Abstract
Autosomal dominant tubulointerstitial kidney disease subtype hepatocyte nuclear factor 1β (ADTKD-HNF1B) is a hereditary disease caused by variants of HNF1B that is characterized by a family history of tubulointerstitial nephropathy with concomitant diabetes mellitus. We report on a Japanese man in his early 40s who had ADTKD-HNF1B diagnosed. He had a reduced glomerular filtration rate, borderline diabetes mellitus, multiple small cysts in his bilateral kidneys, and pancreatic hypoplasia. He also had a family history of diabetes and kidney cystic lesions. These phenotypes represent ADTKD-HNF1B and genetic analysis revealed a missense variant of HNF1B. Kidney biopsy demonstrated not only tubulointerstitial fibrosis but also abnormal mitochondrial morphology in tubular cells, a novel finding.
Index Words: Autosomal dominant tubulointerstitial kidney disease, HNF1B, diabetes mellitus, maturity-onset diabetes of the young type 5 (MODY5)
Introduction
Autosomal dominant tubulointerstitial kidney disease (ADTKD) is a chronic tubulointerstitial kidney disease caused by a gene variant. The subtype hepatocyte nuclear factor 1β (HNF1B) is caused by a variant in the HNF1B gene and is referred to as ADTKD-HNF1B. This subtype has a variety of extrarenal manifestations, such as pancreatic hypoplasia, hyperparathyroidism, hypomagnesemia-like Gitelman syndrome, and genital tract malformation, as well as kidney cysts, unilateral kidney agenesis, and hypoplasia.1,2 It is closely related to early-onset diabetes mellitus with a familial history, in particular maturity-onset diabetes of the young type 5 (MODY5).3 Cystic formation is present in 73% of patients with ADTKD-HNF1B; cysts are usually small and often arise within the kidney cortex.1 However, previous reports have not described the pathology of the kidney in ADTKD-HNF1B.
We report on the kidney disease of an individual with ADTKD-HNF1B and mainly focus on the histology of the kidney.
Case report
A Japanese man in his early 40s was admitted to our hospital for further examination due to a decreased glomerular filtration rate (GFR). The decreased GFR had been detected during a routine health check in his mid-20s, but no further examinations were performed. One year previously, his kidney function had deteriorated. Fetal ultrasonography of his daughter revealed agenesis of the right kidney and multiple cysts in the left kidney, and her estimated GFR was 50 mL/min/1.73 m2. His father has diabetes mellitus and chronic kidney disease, and his mother died of gastric cancer. His history included tonsillitis and appendicitis as a child and depression for the previous 10 years. His depression was treated at an outpatient clinic with paroxetine, olanzapine, and flunitrazepam.
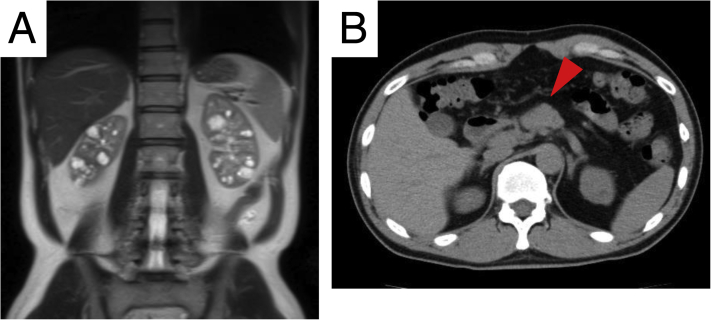
On admission, the patient was 172 cm tall and weighed 61.8 kg. Blood pressure was 123/84 mm Hg; heart rate, 75 beats/min; and body temperature, 35.8 °C. Edema was not present in the lower extremities. Results of laboratory tests were as follows: serum creatinine, 1.64 mg/dL; urea nitrogen, 18 mg/dL; estimated GFR, 38.8 mL/min/1.73 m2; hemoglobin A1c, 6.2%, and total urinary protein excretion, 0.04 g/d. Urine sediment contained fewer than 1 erythrocyte per high-power field. Magnetic resonance imaging showed multiple small cysts in the corticomedullary and medullary areas of bilateral kidneys (Fig 1A). Total kidney volume was 291 mL (right kidney, 143 mL; left kidney, 148 mL). Computed tomography showed pancreatic hypoplasia with deficiency of the pancreatic body and tail (Fig 1B).
Figure 1.
Radiographic images. (A) Magnetic resonance T2-weighted image of the abdomen. Multiple cysts in corticomedullary and medullary legions of bilateral kidneys. (B) Plane computed tomographic image, pancreatic hypoplasia with deficiency of the pancreatic body and tail (red arrowhead).
Due to the patient's decreased GFR, an echo-guided percutaneous kidney biopsy of the right kidney was performed.
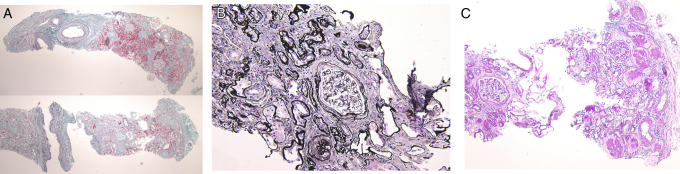
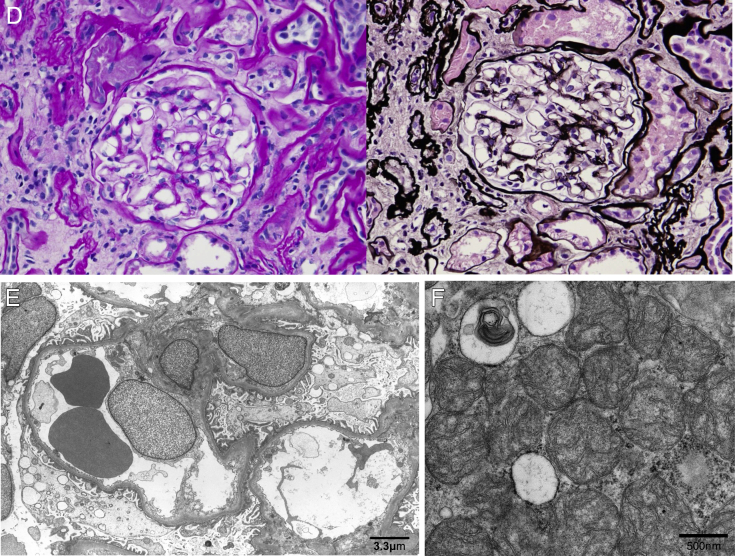
In light microscopy of the biopsy specimen, ∼70% of the total kidney cortical region was replaced by tubulointerstitial fibrosis, and there was thinning of the cortex (Fig 2A and B). Almost all tubules were narrowed, and duplication and swelling of the tubular basement membrane were definite (Fig 2B). Cystic lesions were not present in the specimen. Global sclerosis was observed in 15 of 21 glomeruli, mainly in the outer cortical region (Fig 2C). The remaining glomeruli were intact (Fig 2D). Fibroelastosis of the intralobular arteries was moderate, but arteriolar hyalinosis was not noted. Immunofluorescence was negative for immunoglobulin G (IgG), IgA, IgM, C3, C4, and C1q. Electron microscopy did not reveal any abnormality in glomeruli or tubular basement membranes (Fig 2E) but showed that mitochondria in tubular epithelial cells were small, rounded, and swollen, with shortened cristae (Fig 2F). Although the individual had borderline diabetes mellitus, the glomerular basement membrane was not thickened (width, 330-380 nm; normal width, <430 nm) and was not consistent with diabetic nephropathy.
Figure 2.
Pathology of the kidney biopsy specimen. (A) Light microscopy, Masson trichrome staining. Renal cortical region is replaced by tubulointerstitial fibrosis and is thin. (B) Periodic acid–methenamine silver (PAM) staining. Almost all tubules are contracted and tubular basement membrane shows characteristic duplication and swelling. (C) Periodic acid–Schiff (PAS) staining. Global scleroses exist in 15 of the 21 glomeruli in the outer cortical region. (D) PAS and PAM-Masson staining. The remaining glomeruli were intact. (E) Electron microscopy: no significant changes were seen in glomeruli. (F) Electron microscopy: abnormally small, rounded, and swollen mitochondria were noted in tubular epithelial cells.
To determine the genetic relationships between the patient's and his daughter’s kidneys, we obtained the ethics committee and patient’s approval and genetic analysis of blood samples was performed at the Department of Pediatrics at Kobe University Graduate School of Medicine, in accordance with previous reports.4,5 As a result, a novel heterozygous missense variant of HNF1B (NM_000458.3: c.865A>C, p.(Asn289His)) was detected in both the individual and his daughter.
Discussion
Medullary cystic kidney disease, which is characterized by cyst formation in the corticomedullary and medullary areas, was a morphologic classification based on radiologic features. From the 1990s to 2000s, genetic analyses of medullary cystic kidney and related disease have revealed variants of 5 different genes, including MUC1, uromodulin(UMOD), REN, HNF1B, and more rarely, SEC61A1.6 In 2015, Kidney Disease: Improving Global Outcomes (KDIGO) redefined the disease concept medullary cystic kidney disease as ADTKD, which involved a change from morphologic classification to genetic classification of the disease.
In individuals with ADTKD-HNF1B, hypoplasia of the pancreatic body and tail and a slightly atrophic pancreatic head are involved in the development of diabetes.3 Diabetes with onset before the age of 25 years in families with this autosomal dominant inheritance is called MODY, and the type caused by the variant of HNF1B is called HNF1B-MODY (MODY5). In 2014, Faguer et al7 described an HNF1B score that can be calculated from clinical, imaging, and biological variables. The cutoff threshold for a negative predictive value to rule out HNF1B variants is 8 points. Our patient scored at least 20 points, indicating that our diagnosis of ADTKD-HNF1B was correct. Table 1 shows typical HNF1B-associated phenotypes and compares them with the phenotype of this patient.6
Table 1.
HNF1B-Associated Phenotypes
| Typical HNF1B-Associated Phenotypes | Phenotype of Current Case |
|---|---|
| 1. Neurologic features: autism spectrum disorders; cognitive impairment | On medication for treatment of depression |
| 2. Abnormal liver function: asymptomatic increase in liver enzyme levels; neonatal cholestasis | None |
| 3. Maturity-onset diabetes of the young type 5 (MODY5) | Patient had mild decrease in glucose tolerance; father had type 2 diabetes |
| 4. Pancreatic hypoplasia: hypoplasia of body and tail of pancreas with slightly atrophic head; pancreatic exocrine dysfunction | Patient has pancreatic hypoplasia of body and tail |
| 5. Developmental kidney disease: bilateral hyperechogenic kidney on prenatal ultrasonography; kidney cysts; single kidney; kidney hypoplasia; kidney dysplasia; other (horseshoe or duplex kidneys, etc) | Patient has bilateral kidney cysts; daughter has agenesis of right kidney and cysts in left kidney |
| 6. Genital tract malformations | None |
| 7. Hypomagnesemia | None |
| 8. Hyperuricemia and early-onset gout | None |
Abbreviation: HNF1B, hepatocyte nuclear factor 1β.
Although ADTKD is defined as tubulointerstitial nephropathy, few reports have described the kidney biopsy and pathologic features of ADTKD. Ayasreh Fierro et al8 reported that kidney specimens of patients with ADTKD-UMOD showed interstitial fibrosis, tubular atrophy, normal glomeruli, and tubular dilatation with tubular microcystis. However, kidney biopsy of ADTKD-HNF1B has not been reported.
The findings in the kidney biopsy of this patient may support previous findings. For example, Connor et al9 showed that variants in mitochondrial DNA also caused tubulointerstitial kidney disease. Furthermore, Casemayou et al10 reported that HNF1B regulated transcription factor peroxisome proliferator-activated receptor-γ expression and regulated mitochondrial morphology and respiration in proximal tubule cells in mice.
Through genetic analysis, a novel heterozygous missense variant of HNF1B (NM_000458.3: c.865A>C, p.(Asn289His)) was detected in both the individual and his daughter. Faguer et al11 reported a variant (NM_000458.2: c.865A>G, p.(Asn289Asp)) and submitted the variant to the Human Gene Mutation Database (CM117494). This patient showed the same site variant. According to American College of Medical Genetics and Genomics guidelines, this variant meets PM1, PM2, PP1 and PP3 so that this variant is “likely pathogenic” of ADTKD-HNF1B.
In conclusion, this patient presented with both kidney cystic lesions and diabetes and had a family history of kidney cystic lesion and diabetes, which are HNF1B-associated phenotypes, and genetic analysis revealed a novel variant of HNF1B. We performed a kidney biopsy of his cystic kidney and this is the first report of the kidney biopsy on ADTKD-HNF1B. This case also showed tubulointerstitial fibrosis and abnormal mitochondrial morphology in tubular cells.
Article Information
Authors’ Full Names and Academic Degrees
Yuki Oba, MD, Naoki Sawa, MD, Hiroki Mizuno, MD, Junichi Hoshino, MD, PhD, Keiichi Kinowaki, MD, Kenichi Ohashi, MD, PhD, Naoya Morisada, MD, PhD, Kazumoto Iijima, MD, PhD, Yutaka Yamaguchi, MD, PhD, and Yoshifumi Ubara, MD.
Support
This study was funded by the Okinaka Memorial Institute for Medical Research.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Patient Consent
The authors declare that they have obtained consent from the patient discussed in the report.
Peer Review
Received April 28, 2020. Evaluated by 1 external peer reviewer, with direct editorial input by an Associate Editor and the Editor-in-Chief. Accepted in revised form October 4, 2020.
Footnotes
Complete author and article information provided before references.
Contributor Information
Yuki Oba, Email: pugpug.yuki008@gmail.com.
Yoshifumi Ubara, Email: ubara@toranomon.gr.jp.
References
- 1.Clissold R.L., Hamilton A.J., Hattersley A.T., Ellard S., Bingham C. HNF1B-associated renal and extra-renal disease - an expanding clinical spectrum. Nat Rev Nephrol. 2015;11:102–112. doi: 10.1038/nrneph.2014.232. [DOI] [PubMed] [Google Scholar]
- 2.Verhave J.C., Bech A.P., Wetzels J.F.M., Nijenhuis T. Hepatocyte nuclear factor 1β-associated kidney disease: more than renal cysts and diabetes. J Am Soc Nephrol. 2016;27:345–353. doi: 10.1681/ASN.2015050544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horikawa Y. Maturity-onset diabetes of the young as a model for elucidating the multifactorial origin of type 2 diabetes mellitus. J Diabetes Investig. 2018;9:704–712. doi: 10.1111/jdi.12812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishiwa S., Sato M., Morisada N. Association between the clinical presentation of congenital anomalies of the kidney and urinary tract (CAKUT) and gene mutations: an analysis of 66 patients at a single institution. Pediatr Nephrol. 2019;34:1457–1464. doi: 10.1007/s00467-019-04230-w. [DOI] [PubMed] [Google Scholar]
- 5.Nagano C., Morisada N., Nozu K. Clinical characteristics of HNF1B-related disorders in a Japanese population. Clin Exp Nephrol. 2019;23:1119–1129. doi: 10.1007/s10157-019-01747-0. [DOI] [PubMed] [Google Scholar]
- 6.Devuyst O., Olinger E., Weber S. Autosomal dominant tubulointerstitial kidney disease. Nat Rev Dis Prim. 2019;5:1–20. doi: 10.1038/s41572-019-0109-9. [DOI] [PubMed] [Google Scholar]
- 7.Faguer S., Chassaing N., Bandin F. The HNF1B score is a simple tool to select patients for HNF1B gene analysis. Kidney Int. 2014;86:1007–1015. doi: 10.1038/ki.2014.202. [DOI] [PubMed] [Google Scholar]
- 8.Ayasreh Fierro N., Miquel Rodríguez R., Matamala Gastón A., Ars Criach E., Torra Balcells R. A review on autosomal dominant tubulointerstitial kidney disease. Nefrologia. 2017;37:235–243. doi: 10.1016/j.nefro.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 9.Connor T.M., Hoer S., Mallett A. Mutations in mitochondrial DNA causing tubulointerstitial kidney disease. PLoS Genet. 2017;13:1–17. doi: 10.1371/journal.pgen.1006620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casemayou A., Fournel A., Bagattin A. Hepatocyte nuclear factor-1b controls mitochondrial respiration in renal tubular cells. J Am Soc Nephrol. 2017;28:3205–3217. doi: 10.1681/ASN.2016050508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faguer S., Decramer S., Chassaing N. Diagnosis, management, and prognosis of HNF1B nephropathy in adulthood. Kidney Int. 2011;80:768–776. doi: 10.1038/ki.2011.225. [DOI] [PubMed] [Google Scholar]