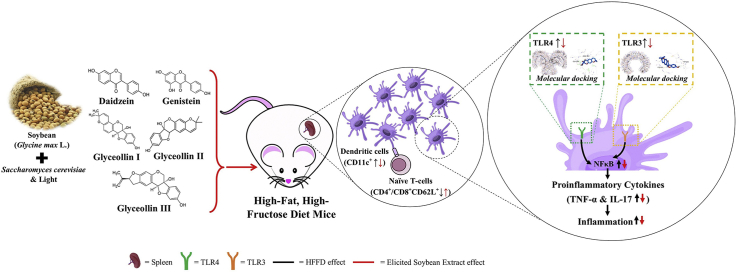
Abstract
Background
The high−fat, high−fructose diet (HFFD) provokes overnutrition and inflammation directly, mainly through Toll−like receptors (TLRs). Soybean (Glycine max L.) contains isoflavone that can be transformed into glyceollin by microbial and physical stimuli. Glyceollin possesses many beneficial effects on health.
Objective
This study evaluates the beneficial effect of soybean extract elicited by Saccharomyces cerevisiae and light (ESE) on dendritic cells (DCs) profile and naïve T cells in HFFD mice.
Materials and methods
Female Balb/C mice were fed with HFFD for 24 weeks then orally administered with simvastatin 2.8 mg/kg BW or ESE 78, 104, and 130 mg/kg BW at the last four weeks. The expression of splenic CD11c+TLR3+, CD11c+TLR4+, NFκB+, CD11c+IL-17+, CD11c+TNF−α+, CD4+CD62L+, and CD8+CD62L+ subsets was measured by flow cytometry. The molecular docking has been measured using Pyrx 0.8, displayed in PyMol and Biovia Discovery Studio.
Result
HFFD significantly increased CD11c+TLR3+, CD11c+TLR4+, NFκB+, CD11c+IL-17+, CD11c+TNF−α+ expression and decreased CD4+CD62L+ and CD8+CD62L+ (p < 0.05) compared to normal diet (ND) groups. ESE reduced CD11c+TLR3+, CD11c+TLR4+, thereby decreasing NFκB+, as well as decreased the CD11c+IL−17+, CD11c+TNF−α+, and restores CD4+CD62L+ and CD8+CD62L+ subsets in HFFD mice. Glyceollin II exhibited the best binding affinity with an average energy of −7.3 kcal/mol to TLR3 and −7.9 kcal/mol to TLR4.
Conclusion
The bioactive compound in ESE act synergistically to modulate TLR3/TLR4 activation, reduced NFκB, IL−17, and TNF−α, and restores naïve T cells expression in HFFD mice. ESE was a favorable candidate to mitigate chronic inflammation.
Keywords: Glyceollin, High−fat, High−fructose, IL−17, TLR3/TLR4, TNF−α
Graphical abstract
Highlights
-
•
HFFD activate TLR3/TLR4, NFκB, TNF-α, and IL-17.
-
•
HFFD reduce naïve T cells.
-
•
ESE improves inflammation caused by HFFD.
1. Introduction
Obesity and its co-morbidities are global health issues that reduce life expectancy and raise healthcare costs [1,2]. Obesity is primarily due to the high-fat diet that causes glucose and free fatty acid (FFA) to rise in circulation and generates inflammation [3]. Inflammation is initiated by an innate immune response by activating pattern recognition receptors (PRRs), primarily by TLRs [4,5]. TLRs are transmembrane PRRs which highly conserved to detect pathogen-associated molecular patterns (PAMPs) derived from pathogens or damaged-associated molecular patterns (DAMPs) as danger signals produced by the non−microbial agent [6]. Fructose, FFA, and modified LDL are potential DAMPs for TLR4, while TLR3 detects mRNA during tissue necrosis. Those DAMPs are increased by HFFD [7,8].
Dendritic cells (DCs) are primary antigen-presenting cells (APCs) that recognize the pathogen and activate early immune response via TLRs signaling pathway [9]. Once DCs are activated, naïve T cells are differentiated to Th1, and CD8 T cells, and balance is switched between Th17/Treg ratio [10,11]. CD4+CD25+ regulatory T cells were reported to involve in regulating proinflammatory cytokines production [12]. However, Treg function is suppressed in excess proinflammatory conditions [13]. Our previous study suggested that ESE enhanced Treg activity in HFFD mice [14]. Based on that, ESE may also have advantages for inhibiting DCs maturation through preventing TLR3/TLR4 activation in HFFD mice.
Soybean (Glycine max L.) is one of the most consumed nutritious commodities in many Asian countries due to its high content of isoflavone [15,16]. Daidzein and genistein are the most well-known isoflavones in soybean, and both were increased by fermentation. However, glyceollin was not found in the fermented soybean product [17]. Glyceollin is derived from daidzein, produced through the stimulation of plant defense response using bacterial or fungal elicitors [18]. A recent study reported that the combination of Saccharomyces cerevisiae with light enhances daidzein, genistein, and glyceollin accumulation [19].
Glyceollin is a major phytoalexin in soybean, which has many beneficial properties. Glyceollin act as antioxidant [20], anti-inflammatory [21], and anti-obesity [22]. Glyceollin as anti-inflammation works through inhibition of IKKα/β phosphorylation [21]. IKKβ is the primary kinase downstream in the Myd88-dependent signaling pathway [23]; thereby glyceollin has the potency for modulating TLR4 activity. However, it remains unclear whether TLR3 activation is modulated by glyceollin and another isoflavone compound. This study aimed to examine the role of glyceollin-rich soybean in the modulation of activation of TLR3/TLR4.
In addition, we also investigated proinflammatory cytokines production as downstream in this pathway. Our study will provide a better understanding of how phytochemical dietary products could modulate TLRs activation in DCs and would be an alternative strategy for reducing the risk of an inflammatory condition in obesity caused by HFFD.
2. Materials and methods
2.1. Soybean preparation
The seeds of Soybean var. Anjasmoro was collected from the Indonesian Legumes and Tuber Crop Research Institute (ILETRI) in Malang, Indonesia. One hundred grams of soybean were soaked for 10 min in 70% alcohol and washed with sterile distilled water three times. Soybean seeds were sown and stored in a dark room for one day in sterile distilled water.
2.2. Soybean elicitation and extraction
Soybean is then cultivated and infected with 1 × 107 S. cerevisiae with 16 h light exposure to a bulb lamp for three days [19]. Soybean was then extracted with 80% ethanol, heated to 50 °C for one hour while stirred slightly. Soybean was then kept at room temperature, centrifuged at 14,000 rpm for 10 min, and then filtered using sterile 0.45 μm pore-size Minisart® filter (Sartorius Biotech GmbH, Goettingen, Germany). The resulting filtrate is concentrated with a rotary evaporator and freeze-dried. The crude extract obtained is then kept at −20 °C before use.
2.3. Experimental animals
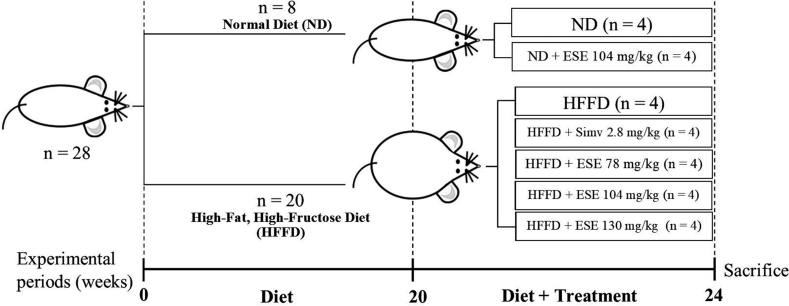
Balb/C mice at six weeks old were bred in the Laboratory of Structure, Physiology, and Animal Development, Brawijaya University. Females offspring mice at three weeks old were then selected for further study. Mice were placed individually after post−weaning in a plastic cage at a free pathogen chamber with controlled temperature (23-25 °C), humidity (50-60%), and 12 h dark/light cycle. Mice were adapted for one week before the experiment begins with food and drink ad libitum. In the pretreatment phase, mice were randomly grouped into two major groups, the normal diet (ND) and high−fat, high−fructose diet (HFFD). Mice in ND groups were fed with normal chow diet consist of 67.27% carbohydrate, 12.73% protein, and 5.33% fat. Mice in HFFD groups were fed with a high−fat diet based on beef tallows consisting of 53.46% carbohydrate, 8.57% protein, 21.06% fat, and 10% fructose in the drinking water. HFFD groups have consumed the diet for 20 weeks to induced obesity.
2.4. Soybean extract administration
At the beginning of 20th week, mice in the ND group and obese mice in the HFFD group were randomly sub-grouped into seven groups (n = 4), as follows:
Group I: normal diet without additional treatment (ND).
Group II: ND treated with ESE 104 mg/kg BW (ND + ESE−104).
Group III: High−fat, high−fructose diet without additional treatment (HFFD).
Group IV: HFFD treated with simvastatin 2.8 mg/kg BW (HFFD + Simv).
Group V: HFFD treated with ESE 78 mg/kg BW (HFFD + ESE−78).
Group VI: HFFD treated with ESE 104 mg/kg BW (HFFD + ESE−104).
Group VII: HFFD treated with ESE 130 mg/kg BW (HFFD + ESE−130).
Either simvastatin or ESE was administered each day intragastrically for an additional four weeks. The detailed experiment is shown in Fig. 1.
Fig. 1.
Experimental design. ESE: elicited soybean extract; Simv = simvastatin. The animal experiment was performed over 24 weeks, where the ESE or simv was treated at the last four weeks. At the end of the 24th week, the animal was sacrificed, and the ESE effects were investigated.
2.5. Immunofluorescence staining and flowcytometry
Mice were kept on overnight fasting with free access to water before being sacrificed by cervical dislocation. The spleen samples were taken, washed twice with sterile PBS, and smashed into a single-cell suspension. A single-cell suspension of the spleen has been prepared as per routine laboratory procedures [24]. Subsequent splenic lymphocytes were centrifuged at 2500 rpm for 5 min at 10 °C. The supernatant was removed, and the pellet was stained with FITC anti−mouse CD4 (GK1.5, Biolegend, San Diego, CA), PE anti−mouse CD8a (53–6.7, Biolegend, San Diego, CA), PE/Cy5 anti−mouse CD62L (MEL-14) as a naïve T cells marker, and FITC anti−mouse CD11c (N418, Biolegend, San Diego, CA) as a DCs cell-surface marker for 30 min at 4°C. According to the manufacturing instruction, splenic lymphocytes stained with CD11c antibody are then fixed and permeabilized with a cytofix/cytoperm buffer (BD−Biosciences, Pharmingen) and washed with a perm/wash buffer. Cells were then stained for intracellular staining with PE anti−mouse TLR3 (11F8, Biolegend, San Diego, CA), PE/Cy7 anti−mouse TLR4 (SA15−21, Biolegend, San Diego, CA), PE anti−mouse IL17A (TC11-18H10.1, Biolegend, San Diego, CA), and PerCP/Cy5.5 anti−mouse TNF−α (MP6−XT22, Biolegend, San Diego, CA). Splenic lymphocytes stained with PE/Cy5 NFκB (bs-17502R-Cy5, Bioss Inc., Massachusetts, USA) for intracellular staining were not stained for extracellular cell−surface markers. Cells stained with antibodies to an intracellular specific marker were then incubated at 4 °C for 30 min. The stained cells with a total of 10,000 cell events were obtained for each sample using FACS Calibur™ (BD−Biosciences, San Jose, CA). The cells were then analyzed using FlowJo v10 for Windows (FlowJo LLC, Ashland, OR). The percentage of TLR3, TLR4, TNF-α, and IL-17 expression by DCs were calculated according to the formula:
UR = upper right.
LR = lower right.
The percentage of CD62L positive expressed by CD4 and CD8 T cells was the first gate for lymphocytes, then determined the CD4 or CD8 positive cells using a histogram. The gating processes are described in Supplementary Figs. S1–S4.
2.6. Molecular docking between selected ESE active compound with TLR3 and TLR4 complexes
Human TLR3 (1ZIW) and TLR4/MD2 complex (3FXI) were collected from the Protein Data Bank (PDB). Ligand docking to the TLR3 complex was set to x = 72.0554, y = −17.5710, z = 9.3968 with dimension (Angstrom) 18.0569, 15.7276, and 16.7383, respectively. Ligand docking to TLR4/MD2 complex was set to x = 12.9798, y = −10.3865, and z = −1.1131 with dimension (Angstrom) 26.7464, 26.2213, and 26.2905, respectively [25]. Chemical structure of simvastatin (54454), glyceollin I (162807), glyceollin II (181883), glyceollin III (11954193), genistein (5280961), and daidzein (5281708) was collected from PubChem ID. CUPCT4A (53242268) was used as drug control for TLR3, while TAK−242 (11703255) was used as drug control for TLR4. Autodock vina in Pyrx 0.8 (The Scripps Research Institute, California) was used as an assessment to evaluate the binding affinity between TLR3 and TLR4 ligands. Open Babel in Pyrx 0.8 has been used for minimizing the ligand then visualized in PyMol (Schrӧdinger Inc., LLC). Biovia Discovery Studio v20 (Dassault System, Biovia corp.) has been used for analyzed protein and ligand interaction.
2.7. Statistical analysis
The result is reported as the mean ± standard deviation (SD). Data were presented as percentage of CD11c+TLR3+, CD11c+TLR4+, NFκB+, CD11c+IL−17+, CD11c+TNF−α+, CD4+CD62L+, and CD8+CD62L+ were analyzed by GraphPad Prism 8 (GraphPad Software Inc., La Jolla, CA). In order to compare significant differences among groups, the statistical analysis was performed by one-way analysis of variance (ANOVA) with Tukey’s HSD post hoc test. P < 0.05 was signified a statistically significant result.
3. Result
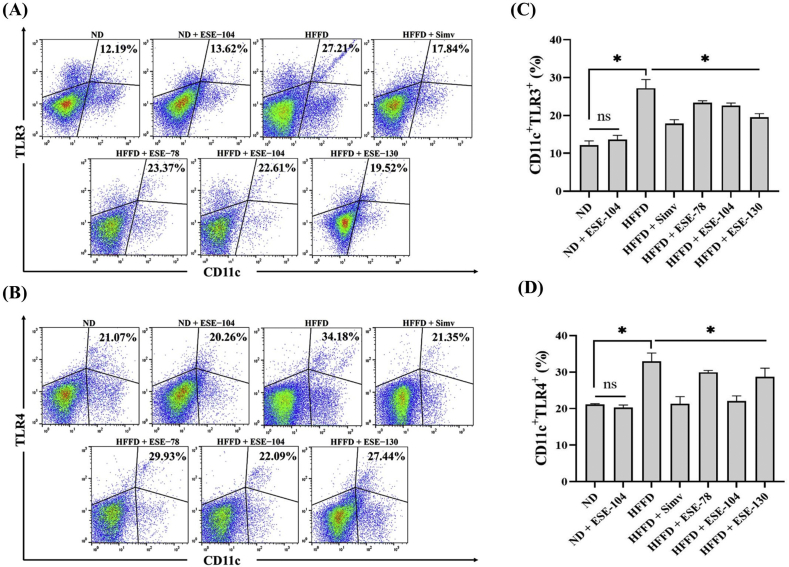
3.1. Elicited soybean extract inhibit dendritic cells maturation through suppress TLR3/TLR4 activation in HFFD mice
HFFD over 24 weeks alter the CD11c+TLR3+ (Fig. 2A) and CD11c+TLR4+ (Fig. 2B) subsets in HFFD mice compared to ND mice. The expression of CD11c+TLR3+ and CD11c+TLR4+ were increased in HFFD mice, while the treatment of ESE decreased significantly (p < 0.05) the expression of CD11c+TLR3+ (Fig. 2C) and CD11c+TLR4+ (Fig. 2D) in HFFD mice. Interestingly, the ESE dose 104 mg/kg BW which given in ND mice did not alter CD11c+TLR3+ and CD11c+TLR4+ expression (p < 0.05) compared to ND mice only (Fig. 2C−D).
Fig. 2.
DCs expression on ND or HFFD mice treated/untreated with ESE. (A) Dot plot analysis of CD11c+TLR3+ subsets by flow cytometry. (B) Dot plot analysis of CD11c+TLR4+ subsets by flow cytometry. The value upright indicates the percentages of CD11c+TLR3+/TLR4+ subsets. (C) ESE declines CD11c+TLR3+ expression on splenocytes. (D) ESE declines CD11c+TLR4+ expression on splenocytes. The values in the chart are mean ± SD. Statistical analysis is based on one-way ANOVA followed by post hoc test using Tukey’s HSD test. ∗p < 0.05 displayed a significant difference in ND and treatment groups compared to the HFFD group. ns, not significant (p > 0.05) indicate not significantly differ between the ND group and the ND + ESE−104 group.
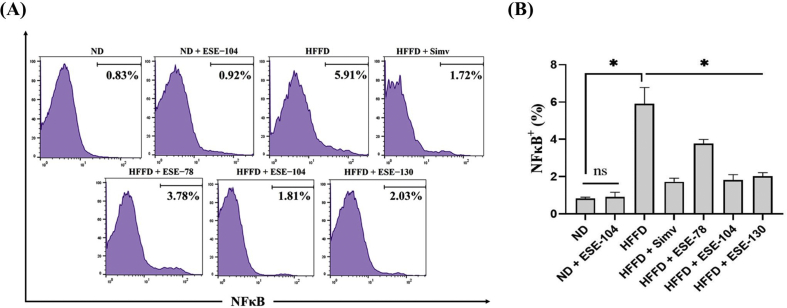
3.2. Elicited soybean extract declines NFκB activation in HFFD mice
NFκB is the main transcription factor for proinflammatory cytokines. NFκB+ expression was increased in mice after HFFD was given over 24 weeks (Fig. 3A). Our result showed that ESE given in HFFD mice declined the expression of NFκB+ (Fig. 3A). The ESE dose 104 mg/kg BW, which was given in ND mice, did not alter NFκB+ expression (p < 0.05) when compared to ND groups (Fig. 3B). interestingly, HFFD mice treated with either ESE or simvastatin did not significantly differ from declining NFκB+ expression.
Fig. 3.
NFκB expression on ND or HFFD mice treated/untreated with ESE. (A) Histogram analysis of NFκB+ expression by flow cytometry. (B) ESE suppressed NFκB+ expression on splenocytes. The values in the chart are mean ± SD. Statistical analysis is based on one-way ANOVA followed by post hoc test using Tukey’s HSD test. ∗p < 0.05 displayed a significant difference in treatment groups when compared to the HFFD group. ns, not significant (p > 0.05) indicate not significantly differ between the ND group and the ND + ESE−104 group.
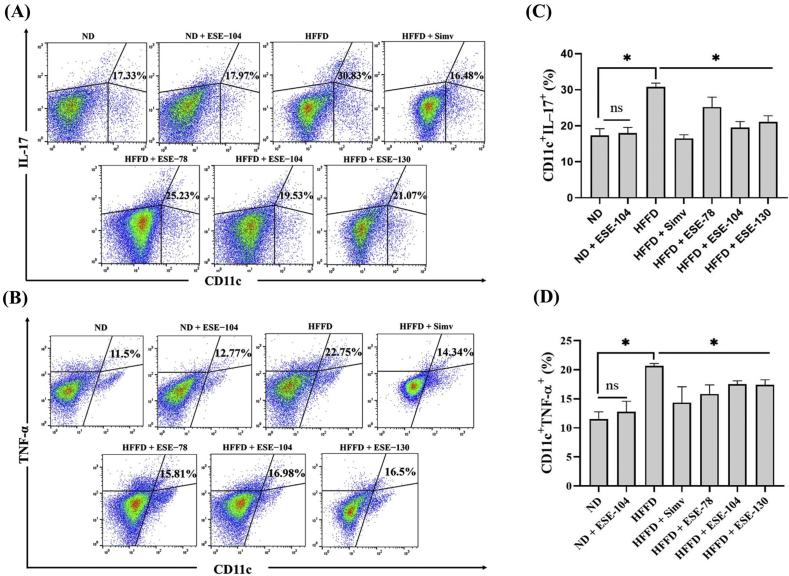
3.3. Elicited soybean extract inhibit IL−17 and TNF−α proinflammatory cytokines in dendritic cells in HFFD mice
A proinflammatory cytokine is the main product in an inflammatory signaling pathway. Our result showed HFFD caused the increased of CD11c+IL−17+ (Fig. 4A) and CD11c+TNF−α+ (Fig. 4B) expression (p < 0.05) compared to ND, while treatment with ESE cause significantly decreases the expression of CD11c+IL−17+ (Fig. 4C) and CD11c+TNF−α+ (Fig. 4D) in HFFD mice. The ESE dose 104 mg/kg BW, which was given in ND mice, did not alter CD11c+IL−17+ and CD11c+TNF−α+ expression (p < 0.05) compared to ND mice only (Fig. 4C−D).
Fig. 4.
DCs expression on ND or HFFD mice treated/untreated with ESE. (A) Dot plot analysis of CD11c+IL−17+ subsets by flow cytometry. (B) Dot plot analysis of CD11c+TNF−α+ subsets by flow cytometry. The value upright indicates the percentages of CD11c+IL−17+/TNF−α+. (C) ESE declines CD11c+IL−17+ expression on splenocytes. (D) ESE declines CD11c+TNF−α+ expression on splenocytes. The values in the chart are mean ± SD. Statistical analysis is based on one-way ANOVA followed by post hoc test using Tukey’s HSD test. ∗p < 0.05 displayed a significant difference in treatment groups when compared to the HFFD group. ns, not significant (p > 0.05) indicate not significantly differ between the ND group and the ND + ESE−104 group.
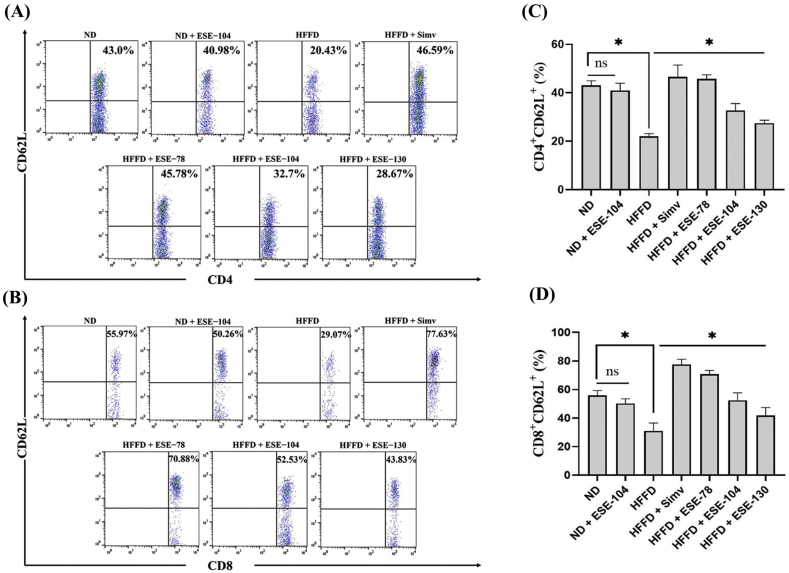
3.4. Elicited soybean extract restores naïve T cells in HFFD mice
Naïve T cells, in general, express CD62L (L-selectin) in the cell-surface and will lost CD62L expression when differentiating into memory T cells. HFFD over 24 weeks declined the percentage of naïve T cells, CD4+CD62L+ (Fig. 5A) and CD8+CD62L+ (Fig. 5B) around 2-fold compared to ND mice. Our result demonstrated that HFFD significantly declined (p < 0.05) the expression of CD4+CD62L+ (Fig. 5C) and CD8+CD62L+ (Fig. 5D) compared to ND mice. ESE treatment restored significantly (p < 0.05) the expression of CD4+CD62L+ and CD8+CD62L+. There is no difference between CD4+CD62L+ and CD8+CD62L+ expression in ND mice and ND supplemented with ESE 104 mg/kg BW (Fig. 5C and D).
Fig. 5.
Naïve T cells expression on ND or HFFD mice treated/untreated with ESE. (A) Dot plot analysis of CD4+CD62L+ subsets by flow cytometry. (B) Dot plot analysis of CD8+CD62L+ subsets by flow cytometry. The value upright indicates the percentages of CD4+/CD8+CD62L+. (C) ESE restores CD4+CD62L+ expression on splenocytes. (D) ESE restores CD8+CD62L+ expression on splenocytes. The values in the chart are mean ± SD. Statistical analysis is based on one-way ANOVA followed by post hoc test using Tukey’s HSD test. ∗p < 0.05 displayed a significant difference in treatment groups when compared to the HFFD groups. ns, not significant (p > 0.05) indicate not significantly differ between the ND group and the ND + ESE−104 group.
3.5. The direct binding and energy affinity of ESE compound with TLR3 and TLR4 complex
In the current study, our docking result demonstrated that glyceollin II has the best binding affinity for binding with TLR3 and TLR4/MD−2 complexes, respectively (Table 1). Interestingly, glyceollin II served the highest ability to bind with TLR3 and TLR4/MD−2 complexes compared to drug control (Table 1). The docking analysis supports our in vivo result, which illustrated that glyceollin-rich in ESE positively affected TLR3 and TLR4/MD−2 complex.
Table 1.
The calculated binding affinity between TLR3/TLR4 complex and ESE selected compounds.
| Receptor | Ligand | Binding affinity (kcal/mol) | Hydrogen Bond Interaction | Van der Waals Interaction |
|---|---|---|---|---|
| TLR3 (1ZIW) | Glyceollin II | −7.3 | Cys 651, Trp 660 | Glu 652, Ile 661, Thr 664, Val 625 |
| Glyceollin I | −6.7 | Thr 650 | Asp 648, Cys 651, Glu 652, Phe 634, Ser 653, Thr 664 | |
| Glyceollin III | −6.7 | Cys 651, Trp 660 | Asp 648, Glu 652, Ile 661, Thr 664, Val 625 | |
| Daidzein | −6.1 | – | Leu 640, Met 642, Phe 647, Ser 653, Thr 650, Thr 664, Val 625, Val 658 | |
| CUCPT4A | −6.1 | Ser 653 | Cys 651, Glu 652, Ile 661, Leu 640, Phe 634, Thr 650, Trp 660 | |
| Genistein | −6.0 | – | Cys 651, Leu 640, Met 642, Phe 647, Ser 653, Thr 664, Val 625, Val 658, | |
| Simvastatin | −5.5 | Ser 653 | Asp 648, Cys 651, Glu 652, Leu 640, Met 642, Phe 634, Thr 650, Thr 664, Val 625, Val 658 | |
| TLR4 (3FXI) | Glyceollin II | −7.9 | Asn 383, Gly 363 | Gly 364, Gly 384, His 431, Lys 435 |
| Glyceollin I | −7.8 | Asn 383, Gly 363, Gly 364 | Arg 382, Gly 384, Lys 435, Phe 342, Phe 408, | |
| Glyceollin III | −7.7 | Gly 363, Gly 364 | Asn 361, Asn 365, Gly 384 | |
| Simvastatin | −7.1 | Asn 383, Gly 363 | Asn 361, Asn 365, Gln 436, Gly 364, Lys 362, Lys 388, Ser 360, Thr 413, Val 411 | |
| Genistein | −7.0 | Gly 363, Gly 364, Lys 435, Thr 413 | Asn 365, Asn 383, Asn 409, Gly 384, His 431, Phe 408, Val 411 | |
| Daidzein | −6.9 | Lys 435 | Asn 365, Asn 409, Gly 384, Gly 410, Lys 388, Phe 387, Phe 408, Ser 386, | |
| TAK-242 | −6.5 | Lys 435, Thr 413 | Asn 365, Gln 436, Gly 363, Gly 410, Lys 362, Lys 388, Phe 387, Ser 386 |
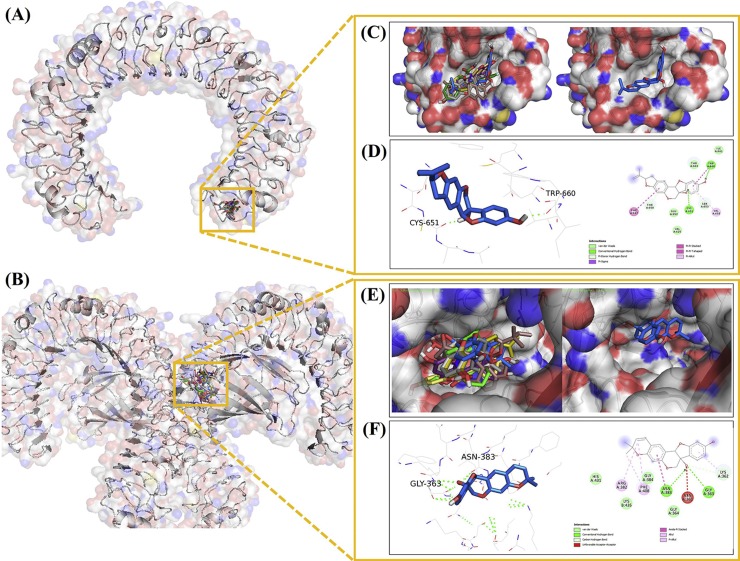
The molecular docking analysis revealed that all ligand has bound in the same site in the TLR3 complex (Fig. 6A) and TLR4/MD−2 complex (Fig. 6B). interestingly, the selective ESE compounds and simvastatin have the same binding site with selective TLR3 inhibitor, CUCPT4A (Fig. 6C). Further, we pointed to glyceollin II as the best model in the TLR3 complex with the lowest binding energy and formed a hydrogen bond with Cys 651 and Trp 660 (Fig. 6D). We assumed Cys651 as a main binding site in all of the TLR3 complexes, except in the daidzein−TLR3 complex. Interestingly, Cys 651 formed hydrogen bond interaction in glyceollin II and glyceollin III, while in other ligands formed van der Waals interaction in TLR3 complexes. In the TLR4/MD−2 complex, the selective ESE compounds and simvastatin also have the same binding site as the selective TLR4 inhibitor, TAK−242 (Fig. 6E). Further, we pointed to glyceollin II as the best model in the TLR4/MD−2 complex with the lowest binding energy and formed a hydrogen bond with Asn 383 and Gly 363 (Fig. 6F). We assumed that Gly 363 was the critical binding site that appeared in all complexes, except in the daidzein−TLR4/MD−2 complex. Interestingly, Gly 363 formed van der Waals interaction only with TAK−242−TLR4/MD−2 complex.
Fig. 6.
Three-dimensional structure of ESE active compound, simvastatin, and drug inhibitor as control with (A) TLR3 complex, (B) TLR4/MD−2 complex, (C) Molecular docking result with the TLR3 complex portrays that all ligands bind in the same site with control, (D) Visualization of amino acid interaction between the TLR3 complex with glyceollin II, (E) Molecular docking result with TLR4/MD−2 complex portrays all ligands bind in the same site with control, (F) Visualization of amino acid interaction between TLR4/MD−2 complex with glyceollin II. Note: daidzein = yellow−stick orange, genistein = violet−purple stick, glyceollin I = gray 80 stick, glyceollin II = marine blue stick, glyceollin III = olive stick, simvastatin = dark salmon stick, CUCPT4A/TAK−242 = green stick.
4. Discussion
TLRs dimerization is a critical step to initiate the downstream of the inflammation signaling pathway. High glucose and FFA from the high-fat diet could amplify the inflammatory signals via TLRs. Engagement of TLRs with DAMPs will trigger intracellular signaling cascades through activation of NFκB, as the main transcription factor to generate proinflammatory cytokines [26,27]. Based on that, dysregulation of TLRs activity could develop the risk of chronic inflammatory and immune disease. Recent studies indicate that phytochemicals based on natural products possessed an anti−inflammatory effect [28,29], make it possible to target TLRs and their downstream component [30].
Our present study suggested that multi−compound contain in elicited soybean suppress DCs activation through inhibiting the interaction between ligand and TLR3/TLR4 (Fig. 2A–D). Genistein interferes with the interaction between TLR4 with lipopolysaccharide (LPS) by preventing the binding of LPS to TLR4 [31]. Daidzein, another isoflavone compound in soybean, can suppress TLR4 expression through the TLR4−Myd88−NFκB signaling pathway [32]. Atho’illah et al. demonstrated that ESE decline TLR3 and TLR4 in B cells of HFFD mice [24]. Another phytoalexin contains in grape, resveratrol, inhibits TBK1, and RIP1 in TRIF complex [33]. TRIF is the main adaptor for NFκB and IRF3 via the TRIF−dependent signaling pathway [23]. We assumed that glyceollin, phytoalexin containing in ESE has a similar mechanism with resveratrol, although it still needs to be further investigated.
To gain mechanism insight for the possibility of glyceollin could modulate TLR3 and TLR4, we used molecular docking as an approach (Fig. 6A–F). As a result, glyceollin II has the lowest binding affinity than other compounds to TLR3 and TLR4−MD2 complex (Table 1). Interestingly, glyceollin II has the lowest binding energy to the TLR3 complex compared to CUCPT4A. In TLR3, glyceollin II binds to Cys 651, which is involved in disulfide bond formation and essential for the TLR3 function [34]. In the TLR4−MD2 complex, glyceollin II binds with Asn 383 and Gly 363. Gly 363 was reported as a conserved residue in human TLR4 at LRR13 and suggested the potential role in TLR4−TLR4 contacts [35]. However, there is still a need for further investigation about the potential role of Gly 363 to interfere with the TLR4/MD2 complex. Interestingly, glyceollin II and genistein formed van der Waals interaction with His 431 (Table 1). His 431 was reported to have a crucial role in triggering MD2 and TLR4 dimerization for cellular activation [36]. Based on the docking result, we hypothesize that glyceollin in ESE may disrupt dimerization between DAMPs and TLR3/TLR4, which makes the possibility interfere in the upstream of TLR3/TLR4 signaling pathway and delay inflammation.
Our present study suggested that ESE declines the expression of NFκB in HFFD mice (Fig. 3A and B). The result was consistent with the previous result, CD11c+TLR3+, and CD11c+TLR4+ lower expression after treated with ESE. NFκB is the main inflammatory signaling pathway linked to metabolism and immunity at cellular levels, which increased in obesity [37]. NFκB is a main proinflammatory signaling pathway for IL−17, TNF−α synthesis, and NFκB triggered by TLR3/TLR4 activation [38]. The activation of NFκB further stimulated an excessive proinflammatory cytokine released from cells. Genistein was reported to acts through inhibition of NFκB translocation, while glyceollin inhibits IKKα/β phosphorylation, an upstream kinase for IκB in NFκB signaling pathway after LPS stimulation [21]. Genistein and glyceollin act through inhibition of NFκB phosphorylation. Genistein inhibits NFκB−IκB phosphorylation complex and NFκB translocation into the nucleus for encoding proinflammatory cytokine genes [31], while glyceollin inhibits IκB kinase, degradation of IκB, prevent NFκB/DNA formation complex [21], and suppressed phosphorylation of p65 NFκB [39]. Another compound in ESE, daidzein, disrupts the NFκB signaling pathway leads to delay DCs maturation, caused DCs not to release the excess of a proinflammatory mediator to trigger a specific immune response [40].
In line with our result, IL−17 and TNF−α expression in DCs reduces after treatment both with ESE and simvastatin (Fig. 4A–D). TNF−α is the primary proinflammatory cytokine produced by DCs during inflammation via activation of MAPK and NFκB signaling pathway and provoked other proinflammatory cytokines such as IL−1β and IL−6 to release in circulation [41]. The previous studies demonstrated that CD11c+ subsets increase around two-fold or higher in HFD mice than ND mice [[42], [43], [44]]. Interestingly, the study conducted by Nguyen et al. demonstrated that diet-induced obesity elicits CD11c+ in adipose tissue around 5-fold in 12-weeks HFD mice, or 23.8% in HFD mice compared to 4.4% in ND mice [45]. The upregulation of CD11c+ is also followed by increasing TNF−α mRNA expression around 2-fold or higher [46,47]. In line with our finding, HFFD over 24-weeks upregulated the CD11c+TNF−α+ expression around 2-fold compared to ND, while ESE supplementation could reverse TNF−α expression by CD11c positive cells (Fig. 4A).
IL−17 is mainly produced by Th17, but other immune cells such as DCs are also known to produce IL−17 [48]. The increased of IL−17 also followed by IL−6 elevation, which act as a downstream of IL−17 signaling pathway, resulting in the differentiation of the Th17 cell during obesity [49,50]. Furthermore, the upregulation of IL−17 expression may display an increase in Th17 cells [51]. The combination of TNF−α with IL−17 enhanced other proinflammatory cytokine syntheses [52]. The excess production of proinflammatory cytokine has been associated with the development of the chronic inflammatory disease. Interestingly, treatment with ESE displays the same better effect with simvastatin. The decrease of TNF−α and IL−17 expression as proinflammatory cytokine is expected to reduced inflammation caused by HFFD.
Our next finding revealed that HFFD down-regulates CD4+CD62L+ and CD8+CD62L+ as a marker for naïve T cells (Fig. 5A–D). This result supports our finding that HFFD triggers the activation of DCs, resulting in the higher expression of proinflammatory cytokines. DCs commonly expressed CD11c and MHC class II, and the expression was increased once DCs is activated to activate naïve T cells [9]. Interestingly, memory CD4+ T cells mainly migrated to inflammatory sites, driving obesity−associated insulin resistance together with CD8+ in adipose tissue [53,54]. In line with our result, HFD resulting in the lower expression of CD4+CD62L+, thus interrupt the lymphocyte and erythrocyte homeostasis [53,55]. Studies demonstrated by Nur’aini et al. demonstrated that ESE administration suppressed the proinflammatory mediators expressed by CD4 and CD8 T cells [56]. These findings are consistent with our present result, in which the inhibition of CD11c+ activity is followed by the restoration of naïve T cells after ESE administration (Fig. 5A–D). The inhibition mechanism by ESE caused diminished inflammation downstream signaling cascade in DCs. The bioactive compound of ESE acts synergistically to modulate DCs activation via TLR3/TLR4 signaling pathway, thus balancing the innate and adaptive immune system in HFFD.
5. Conclusion
ESE ameliorates inflammation through modulating TLR3/TLR4 in DCs, reduce NFκB in splenocytes, IL−17 and TNF−α expression in DCs, and restores naïve T cells. ESE supplementation did not alter the DCs profile and naïve T cells in ND mice. ESE could be a promising agent in the future for alleviating obesity and its complication due to inflammation caused by HFFD.
Ethical approval
Animal care and experimental procedures have been followed in compliance with the Guide to the Care and Use of Laboratory Animals (National Institutes of Health, United States) and the regulations of the Institutional Ethics Committee, Brawijaya University (Approval number: 647-KEP-UB).
Source(s) of funding
None declared.
Conflict of interest
None.
Acknowledgements
We thanks to Prof. Nashi Widodo, Ph.D. Med.Sc. for granted S. cerevisiae. The Authors also thanks to Galuh Wening Permatasari, M. Eng., Yuyun Ika Christina, M.Si., and Siti Nur Arifah, M.Si for helpful discussion.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jaim.2021.01.003.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Jin C., Henao-mejia J., Flavell R.A. Perspective innate immune receptors: key regulators of metabolic disease progression. Cell Metabol. 2013;17:873–882. doi: 10.1016/j.cmet.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Lobstein T., Jackson-Leach R. Planning for the worst: estimates of obesity and comorbidities in school-age children in 2025: planning for the worst. Pediatr Obes. 2016;11:321–325. doi: 10.1111/ijpo.12185. [DOI] [PubMed] [Google Scholar]
- 3.Lee Y.C. The effect of high-fat diet-induced pathophysiological changes in the gut on obesity: what should be the ideal treatment? Clin Transl Gastroenterol. 2013;4:e39. doi: 10.1038/ctg.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McArdle M.A., Finucane O.M., Connaughton R.M., McMorrow A.M., Roche H.M. Mechanisms of obesity-induced inflammation and insulin resistance: insights into the emerging role of nutritional strategies. Front Endocrinol. 2013;4:1–23. doi: 10.3389/fendo.2013.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin J., Peng Y., Wu J., Wang Y., Yao L. Toll-like receptor 2/4 links to free fatty acid-induced inflammation and -cell dysfunction. J Leukoc Biol. 2014;95:47–52. doi: 10.1189/jlb.0313143. [DOI] [PubMed] [Google Scholar]
- 6.Jialal I., Kaur H., Devaraj S. Toll-like receptor status in obesity and metabolic syndrome: a translational perspective. J Clin Endocrinol Metab. 2014;99:39–48. doi: 10.1210/jc.2013-3092. [DOI] [PubMed] [Google Scholar]
- 7.Spruss A., Bergheim I. Dietary fructose and intestinal barrier: potential risk factor in the pathogenesis of nonalcoholic fatty liver disease. J Nutr Biochem. 2009;20:657–662. doi: 10.1016/j.jnutbio.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Woolard M.D., Kevil C.G. Paying the toll for glucose regulation: a central role for TLR3. Diabetes. 2015;64:3345–3346. doi: 10.2337/db15-0679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pearce E.J., Everts B. Dendritic cell metabolism. Nat Rev Immunol. 2015;15:18–29. doi: 10.1038/nri3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertola A., Ciucci T., Rousseau D., Bourlier V., Duffaut C., Bonnafous S. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes. 2012;61:2238–2247. doi: 10.2337/db11-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee B.C., Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2014;1842:446–462. doi: 10.1016/j.bbadis.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rifa’i M., Widodo N. Significance of propolis administration for homeostasis of CD4+CD25+ immunoregulatory T cells controlling hyperglycemia. SpringerPlus. 2014;3:526. doi: 10.1186/2193-1801-3-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang B., Sun J., Ma Y., Wu G., Shi Y., Le G. Increased oxidative stress and the apoptosis of regulatory T cells in obese mice but not resistant mice in response to a high-fat diet. Cell Immunol. 2014;288:39–46. doi: 10.1016/j.cellimm.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Atho’illah M.F., Widyarti S., Rifa’i M. Elicited soybean (Glycine max L.) extract improves regulatory T cell activity in high fat-fructose diet mice. AIP Conf Proc 1844. 2017 doi: 10.1063/1.4983415. 020004. [DOI] [Google Scholar]
- 15.Cheong S.H., Furuhashi K., Ito K., Nagaoka M., Yonezawa T., Miura Y. Daidzein promotes glucose uptake through glucose transporter 4 translocation to plasma membrane in L6 myocytes and improves glucose homeostasis in Type 2 diabetic model mice. J Nutr Biochem. 2014;25:136–143. doi: 10.1016/j.jnutbio.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Sankar P., Zachariah B., Vickneshwaran V., Jacob S.E., Sridhar M.G. Amelioration of oxidative stress and insulin resistance by soy isoflavones (from Glycine max) in ovariectomized Wistar rats fed with high fat diet: the molecular mechanisms. Exp Gerontol. 2015;63:67–75. doi: 10.1016/j.exger.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Park S., Kim D.S., Kim J.H., Kim J.S., Kim H.J. Glyceollin-containing fermented soybeans improve glucose homeostasis in diabetic mice. Nutrition. 2012;28:204–211. doi: 10.1016/j.nut.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 18.Boué S.M., Carter C.H., Ehrlich K.C., Cleveland T.E. Induction of the soybean phytoalexins coumestrol and glyceollin by aspergillus. J Agric Food Chem. 2000;48:2167–2172. doi: 10.1021/jf9912809. [DOI] [PubMed] [Google Scholar]
- 19.Atho’illah M.F., Safitri Y.D., Nur’aini F.D., Savitri R.U., Rahayu S., Widyarti S. Evaluation of glyceollin accumulation and antioxidant properties on soybean (Glycine max L.) through combination of different biotic elicitor and light. Sci Study Res Chem Chem Eng Biotechnol Food Ind. 2019;20:199–208. [Google Scholar]
- 20.Kim H.J., Suh H.-J., Kim J.H., Park S., Joo Y.C., Kim J.S. Antioxidant activity of glyceollins derived from soybean elicited with Aspergillus sojae. J Agric Food Chem. 2010;58:11633–11638. doi: 10.1021/jf102829z. [DOI] [PubMed] [Google Scholar]
- 21.Yoon E.K., Kim H.K., Cui S., Kim Y.H., Lee S.H. Soybean glyceollins mitigate inducible nitric oxide synthase and cyclooxygenase-2 expression levels via suppression of the NF-κB signaling pathway in RAW 264.7 cells. Int J Mol Med. 2012;29:711–717. doi: 10.3892/ijmm.2012.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang H., Xie Z., Boue S.M., Bhatnagar D., Yokoyama W., Yu L. Cholesterol-lowering activity of soy-derived glyceollins in the golden Syrian hamster model. J Agric Food Chem. 2013;61:5772–5782. doi: 10.1021/jf400557p. [DOI] [PubMed] [Google Scholar]
- 23.Lee J.Y., Hwang D.H. The modulation of inflammatory gene expression by lipids: mediation through Toll-like receptors. Mol Cell. 2006;21:174–185. https://doi.org/957 [pii] [PubMed] [Google Scholar]
- 24.Atho’illah M.F., Safitri Y.D., Nur’aini F.D., Widyarti S., Hideo T., Rifa’i M. Soybean extract suppresses B cell activation through TLR3/TLR4 in high fat-high fructose diet mice. Turk J Immunol. 2018;6:95–103. doi: 10.25002/tji.2018.866. [DOI] [Google Scholar]
- 25.Resman N., Oblak A., Gioannini T.L., Jerrold P., Jerala R. 2014. Tetraacylated lipid A and paclitaxel-selective activation of TLR4/MD-2 conferred through hydrophobic interactions; pp. 1–9. [DOI] [PubMed] [Google Scholar]
- 26.Yu L., Wang L., Chen S. Endogenous toll-like receptor ligands and their biological significance. J Cell Mol Med. 2010;14:2592–2603. doi: 10.1111/j.1582-4934.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dasu M.R., Jialal I. Free fatty acids in the presence of high glucose amplify monocyte inflammation via Toll-like receptors. Am J Physiol Endocrinol Metab. 2011;300:E145–E154. doi: 10.1152/ajpendo.00490.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arifah S.N., Atho’illah M.F., Lukiati B., Lestari S.R. Herbal medicine from single clove garlic oil extract ameliorates hepatic steatosis and oxidative status in high fat diet mice. Malays J Med Sci. 2020;27:46–56. doi: 10.21315/mjms2020.27.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shengule S.A., Mishra S., Joshi K., Apte K., Patil D., Kale P. Anti-hyperglycemic and anti-hyperlipidaemic effect of Arjunarishta in high-fat fed animals. J Ayurveda Integr Med. 2018;9:45–52. doi: 10.1016/j.jaim.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pavlova S.I., Albegova D.Z., Vorob’eva Y.S., Laptev O.S., Kozlov I.G. Flavonoids as potential immunosuppressants affecting intracellular signaling pathways (a review) Pharm Chem J. 2016;49:645–652. doi: 10.1007/s11094-016-1345-x. [DOI] [Google Scholar]
- 31.Jeong J.W., Lee H.H., Han M.H., Kim G.Y., Kim W.J., Choi Y.H. Anti-inflammatory effects of genistein via suppression of the toll-like receptor 4-mediated signaling pathway in lipopolysaccharide-stimulated BV2 microglia. Chem Biol Interact. 2014;212:30–39. doi: 10.1016/j.cbi.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 32.Feng G., Sun B., Li T.Z. Daidzein attenuates lipopolysaccharide-induced acute lung injury via toll-like receptor 4/NF-kappaB pathway. Int Immunopharm. 2015;26:392–400. doi: 10.1016/j.intimp.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Youn H.S., Lee J.Y., Fitzgerald K.A., Young H.A., Akira S., Hwang D.H. Specific inhibition of MyD88-independent signaling pathways of TLR3 and TLR4 by resveratrol: molecular targets are TBK1 and RIP1 in TRIF complex. J Immunol Baltim Md 1950. 2005;175:3339–3346. doi: 10.4049/jimmunol.175.5.3339. [DOI] [PubMed] [Google Scholar]
- 34.Ranjith-Kumar C.T., Miller W., Xiong J., Russell W.K., Lamb R., Santos J. Biochemical and functional analyses of the human Toll-like receptor 3 ectodomain. J Biol Chem. 2007;282:7668–7678. doi: 10.1074/jbc.M610946200. [DOI] [PubMed] [Google Scholar]
- 35.Walsh C., Gangloff M., Monie T., Smyth T., Wei B., McKinley T.J. Elucidation of the MD-2/TLR4 interface required for signaling by lipid IVa. J Immunol. 2008;181:1245–1254. doi: 10.4049/jimmunol.181.2.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lonez C., Irvine K.L., Pizzuto M., Schmidt B.I., Gay N.J., Ruysschaert J.M. Critical residues involved in Toll-like receptor 4 activation by cationic lipid nanocarriers are not located at the lipopolysaccharide-binding interface. Cell Mol Life Sci CMLS. 2015;72:3971–3982. doi: 10.1007/s00018-015-1915-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mraz M., Haluzik M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J Endocrinol. 2014;222:R113–R127. doi: 10.1530/JOE-14-0283. [DOI] [PubMed] [Google Scholar]
- 38.Kawai T., Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009;21:317–337. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim H.J., Sung M.K., Kim J.S. Anti-inflammatory effects of glyceollins derived from soybean by elicitation with Aspergillus sojae. Inflamm Res. 2011;60:909–917. doi: 10.1007/s00011-011-0351-4. [DOI] [PubMed] [Google Scholar]
- 40.Yum M.K., Jung M.Y., Cho D., Kim T.S. Suppression of dendritic cells’ maturation and functions by daidzein, a phytoestrogen. Toxicol Appl Pharmacol. 2011;257:174–181. doi: 10.1016/j.taap.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Makki K., Froguel P., Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. 2013;2013:1–12. doi: 10.1155/2013/139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stefanovic-Racic M., Yang X., Turner M.S., Mantell B.S., Stolz D.B., Sumpter T.L. Dendritic cells promote macrophage infiltration and comprise a substantial proportion of obesity-associated increases in CD11c+ cells in adipose tissue and liver. Diabetes. 2012;61:2330–2339. doi: 10.2337/db11-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singer K., DelProposto J., Lee Morris D., Zamarron B., Mergian T., Maley N. Diet-induced obesity promotes myelopoiesis in hematopoietic stem cells. Mol Metabol. 2014;3:664–675. doi: 10.1016/j.molmet.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cho K.W., Morris D.L., Lumeng C.N. vol. 537. Elsevier; 2014. Flow cytometry analyses of adipose tissue macrophages; pp. 297–314. (Methods enzymol). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen M.T.A., Favelyukis S., Nguyen A.-K., Reichart D., Scott P.A., Jenn A. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 46.Patsouris D., Li P.-P., Thapar D., Chapman J., Olefsky J.M., Neels J.G. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metabol. 2008;8:301–309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng K., Song Z., Chen Y., Li S., Zhang Y., Zhang H. Resveratrol protects against renal damage via attenuation of inflammation and oxidative stress in high-fat-diet-induced obese mice. Inflammation. 2019;42:937–945. doi: 10.1007/s10753-018-0948-7. [DOI] [PubMed] [Google Scholar]
- 48.Onishi R.M., Gaffen S.L. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology. 2010;129:311–321. doi: 10.1111/j.1365-2567.2009.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu R., Tao A., Zhang S., Zhang M. Neutralization of interleukin-17 attenuates high fat diet-induced non-alcoholic fatty liver disease in mice. Acta Biochim Biophys Sin. 2013;45:726–733. doi: 10.1093/abbs/gmt065. [DOI] [PubMed] [Google Scholar]
- 50.Endo Y., Asou H.K., Matsugae N., Hirahara K., Shinoda K., Tumes D.J. Obesity drives Th17 cell differentiation by inducing the lipid metabolic kinase. ACC1 Cell Rep. 2015;12:1042–1055. doi: 10.1016/j.celrep.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 51.Aarts S., Reiche M., den Toom M., Gijbels M., Beckers L., Gerdes N. Depletion of CD40 on CD11c+ cells worsens the metabolic syndrome and ameliorates hepatic inflammation during NASH. Sci Rep. 2019;9:14702. doi: 10.1038/s41598-019-50976-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Astry B., Venkatesha S.H., Moudgil K.D. Involvement of the IL-23/IL-17 axis and the Th17/Treg balance in the pathogenesis and control of autoimmune arthritis. Cytokine. 2015;74:54–61. doi: 10.1016/j.cyto.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lestari S.R., Atho’illah M.F., Christina Y.I., Rifa’i M. Single garlic oil modulates T cells activation and proinflammatory cytokine in mice with high fat diet. J Ayurveda Integr Med. 2020;11:414–420. doi: 10.1016/j.jaim.2020.06.009. S0975947618311173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mauro C., Smith J., Cucchi D., Coe D., Fu H., Bonacina F. Obesity-induced metabolic stress leads to biased effector memory CD4 + T cell differentiation via PI3K p110δ-Akt-mediated signals. Cell Metabol. 2017;25:593–609. doi: 10.1016/j.cmet.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Safitri Y.D., Atho’illah M.F., Nur’aini F.D., Widyarti S., Rifa’i M. The effects of elicited soybean (Glycine max) extract on hematopoietic cells of high fat-fructose diet Balb/C mice model. Jordan J Biol Sci. 2018;11:241–246. [Google Scholar]
- 56.Nur’aini F.D., Rahayu S., Rifa’i M. Anti-inflammatory activity of elicited soybean (Glycine max) extract on Balb/C mice (Mus musculus) with high-fat and -fructose diet. Cent-Eur J Immunol. 2019;44:7–14. doi: 10.5114/ceji.2019.84010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.