Abstract
Background:
Toxoplasmosis is a zoonotic disease caused by the obligate intracellular parasite, Toxoplasma gondii. This global infectious disease has been associated with behavioral changes in rodents and can result in humans’ neuropsychiatric symptoms. Since the neurotransmitters alteration can cause a behavioral change, in this study, tyrosine level, as a precursor of dopamine, was evaluated in acute murine toxoplasmosis during 2015 and 2016 in Shiraz, Iran.
Methods:
At the first, 105 tachyzoites of T. gondii were subcutaneously inoculated to 50 BALB/c mice as experimental groups and 10 mice inoculated by PBS considered as the control group. After that, daily, one group of mice was bled, and sera were collected. Then, their serum tyrosine level was evaluated by HPLC method.
Results:
After data analysis, the maximum mean serum tyrosine level was seen at 2th day of post parasite inoculation (0.0194 mg/ ml), with a significant difference compared to the control group (0.0117 mg/ ml, P=0.025). Moreover, the least quantity of serum tyrosine (0.076 mg/ml) was seen on the 5th day, after parasite inoculation, however, no significant difference was seen.
Conclusion:
Serum tyrosine level increased in 2 d after inoculation of Toxoplasma, but the level regularly decreased in successive days. Tyrosine level increased by phenylalanine hydroxylase 2 days after inoculation, then tyrosine decreased by tyrosine hydroxylase in the next days. Toxoplasma tyrosine hydroxylase enzymes, at primary days of toxoplasmosis, effect on tyrosine production, and after that, the most effect on tyrosine consumption.
Keywords: Toxoplasma gondii, Tyrosine, Mice, High-performance liquid chromatography
Introduction
Toxoplasma gondii is an obligate intracellular parasite of phylum Apicomplexa. The variety of warm-blooded animals as intermediate hosts and felines as definitive hosts are known (1). The disease has two forms. The congenital condition leads to abortion, chorioretinitis, hydrocephaly, microcephaly, jaundice, and cutaneous rashes (1, 2) The acquisitive form resulted in fever, lymphadenopathy, headache, and fatigue in immunocompetent individuals and encephalitis in HIV patients (1, 3). Due to the parasite’s cyst’s placement in the brain, this organ has great potential in psychiatric complications from the disease (4). Relevance between toxoplasmosis and such disorders, including hyperactivity, obsessive-compulsive, and schizophrenia, has been shown (5, 6). Toxoplasmosis in people with mental disorders is more common than in healthy people. Therefore, T. gondii can be introduced as an etiological agent in mental disorders (7). Moreover, psychological complications, including personality disorder, bipolar disorder, obsessive-compulsive disorder, migraine, and autism, have related to chronic toxoplasmosis (4, 8). This parasite has a trigger agent on the process of Parkinson’s and Alzheimer’s disease (9, 10).
The role of T. gondii in the synthesis of dopamine and other neurotransmitters has been verified (11). Homologous tyrosine hydroxylase and dopamine receptors have been found in the parasite’s protein-coding genes (11, 12). Tyrosine hydroxylase is an enzyme that catalyzes the conversion of tyrosine to L-dopamine. Finally, the dopamine decarboxylase enzyme changed L-dopamine to dopamine, the parasite’s final product (13).
In chronic toxoplasmosis, the produced dopamine can have a dual role in different neurological disorders. In the association between Toxoplasma infection and schizophrenia, the extra produced dopamine due to the parasite’s metabolism can negatively affect individuals. Moreover, most antipsychotic drugs decrease the dopamine level or inhibit dopamine receptors (4, 14). On the other hand, in patients with Parkinson’s disease, tyrosine hydroxylase induced by Toxoplasma increases dopamine production, causes Parkinson’s symptoms to be milder (15).
Many studies conducted to determine the role of T. gondii in brain disorders found the variation of brain neurotransmitters, such as dopamine. Tyrosine is the essential amino-acid for the synthesis of dopamine. So, the present study was performed to assess the tyrosine levels in consecutive days post-infection.
Materials and Methods
The present study evaluated the serum tyrosine levels in acute murine toxoplasmosis during 2015 and 2016 in Shiraz, Iran.
Ethics approval
This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals (16). The research project was approved by the Ethics Committee of Animal Experiments of the Shiraz University of Medical Sciences (permit number 11598), Shiraz, Iran.
Parasite’s preparation
RH strain of T. gondii was injected intraperitoneally into BALB/c mice. After 72 h, mice were killed with considering ethical standards, and then parasites were collected from peritoneum by flushing of Phosphate Buffered Saline (PBS). Parasites were mechanically isolated from the host cells via passing aspirated fluid from high gauge needles. Then for 10 min in 200 g were centrifuged to separate cellular debris. The upper part was separated and was centrifuged for 10 min with 800 g. The sediment was washed three times with PBS at a pH of 7.2, and finally, the pure tachyzoites form was isolated (17).
Animals
Sixty BALB/c mice, aged 6 wk, and weight 18–24 gr were prepared from Pasteur Institute in Tehran. All animals were kept in standard conditions, including temperature 22±2 °C and humidity (40%–60%), 12-h darkness-lighting cycle, optimum ventilation, and sufficient food and water access. Sixty mice were divided into six groups (10 mice per group). At first, 105 tachyzoites (in 200 μl PBS) were injected subcutaneously (17, 18) into experimental groups. Considered the last group as the control group, injected PBS (without parasite). Sampling was carried out from 5 differences groups due to 5 consecutive days. About 24 h after injection, daily, one group of 10 mice anesthetized with chloroform, and then bled was performed from their heart. Two hundred μl of serum were separated and kept in −70° centigrade until use. Sampling from the control group was carried out on the third day after injection.
HPLC
Chromatography conditions
HPLC assay was performed to determine serum Tyrosine level (Waters, USA). The samples were chromatographed on a reversed-phase column (Spherisorb C1; Waters) with a C18 column in isocratic mode. The mobile phase consisted of 5% acetonitrile in water with a flow rate of 1ml/min. The absorbance of the diluted sera at 225 nm was monitored in a UV detector (model LC 95; Perkin-Elmer, Überlin, Germany).
Standard curve preparation
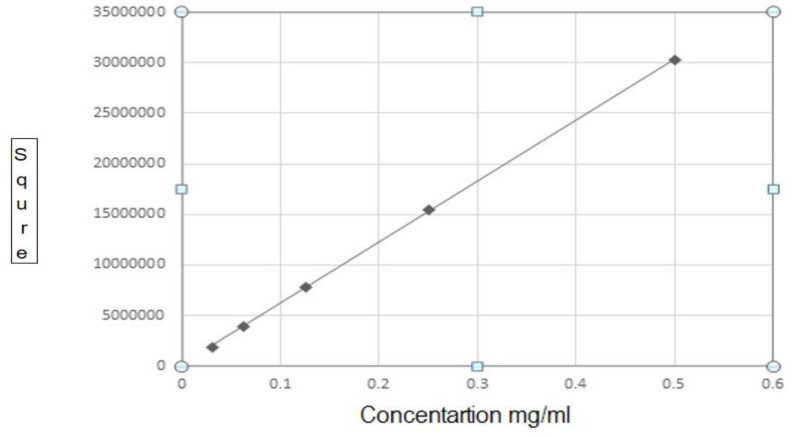
To assessment of tyrosine in the samples, High-performance liquid chromatography (HPLC) device was set up for standard solutions with two-fold dilutions of L-Tyrosine (1, 0.5, 0.25, 0.125, 0.0625, 0.3125 mg/ml). For this purpose, 0.01 g of tyrosine (L-Tyrosine, Sigma-Aldrich-T3754) was poured in 1ml perchloric acid 5% and mixed enough to reach the uniform solution. The sample volume was increased to 10 ml by perchloric acid and passed through a syringe tip filter (0.22 μm). Then mentioned above concentrations were prepared with the solvent. At this point, each of the dilutions was injected three times into the HPLC device, and standard curves were drawn using software (SQS 98; Perkin-Elmer) by different concentrations (Fig. 1).
Fig. 1:
Tyrosine standard curve obtained of HPLC
Samples preparation
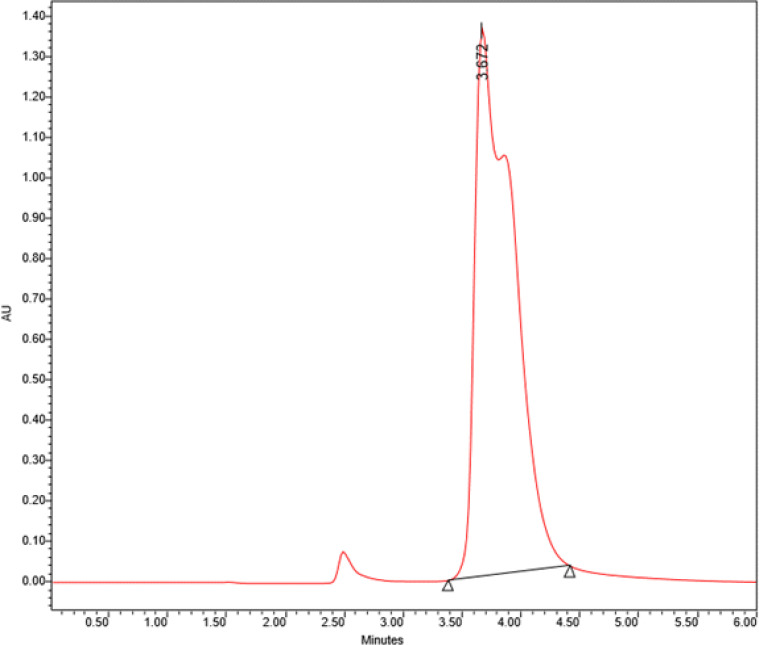
The frozen sera melt at room temperature, and then 50 μl of each serum was mixed with an equal amount of 5% perchloric acid and filtered. This stage was done for all 60 samples. Then 50 μl of the serum dilutions were injected into the devices via a specific needle. After that, in 6 min and wavelength 225 nm, the curves of each sample were obtained. The peaks of tyrosine were identified by comparison with the retention times of standard compounds (Sigma) (Fig. 2).
Fig. 2:
Retention time of the standard sample of tyrosine using HPLC.
(Concentration: 0.5 mg/ ml), (Flow: 1ml/minute), (Absorbance = 224A°)
Statistical analysis
Statistical data were evaluated by ANOVA and Post Hoc test in software SPSS version 20 (Chicago, IL, USA). P-value ≤ 0.05 was considered as statistical differences.
Results
Calculating the concentration of tyrosine in serum samples based on the standard curve
After preparation of standard tyrosine solutions in different concentrations, they were injected into the device. The related curve was drawn based on the area under the curve, and the Standard equation was calculated.
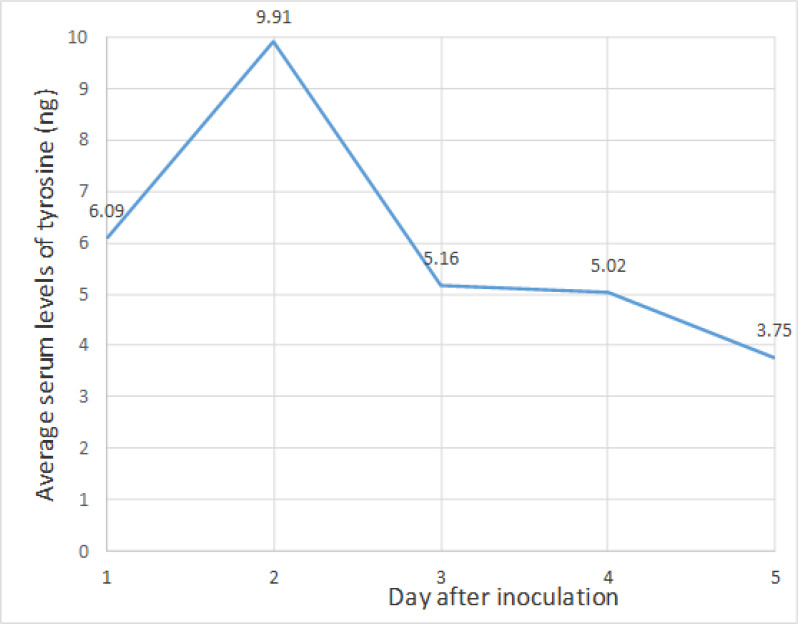
The tyrosine level in each unknown sample was calculated; based on the area under the curve and obtained slope from the standard curve equation. The average serum levels of tyrosine on the second day after parasite injection show the highest level compared with other days, and based on statistical; it was significant (Fig. 3). Comparing the mean serum levels of tyrosine on the second day after the inoculation of parasites with the control group level shows a statistically significant difference (Table 1). (P=0.025).
Fig. 3:
The average of serum tyrosine levels in mice according to the days after parasite inoculation
Table 1:
levels of serum tyrosine in consecutive days in mice inoculated toxoplasma tachzoites
| Days after tachyzoites injection | Number of mice | Minimum level | Maximum level | Mean level | Standard deviation |
|---|---|---|---|---|---|
| Group: 1 | 10 | 0.0057 | 0.0169 | 0.0121 | 0.00331 |
| Group: 2 | 8 | 0.008 | 0.0388 | 0.0194 | 0.01119 |
| Group: 3 | 9 | 0.0025 | 0.0189 | 0.0103 | 0.00511 |
| Group: 4 | 10 | 0.0041 | 0.0144 | 0.0101 | 0.00350 |
| Group: 5 | 10 | 0.0057 | 0.0123 | 0.076 | 0.00199 |
| Control Group: | 10 | 0.0063 | 0.0164 | 0.0117 | 0.00297 |
Discussion
Changes in the level of brain neurotransmitters like dopamine can be the reason to justify behavioral disorders. To understand the behavioral disorders in toxoplasmosis patients, stressed out many studies on the potential role of Toxoplasma infection because of its neurotransmitters level conversion (13, 19). Chronic toxoplasmosis can lead to impaired learning, memory functions, and Alzheimer-like symptoms among infected BALB/c mice (20). The studies have shown an association between Toxoplasma and elevated dopamine levels (4). According to dopamine level analysis and its role in behavioral changes induction, several studies have been performed over laboratory animals and human (21–25). However, tyrosine levels as a main precursor of dopamine during toxoplasmosis need to be more investigated. In this study, we have evaluated the serum tyrosine level, as the precursor of dopamine, in acute murine toxoplasmosis. In our study, the highest level of serum tyrosine was shown on the second day after injection. Moreover, tyrosine assessment levels in the consecutive days after injection of parasites show a decrease in tyrosine levels. This finding indicated a functional enzyme in the T. gondii parasite, which can decrease the tyrosine level in their host. There were two encoding tyrosine hydroxylase genes (AaaH1 and AaaH2) in the T. gondii genome. This enzyme has an important role in producing dopamine’s precursor molecule L-dihydroxyphenylalanine (L-DOPA) from L-tyrosine (26). The TgAaaH1 enzyme tyrosine hydroxylase gene is active in tachyzoite form, whereas the TgAaaH2 gene is activated during bradyzoite conversion (13). In the current study, tyrosine levels on the second day after parasite inoculation was increased. Then, tyrosine hydroxylase affects tyrosine and convert it to dopamine. However, the role of tyrosine hydroxylase and behavioral change during toxoplasmosis remains controversial. Behavioral changes in infected-mice with the knock-out AaaH2 gene strains compared to the wild strain of Toxoplasma parasites have been similar in both groups’ chronic toxoplasmosis phase. (27). Some studies point out another mechanism for altering dopamine levels in infected mice. For example, cytokines can convert neuromodulator’s levels, such as dopamine. The brain cells, such as microglia and astrocytes, generate the cytokines that cause parasite encystment in the brain (28, 29). Although the Follicular helper T cells (TFH) are highly required for efficacy in the immunological synapse use dopamine (30). Considering the above data and the role of dopamine in the inflammatory response, it is possible that the increase of tyrosine production on the second day of infection and then its decrease in the next days could be reactive by the immune system against the presence of parasites.
The elevated level of dopamine due to infection with the Toxoplasma parasite could affect some diseases, including schizophrenia, Alzheimer’s, and Parkinson’s (10, 19). The existence of a 2-tyrosine hydroxylase enzyme in the Toxoplasma parasite previously had been shown (13, 19). This enzyme could effectively convert phenylalanine to tyrosine in infected patients. It is also necessary to catalyze the dopamine from tyrosine and tyrosine from phenylalanine (13). Accordingly, elevated tyrosine levels on the second day after parasite injection may be due to the presence of phenylalanine hydroxylase. During a stressful period, increases in sympathoadrenal system response lead to tyrosine hydroxylase enzyme activity. This situation causes the synthesis of additional catecholamine by affecting tyrosine, coping with a stressful situation (31). In the current study, tyrosine hydroxylase decreases the total tyrosine levels in the days post-infection. It is considered one of the stressful situations. Dopamine has been shown as an essential elevated neurotransmitter in toxoplasmosis patients, especially in the chronic stage. Acute toxoplasmosis did not increase the dopamine levels, but chronic disease could increase dopamine levels up to 14% more than the control group in animal models (32). Moreover, it has been shown to increase dopamine and serotonin in mice’s brains in the third week post-infection, but these changes returned to normal in the sixth week (33). In our study, due to using lethal strain RH, the possibility of mice survival over a week was not possible, so the tyrosine level survey was done in acute toxoplasmosis.
Conclusion
Toxoplasma tyrosine hydroxylase enzymes, at primary days of toxoplasmosis, affect tyrosine production. After that, the most effect on tyrosine consumption leads to dopamine synthesis.
Acknowledgements
The authors would like to thank the Office of Vice-Chancellor for Research of Shiraz University of Medical Sciences, Shiraz, Iran, to support this project. This article was extracted from an MD thesis by Mina Mousaei Sisakht.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Dubey J, Jones J. Toxoplasma gondii infection in humans and animals in the United States. Int J Parasitol. 2008;38(11):1257–78. [DOI] [PubMed] [Google Scholar]
- 2.Dunn D, Wallon M, Peyron F, et al. Mother-to-child transmission of toxoplasmosis: risk estimates for clinical counselling. Lancet. 1999;353(9167):1829–33. [DOI] [PubMed] [Google Scholar]
- 3.Weiss LM, Dubey JP. Toxoplasmosis: A history of clinical observations. Int J Parasitol. 2009;39(8):895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flegr J. How and why Toxoplasma makes us crazy. Trends Parasitol. 2013;29(4):156–63. [DOI] [PubMed] [Google Scholar]
- 5.Fekadu A, Shibre T, Cleare AJ. Toxoplasmosis as a cause for behavior disorders-overview of evidence and mechanisms. Folia Parasitol (Praha). 2010;57(2):105–13. [DOI] [PubMed] [Google Scholar]
- 6.Alipour A, Shojaee S, Mohebali M, et al. Toxoplasma infection in schizophrenia patients: a comparative study with control group. Iran J Parasitol. 2011;6(2):31–37. [PMC free article] [PubMed] [Google Scholar]
- 7.Torrey EF, Bartko JJ, Lun Z-R, et al. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull. 2007;33(3):729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chegeni TN, Sarvi S, Amouei A, et al. Relationship between toxoplasmosis and obsessive compulsive disorder: A systematic review and meta-analysis. PLoS Negl Trop Dis. 2019;13(4):e0007306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torres L, Robinson S-A, Kim D-G, et al. Toxoplasma gondii alters NMDAR signaling and induces signs of Alzheimer’s disease in wild-type, C57BL/6 mice. J Neuroinflammation. 2018;15(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bayani M, Riahi SM, Bazrafshan N, et al. Toxoplasma gondii infection and risk of Parkinson and Alzheimer diseases: A systematic review and meta-analysis on observational studies. Acta Trop. 2019; 196: 165–171. [DOI] [PubMed] [Google Scholar]
- 11.Henriquez S, Brett R, Alexander J, et al. Neuropsychiatric disease and Toxoplasma gondii infection. Neuroimmunomodulation. 2009;16(2):122–33. [DOI] [PubMed] [Google Scholar]
- 12.Zhu S. Psychosis may be associated with toxoplasmosis. Med Hypotheses. 2009;73(5):799–801. [DOI] [PubMed] [Google Scholar]
- 13.Gaskell EA, Smith JE, Pinney JW, et al. A unique dual activity amino acid hydroxylase in Toxoplasma gondii. PLoS One. 2009;4(3):e4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikam SS, Awasthi AK. Evolution of schizophrenia drugs: a focus on dopaminergic systems. Curr Opin Investig Drugs. 2008;9(1):37–46. [PubMed] [Google Scholar]
- 15.Miman O, Kusbeci OY, Aktepe OC, et al. The probable relation between Toxoplasma gondii and Parkinson’s disease. Neurosci Lett. 2010;475(3):129–31. [DOI] [PubMed] [Google Scholar]
- 16.National Research Council .Guide for the Care and Use of Laboratory Animals. National Academies Press (US); 2010: 10.17226/12910. [Google Scholar]
- 17.Paulino J, Vitor R. Experimental congenital toxoplasmosis in Wistar and Holtzman rats. Parasite. 1999;6(1):63–6. [DOI] [PubMed] [Google Scholar]
- 18.Asgari Q, Keshavarz H, Shojaee S, et al. In Vitro and In Vivo Potential of RH Strain of Toxoplasma gondii (Type I) in Tissue Cyst Forming. Iran J Parasitol. 2013;8(3):367–75. [PMC free article] [PubMed] [Google Scholar]
- 19.Webster JP, McConkey GA. Toxoplasma gondii-altered host behaviour: clues as to mechanism of action. Folia Parasitol (Praha). 2010;57(2):95–104. [DOI] [PubMed] [Google Scholar]
- 20.Mahmoudvand H, Sheibani V, Shojaee S, et al. Toxoplasma gondii infection potentiates cognitive impairments of Alzheimer’s disease in the BALB/c mice. J Parasitol. 2016;102(6):629–35. [DOI] [PubMed] [Google Scholar]
- 21.Dalimi A, Abdoli A. Latent toxoplasmosis and human. Iran J Parasitol. 2012;7(1):1–17. [PMC free article] [PubMed] [Google Scholar]
- 22.Berenreiterová M, Flegr J, Kuběna AA, et al. The distribution of Toxoplasma gondii cysts in the brain of a mouse with latent toxoplasmosis: implications for the behavioral manipulation hypothesis. PLoS One. 2011;6(12):e28925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang ZT, Harmon S, O’Malley KL, et al. Reassessment of the role of aromatic amino acid hydroxylases and the effect of infection by Toxoplasma gondii on host dopamine. Infect Immun. 2015;83(3):1039–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Babaie J, Sayyah M, Fard-Esfahani P, et al. Contribution of dopamine neurotransmission in proconvulsant effect of Toxoplasma gondii infection in male mice. J Neurosci Res. J Neurosci Res. 2017;95(10):1894–905. [DOI] [PubMed] [Google Scholar]
- 25.Parlog A, Schlüter D, Dunay IR. Toxoplasma gondii-induced neuronal alterations. Parasite Immunol. 2015;37(3):159–70. [DOI] [PubMed] [Google Scholar]
- 26.Prandovszky E, Gaskell E, Martin H, et al. The neurotropic parasite Toxoplasma gondii increases dopamine metabolism. PLoS One. 2011;6(9):e23866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Afonso C, Paixão VB, Klaus A, et al. Toxoplasma-induced changes in host risk behaviour are independent of parasite-derived AaaH2 tyrosine hydroxylase. Sci Rep. 2017;7(1):13822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindová J, Novotná M, Havlíček J, et al. Gender differences in behavioural changes induced by latent toxoplasmosis. Int J Parasitol. 2006;36(14):1485–92. [DOI] [PubMed] [Google Scholar]
- 29.Carruthers VB, Suzuki Y. Effects of Toxoplasma gondii infection on the brain. Schizophr Bull. 2007;33(3):745–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papa I, Saliba D, Ponzoni M, et al. T FH-derived dopamine accelerates productive synapses in germinal centers. Nature. 2017;547(7663):318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armando I, Jezova M, Bregonzio C, et al. Angiotensin II AT1 and AT2 Receptor Types Regulate Basal and Stress-Induced Adrenomedullary Catecholamine Production through Transcriptional Regulation of Tyrosine Hydroxylase. Ann N Y Acad Sci. 2004;1018:302–9. [DOI] [PubMed] [Google Scholar]
- 32.Stibbs H. Changes in brain concentrations of catecholamines and indoleamines in Toxoplasma gondii infected mice. Ann Trop Med Parasitol. 1985;79(2):153–7. [DOI] [PubMed] [Google Scholar]
- 33.Gatkowska J, Wieczorek M, Dziadek B, et al. Sex-dependent neurotransmitter level changes in brains of Toxoplasma gondii infected mice. Exp Parasitol. 2013;133(1):1–7. [DOI] [PubMed] [Google Scholar]