Abstract
Caffeine is well known for its central nervous system–stimulating effect. Toxicity may occur following high-dose caffeine ingestions. We describe a case of caffeine intoxication secondary to reported ingestion of a large dose of caffeine (60,000 mg in tablet form) with an initial serum caffeine level of 608 μmol/L (known lethal serum level starting from 412 μmol/L). This case demonstrates the key clinical manifestations of caffeine intoxication and the effect of its associated massive adrenergic surge with neurologic symptoms, cardiovascular instability, metabolic abnormalities, and the significant risk of mortality. We highlight important kidney management considerations, including protective measures against electrolyte disturbances such as hypokalemia and hypophosphatemia, and the use of prolonged hemodialysis for caffeine elimination. We share our practical decision making and approach to dialysis discontinuation if serum caffeine level reporting is unavailable or delayed.
Index Words: Caffeine, intoxication, toxicity, elimination, hemodialysis
Introduction
Caffeinated beverages are consumed by 89% of the US population,1 with an average daily consumption of 165 mg/d.2 The limit of safe consumption is considered <400 mg/d for adults and 2.5 to 3 mg/kg per day for pediatrics, with significant individual variability.3,4 Doses > 150 mg/kg are considered lethal.5 We share our management strategies and kidney-related considerations in the following case of caffeine intoxication.
Case report
An individual ingested 60,000 mg of caffeine in tablet form. This individual was not receiving any regular medications and had normal baseline kidney function. On arrival to the emergency department 2 hours after ingestion, this individual was extremely agitated, irritable, diaphoretic, and hyperventilating and had episodes of repeated vomiting. There was no history of alcohol or other recreational drug ingestion. Vital signs were blood pressure, 155/88 mm Hg; heart rate, 132 beats/min (later accelerated to >200 beats/min); respiratory rate, 32 breaths/min; temperature, 35.8 °C; and oxygen saturation, 97% while breathing room air. This individual was of average size, and although the initial Glasgow Coma Scale score was 15, incoherence and confusion soon developed. The electrocardiogram showed sinus tachycardia, with subsequent development of supraventricular tachycardia. Initial laboratory results are shown in Table 1. Screening for other toxic ingestion was done and was reported as all negative.
Table 1.
Laboratory Results
| Reference Range | Presentation (day 0) | Day 0 Before 1st HD | Day +1 Before 2nd HD | Day +2 | Day +3 | |
|---|---|---|---|---|---|---|
| Potassium, mmol/L | 3.2-5 | 2.6 | 4.8 (after replacement) | 3.6 with replacement | 3.8 without replacement | 3.5 without replacement |
| Bicarbonate, mmol/L | 23-29 | 12 | 18 (after replacement) | 24 | 26 | 26 |
| Creatinine, mg/dL | 0.72-1.24 | 0.86 | 0.60 | 0.62 | 0.66 | 0.62 |
| Corrected calcium, mg/dL | 8.8-10.4 | 8.5 | 8.7 | 9.0 | 8.8 | 8.6 |
| Phosphorus, mg/dL | 2.5-4.3 | 3.6 | 2.9 | 2.5 | 3.5 | 3.8 |
| Creatinine kinase, U/L | <240 | 713 | 500 | 291 | 168 | 158 |
| Lactate, mmol/L | 0.5-2 | 8.2 | 3.7 | n/a | 1.3 | n/a |
Note: Results of all other routine blood work, including complete blood cell count and liver enzymes, were normal. Conversion factors for units: creatinine in mg/dL to μmol/L, ×88.4; calcium in mg/dL to mmol/L, ×2495; phosphorus in mg/dL to mmol/L, ×0.3229.
Abbreviation: HD, hemodialysis.
Critical care, nephrology, and poison control teams were involved immediately. The patient received lorazepam, ondansetron, and intravenous (IV) potassium chloride in the emergency department and then transferred to the intensive care unit. Despite initial hemodynamic stability, rapid response intubation was performed due to deterioration in the level of consciousness, recurrent episodes of supraventricular tachycardia, and the risk for aspiration. This required large doses of propofol, midazolam, and fentanyl. Fifty grams of activated charcoal was given through a nasogastric tube to facilitate gastrointestinal elimination. Another peripheral IV line was established, and a temporary dialysis catheter was inserted. The decision to initiate hemodialysis (HD) was based on the reported high dose of caffeine ingestion. The goal was to help with drug elimination given the patient’s clinical deterioration. Dialysis was initiated 5 hours after presentation (7 hours after presumed ingestion time). Details of the dialysis session and dialysate composition are illustrated in Table 2. We aimed to maintain urine output at >100 mL/h by an infusion of Ringer’s lactate at 125 to 150 mL/h.
Table 2.
Dialysis Session Details and Dialysate Composition
| Duration, h | 8 |
|---|---|
| Anticoagulation | No anticoagulation |
| Dialyzer | Fresenius FX 120 CorDiax dialyzer |
| KUf 87 mL/h.mm Hg | |
| Surface area 2.5 m2 | |
| Polysulfone, single-use high-flux dialyzer | |
| Dialysis machine | Bellco formula 2000 |
| Blood flow, mL/min | 350 |
| Dialysate flow, mL/min | 700 |
| Ultrafiltration | No ultrafiltration |
| Dialysate composition (5 L) | Acidic bicarbonate hemodialysis concentrates mixing ratio 1+44 |
| Acetate, mmol/L | 4 |
| Sodium, mmol/L | 140 |
| Chloride, mmol/L | 105.8 |
| Potassium, mmol/L | 4 |
| Bicarbonate, mmol/L | 40 |
| Calcium, mmol/L | 1.5 |
| Magnesium, mmol/L | 0.375 |
| Dextrose, mmol/L | 11.1 |
| Added phosphate sodium 120 mL, mmol/L (each 100 mL contains 16 g of monobasic sodium phosphate and 6 g of of dibasic sodium phosphate) | 0.95 |
Abbreviation: KUf, ultrafiltration coefficient.
Blood was drawn for serum caffeine levels and sent to an external laboratory to measure levels at presentation, immediately before dialysis, and after finishing the first dialysis session; however, results would not be available for up to 48 hours. It was measured using a high-performance liquid chromatography method.
Our patient tolerated the first HD session without interruption. We followed up serum potassium levels (Table 1) as a marker of adrenergic activity (in consideration of concurrent losses from high urinary flows and against a high dialysate potassium concentration); the patient needed large doses of IV potassium replacement (200 mmol of IV potassium chloride in the first 24 hours). This suggested continued activity of the drug (caffeine) or one of its metabolites.
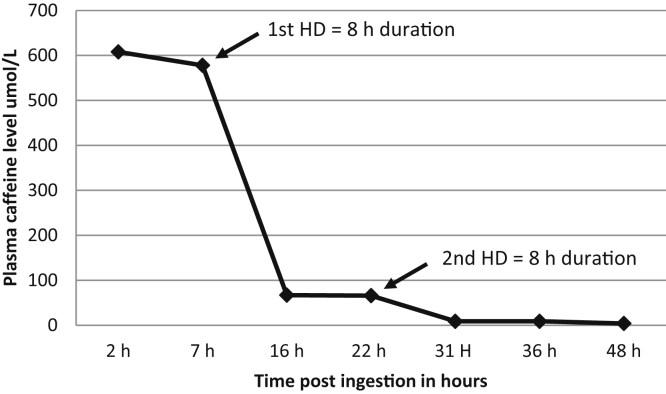
Caffeine levels were redrawn 6 hours after the first HD session, and a second HD session was performed with similar parameters. After the second HD session, serum potassium levels normalized without needing further potassium supplementation. Because both cardiovascular (normal sinus rhythm and blood pressure) and neurologic symptoms improved, we discontinued further HD. The patient subsequently maintained urine output of 100 to 200 mL/h and received appropriate calcium and magnesium replacement. Metabolic acidosis had been corrected by dialysis. Serum caffeine levels were reported on day 3 following the initial presentation, as shown in Table 3 and Fig 1. The patient was discharged after an approximately week-long hospitalization.
Table 3.
Serum Caffeine Levels Relative to Dialysis Administration
| Time Point | At Presentation (2 h post PIT) | 7 h Post PIT (immediately before 1st HD) | 16 h Post PIT (post 1st HD) | 22 h After PIT (before 2nd HD) | 31 h Post PIT (post 2nd HD) | 36 h Post PIT | 48 h Post PIT |
|---|---|---|---|---|---|---|---|
| Serum caffeine level, μmol/L | 608 | 578 | 67 | 66 | 9 | 9 | 4 |
Note: Serum caffeine levels are shown at different time points during the hospital course, demonstrating significant elimination with dialysis. Reference level, 30 to 100 μmol/L.
Abbreviations: HD, hemodialysis; PIT, presumed ingestion time.
Figure 1.
Serum caffeine level and elimination by dialysis. Reference level, 30 to 100 μmol/L. Abbreviation: HD, hemodialysis.
Discussion
Caffeine is rapidly and completely absorbed from the small intestine, reaching peak plasma concentration 30 to 60 minutes after ingestion. It is primarily metabolized to paraxanthine (80%), theobromine (11%), and theophylline (5%), mainly through the cytochrome P450 1A2 isozyme. These compounds are further metabolized and finally excreted by the kidneys with an average elimination half-life of 3 to 6 hours. Less than 5% of the ingested caffeine is excreted unchanged in urine.6, 7
Caffeine has a nonselective adenosine receptor antagonistic effect and causes increased catecholamine release through acting at presynaptic adenosine A1 receptors and the adrenal medulla.4 At higher toxic levels, caffeine may have phosphodiesterase inhibition, increased intracellular calcium release, and cholinergic and γ-aminobutyric acid type A receptor antagonistic effects.8
Favorable effects of caffeine, such as increased arousal and alertness, occur with average consumption of a 250-mg dose (a 235-mL cup of coffee contains 103 to 200 mg of caffeine), while nondesirable effects such as increased tension, nausea, and palpitation occur with doses of 500 to 750 mg.9 Clinical features of intoxication include hypotension, arrhythmias, neurologic symptoms (eg, agitation, excitation, seizures, and coma), and metabolic abnormalities (eg, hypokalemia, hypocalcemia, and lactic acidosis). Acute kidney injury can occur secondary to shock or rhabdomyolysis.10
Management should be directed by clinical manifestation because caffeine levels are not always available. At presentation, our patient’s serum caffeine level was 608 μmol/L (118 mg/L); in a previous report, the median and 10th percentile lethal serum caffeine concentrations were 927 μmol/L (180 mg/L) and 433 μmol/L (84 mg/L), respectively.4,11 The average serum caffeine level in a moderate to heavy caffeine drinker is 22.7 μmol/L (4.4 mg/L).12,13 Our patient was at high risk for arrhythmia and hemodynamic instability. The first aim of management was sedation and intubation to protect the airways and establish ventilation. The next step was to establish peripheral and central vessel access for fluid and electrolyte management and insert a dialysis catheter to help drug elimination through HD.
Caffeine is an ideal dialyzable drug because of its low molecular weight (194 g/mol), water solubility, small volume of distribution (0.7 L/kg), and low percentage of protein binding (10%-35%). Given the patient’s clinical state and consenting challenges, the interval from presentation to HD initiation was deemed to be acceptable.
We performed prolonged dialysis sessions (8 hours) because higher doses of HD with prolonged duration are ideal for effective drug removal,14 unlike other slower modalities that are used in more unstable patients in the intensive care unit setting, including slow low-efficiency dialysis or continuous kidney replacement therapy. This is reflected after the first dialysis session by the reduced caffeine level to 67 μmol/L (11% of predialysis level).
Because of practical issues, samples for caffeine measurements were not sent from the dialysate or urine. Therefore, the exact contribution of HD to caffeine elimination in our case is unknown. However, we think it is significant based on 2 factors: first, the difference in the decrease in caffeine levels in the first 7 hours before dialysis initiation (endogenous clearance only) was minimal (<5% decrease) compared with the subsequent 8 hours after dialysis (endogenous clearance + dialysis; 88.4% decrease). Second, as previously described, endogenous clearance is substantially decreased with high toxic concentrations due to saturation of the metabolizing isozymes,4,9 thus elimination is greatly augmented by HD. In addition to the higher potassium dialysis bath (4 mmol/L) and IV potassium chloride given intermittently, the dialysate was supplemented with a phosphate sodium solution to guard against hypophosphatemia. Protective management of hypokalemia and hypophosphatemia is important due to the patient’s observed supraventricular tachycardia and high risk for subsequent arrhythmias.
Last, knowing that there would be a 48-hour delay in receiving plasma caffeine levels prompted a decision to start dialysis based on the history of caffeine ingestion and the clear clinical presentation, after ruling out other intoxications. In the absence of timely caffeine levels, the decision to resume dialysis was clinically based on the cardiac and neurologic manifestations and on the trend in serum potassium levels. Hypokalemia in caffeine intoxication is mainly secondary to an intracellular potassium shift, which is due to increased release of catecholamines. The second HD session was in response to decreasing serum potassium to levels requiring replacement, likely representing ongoing increased catecholamine release secondary to high levels of caffeine or one of its active metabolites (other causes of hypokalemia were ruled out). We recognize that this may have been confounded by the high urinary flows but interestingly, our patient did not require further potassium replacement after the second HD session.
Few case reports demonstrate the use of HD, hemoperfusion, and/or continuous venovenous hemodiafiltration therapies for the treatment of caffeine intoxications.15, 16, 17 In one report, an initial 4-hour HD session was successful in reducing caffeine levels by 66%, but the patient was hemodynamically unstable and subsequently transitioned to continuous hemodiafiltration.5 In our case, 1 session of intensive prolonged HD contributed to increasing and expediting the elimination of caffeine in a patient with ingestion of a potentially toxic dose.
In conclusion, management of caffeine intoxication can be challenging. Early initiation of prolonged HD is effective for caffeine elimination even from 1 session. Meticulous electrolyte replacements and high dialysate potassium and phosphorus concentrations are important considerations when HD is used for treating caffeine toxicity. Serum potassium level can be used as a surrogate marker for ongoing caffeine toxicity in case of a delay in reporting caffeine levels.
Article Information
Authors’ Full Names and Academic Degrees
Mohamed Elbokl, MBBCH, Ian Randall, MD, MPH, and Charmaine E. Lok, MD, MSc.
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Acknowledgements
The authors thank the HD nurses for expedient care of this individual.
Patient Consent
The authors were unable to obtain consent from the patient, and thus have removed all identifying information from this Case Report.
Peer Review
Received June 1, 2020. Evaluated by 1 external peer reviewer, with direct editorial input from the Editor-in-Chief. Accepted in revised form November 8, 2020.
Footnotes
Complete author and article information provided before references.
References
- 1.Verster J.C., Koenig J. Caffeine intake and its sources: a review of national representative studies. Crit Rev Food Sci Nutr. 2018;58(8):1250–1259. doi: 10.1080/10408398.2016.1247252. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell D.C., Knight C.A., Hockenberry J., Teplansky R., Hartman T.J. Beverage caffeine intakes in the U.S. Food Chem Toxicol. 2014;63:136–142. doi: 10.1016/j.fct.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 3.Nawrot P., Jordan S., Eastwood J., Rotstein J., Hugenholtz A., Feeley M. Effects of caffeine on human health. Food Addit Contam. 2003;20(1):1–30. doi: 10.1080/0265203021000007840. [DOI] [PubMed] [Google Scholar]
- 4.Willson C. The clinical toxicology of caffeine: a review and case study. Toxicol Rep. 2018;5:1140–1152. doi: 10.1016/j.toxrep.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fausch K., Uehlinger D.E., Jakob S., Pasch A. Haemodialysis in massive caffeine intoxication. Clin Kidney J. 2012;5(2):150–152. doi: 10.1093/ckj/sfs020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnaud M.J. Pharmacokinetics and metabolism of natural methylxanthines in animal and man. Handb Exp Pharmacol. 2011;200:33–91. doi: 10.1007/978-3-642-13443-2_3. [DOI] [PubMed] [Google Scholar]
- 7.Carrillo J.A., Benitez J. Clinically significant pharmacokinetic interactions between dietary caffeine and medications. Clin Pharmacokinet. 2000;39(2):127–153. doi: 10.2165/00003088-200039020-00004. [DOI] [PubMed] [Google Scholar]
- 8.Magkos F., Kavouras S.A. Caffeine use in sports, pharmacokinetics in man, and cellular mechanisms of action. Crit Rev Food Sci Nutr. 2005;45(7-8):535–562. doi: 10.1080/1040-830491379245. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan G.B., Greenblatt D.J., Ehrenberg B.L., Goddard J.E., Cotreau M.M., Harmatz J.S. Dose-dependent pharmacokinetics and psychomotor effects of caffeine in humans. J Clin Pharmacol. 1997;37(8):693–703. doi: 10.1002/j.1552-4604.1997.tb04356.x. [DOI] [PubMed] [Google Scholar]
- 10.Campana C., Griffin P.L., Simon E.L. Caffeine overdose resulting in severe rhabdomyolysis and acute renal failure. Am J Emerg Med. 2014;32(1):111. doi: 10.1016/j.ajem.2013.08.042. e3-e4. [DOI] [PubMed] [Google Scholar]
- 11.Jones A.W. Review of caffeine-related fatalities along with postmortem blood concentrations in 51 poisoning deaths. J Anal Toxicol. 2017;41(3):167–172. doi: 10.1093/jat/bkx011. [DOI] [PubMed] [Google Scholar]
- 12.Banerjee P., Ali Z., Levine B., Fowler D.R. Fatal caffeine intoxication: a series of eight cases from 1999 to 2009. J Forensic Sci. 2014;59(3):865–868. doi: 10.1111/1556-4029.12387. [DOI] [PubMed] [Google Scholar]
- 13.Benowitz N.L. Clinical pharmacology of caffeine. Annu Rev Med. 1990;41:277–288. doi: 10.1146/annurev.me.41.020190.001425. [DOI] [PubMed] [Google Scholar]
- 14.Mirrakhimov A.E., Barbaryan A., Gray A., Ayach T. The role of renal replacement therapy in the management of pharmacologic poisonings. Int J Nephrol. 2016;2016:3047329. doi: 10.1155/2016/3047329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishigaki S., Fukasawa H., Kinoshita-Katahashi N., Yasuda H., Kumagai H., Furuya R. Caffeine intoxication successfully treated by hemoperfusion and hemodialysis. Intern Med. 2014;53(23):2745–2747. doi: 10.2169/internalmedicine.53.2882. [DOI] [PubMed] [Google Scholar]
- 16.Kapur R., Smith M.D. Treatment of cardiovascular collapse from caffeine overdose with lidocaine, phenylephrine, and hemodialysis. Am J Emerg Med. 2009;27(2):253. doi: 10.1016/j.ajem.2008.06.028. e3-e6. [DOI] [PubMed] [Google Scholar]
- 17.Kato Y., Kuriyama A., Hata R., Ikegami T. Extracorporeal membrane oxygenation for hypokalemia and refractory ventricular fibrillation associated with caffeine intoxication. J Emerg Med. 2019;58(1):59–62. doi: 10.1016/j.jemermed.2019.09.023. [DOI] [PubMed] [Google Scholar]