93 (1.29-2.87) |
| Cardiovascular disease | No history of cardiovascular disease | 1.68 (1.25-2.27) | 1.38 (0.95-1.98) |
| Current smoker | Not current smoker | 1.08 (0.69-1.62) | 0.97 (0.56-1.61) |
| Total cholesterol (mg/dL)a | 1.06 (1.03,1.09) | 1.04 (1.01-1.08) | |
| Serum albumin (g/dL) | 0.41 (0.31-0.55) | 0.71 (0.46-1.10) | |
| Hemoglobin (g/dL) | 0.76 (0.70-0.83) | 0.89 (0.78-1.01) | |
| Calcium (mg/dL) | 0.61 (0.46-0.82) | 0.94 (0.62-1.42) | |
| Phosphate (mg/dL) | 1.62 (1.32-1.97) | 1.05 (0.79-1.38) | |
| BMI (kg/m2) | 0.94 (0.92-0.96) | 0.92 (0.89-0.94) | |
| Urinary albumin-creatinine ratio (μg/mg)b | 3.21 (2.21-4.62) | 1.57 (0.82-2.86) | |
| eGFR (mL/min/1.73 m2)c | 0.87 (0.83,0.92) | 0.99 (0.92-1.07) | |
| Ankle-brachial index (units) | 0.41 (0.20-0.87) | 0.70 (0.32-1.49) | |
| β-Blocker use | No β-blocker use | 1.70 (1.25-2.32) | 1.52 (1.06-2.19) |
| ACE inhibitor or ARB use | No ACE inhibitor or ARB use | 1.16 (0.84-1.63) | 1.26 (0.86-1.88) |
Note: eGFR calculated using Chronic Renal Insufficiency Cohort Study equation. For continuous variables, estimates per 1 unit change except for variables noted.
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; eGFR, estimated glomerular filtration rate.
Total cholesterol per 10-mg/dL change.
Urinary albumin-creatinine ratio per doubling.
eGFR per 5-mL/min/1.73 m2 change.
After a median follow-up of 7.9 years, 819 participants died, 940 had a composite cardiovascular event, and 1,164 had the composite kidney outcome. In unadjusted analyses, participants with orthostatic hypotension were more likely to experience the cardiovascular outcome (HR, 1.74; 95% CI, 1.45-2.09), kidney outcome (HR, 1.85; 95% CI, 1.56-2.18), and mortality (HR, 1.39; 95% CI, 0.78-1.79) than those without orthostatic hypotension. In models adjusted for clinical center, age, sex, race, diabetes status, seated systolic BP, cardiovascular disease history, smoking status, BMI, ankle-brachial index, β-blocker use, ACE inhibitor or ARB use, eGFR, total cholesterol level, hemoglobin level, calcium level, phosphate level, and urinary albumin-creatinine ratio, the presence of orthostatic hypotension was independently associated with the composite cardiovascular outcome (HR, 1.12; 95% CI, 1.03-1.21), but not the kidney outcome (HR, 1.08; 95% CI, 0.9-1.3) or mortality (HR, 0.87; 95% CI, 0.64-1.14; Table 3). When components of the composite cardiovascular end point were evaluated individually, the presence of orthostatic hypotension was associated with higher risk for myocardial infarction, stroke, and peripheral arterial disease, but not congestive heart failure (Table 3).
Table 3.
Association Between Orthostatic Hypotension at Baseline and Clinical Outcomes
| Outcome | No. of Events | Unadjusted Hazard Ratio (95% CI) (n = 3,873) | Adjusted Hazard Ratio (95% CI)a (n = 3,573) |
|---|---|---|---|
| Composite cardiovascular outcome | No OH = 875, OH = 65 | 1.74 (1.45-2.09) | 1.12 (1.03-1.21) |
| Kidney outcome | No OH = 1,074, OH = 90 | 1.85 (1.56-2.18) | 1.08 (0.9-1.3) |
| All-cause mortality | No OH = 770, OH = 49 | 1.39 (0.78-1.79) | 0.87 (0.64-1.14) |
| Heart failure | No OH = 447, OH = 31 | 1.63 (1.33-1.99) | 1.16 (0.92-1.43) |
| Myocardial infarction | No OH = 212, OH = 12 | 1.49 (1.21-1.84) | 1.15 (1.04-1.27) |
| Stroke | No OH = 126, OH = 6 | 1.46 (1.18-1.81) | 1.13 (1.02-1.25) |
| Peripheral arterial disease | No OH = 156, OH = 13 | 1.58 (1.28-1.95) | 1.13 (1.02-1.24) |
Note: Cardiovascular composite outcome includes heart failure, myocardial infarction, stroke and peripheral arterial disease. Kidney outcomes includes end-stage kidney disease or 50% decline in eGFR.
Abbreviations: eGFR, estimated glomerular filtration rate; OH, orthostatic hypotension.
Cox proportional hazards models adjusted for clinical center, age, sex, race, diabetes, seated systolic blood pressure, cardiovascular disease, smoking status, body mass index, ankle-brachial index, β-blocker use, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use, eGFR, total cholesterol level, hemoglobin level, calcium level, phosphate level, and urinary albumin-creatinine ratio.
Orthostatic Hypertension
Baseline characteristics of participants with (n = 81; 2.1%) and without orthostatic hypertension are presented in Table S1. Participants with orthostatic hypertension were more likely to be older, be of Black race, and have diabetes. In a cross-sectional multivariable binary logistic regression model, Black race and higher BMI were independently associated with the presence of orthostatic hypertension (Table S2).
After a median follow-up of 7.9 years, in unadjusted comparisons, participants with orthostatic hypertension at baseline were more likely to develop the composite cardiovascular end point (HR, 1.34; 95% CI, 1.00-1.8), kidney end point (HR, 1.48; 95% CI, 1.14-1.91), and mortality (HR, 1.5; 95% CI, 1.04-2.17) compared with participants without orthostatic hypertension. In Cox regression models adjusted for clinical center, age, sex, race, diabetes status, seated systolic BP, cardiovascular disease history, smoking status, BMI, ankle-brachial index, β-blocker use, ACE inhibitor or ARB use, eGFR, total cholesterol level, hemoglobin level, calcium level, phosphate level, and urinary albumin-creatinine ratio, orthostatic hypertension was independently associated with increased risk for progression to end-stage kidney disease or 50% decline in GFR (HR, 1.51; 95% CI, 1.14-1.97)], but not associated with adverse cardiovascular outcomes or mortality (Table 4).
Table 4.
Association Between Orthostatic Hypertension at Baseline and Clinical Outcomes
| Outcome | No. of Events | Unadjusted Hazard Ratio (95% CI) (n = 3,873) | Adjusted Hazard Ratio (95% CI)a (n = 3,573) |
|---|---|---|---|
| Composite cardiovascular outcome | No OHT = 913, OHT = 27 | 1.34 (1.00-1.8) | 1.3 (0.93-1.75) |
| Kidney outcome | No OHT = 1,135, OHT = 29 | 1.48 (1.14-1.91) | 1.51 (1.14-1.97) |
| All-cause mortality | No OHT = 793, OHT = 26 | 1.5 (1.04-2.17) | 1.44 (0.94-2.1) |
| Heart failure | No OHT = 469, OHT = 9 | 1.15 (0.83-1.61) | 1.0 (0.68-1.4) |
| Myocardial infarction | No OHT = 217, OHT = 7 | 1.28 (0.92-1.78) | 1.28 (0.88-1.78) |
| Stroke | No OHT = 125, OHT = 7 | 1.33 (0.96-1.86) | 1.34 (0.92-1.87) |
| Peripheral arterial disease | No OHT = 164, OHT = 5 | 1.3 (0.94-1.81) | 1.29 (0.89-1.8) |
Note: Cardiovascular composite outcome includes heart failure, myocardial infarction, stroke, and peripheral arterial disease. Kidney outcome includes end-stage kidney disease or 50% decline in eGFR.
Abbreviations: eGFR, estimated glomerular filtration rate; OHT, orthostatic hypertension.
Cox proportional hazards models adjusted for clinical center, age, sex, race, diabetes, seated systolic blood pressure, cardiovascular disease, smoking status, body mass index, ankle-brachial index, β-blocker use, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use, eGFR, total cholesterol level, hemoglobin level, calcium level, phosphate level, and urinary albumin-creatinine ratio.
Sensitivity analysis showed a greater prevalence of disease when alternative definitions of orthostatic hypotension (decrease in systolic BP > 20 mm Hg or decrease in diastolic BP > 10 mm Hg, n = 270; decrease in systolic BP > 10 mm Hg, n = 619) and hypertension (increase in systolic BP > 20 mm Hg or increase in diastolic BP > 10 mm Hg, n = 444; increase in systolic BP > 10 mm Hg, n = 413) were used (Tables S3 and S4). The point estimates of the HR for the association between orthostatic hypotension and orthostatic hypertension and clinical outcomes were consistent when defined by change in systolic BP > 20 mm Hg or change in systolic BP > 20 mm Hg and/or change in diastolic BP > 10 mm Hg. There were no statistically significant associations between orthostatic hypo- or hypertension and outcomes when defined by change in systolic BP > 10 mm Hg.
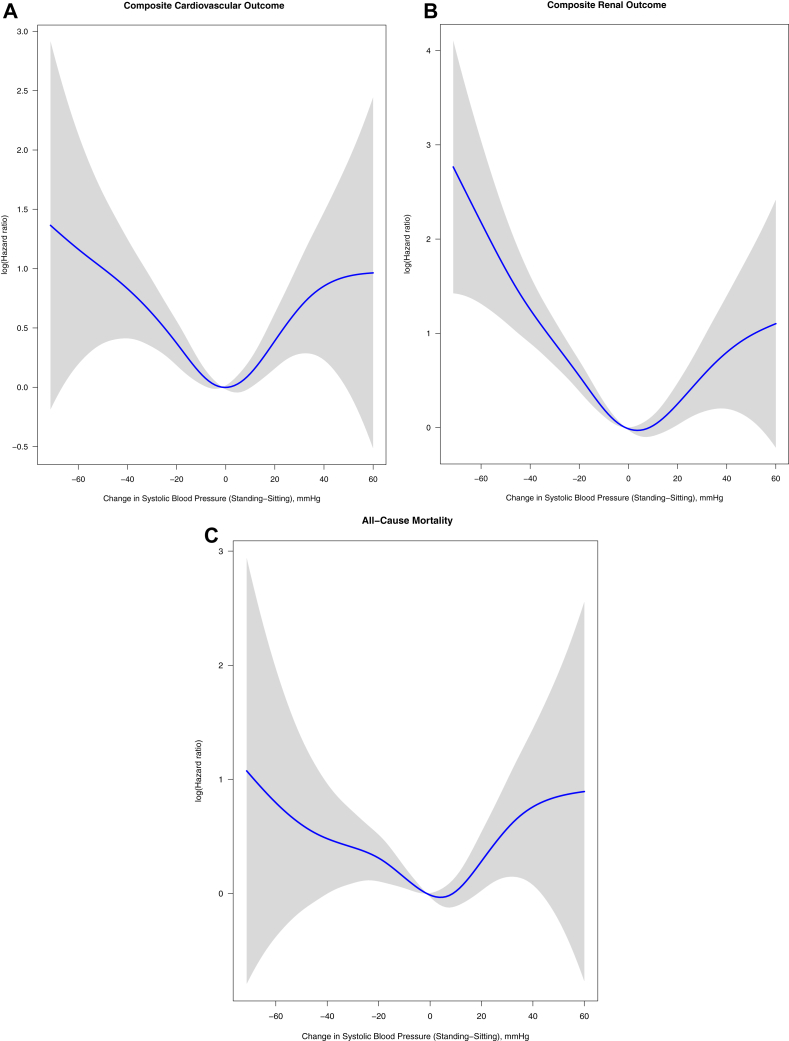
The association between orthostatic change in BP as a continuous variable and clinical outcomes demonstrated higher risk for outcomes at the extremes of decline and increase in BP with standing (Figs 2 and S1).
Figure 2.
Association between orthostatic change in systolic blood pressure (as a continuous variable) and clinical outcomes.
We conducted additional analyses evaluating the association between orthostatic hypo- and hypertension with the cardiovascular and kidney outcomes and mortality adding an interaction term for diabetes. The interaction term for diabetes was not statistically significant, suggesting that the associations were consistent across participants with and without diabetes. We also conducted similar analyses adding an interaction term for GFR (above and below the median). The interaction terms were not statistically significant, suggesting that the associations were consistent across participants with low and high GFRs.
Discussion
Our study demonstrates that there is a wide range of orthostatic changes in BP among patients with mild to moderate CKD. Orthostatic hypotension (4.6%) is more common than orthostatic hypertension (2.1%) in this cohort; diabetes, reduced BMI, and β-blocker use are associated with higher prevalence of orthostatic hypotension. Black race and higher BMI are associated with higher prevalence of orthostatic hypertension. The presence of orthostatic hypotension is associated with higher risk for adverse cardiovascular outcomes, whereas orthostatic hypertension is associated with higher risk for adverse kidney outcomes.
The prevalence of orthostatic hypotension is ∼5%, increasing with age to 16% to 18% in patients older than 65 years.3, 4, 5, 6, 7, 8, 9, 10,12,21 The mechanisms underlying orthostatic hypotension are not fully understood; intravascular volume depletion or impaired autonomic response are thought to mediate the decrease in BP with standing seen in these patients. Various comorbid conditions, such as diabetes, hypertension, and cardiovascular disease, increase the risk for orthostatic hypotension in the general population. Patients with neurologic conditions, such as Parkinson disease and dementia with Lewy bodies, are also more likely to have orthostatic hypotension.16,22,23 Patients with orthostatic hypotension have been shown to be at increased risk for falls, ischemic stroke, coronary artery disease, heart failure, atrial fibrillation, CKD, cognitive function decline, and all-cause mortality, though a recent analysis from the Systolic Blood Pressure Intervention Trial (SPRINT) showed that orthostatic hypotension was not associated with higher risk for cardiovascular disease events.3, 4, 5, 6, 7, 8, 9, 10,12,24,25
Less is known about orthostatic hypotension in patients with CKD. In the Irish Longitudinal Study on Ageing, 11% of participants with GFRs < 60 mL/min/1.73 m2 had orthostatic hypotension.14 In SPRINT, participants with orthostatic hypotension were more likely to have GFRs < 45 mL/min/1.73 m2 (16%) compared with those without orthostatic hypotension (9%), and GFRs < 45 mL/min/1.73 m2 were independently associated with greater decrease in BP standing.11 In the Atherosclerosis Risk in Communities (ARIC) Study, the presence of orthostatic hypotension was associated with increased risk for incident CKD and increased albuminuria.12
Our study makes an important contribution to this field. We demonstrate that the prevalence of orthostatic hypotension in the CRIC population is ∼5%, consistent with other literature in the field. Importantly, we demonstrate for the first time in the setting of CKD that orthostatic hypotension is associated with higher risk for cardiovascular outcomes. Several factors that may contribute to alterations in BP compensation with standing are present in patients with CKD; these include arterial stiffness, anemia, and impaired autonomic function, among other determinants.26, 27, 28 Whether these factors mediate the relationship between orthostatic hypotension and cardiovascular outcomes needs to be evaluated in future studies. As expected, individuals with diabetes have a high prevalence of orthostatic hypotension, likely secondary to autonomic neuropathy and impaired baroreceptor sensitivity. Medication-induced sympathetic blockade may contribute to orthostatic decline in BP with a lower capacity to increase cardiac output in response to decreases in BP with orthostasis.
Our study highlights the importance of orthostatic measurement of BP in patients with CKD. In addition to well-recognized associations between orthostatic hypotension and outcomes such as cognitive function and falls, we now demonstrate that the presence of orthostatic hypotension may be an independent risk factor for cardiovascular disease. Future research must look to further elucidate the mechanism underlying this relationship and evaluate whether interventions to optimize orthostatic changes in BP can improve outcomes.
Orthostatic hypertension has not been as well studied in the general population, though recently there has been increasing interest in this phenotype of orthostatic change in BP.29 The mechanisms of orthostatic hypertension are not known; it is thought that excessive activation of the neurohormonal mechanisms that regulate BP with change in position contribute to this phenomenon.30
The prevalence of orthostatic hypertension in the literature is variable, ranging between 1% and 28%, in part due to lack of a uniform definition.2 The ARIC Study reported systolic orthostatic hypertension in 2.4% of community-dwelling middle-aged individuals moving from seated to standing position.4 Tabara et al31 had similar findings when measuring systolic orthostatic hypertension in community-dwelling individuals (2.7%). Orthostatic hypertension is observed with conditions including hypertension, abnormal diurnal BP variation, dysautonomias, and diabetes.4,30 Some but not all studies that have evaluated long-term outcomes have shown increased risk for cardiovascular morbidity and mortality, as well as all-cause mortality, in patients with orthostatic hypertension.32,33 Patients with orthostatic hypertension have also been shown to have cognitive decline and lower scores in testing of neurobehavioral functioning and activities of daily living.34,35
To our knowledge, no prior study has evaluated orthostatic hypertension in the CKD population. We demonstrate that the prevalence of orthostatic hypertension in CRIC Study participants was low; <3% of the study population has a >20 mm Hg increase in BP with standing. This is lower than what has been reported in other studies, but as discussed next, lack of consistency of definition in studies limits the ability to compare across studies. In addition, our study population was younger compared with other studies that have focused on geriatric patients, who are more likely to have orthostatic hypertension. In our study, African American race and higher BMI were associated with higher prevalence of orthostatic hypertension, which is consistent with risk factors in the general population.15,36
Participants with orthostatic hypertension were shown to be at increased risk for kidney outcomes. The mechanism of how orthostatic hypertension may mediate the progression of kidney disease is unclear; we speculate that because most individuals are ambulatory during the day, participants with orthostatic hypertension are exposed to a higher BP over the course of the day than those without hypertension. This higher burden of hypertension may eventually contribute to progression of kidney disease. It is also possible that there are other confounders that affect the relationship between orthostatic hypertension and kidney disease.
This is a novel finding and future studies will be needed to further evaluate the mechanism underlying these findings. There was no statistically significant association between orthostatic hypertension and cardiovascular events in our study, which is different from other studies, however, our findings may be due to the small number of patients with orthostatic hypertension and future studies with larger numbers of patients with orthostatic hypertension will be needed to definitively address this issue.
One of the challenges in evaluating the literature in this field is lack of a consensus definition of these conditions. Several permutations of systolic and diastolic BP cutoffs have been proposed in previous studies. We conducted sensitivity analyses using other common BP thresholds to define orthostatic hypo- and hypertension. We noted that change in systolic BP of at least 20 mm Hg identified participants at high risk for outcomes and may need to be considered when defining significant orthostatic changes in BP in future studies.
There are several strengths of this study. The CRIC study population is well characterized and diverse and has a large sample size with a long duration of follow-up and carefully adjudicated outcomes. Another strength is that BP was carefully measured using standardized protocols recommended by the American Heart Association by trained providers in a research setting.
However, several limitations need to be considered. We studied a single measure of orthostatic change at baseline; it is plausible that orthostatic changes in BP are variable throughout the follow-up of these patients and that this may affect outcomes. Future research needs to examine the orthostatic change in BP over time and its relationship with outcomes. In addition, we considered only a single measurement of BP after standing; some investigators have measured BP repeatedly every 10 seconds after standing, allowing assessment of “stabilization” of BP. Finally, falls and other symptoms of orthostatic hypotension were not ascertained in CRIC and therefore the association between orthostatic changes in BP and these conditions could not be studied.
In summary, we demonstrate that there is marked variability in the response of BP to change in position from seated to standing. Systolic orthostatic hypotension was observed in 4.6% of individuals compared with 2.1% of individuals with orthostatic hypertension in the CRIC population. Both were independently associated with various demographic and clinical factors that can be used to assess risk for each phenotype of change in BP. The presence of orthostatic hypotension at baseline was independently associated with increased risk for cardiovascular outcomes; the presence of orthostatic hypertension was associated with higher risk for kidney outcomes. These novel findings highlight the importance of measurement of orthostatic BP in practice and the need for future investigation to understand the mechanisms and potential interventions to minimize the risk associated with orthostatic changes in BP.
Article Information
Authors’ Full Names and Academic Degrees
Mohamed Rouabhi, BS, Jared Durieux, MS, MDS, MPH, Sadeer Al-Kindi, MD, Jordana B. Cohen, MD, MSCE, Raymond R. Townsend, MD, and Mahboob Rahman, MD.
Authors’ Contributions
Research idea and study design: MRouabhi, RRT, MRahman; data acquisition: MRahman, RRT; data analysis/interpretation: MRouabhi, JD, RRT, JBC, SA-K, MRahman; statistical analysis: MRouabhi, JD, JBC; supervision or mentorship: MRahman. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This work was supported by National Institutes of Health (NIH) grant 5U01DK061021, the Leonard C Rosenberg Renal Research Foundation, and the Case Western Reserve University Digestive Health Research Institute Case Medical Student Summer Research Program NIH/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants 1T35DK111373-02 and 1T35DK111373-03. The NIH was involved in study design, collection and analysis of data for the CRIC Study but for this report had no involvement in interpretation of data, writing the report, and the decision to submit the report for publication.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Peer Review
Received July 18, 2020. Evaluated by 2 external peer reviewers, with direct editorial input from the Statistical Editor and the Editor-in-Chief. Accepted in revised form October 25, 2020.
Data Sharing Plan
The data set used for these analyses is publicly available through the NIDDK repository.
Footnotes
Complete author and article information provided before references.
Figure S1: Association between orthostatic change in diastolic blood pressure (as a continuous variable) and clinical outcomes
Table S1: Baseline characteristics of patients by presence or absence of orthostatic hypertension
Table S2: Factors independently associated with orthostatic hypertension
Table S3: Sensitivity analyses using alternate criteria to define orthostatic hypotension
Table S4: Sensitivity analyses using alternate criteria to define orthostatic hypertension
Supplementary Material
Figure S1, Tables S1-S4.
References
- 1.Magkas N., Tsioufis C., Thomopoulos C. Orthostatic hypotension: from pathophysiology to clinical applications and therapeutic considerations. J Clin Hypertens. 2019;21:546–554. doi: 10.1111/jch.13521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magkas N., Tsioufis C., Thomopoulos C. Orthostatic hypertension: from pathophysiology to clinical applications and therapeutic considerations. J Clin Hypertens. 2019;21:426–433. doi: 10.1111/jch.13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eigenbrodt M.L., Rose K.M., Couper D.J., Arnett D.K., Smith R., Jones D. Orthostatic hypotension as a risk factor for stroke: the Atherosclerosis Risk in Communities (ARIC) Study, 1987-1996. Stroke. 2000;31(10):2307–2313. doi: 10.1161/01.str.31.10.2307. [DOI] [PubMed] [Google Scholar]
- 4.Yatsuya H., Folsom A.R., Alonso A., Gottesman R.F., Rose K.M., ARIC Study Investigators Postural changes in blood pressure and incidence of ischemic stroke subtypes: the ARIC study. Hypertension. 2011;57(2):167–173. doi: 10.1161/HYPERTENSIONAHA.110.161844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rose K.M., Tyroler H.A., Nardo C.J. Orthostatic hypotension and the incidence of coronary heart disease: the Atherosclerosis Risk in Communities study. Am J Hypertens. 2000;13:571–578. doi: 10.1016/s0895-7061(99)00257-5. [DOI] [PubMed] [Google Scholar]
- 6.Jones C.D., Loehr L., Franceschini N. Orthostatic hypotension as a risk factor for incident heart failure: the Atherosclerosis Risk in Communities Study. Hypertension. 2012;59(5):913–918. doi: 10.1161/HYPERTENSIONAHA.111.188151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agarwal S.K., Alonso A., Whelton S.P. Orthostatic change in blood pressure and incidence of atrial fibrillation: results from a bi-ethnic population based study. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0079030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rose K.M., Couper D., Eigenbrodt M.L., Mosley T.H., Sharrett A.R., Gottesman R.F. Orthostatic hypotension and cognitive function: the Atherosclerosis Risk in Communities Study. Neuroepidemiology. 2010;34(1):1–7. doi: 10.1159/000255459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rose K.M., Eigenbrodt M.L., Biga R.L. Orthostatic hypotension predicts mortality in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2006;114(7):630–636. doi: 10.1161/CIRCULATIONAHA.105.598722. [DOI] [PubMed] [Google Scholar]
- 10.Fedorowski A., Stavenow L., Hedblad B., Berglund G., Nilsson P.M., Melander O. Orthostatic hypotension predicts all-cause mortality and coronary events in middle-aged individuals (The Malmo Preventive Project) Eur Heart J. 2010;31(1):85–91. doi: 10.1093/eurheartj/ehp329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Townsend R.R., Chang T.I., Cohen D.L. Orthostatic changes in systolic blood pressure among SPRINT participants at baseline. J Am Soc Hypertens. 2016;10(11):847–856. doi: 10.1016/j.jash.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franceschini N., Rose K.M., Astor B.C., Couper D., Vupputuri S. Orthostatic hypotension and incident chronic kidney disease: the Atherosclerosis Risk in Communities Study. Hypertension. 2010;56(6):1054–1059. doi: 10.1161/HYPERTENSIONAHA.110.156380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasaki O., Nakahama H., Nakamura S. Orthostatic hypotension at the introductory phase of haemodialysis predicts all-cause mortality. Nephrol Dial Transplant. 2005;20(2):377–381. doi: 10.1093/ndt/gfh614. [DOI] [PubMed] [Google Scholar]
- 14.Canney M., O’Connell M.D.L., Sexton D.J. Graded association between kidney function and impaired orthostatic blood pressure stabilization in older adults. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.005661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nardo C.J., Chambless L.E., Light K.C. Descriptive epidemiology of blood pressure response to change in body position: the ARIC Study. Hypertension. 1999;33(5):1123–1129. doi: 10.1161/01.hyp.33.5.1123. [DOI] [PubMed] [Google Scholar]
- 16.Fleg J.L., Evans G.W., Margolis K.L. Orthostatic hypotension in the ACCORD (Action to Control Cardiovascular Risk in Diabetes) blood pressure trial: prevalence, incidence and prognostic significance. Hypertension. 2016;68:888–895. doi: 10.1161/HYPERTENSIONAHA.116.07474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kario K. Orthostatic hypertension—a new haemodynamic cardiovascular risk factor. Nat Rev Nephrol. 2013;9:726–738. doi: 10.1038/nrneph.2013.224. [DOI] [PubMed] [Google Scholar]
- 18.Lash J.P., Go A.S., Appel L.J. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function [published correction appears in Clin J Am Soc Nephrol. 2011;6(10):2548-2553] Clin J Am Soc Nephrol. 2009;4(8):1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson A.H., Yang W., Hs-u C.Y. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2012;60(2):250–261. doi: 10.1053/j.ajkd.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80(1):27–38. [Google Scholar]
- 21.Rutan G.H., Hermanson B., Bild D.E., Kittner S.J., LaBaw F., Tell G.S. Orthostatic hypotension in older adults: the Cardiovascular Health Study. Hypertension. 1992;19:508–519. doi: 10.1161/01.hyp.19.6.508. [DOI] [PubMed] [Google Scholar]
- 22.Metzler M., Duerr S., Granata R., Krismer F., Robertson D., Wenning G.K. Neurogenic orthostatic hypotension: pathophysiology, evaluation, and management. J Neurol. 2013;260(9):2212–2219. doi: 10.1007/s00415-012-6736-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonell K.E., Shibao C.A., Classen D.O. Clinical relevance of orthostatic hypotension in neurodegenerative disease. Curr Neurol Neurosci Rep. 2015;15(12):78. doi: 10.1007/s11910-015-0599-0. [DOI] [PubMed] [Google Scholar]
- 24.Juraschek S.P., Daya N., Appel L.J. Orthostatic hypotension in middle-age and risk of falls. Am J Hypertens. 2017;30(2):188–195. doi: 10.1093/ajh/hpw108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juraschek S.P., Taylor A.A., Wright J.T. Orthostatic hypotension, cardiovascular outcomes, and adverse events: results from SPRINT. Hypertension. 2020;75:660–667. doi: 10.1161/HYPERTENSIONAHA.119.14309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirkham F.A., Rankin P., Parekh N. Aortic stiffness and central systolic pressure are associated with ambulatory orthostatic BP fall in chronic kidney disease. J Nephrol. 2020;33:317–324. doi: 10.1007/s40620-019-00655-6. [DOI] [PubMed] [Google Scholar]
- 27.Liu W., Wang L., Huang X., He W., Song Z., Yang J. Impaired orthostatic blood pressure stabilization and reduced hemoglobin in chronic kidney disease. J Clin Hypertens. 2019;21:1317–1324. doi: 10.1111/jch.13658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onodugo O., Arodiwe E., Okoye J. Prevalence of autonomic dysfunction among pre-dialysis chronic kidney disease patients in a tertiary hospital, South East Nigeria. Afr Health Sci. 2018;18(4):950–957. doi: 10.4314/ahs.v18i4.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jordan J., Ricci F., Hoffmann F., Hamrefors V., Fedorowski A. Orthostatic hypertension critical appraisal of an overlooked condition. Hypertension. 2020;75:1151–1158. doi: 10.1161/HYPERTENSIONAHA.120.14340. [DOI] [PubMed] [Google Scholar]
- 30.Fessel J., Robertson D. Orthostatic hypertension: when pressor reflexes overcompensate. Nat Clin Pract Nephrol. 2006;2(8):424–431. doi: 10.1038/ncpneph0228. [DOI] [PubMed] [Google Scholar]
- 31.Tabara Y., Masaki M., Ikezoe T. Small degree of lumbar lordosis as an overlooked determinant for orthostatic increases in blood pressure in the elderly: the Nagahama Study. Am J Hypertens. 2019;32(1):61–69. doi: 10.1093/ajh/hpy137. [DOI] [PubMed] [Google Scholar]
- 32.Agnoletti D., Valbusa F., Labat C. Evidence for a prognostic role of orthostatic hypertension on survival in a very old institutionalized population. Hypertension. 2016;67(1):191–196. doi: 10.1161/HYPERTENSIONAHA.115.06386. [DOI] [PubMed] [Google Scholar]
- 33.Veronese N., De Rui M., Bolzetta F. Orthostatic changes in blood pressure and mortality in the elderly: the Pro.V.A Study. Am J Hypertens. 2015;28(10):1248–1256. doi: 10.1093/ajh/hpv022. [DOI] [PubMed] [Google Scholar]
- 34.Curreri C., Giantin V., Veronese N. Orthostatic changes in blood pressure and cognitive status in the elderly: the Progetto Veneto Anziani Study. Hypertension. 2016;68(2):427–435. doi: 10.1161/HYPERTENSIONAHA.116.07334. [DOI] [PubMed] [Google Scholar]
- 35.Matsubayashi K., Okumiya K., Wada T. Postural dysregulation in systolic blood pressure is associated with worsened scoring on neurobehavioral function tests and leukoaraiosis in the older elderly living in a community. Stroke. 1997;28(11):2169–2173. doi: 10.1161/01.str.28.11.2169. [DOI] [PubMed] [Google Scholar]
- 36.Fedorowski A., Ostling G., Persson M. Orthostatic blood pressure response, carotid intima-media thickness, and plasma fibrinogen in older nondiabetic adults. J Hypertens. 2012;30(3):522–529. doi: 10.1097/HJH.0b013e32834fa860. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1, Tables S1-S4.