Abstract
Rationale & Objective
Despite growing interest in individualizing care, routine dialysis processes, including the interdisciplinary plan of care, often fail to account for patient-identified priorities. To better align dialysis care with patient priorities and improve care planning experiences, we implemented a person-centered care plan program at a single clinic. We also sought to gain insight into key implementation considerations and areas for program improvement.
Study Design
6-month quality improvement project with research substudy.
Setting & Participants
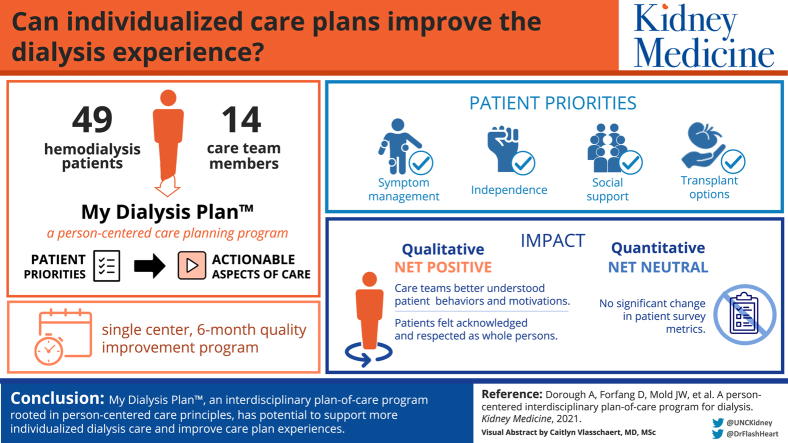
49 hemodialysis patients and 14 care team members at a North Carolina dialysis clinic.
Quality Improvement Activities
Implementation of My Dialysis Plan, a person-centered care plan program.
Outcome(s)
Participant perspectives and care plan meeting characteristics (quality improvement); pre- to postprogram change in patient-reported autonomy support, patient-centeredness of care, and dialysis care individualization (research).
Analytical Approach
We used the Consolidated Framework for Implementation Research to guide implementation and evaluation. We conducted pre-, intra-, and post-project interviews with clinic stakeholders (patients, clinic personnel, and medical providers) to identify implementation barriers, facilitators, and perceptions. We compared pre- and post-project care plan meeting content and patient-reported outcome survey scores.
Results
We conducted 54 care plans with 49 patients. Overall, care teams successfully used My Dialysis Plan to elicit and link patient priorities to actionable aspects of dialysis care. Participants identified interdisciplinary team commitment, accountability, and the structured yet flexible meeting approach as key implementation elements. Throughout the project, stakeholder input guided program modifications (eg, implementation practices and resources) to better meet clinic needs, but follow-up on care plan–identified action items remained challenging. Among the 28 substudy participants, there was no difference in pre- to post-project patient-reported outcome survey scores.
Limitations
Single clinic implementation.
Conclusions
My Dialysis Plan has the potential to enhance dialysis care individualization and care plan experiences. Evaluation of program impact on patient-reported and clinical outcomes is needed.
Index Words: Dialysis, hemodialysis, person-centered care, interdisciplinary plan of care, implementation
Graphical abstract
PLAIN-LANGUAGE SUMMARY.
There is growing interest in making dialysis care less protocolized and more individualized. The required interdisciplinary plan of care is an opportunity to incorporate patient priorities more meaningfully into dialysis care. We implemented My Dialysis Plan, a person-centered care planning program that equips patients and care team members with tools to hold patient priority–driven and shared decision-making–focused care plan meetings, in a single outpatient hemodialysis clinic. We demonstrated that this program has potential to enhance patient and care team experiences and is feasible to incorporate into existing care processes. Future studies should assess the program’s impact on outcomes and costs.
Individuals receiving dialysis prioritize well-being and quality of life over laboratory values and even death, yet most dialysis clinical and quality measures focus on the latter.1, 2, 3, 4, 5 In 2017, a Centers for Medicare & Medicaid Services (CMS) Technical Expert Panel with 50% patient representation recommended using patient “life goals” to guide dialysis care.6 International experts suggested a similar approach in 2018 when KDIGO (Kidney Disease: Improving Global Outcomes) proposed replacing the traditional concept of “dialysis adequacy’” with “goal-directed dialysis.”7 The CMS Conditions of Coverage support the use of patient goals to inform dialysis care, mandating the development of interdisciplinary plans of care that consider the “patient’s needs, wishes, and goals.”8 Despite this intention, patients and providers believe that dialysis care plans are often formulaic, focusing on biochemical markers and failing to capture patient priorities.
Incorporating the philosophy of person-centered care may promote better alignment of dialysis care and patient priorities. In person-centered care, clinicians and patients act as partners, engaging in shared decision making to coordinate care that is effective for and meaningful to the whole person over time.9, 10, 11 This approach has been shown to strengthen patient–care team relationships and promote patient engagement, better health outcomes, improved quality of life, and greater care and job satisfaction in primary care, mental health, and geriatric populations.12, 13, 14, 15
However, there are barriers to person-centered care adoption in the dialysis setting, including lack of resources, regulatory factors, and infrastructure, as well as rigid interdisciplinary roles.16 Integrating person-centered care into existing dialysis care processes, such as the development of interdisciplinary plans of care, may be one strategy to center care around patient priorities without overburdening care teams and patients. However, we lack models to guide such an approach.
To address this need, we developed My Dialysis Plan (UNC Kidney Center), a person-centered care dialysis interdisciplinary plan-of-care program, with input from clinic stakeholders.17 We then implemented My Dialysis Plan at a large suburban hemodialysis clinic with the aims of better aligning dialysis care with patient priorities and improving the dialysis care plan experience. We also sought to gain insight into key implementation considerations and areas for program improvement.
Methods
Overview
We implemented My Dialysis Plan in a North Carolina dialysis clinic as a quality improvement (QI) project and conducted a research substudy to assess program potential for improving patient perceptions of care. The QI project was approved by the dialysis clinic’s leadership and determined to be nonhuman subjects research by the University of North Carolina Institutional Review Board (17-0193). We performed, analyzed, and reported the QI project in accordance with Standards for Quality Improvement Reporting Excellent Guidelines (SQUIRE; Table S1).18 The research substudy was approved by the University of North Carolina Institutional Review Board (19-0743), and participants provided informed consent.
Intervention: My Dialysis Plan
My Dialysis Plan is an interdisciplinary plan-of-care program designed to align dialysis care with patient priorities, enhance the care-planning experience, and improve health through better education, patient–care team communication, and shared decision making.17 This person-centered care program provides a flexible yet tailorable structure to assist care teams in individualizing care plans. Supporting program materials include patient education (informational video to encourage active participation in care planning and brochure about what to expect and how to prepare for the care plan meeting) and care team resources (training and care plan meeting materials). Open-source program resources are available at go.unc.edu/MyDialysisPlan.
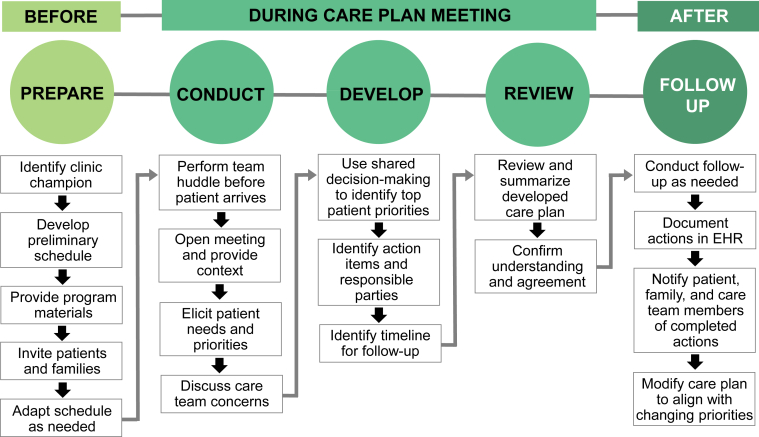
Figure 1 displays an overview of My Dialysis Plan. In summary, care teams invite patients to participate in their care plan meetings in a private setting, and before the meeting, the care team collectively reviews individual assessments. During the meeting, the team elicits patient-identified priorities to guide the collaborative development of an individualized care plan with specific follow-up action items for care teams and patients. At the meeting conclusion, the care team reviews the plan with the patient to confirm understanding and agreement. After the meeting, designated care team members perform and document assigned actions, provide progress updates, and follow up with the patient to identify changing priorities.
Figure 1.
My Dialysis Plan care planning approach, depicted in 3 phases: before, during, and after the care plan meeting. In the weeks before the care plan meeting, a designated care team member issues invitations and schedules meetings. Just before the meeting, the care team huddles to review individual assessments. During the meeting, the care team elicits patient needs and priorities and uses shared decision making to develop an individualized plan of care with specific action items for care team members and the patient. At meeting conclusion, the care team reviews the care plan with the patient to confirm understanding and agreement. After the meeting, care team members perform assigned actions, provide updates on progress, and follow up with the patient to identify changing priorities. Abbreviation: EHR, electronic health record.
Setting and Participants
The participating dialysis clinic, a joint venture between the University of North Carolina and a large dialysis organization, serves approximately 130 in-center hemodialysis patients and operates 2 daytime shifts. All adult hemodialysis patients (end-stage kidney disease [ESKD] or acute kidney injury) due for a care plan during the 6-month project period were eligible to participate. Patients received written letters about the QI project and its opt-out option (N = 1). All clinic personnel and nephrology providers participated in the QI project. All patient QI participants were eligible to participate in the research substudy except for non–English speakers. Research recruitment methods included dialysis clinic fliers and in-person clinic interactions with research personnel. Research participants received $75 remuneration.
Implementation Approach and Data Collection
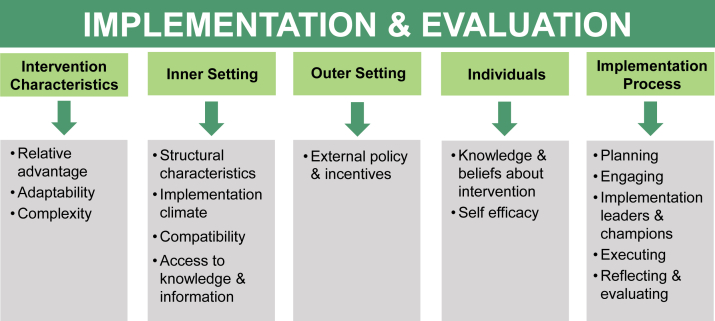
Before the 6-month QI project, we conducted interviews with clinic stakeholders to identify barriers to, facilitators of, and strategies for My Dialysis Plan implementation. We then created a preliminary implementation guide rooted in principles of the Consolidated Framework for Implementation Research (CFIR). The CFIR is a conceptual framework to guide program design, implementation, and evaluation, as well as identify factors that influence intervention effectiveness.19 The framework has 5 domains (intervention characteristics, outer setting, inner setting, characteristics of the individuals involved, and implementation process), each with constructs associated with effective implementation (Table S2). Throughout the project, we collected data on implementation practices and perceptions through interviews, direct observation, and surveys.
Interviews and Observations
A trained interviewer conducted semi-structured interviews with participating patients, clinic personnel, and medical providers before, during, and after program implementation (Table S3). Interviews occurred in person and responses were recorded on standardized note templates. Pre-project interviews assessed clinic needs, resource availability, and program perceptions. Monthly intra-project interviews assessed program barriers and facilitators, acceptability, and feasibility. Post-project interviews assessed participants’ perceptions of program impact and sustainability potential. We supplemented interview data with field observations of clinic personnel on the treatment floor and during care team meetings to assess clinic workflow, culture, and team dynamics. Observations were recorded on standardized templates.
Participant Characteristics
We abstracted demographic, health, and prior care plan data from the electronic health records (EHRs) of all patient participants.
Surveys
Research participants completed the following questionnaires before and after project implementation: Modified Health Care Climate Questionnaire (MHCCQ), Client-Centered Care Questionnaire (CCCQ), and Dialysis Care Individualization Questionnaire. The MHCCQ is a 6-item instrument measuring patient-perceived autonomy support from a single clinician or group of care providers; it has been validated in primary care and breast cancer populations.20, 21, 22 The CCCQ is a 15-item instrument evaluating the client-centeredness of care and services of a new intervention; it has undergone reliability testing in medically frail populations.23,24 We modified select MHCCQ and CCCQ items to correspond with dialysis care processes (Table S4). The Dialysis Care Individualization Questionnaire is a research team–developed 5-item measure assessing patient-perceived individualization of dialysis care.
Data Analyses
Qualitative Data
We analyzed interview and observation data to identify barriers to and facilitators of My Dialysis Plan implementation. Data were entered into tables organized by time of interview (pre-QI, intra-QI, and post-QI), interviewee type (patient, clinic personnel, and medical provider), and content (implementation practices, program components, participant perceptions, and experiences). Using the CFIR as our analytic framework, we coded text according to the 5 CFIR domains and 15 selected constructs, evaluating for patterns or themes in the data.25,26 Findings were used to iteratively update our implementation approach and program materials.
Quantitative Data
Descriptive statistics (eg, count and percentage and median with interquartile range) were used to report participant and pre- and post-program care plan characteristics. We calculated pre- and post-project MHCCQ and CCCQ scores according to instrument scoring instructions, and we calculated Dialysis Care Individualization Questionnaire scores according to the team-developed scoring system. We used paired t tests to compare pre- and post-project survey scores.
Results
Participant Characteristics
Table 1 displays participant characteristics. There were 63 QI participants: 49 patients and 14 care team members (6 medical providers, 4 nurses, 2 dietitians, and 2 social workers). Patient participant mean age was 60 ± 16 years, with mean dialysis vintage of 4 years, 17 (35%) women, 27 (55%) of Black race, and 9 (18%) of Hispanic ethnicity. Of the 42 eligible patients, 28 (67%) enrolled in the research substudy. Overall, substudy participants had similar characteristics to patient QI participants.
Table 1.
Participant Characteristics
| Characteristic | QI Project | Research Substudy |
|---|---|---|
| Patients | ||
| No. of participants | 49 | 28 |
| Age, y | 60 [49-73] | 59 [49-70] |
| Female sex | 17 (35%) | 7 (25%) |
| Race | ||
| Black | 27 (55%) | 18 (64%) |
| White | 20 (41%) | 8 (29%) |
| Other | 2 (4%) | 2 (7%) |
| Ethnicity | ||
| Hispanic | 9 (18%) | 1 (4%) |
| Not Hispanic | 40 (82%) | 27 (96%) |
| Non–English speaking | 7 (14%) | 0 (0%) |
| Highest level of education completed | ||
| <High school | — | 10 (36%) |
| High school graduate or GED | — | 10 (36%) |
| Some college | — | 2 (7%) |
| ≥4-y college degree | — | 6 (21%) |
| Acute kidney injury | 3 (6%) | 1 (4%) |
| Dialysis vintage, y | ||
| <1 | 6 (12%) | 4 (14%) |
| 1-5 | 26 (53%) | 14 (50%) |
| ≥6 | 17 (35%) | 10 (36%) |
| Comorbid medical conditions | ||
| Diabetes | 24 (49%) | 12 (43%) |
| Heart failure | 23 (47%) | 9 (32%) |
| Heart disease | 17 (35%) | 6 (21%) |
| Cancer | 12 (24%) | 1 (4%) |
| History of transplant | 3 (6%) | 3 (11%) |
| Transplant status | ||
| Listed | 5 (10%) | 5 (18%) |
| Evaluation in process | 3 (6%) | 2 (7%) |
| Evaluated and did not qualify | 20 (41%) | 12 (43%) |
| Not under evaluation | 21 (43%) | 9 (32%) |
| Clinic personnel and medical providers | ||
| No. of participants | 14 | — |
| Professional role | — | |
| Medical provider | 6 (43%) | |
| Nurse | 4 (29%) | |
| Dietitian | 2 (14%) | |
| Social worker | 2 (14%) | |
Note: Participant characteristics at time of QI project start. Values are listed as number (percent) or median [interquartile range].
Abbreviations: GED, general education diploma; QI, quality improvement.
My Dialysis Plan Care Plan Meetings
During the 6-month program, we conducted 54 care plans with 49 unique patients: 6 with acute kidney injury, 2 with initial ESKD, 3 with 90-day ESKD, and 43 with annual ESKD care plans. Meetings averaged 23 ± 7 minutes, 43 (80%) occurred off the treatment floor, and 8 (15%) used interpreter services.
Table 2 provides an overview of elicited priorities. Those most frequently elicited were related to symptom management (32 [59%] meetings), social support (25 [46%]), transplantation (16 [30%]), and maintaining or cultivating independence (13 [24%]).
Table 2.
Priorities and Needs Elicited in My Dialysis Plan Care Plan Meetings
| Topics | Meetings (N = 54) |
|---|---|
| Medical | 45 (83%) |
Physical symptoms
|
27 (50%) |
Transplant
|
16 (30%) |
Services
|
10 (19%) |
Mood symptoms
|
9 (17%) |
Medications
|
7 (13%) |
Medical concerns
|
7 (13%) |
Care coordination
|
4 (7%) |
| Psychosocial | 34 (63%) |
Social support
|
25 (46%) |
Independence
|
16 (30%) |
Financial
|
8 (15%) |
Transportation
|
8 (15%) |
Housing and food
|
7 (13%) |
| Personal | 23 (43%) |
Hobbies
|
16 (30%) |
Physical activity
|
12 (22%) |
Travel
|
9 (17%) |
Note: Data reflective of both patient-identified and care team–identified priorities, all discussed during the care plan meeting.
Abbreviations: SSDI, Social Security Disability Insurance; SSI, Supplemental Security Income.
Table 3 displays examples of patient-identified priorities, action items, and responsible parties. Action steps were most often assigned to medical providers (eg, specialist referral and medication or dialysis prescription change), social workers (eg, insurance coordination and financial assistance), and patients (eg, attend appointments and communicate about symptoms). Of the 78 identified care team action items, 41 (53%) had evidence of follow-up in the EHR or clinic-based electronic communication system.
Table 3.
Patient-Identified Priorities, Responsive Action Items, and Responsible Parties
| Patient Priority | Responsive Action Item(s) | Responsible Party |
|---|---|---|
| Increase energy level | Refer to cardiologist, change dialyzer size, monitor Kt/V | Nephrologist |
| Address anxiety and forgetfulness | Refer for neuropsychological evaluation, prescribe antidepressant and monitor effects | Nephrologist and nurse |
| Eat more diverse foods | Discuss alternative protein options that: (1) improve appetite and (2) fit within patient budget to ensure sustainable provision | Dietitian and social worker |
| Spend more time at home | Schedule home dialysis education class | Social worker |
| Obtain eyeglasses | Refer to ophthalmologist to update prescription, confirm insurance benefits | Nephrologist and social worker |
| Maintain independence | Refer to vocational rehabilitation services, follow up on status of transplant evaluation | Social worker |
| Attend monthly family gatherings | Communicate family gathering schedule to care team, modify treatment start time or day of week to facilitate attendance | Patient and nurse |
| Play piano | Refer to hand specialist for pain and numbness in left hand | Nephrologist |
Of the 37 patients who had EHR evidence of a care plan meeting at the participating clinic both before and during My Dialysis Plan implementation, we found that 6 (16%) patients were accompanied by a care partner or family member with My Dialysis Plan (vs 0 prior) and 33 (89%) meetings were held off the treatment floor (vs 2 [5%] prior). Moreover, 26 (70%) My Dialysis Plan care plans documented a nonmedical patient priority and associated action item (vs 4 [11%] prior). However, there was no change in documented advanced care planning (eg, advance directives and end-of-life preferences) discussions pre- to post-program, with no evidence of such discussions in any of the 37 pre- or post-program care plan notes.
Application of CFIR Constructs in Program Implementation and Evaluation
Table 4 displays project-tailored definitions and applications of the 15 CFIR constructs that guided My Dialysis Plan implementation. Key implementation features (CFIR domain) included clinic stakeholder buy-in to the care plan approach and the underlying person-centered care philosophy (characteristics of individuals and inner setting), clinic program champion identification (process), stakeholder involvement in development and modification of implementation strategies and resources (process and intervention characteristic), and program alignment with existing CMS guidance and regulations for interdisciplinary care plans (outer setting).
Table 4.
Project-Tailored Definitions and Application of CFIR Constructs Guiding Implementation and Evaluation
| Construct19 | Project-Tailored Definition | Application |
|---|---|---|
| Intervention Characteristic | ||
| Relative advantage | Perceived advantages of My Dialysis Plan compared with the clinic’s existing care plan approach | Presented an opportunity to provide more individualized dialysis care that was responsive to patient-identified priorities and consistent with care team members’ desired practice |
| Adaptability | Ability to modify and tailor My Dialysis Plan program components and resources to fit changing clinic needs | Iteratively updated program throughout implementation in response to stakeholder feedback |
| Complexity | Perceived difficulty, burden, learning curve, and/or workflow disruption associated with My Dialysis Plan implementation | Assigned program responsibilities to align with existing job roles; minimized additional responsibilities; provided program trainings |
| Outer Setting | ||
| External policy & incentives | Alignment of My Dialysis Plan with CMS guidance and regulations | Developed program to support CMS Conditions of Coverage |
| Inner Setting | ||
| Structural characteristics | Clinic size, characteristics, and social architecture | Selected large suburban dialysis clinic to enhance transferability of developed implementation processes |
| Implementation climate | Clinic stakeholders’ readiness for My Dialysis Plan implementation (ie, buy-in from all clinic stakeholders, cultural norms and values) | Interviewed clinic stakeholders throughout program implementation; discussed program logistics at monthly staff meetings |
| Compatibility | Clinic stakeholders’ desire for a person-centered care planning; fit of My Dialysis Plan with existing clinic workflows | Collaboratively developed program with patients, care teams, and medical providers to enhance relevance; refined implementation processes with clinic stakeholders to ensure local fit |
| Access to information | Readily available health-literacy level appropriate My Dialysis Plan materials for patients and care teams | Developed mixed-media education/implementation resources; updated program resources in response to stakeholder input |
| Characteristics of Individuals | ||
| Knowledge & beliefs about the intervention | Clinic stakeholders’ attitudes and beliefs about person-centered care planning and dialysis care planning experiences | Provided education on person-centered care planning; collected and incorporated clinic personnel feedback on program components |
| Self-efficacy | Care team members’ beliefs in their abilities to elicit and align care with patient priorities and document appropriately | Provided initial administrative support and training materials to ease implementation; sought guidance from goal-directed care expert |
| Process | ||
| Planning | Degree to which tasks for implementing My Dialysis Plan were developed in advance, and the quality of the methods | Collaboratively developed implementation plan with clinic personnel; assigned responsibilities to align with individual skillsets/comfort |
| Engaging | Winning clinic stakeholder buy-in through education and training | Conducted clinic personnel informational and training sessions; proactively sought stakeholder feedback |
| Implementation leaders & champions | Engaging individuals with influence on attitudes and beliefs of care team members and identifying care team members to take primary responsibility for My Dialysis Plan implementation | Engaged clinic operations manager in implementation plan development; identified dietitian as program champion |
| Executing | Implementing My Dialysis Plan according to the collaboratively developed implementation plan | Adhered to implementation plan when feasible; iteratively modified resources and implementation plan as needed |
| Reflecting & evaluating | Obtaining feedback about My Dialysis Plan implementation via monthly debriefing interviews with clinic stakeholders | Held routine care team and QI support team meetings to address barriers/facilitators; interviewed clinic stakeholders |
Abbreviations and Definitions: care team, social workers, dietitians, nurses, and medical providers; CFIR, Consolidated Framework for Implementation Research; clinic stakeholders, patients, clinic personnel, and medical providers; CMS, Centers for Medicare & Medicaid Services; QI, quality improvement.
My Dialysis Plan Implementation Experience and Findings
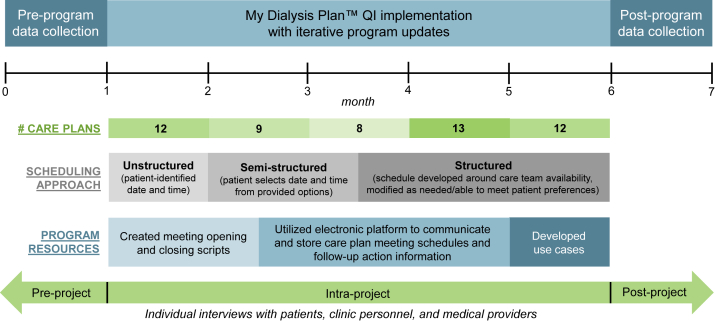
Figure 2 displays the project timeline. Before My Dialysis Plan implementation, we built clinic capacity through program presentations and training sessions. At project start, the QI support team provided on-site administrative assistance and transitioned to no support by project end. Table 5 displays interview findings and responsive program updates.
Figure 3.
Consolidated Framework for Implementation Research (CFIR) domains and constructs that guided My Dialysis Plan implementation and evaluation. The figure outlines the 5 CFIR domains (light green boxes) and the 15 selected constructs (gray boxes) that guided My Dialysis Plan implementation and evaluation.
Figure 2.
Quality improvement (QI) project implementation timeline with iterative program updates. Pre-/post-program data were collected through individual interviews with patients, clinic personnel, and medical providers in the months preceding and following the 6-month project period. Iterative program changes were made in response to intra-project feedback from clinic stakeholders (eg, scheduling approach, program resources).
Table 5.
Interview Findings, Responsive Program Updates, and Future Recommendations
| Component | Key Findings | Responsive Update(s)/Recommendation(s) |
|---|---|---|
| Before Implementation | ||
| Overall impressions |
|
— |
|
||
|
||
|
||
| Barriers |
|
|
|
|
|
|
|
|
|
|
|
| Facilitators |
|
|
|
|
|
|
|
|
| During Implementation | ||
| Overall impressions |
|
|
|
||
|
|
|
|
||
|
|
|
| Barriers | Communication | |
|
|
|
|
|
|
|
|
|
|
|
|
| Process | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Facilitators |
|
— |
|
||
|
||
|
||
|
||
| After Implementation | ||
| Overall impressions |
|
— |
|
||
|
||
|
||
| Remaining barriers |
|
|
|
|
|
|
|
|
|
|
|
| Facilitators |
|
|
|
|
|
|
— | |
Note: Data ascertained from semi-structured interviews with hemodialysis patients, social workers, dietitians, nurses, PCTs, and medical providers at participating clinic. Data summarized and reported in aggregate to protect participant privacy.
Abbreviations: CP, care plan; PCT, patient care technician; RN, registered nurse.
Before implementation, care team members expressed enthusiasm about My Dialysis Plan, viewing it as an opportunity to apply underused clinical skills, build relationships, and individualize care. There was apprehension around care plan meeting duration, scheduling challenges, language barriers, and patient transportation. Some questioned whether patients would participate in meetings held outside of dialysis treatment times. However, most patients were willing, citing the significance of fewer distractions (eg, intradialytic symptoms and beeping machines) and a more private environment. In addition, the “newness” of My Dialysis Plan was attractive to some, offering a change from everyday clinic routines. A few patients doubted the program could meaningfully alter their care, with one stating “...there’s not a whole heck of a lot you can do after 5 years [on dialysis]. I’ve already established what I’m willing to do and what I’m not willing to do.”
Throughout program implementation, stakeholders described improved interdisciplinary teamwork and patient partnerships. Compared with previous care plan meetings, My Dialysis Plan meetings yielded greater insight into the patient as a whole person, providing context to support shared decision making and build rapport for subsequent interactions. In general, care teams found meetings less time-burdensome than expected, noting increased efficiency with experience. However, challenges with scheduling, communication, meeting content, and follow-up necessitated program modifications.
Initially, patients self-selected a care plan meeting time, but this was impractical to coordinate due to care team schedules, monthly meeting burden (often >10 care plan meetings per month), and an unexpectedly high volume of patients opting for private meetings which required additional planning compared to chairside meetings. As such, patients were offered multiple meeting times from which to choose, but scheduling remained arduous. In project month 3, patients were offered specific meeting times and were encouraged to propose alternatives if they or other planned attendees had conflicts. This approach was acceptable to most patients because the clinic coordinated transportation.
Care teams experienced some initial discomfort facilitating meetings (ie, initiating and closing conversations, embracing silence, and eliciting priorities). In response, clinic personnel and the QI support team developed scripts to simplify meeting introductions (ie, set expectations for meeting purpose, approach, and length) and closings (ie, review meeting notes, assess patient agreement, and adjourn) and sample “use” cases tying frequently cited patient priorities to actionable aspects of care. Care teams also found that acute kidney injury care plans warranted greater focus on laboratory values than ESKD care plans because patient priorities were often related to kidney injury recovery status. Finally, care plan follow-up was occasionally missed or not communicated to others. In response, the clinic adopted a Health Insurance Portability and Accountability Act–compliant electronic platform to share information.
Despite intermittent scheduling and communication hurdles, care teams thought that program advantages outweighed the challenges, electing to continue My Dialysis Plan post-program. Overall, participants found the program’s educational resources to be helpful, observing that the brochure adequately described care plan meetings and the video equipped patients for active participation. Patients reported feeling heard and better informed about their dialysis care. One patient commented, “I feel like [the care team] listened and if they needed clarification, they asked for it. If I needed clarification, it was provided [to me].” Although care team members were occasionally frustrated by their inability to address some patient priorities due to limited resources or interventions, they recognized that heightened awareness supported deeper patient–care team relationships. One care team member said, “It enhances relationships.... Getting to know [patients] and understanding them, helping them see we are invested…that’s a valuable end point.” A patient described, “I love talkin’ just like we did in that meeting. It showed that they care, and they will do something to try to help. That’s what matters to me. I know it’s not always possible for things to go the way I want them to go.” Despite program benefits, care teams continued to struggle with completing and communicating all follow-up action items. Despite this lack of follow-up, most patients identified the team’s interest in their priorities as a program benefit.
Research Findings
Among the 25 research participants with pre- and post-project data, there was no significant change in pre- to post-project survey scores of perceived autonomy support from providers (0.3 ± 1.3; P = 0.3), patient-centeredness (1.2 ± 5.2; P = 0.2), or dialysis care individualization (0.1 ± 0.8; P = 0.5).
Discussion
Our findings suggest that My Dialysis Plan, an interdisciplinary plan-of-care program rooted in person-centered care principles, has the potential to support more individualized dialysis care and improve care plan experiences. Project participants identified interdisciplinary team commitment, accountability, and the structured yet flexible care plan meeting approach as key implementation elements. Our report also underscores the significance of incorporating diverse stakeholder input throughout implementation of new programs to promote buy-in, feasibility, and sustainability.
Despite initial implementation concerns raised by project stakeholders and others,16 we found that in most cases, the dialysis care team could use My Dialysis Plan to elicit and link patient priorities to actionable aspects of dialysis care. In shifting the care plan focus from problems to priorities, patients felt acknowledged and respected as whole persons, and care teams better understood patient behaviors (eg, tardiness due to lack of driver’s license) and motivations (eg, symptom management). As such, care teams could more easily connect medical advice to patient priorities, engendering patient buy-in and increasing the likelihood of adherence.
Care plan meetings did not have to be long to garner rich information, as evidenced by brief discussions with skeptical patients who chose not to deeply engage in the process. In these instances, care team members still found the person-centered care approach worthwhile, citing that even a small amount of new information (eg, life experiences and stressors) benefitted future interactions. In cases without simple solutions or overt connections between patient priorities and dialysis, patients still felt empowered from the opportunity to be heard. These experiences fostered trust, improved care experiences, and reminded care team members why they chose to work in dialysis.
In addition, these conversations cultivated shared decision making, a process in which clinicians support patient autonomy by providing comprehensive information (eg, education and treatment options) and working with patients to reach informed decisions that match their individual preferences.27,28 Studies in other chronic illness populations have shown that shared decision making increases patient knowledge and self-efficacy, strengthens care team–patient relationships, fosters patient activation, improves patient-reported outcomes, and decreases health care use.29, 30, 31
In My Dialysis Plan care plan meetings, shared decision making often manifested as trade-off discussions. For example, a patient with a history of high interdialytic weight gains described feeling too fatigued to eat lunch with their grandchild after treatment. In response, the care team provided salt and fluid intake counseling, explaining that lower weight gains and the resultant gentler fluid removal might mitigate treatment-associated fatigue. Upon understanding the potential link between fluid control and post-dialysis fatigue, the patient was more receptive and ultimately adherent to the suggested dietary restrictions. Care team members found program resources helpful in supporting these conversations, appreciating the structured conversation guide and case examples. Moreover, and consistent with the existing literature,32,33 engaging in shared decision making left patients and care team members feeling more connected and aligned in care goals.
These findings are particularly relevant given the recent release of the CMS End-Stage Renal Disease Treatment Choices payment model.34 The model aims to give ESKD beneficiaries enhanced freedom and choice and encourage greater use of home dialysis and kidney transplantation. These goals align closely with the intent of My Dialysis Plan. As such, program resources may be useful in facilitating patient-care team conversations about modality selection and transplantation. More broadly, the resources could support additional goals of the model by fostering shared decision making and promoting patient activation.
Despite program successes and care team commitment to the person-centered care philosophy, there were challenges with incorporating My Dialysis Plan into clinical practice. Some were easily resolved by collaboratively modifying program resources, such as developing scripts to ease meeting facilitation and adding resources to support shared decision making. Conversely, care plan meeting scheduling required iterative attempts to establish a sustainable approach. Still other challenges persisted throughout the project, namely insufficient interteam communication and lack of infrastructure for care plan follow-up. These require additional attention in future implementations. Integrating My Dialysis Plan–elicited information into the EHR would be one way to streamline communication, document priorities, and promote accountability for follow-up.
Finally, we observed no change in documented advance care planning discussions pre- to post-program, suggesting that patients and/or care teams may need additional support to comfortably engage in these conversations. As such, future program iterations could place greater emphasis on the topic and equip participants with existing serious illness conversation resources.35,36
Project strengths include incorporation of stakeholder input throughout My Dialysis Plan implementation, selection of a large dialysis clinic in which logistical barriers were likely to be encountered, and collection of end-user experiential data throughout the project.
Limitations relate to the transferability of findings due to study implementation at a single clinic, since varying clinic sizes and cultures, patient populations, and/or ownership structures may present different implementation climates, facilitators, and barriers. In addition, we relied on qualitative data to support our conclusion that My Dialysis Plan has the potential to individualize dialysis care and promote more meaningful care plan experiences. Our research substudy findings showed no significant pre- to post-project change in patient-reported perceived autonomy support from providers, patient-centeredness of care, or dialysis care individualization. These results stand in contrast to the overall positive sentiments expressed in participant interviews.
Potential explanations for these somewhat discrepant findings may relate to differences in participants in the research substudy and overall QI project or biases in survey responses. For example, response-shift bias occurs in settings in which a respondent’s frame of reference for a measured construct(s) changes between pre- and posttesting.37 In our project, many patients answered the pre-implementation survey questions with the highest possible level of agreement, verbalizing unawareness of the possibility or need for improvement in the measured constructs. However, during post-project interviews, many explicitly described a meaningful change in these areas. Because there were not more positive response options available on the post-project surveys, respondents indicated the same highest level of agreement as they did on preproject surveys. Other types of response bias related to social desirability (eg, lack of privacy during administration of surveys) and/or acquiescence bias (eg, survey fatigue) are also possible.38 Finally, our small pilot study was not designed or powered to evaluate for statistically significant differences in outcomes in the pre- and post-project periods. Future studies examining program impact on patient-reported outcomes such as care satisfaction, activation, and health-related quality of life, as well as clinical outcomes such as hospitalizations and use of palliative and hospice services, are needed.
In conclusion, we demonstrated that a person-centered care planning approach has the potential to enhance patient and care team experiences and is feasible to incorporate into the current structure of care. Future studies are needed to assess program sustainability and effect on patient-reported and clinical outcomes, as well as develop implementation practices for diverse clinics.
Article Information
Authors’ Full Names and Academic Degrees
Adeline Dorough, MPH, Derek Forfang, James W. Mold, MD, MPH, Abhijit V. Kshirsagar, MD, MPH, Darren A. DeWalt, MD, MPH, and Jennifer E. Flythe, MD, MPH.
Authors’ Contributions
Research idea and study design: JEF, DF; data acquisition: AD; supervision: JEF; all authors were involved in data analysis/interpretation and intellection content. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This project was funded by the American Institutes for Research, with support from the Robert Wood Johnson Foundation, for a patient-centered measurement pilot study (grant 044970001). The funder had no role in the study design, data collection, analysis, manuscript writing or the decision to submit the report for publication. Dr Flythe is supported by the National Institute of Diabetes, Digestive and Kidney Diseases (K23 DK109401).
Financial Disclosure
In the last 2 years, Dr Flythe has received speaking honoraria from the American Society of Nephrology, National Kidney Foundation, and multiple universities, as well as investigator-initiated research funding unrelated to this project from the Renal Research Institute, a subsidiary of Fresenius Kidney Care, North America; is on the medical advisory board to NxStage Medical, now owned by Fresenius Kidney Care, North America; and has received consulting fees from Fresenius Kidney Care, North America and AstraZeneca. Dr Mold has written and published a book for patients on goal-oriented care from which he receives royalties. The remaining authors have no relevant financial interests to disclose.
Acknowledgements
The authors thank our collaborating clinic’s patients, personnel, and medical providers for their enthusiasm, flexibility, and dedication throughout program development and implementation; Kevin Fowler, David White, Lucy Menefee, Bradley Manton, Lisa Harvey, Shirley Franks, and Colleen Chavis (in memoriam) for contributions while serving on the stakeholder panel; Matthew Tugman for assistance with data abstraction and manuscript editing; and Karin True for assistance with data abstraction.
Peer Review
Received June 2, 2020. Evaluated by 1 external peer reviewer, with direct editorial input from the Editor-in-Chief. Accepted in revised form November 1, 2020.
Footnotes
Complete author and article information provided before references.
Table S1. SQUIRE guidelines and manuscript section with the relevant content.
Table S2. CFIR constructs and definitions.
Table S3. Interview guide topics, questions, and probes.
Table S4. Research substudy surveys with source and adapted questions.
Supplementary Material
Tables S1-S4
References
- 1.Weiner D., Watnick S. The ESRD Quality Incentive Program-can we bridge the chasm? J Am Soc Nephrol. 2017;28(6):1697–1706. doi: 10.1681/ASN.2016101079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kliger A.S. Quality measures for dialysis: time for a balanced scorecard. Clin J Am Soc Nephrol. 2016;11(2):363–368. doi: 10.2215/CJN.06010615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finkelstein F.O. Performance measures in dialysis facilities: what is the goal? Clin J Am Soc Nephrol. 2015;10(1):156–158. doi: 10.2215/CJN.04780514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tong A., Manns B., Hemmelgarn B. Establishing core outcome domains in hemodialysis: report of the Standardized Outcomes in Nephrology-Hemodialysis (SONG-HD) Consensus Workshop. Am J Kidney Dis. 2017;69(1):97–107. doi: 10.1053/j.ajkd.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evangelidis N., Tong A., Manns B. Developing a set of core outcomes for trials in hemodialysis: an international Delphi survey. Am J Kidney Dis. 2017;70(4):464–475. doi: 10.1053/j.ajkd.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Medicare & Medicaid Services. End-Stage Renal Disease Patient-Reported Outcomes Technical Expert Panel Final Report. Accessed May 31, 2020. https://dialysisdata.org/sites/default/files/content/ESRD_Measures/ESRD_Patient_Reported_Outcomes_TEP_Summary_Report.pdf
- 7.Chan C.T., Blankestijn P.J., Dember L.M. Dialysis initiation, modality choice, access, and prescription: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019;96(1):37–47. doi: 10.1016/j.kint.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Department of Health and Human Services. 42 CFR Parts 405, 410, 413 et al. Medicare and Medicaid Programs; Conditions for Coverage for End-Stage Renal Disease Facilities; Final Rule. April 15, 2008. Accessed May 31, 2020. https://www.cms.gov/Regulations-and-Guidance/Legislation/CFCsAndCoPs/Downloads/ESRDfinalrule0415.pdf [PubMed]
- 9.American Geriatrics Society Expert Panel on Person-Centered Care Person-centered care: a definition and essential elements. J Am Geriatr Soc. 2016;64(1):15–18. doi: 10.1111/jgs.13866. [DOI] [PubMed] [Google Scholar]
- 10.Kogan A.C., Wilber K., Mosqueda L. Person-centered care for older adults with chronic conditions and functional impairment: a systematic literature review. J Am Geriatr Soc. 2016;64(1):e1–e7. doi: 10.1111/jgs.13873. [DOI] [PubMed] [Google Scholar]
- 11.Morton R.L., Sellars M. From patient-centered to person-centered care for kidney diseases. Clin J Am Soc Nephrol. 2019;14(4):623–625. doi: 10.2215/CJN.10380818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Pol-Grevelink A., Jukema J.S., Smits C.H. Person-centred care and job satisfaction of caregivers in nursing homes: a systematic review of the impact of different forms of person-centred care on various dimensions of job satisfaction. Int J Geriatr Psychiatry. 2012;27(3):219–229. doi: 10.1002/gps.2719. [DOI] [PubMed] [Google Scholar]
- 13.Valentijn P.P., Pereira F.A., Ruospo M. Person-centered integrated care for chronic kidney disease: a systematic review and meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol. 2018;13(3):375–386. doi: 10.2215/CJN.09960917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim S.K., Park M. Effectiveness of person-centered care on people with dementia: a systematic review and meta-analysis. Clin Interv Aging. 2017;12:381–397. doi: 10.2147/CIA.S117637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossom R.C., Solberg L.I., Vazquez-Benitez G. The effects of patient-centered depression care on patient satisfaction and depression remission. Fam Pract. 2016;33(6):649–655. doi: 10.1093/fampra/cmw068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis R.A., Benzies K.M., MacRae J., Thomas C., Tonelli M. An exploratory study of person-centered care in a large urban hemodialysis program in Canada using a qualitative case-study methodology. Can J Kidney Health Dis. 2019;6 doi: 10.1177/2054358119871539. 2054358119871539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dorough A., Forfang D., Murphy S.L. Development of a person-centered interdisciplinary plan-of-care program for dialysis. Nephrol Dial Transplant. 2020;35(8):1426–1435. doi: 10.1093/ndt/gfaa018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogrinc G., Davies L., Goodman D., Batalden P., Davidoff F., Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986–992. doi: 10.1136/bmjqs-2015-004411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Damschroder L.J., Aron D.C., Keith R.E., Kirsh S.R., Alexander J.A., Lowery J.C. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50–65. doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams G.C., Deci E.L. Activating patients for smoking cessation through physician autonomy support. Med Care. 2001;39(8):813–823. doi: 10.1097/00005650-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Shumway D., Griffith K.A., Jagsi R., Gabram S.G., Williams G.C., Resnicow K. Psychometric properties of a brief measure of autonomy support in breast cancer patients. BMC Med Inform Decis Mak. 2015;15:51. doi: 10.1186/s12911-015-0172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt K., Gensichen J., Petersen J.J. Autonomy support in primary care--validation of the German version of the Health Care Climate Questionnaire. J Clin Epidemiol. 2012;65(2):206–211. doi: 10.1016/j.jclinepi.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 23.de Witte L., Schoot T., Proot I. Development of the client-centred care questionnaire. J Adv Nurs. 2006;56(1):62–68. doi: 10.1111/j.1365-2648.2006.03980.x. [DOI] [PubMed] [Google Scholar]
- 24.Muntinga M.E., Mokkink L.B., Knol D.L., Nijpels G., Jansen A.P. Measurement properties of the Client-centered Care Questionnaire (CCCQ): factor structure, reliability and validity of a questionnaire to assess self-reported client-centeredness of home care services in a population of frail, older people. Qual Life Res. 2014;23(7):2063–2072. doi: 10.1007/s11136-014-0650-7. [DOI] [PubMed] [Google Scholar]
- 25.Braun V., Clarke V. Sage; 2012. Successful Qualitative Research: A Practical Guide for Beginners. [Google Scholar]
- 26.Braun V., Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101. [Google Scholar]
- 27.Makoul G., Clayman M.L. An integrative model of shared decision making in medical encounters. Patient Educ Couns. 2006;60(3):301–312. doi: 10.1016/j.pec.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Clayman M.L., Gulbrandsen P., Morris M.A. A patient in the clinic; a person in the world. Why shared decision making needs to center on the person rather than the medical encounter. Patient Educ Couns. 2017;100(3):600–604. doi: 10.1016/j.pec.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 29.Hughes T.M., Merath K., Chen Q. Association of shared decision-making on patient-reported health outcomes and healthcare utilization. Am J Surg. 2018;216(1):7–12. doi: 10.1016/j.amjsurg.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Braddock C.H. The emerging importance and relevance of shared decision making to clinical practice. Med Decis Making. 2010;30(5(suppl)):5S–7S. doi: 10.1177/0272989X10381344. [DOI] [PubMed] [Google Scholar]
- 31.Pirhonen L., Olofsson E.H., Fors A., Ekman I., Bolin K. Effects of person-centred care on health outcomes-a randomized controlled trial in patients with acute coronary syndrome. Health Policy. 2017;121(2):169–179. doi: 10.1016/j.healthpol.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Tinetti M.E., Naik A.D., Dodson J.A. Moving from disease-centered to patient goals-directed care for patients with multiple chronic conditions: patient value-based care. JAMA Cardiol. 2016;1(1):9–10. doi: 10.1001/jamacardio.2015.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tinetti M.E., Esterson J., Ferris R., Posner P., Blaum C.S. Patient priority-directed decision making and care for older adults with multiple chronic conditions. Clin Geriatr Med. 2016;32(2):261–275. doi: 10.1016/j.cger.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 34.Department of Health and Human Services, Centers for Medicare & Medicaid Services 42 CFR Part 512. Medicare Program; Specialty Care Models to Improve Quality of Care and Reduce Expenditures. Fed Regist. 2020;(189):85. https://www.govinfo.gov/content/pkg/FR-2020-09-29/pdf/2020-20907.pdf [Google Scholar]
- 35.Mandel E.I., Bernacki R.E., Block S.D. Serious illness conversations in ESRD. Clin J Am Soc Nephrol. 2017;12(5):854–863. doi: 10.2215/CJN.05760516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schell J.O., Cohen R.A. A communication framework for dialysis decision-making for frail elderly patients. Clin J Am Soc Nephrol. 2014;9(11):2014–2021. doi: 10.2215/CJN.02190314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howard G. Response-shift bias: a problem in evaluating interventions with pre/post self-reports. Eval Rev. 1980;4(1):93–106. [Google Scholar]
- 38.Bowling A. Mode of questionnaire administration can have serious effects on data quality. J Public Health (Oxf) 2005;27(3):281–291. doi: 10.1093/pubmed/fdi031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1-S4