Abstract
Background:
We aimed to compare parasite burden in BALB/c mice, using three methods including the direct fluorescent microscopic using recombinant Leishmania major expressing an enhanced green fluorescent protein, limiting dilution assay, and real-time PCR technique.
Methods:
The current study was carried out in 2018, to induce stable enhanced green fluorescent protein (EGFP) production. Initially, the linearized DNA expression cassette (pLEXSY-egfp-sat2) was integrated into the ssu locus of L. major. The expression of EGFP in recombinant parasite was analyzed using direct fluorescent microscopy. Afterward, BALB/c mice were infected with the L. major EGFP, and the infection was evaluated in the foot-pads and inguinal lymph-nodes using an in vivo imaging system. Subsequently, eight BALB/c mice were infected with L. major EGFP, and the results of evaluating parasite burden by a SYBR-Green based real-time PCR analysis and the limiting dilution assays were compared to the results obtained from the direct fluorescent microscopy.
Results:
The results of the direct fluorescent microscopy showed that EGFP gene stably was expressed in parasites. Moreover, the in vivo imaging analysis of foot-pad lesions revealed that the infection caused by L. major EGFP was progressing over time. Additionally, significant correlations were observed between the results of parasite burden assay using the direct fluorescent microscopy and either limiting dilution assay (r=0.976, P<0.0001) or quantitative real-time PCR assay (r=0.857, P<0.001).
Conclusion:
Ultimately, the utilization of the direct fluorescent microscopy by employing a stable EGFP-expressing L. major is a suitable substitution for the existing methods to quantify parasite burden.
Keywords: Green fluorescent protein, Leishmania major, Limiting dilution assay, Parasite burden
Introduction
The precise determination of parasite burden is quite indispensable for evaluation of the effect of any compound with leishmanicidal activity, immunoprophylactic procedure, and immunological manipulation in experimental leishmaniasis. So far, evaluating the parasite burden has been performed by using different techniques including measuring the diameter of the lesions (1) and direct parasite counting (2). Limiting Dilution Assay (LDA) is among the most used methods for the assessment of parasite burden in the experimental models of Leishmania infection (3). LDA is a culture-based method in which in vitro incubation of infected tissues in the culture medium is used to enumerate the motile promastigotes (4). To estimate the parasite burden, the raw data should be analyzed by using a software, such as ELIDA (5). Using LDA, the parasite could be detected at the site of infection as early as 3 d after the inoculation of L. major (4).
In recent years, investigators have developed a molecular method based on real-time PCR to diagnose and quantify Leishmania parasites in clinical and experimental settings (6–8). The real-time PCR assay is a rapid test for counting parasites in any tissues which, has high sensitivity, rapidity, reproducibility, and feasibility. There is a strong association between the results of real-time PCR and LDA. However, the absolute parasite burden may be overestimated, when using real-time PCR assay, due to the amplification of DNA from both viable and dead parasites (6).
To overcome the drawbacks of LDA and real-time PCR methods, direct fluorescent microscopy (DFM) to count the transgenic promastigotes expressing reporter genes is useful. Up to now, many reporter genes including green fluorescent protein (GFP) (for fluorimetric methods), chloramphenicol acetyltransferase, β-galactosidase and alkaline phosphatase (for colorimetric methods), and firefly luciferase (for bioluminescent based assay) have been introduced (9). Methods based on fluorescent reporter proteins profit by some advantages such as high sensitivity, no radioactivity, rapidity, and high efficiency (9).
The detection of GFP activity simply is done with minimum manipulation by utilizing a fluorometer (10), a fluorescence microscope, or flow cytometer (11); additionally, GFP expression assay has the potential to be monitored by the in vivo imaging technique (12).
Despite the growing interest in using the GFP-expressing Leishmania for assessment of the parasite burden, the correlation between parasite burden measurement using DFM and widely used methods i.e. LDA and real-time PCR, have not been assessed yet. The objective of the current study was to compare the correlation coefficient within these three methods for assessing the parasite burden in experimentally infected mice. We used a transgenic Leishmania major that constitutively expresses the enhanced green fluorescent protein (EGFP) (L. majorEGFP) in its cytoplasm.
Materials and Methods
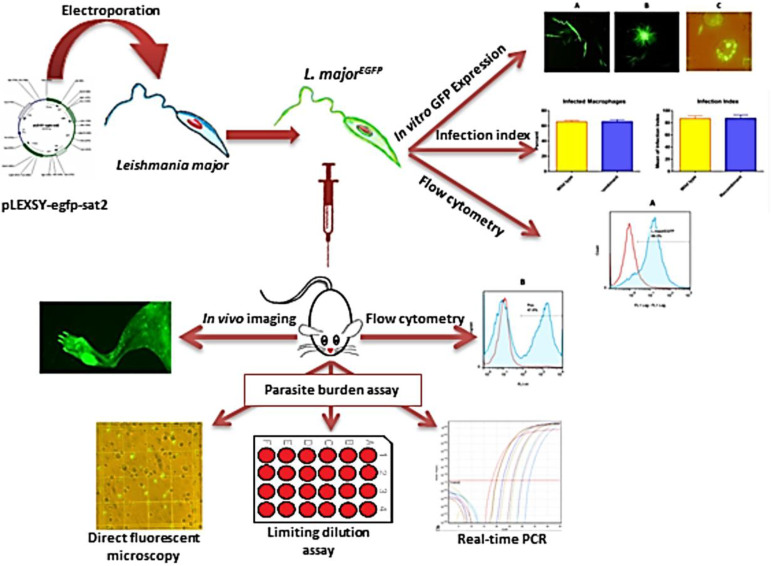
The present study was carried out at the Department of Immunology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran in 2018 (Fig. 1).
Fig. 1:
The figure demonstrates the design of the study and steps beginning with cloning the EGFP gene into the ssu locus, followed by evaluating the homologous recombination and EGFP expression, and then comparison DFM, LDA and real-time PCR to assess the parasite burden in experimentally infected mice
Parasite cultivation
Promastigotes of Leishmania major strain (MRHO/IR/75/ER) were cultivated in RPMI 1640 medium (Gibco, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Gibco, USA) and 100 μg/mL penicillin-streptomycin (Thermo Fisher Scientific, USA). To maintain the infectiousness, parasites (2 × 105) were continuously passaged in BALB/c mice (purchased from the Pasteur Institute of Iran).
Preparation of recombinant L. major expressing EGFP
For stable and constitutive expression of the target protein, a Leishmania expression system has been developed; in this system, the integration of the DNA expression cassette into the chromosomal 18S rRNA locus (ssu) causes high yield protein production (13). Initially, pLEXSY-egfp-sat2 vector (Jena Bioscience, Germany) was transformed and replicated in Escherichia coli Top10 strain. Then the plasmid DNA was extracted from the recombinant colonies (Qiagen Plasmid Midi Kit). Afterward, the pLEXSY-egfp-sat2 plasmid was digested with SwaI enzyme (Thermo Fisher Scientific, USA). Plasmid linearization was checked by gel electrophoresis. The longer fragment containing egfp and nourseothricin (NTC) resistance genes were purified from agarose gel (QIAquick gel extraction kit, Qiagen, Germany). Subsequently, the target DNA was ligated into the ssu locus of L. major chromosome, through homologous recombination using the electroporation method. For transfection, 4.5×107 logarithmic phase parasites were re-suspended in 300 μL cold electroporation buffer (EPB; consist of 21 mM HEPES, 137 mM NaCl, 5 mM KCl, 0.7 mM Na2HPO4, 6 mM glucose; pH= 7.5). After incubation on ice (10 min) and addition of 2 μg linearized plasmid, parasites were electroporated with two pulses (2500 V for 5 min and 10 sec interval) with an electroporator (Multiporator™ Electroporation System, Eppendorf, USA). Electroporated parasites were incubated for 10 min on ice; subsequently, were transferred into 1 mL RPMI consist of 10% FBS, and were cultured at 26 °C. The negative control sample was electroporated under the same conditions without the use of plasmid. NTC antibiotic (30 μg/mL) was added to cultures media, after 48 h; and its concentration gradually increased to 100 μg/mL. EGFP expression was directly evaluated in promastigotes, by fluorescence microscopy (E8400, Nikon, Japan).
Evaluation of the genomic integration of EGFP
To evaluate the genomic integration of EGFP and NTC resistance genes in ssu locus, the real-time PCR assay was used. Specific primers were designed for the inserted sequence and 18s rRNA locus by primer design software (Beacon Designer 7, USA). The sequences of the forward and reverse primers respectively were as follows: cttagccatgcatgcctcag and gctgtaacgccttgatgtgt. PCR reaction was carried out on a Rotor-gene Q real-time PCR (Qiagen, Germany) using the following process: initially, holding for 15 min at 94 °C followed by 40 cycles, which comprised of denaturation at 95 °C for 15 sec, and annealing at 55 °C for 30 sec followed by the extension at 72 °C for 30 seconds. The analysis of the melt curve was performed to distinguish difference between PCR products.
Peritoneal macrophage preparation and infection
Peritoneal macrophage isolation and infection with either wild type (WT) or recombinant L. major parasites were carried out as described previously (14). Macrophages were infected with metacyclic promastigotes of L. majorEGFP (macrophage: parasite ratio of 1:15), and were incubated overnight at 37 °C with 5% CO2. At the end of the incubation time, infected macrophages were directly monitored by using fluorescence microscopy regarding the EGFP expression of the transfected L. major amastigotes. Furthermore, mouse peritoneal macrophages were treated with WT parasites and stained with Giemsa. To calculate the percent of infected macrophages, each coverslip was divided into four areas and in each area, 100 macrophages were counted and the number of the infected macrophages was determined. The average number of amastigote per macrophage was identified by enumerating the total number of intracellular parasites in 400 macrophages. Additionally, the infection index was calculated by multiplying the percentage of infected macrophage in the average number of the amastigotes per macrophage (14).
In vivo imaging
Initially, 2×105 L. majorEGFP parasites in the total volume of 50 μL were injected subcutaneously to the left hind foot-pad of BALB/c mice. In order to capture the in vivo images at different time intervals, the mice were anesthetized with isoflurane, and the left foot-pads were imaged using a small animal imaging system (KODAK Image Station 4000 Digital Imaging System, USA).
Flow cytometry analysis
The EGFP-expression was analyzed in the transfected promastigotes by using flow cytometry. Parasites at logarithmic and stationary phases (106 cell/mL in PBS) were analyzed with a FACScalibur flow cytometer (BD: Becton Dickinson, Franklin Lakes, NJ) The EGFP expression in L. majorEGFP (10,000 events) was measured in comparison to WT parasites. Furthermore, to analyze L. majorEGFP infected macrophages by flow cytometry, the macrophage population was gated using forward (FCS) vs. side scatters (SCC). Extracellular parasites and cell debris were excluded from the analysis based on FCS- and SCC parameters. The fluorescence of the cells was quantified with an FL1 histogram. The flow cytometry results were analyzed using FlowJo software (TreeStar. Inc., USA, version 7.5.3).
Parasite burden measurements
To evaluate the parasite burden, eight BALB/c mice were infected with different numbers of metacyclic L. majorEGFP ranging from 2 × 104 to 5 × 105. Eight weeks after the challenge, the mice were sacrificed and the parasite burden was evaluated in the draining lymph nodes by three methods, namely DFM, Real-time PCR, and LDA. LDA was done according to the method described before (6). In brief, the cells of lymph nodes were suspended in Schneider medium supplemented with 12% heat-inactivated FBS and 1% Penstrep. After counting the cells, twelve serial dilutions were made; for each dilution, eight wells (100 μL) were prepared in 96-well microtiter plates. The cells were incubated at 26 °C for 7 d; then, the total number of positive wells (presence of motile promastigotes) and negative wells (absence of motile promastigotes) was identified by an inverted light microscope. The total number of amastigotes in the lymph nodes was estimated, by using the Leishmania LDA software (15).
We also used real-time PCR assay to quantify the parasite burden, according to the aforementioned procedure (6). Concisely, to prepare a standard curve, DNA of 5×106 WT L. major promastigotes were extracted and ten-fold serial dilutions of L. major DNA, corresponding to 5 × 106 to 5 × 10−1 parasites per reaction were prepared. The average cycle threshold (CT) of duplicates in each dilution was plotted against the number of parasites (16). The number of parasites per lymph node was calculated by interpolating the CT of samples in the standard curve.
DFM analysis was performed on the cells extracted from draining lymph nodes of the lesion site. Lymph node cells were isolated and counted. Secondly, 0.1 mL of cell suspension was transferred on a Neubauer slide. The green dots were viewed and counted in the area of one centimeter using a fluorescence microscope at 400x magnification with a dichromatic mirror cut-on 505 nm excitation filter 450–490 nm; and barrier filter 520 nm (Nikon, NY, USA). The photos were taken with a Nikon digital camera (DXM1200F). The data was adjusted according to the absolute counts of the number of cells from each lymph node. The counting was done in triplicate. All the parasite burden assays were done as duplicate.
Data Analyses
Statistical analyses were done with the statistical program SPSS (ver.17; Chicago, IL, USA). To compare the means of groups, Univariate two-way ANOVA was performed. Spearman’s Rho test was used to estimate the correlation between the three quantification methods. Statistical differences between samples were considered significant at P<0.05.
Results
Development of EGFP–expressing transgenic Leishmania major
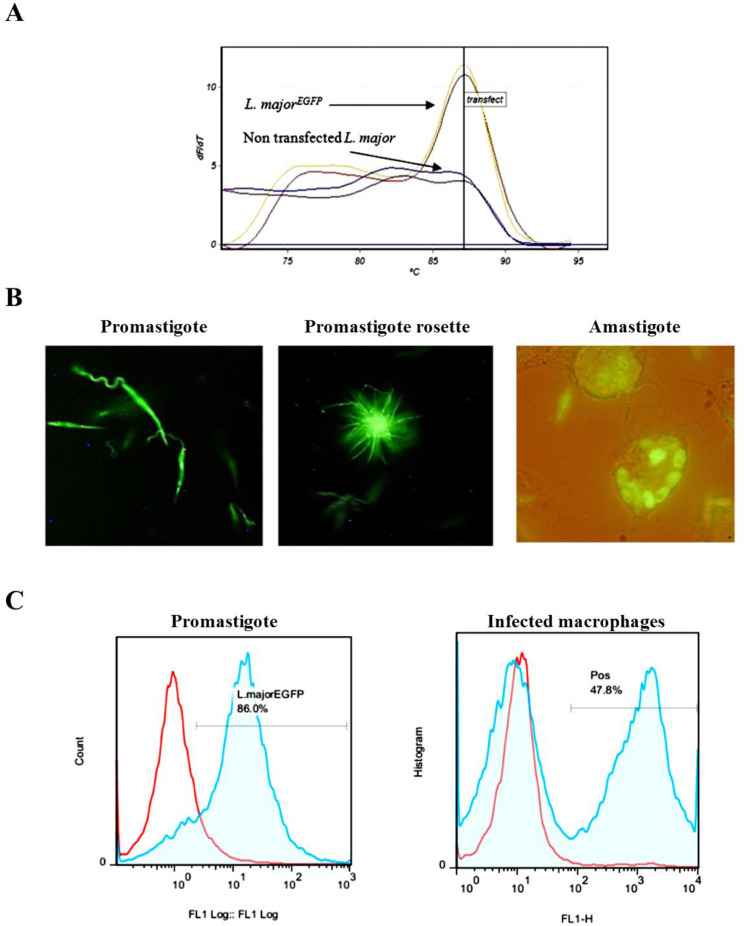
In order to confirm the correct integration, the genomic DNA was used for real-time PCR assay. If the DNA cassette is inserted into the 18S rRNA gene through homologous recombination, this fragment could be amplified (Fig. 2A). The constant expression of EGFP in the recombinant parasite was observed during the twelve months’ post-electroporation; moreover, even in the absence of the antibiotic pressure, L. majorEGFP could stably express and preserve the reporter green fluorescent gene over the aforementioned time.
Fig. 2:
Evaluation of the EGFP–expression in the transgenic Leishmania major. (A) The expected melting point for EGFP (87.5 °C) was observed in real-time PCR product of EGFP positive L. major. (B) The visual evaluation of the recombinant L. major expressing green fluorescent protein. Live promastigotes and intracellular amastigotes in the peritoneal macrophages were imaged using florescent microscope. (C) Flow cytometry analyses of L. majorEGFP and WT parasites. (Left panel) L. majorEGFP (blue line) and WT (Redline) promastigotes in logarithmic phase. (Right panel) The comparison between the fluorescence of splenocytes from the infected mice with L. majorEGFP (blue line) and non-infected (red line)
EGFP expression in Leishmania promastigotes and amastigotes were observed by DFM (Fig. 2B). Furthermore, flow cytometry results showed that in both stationary and logarithmic phases, high levels of green fluorescence emission were detected (Fig. 2C). Besides, the presence of EGFP positive cells in splenocytes of the infected mice was detected using flow cytometry (Fig. 2C).
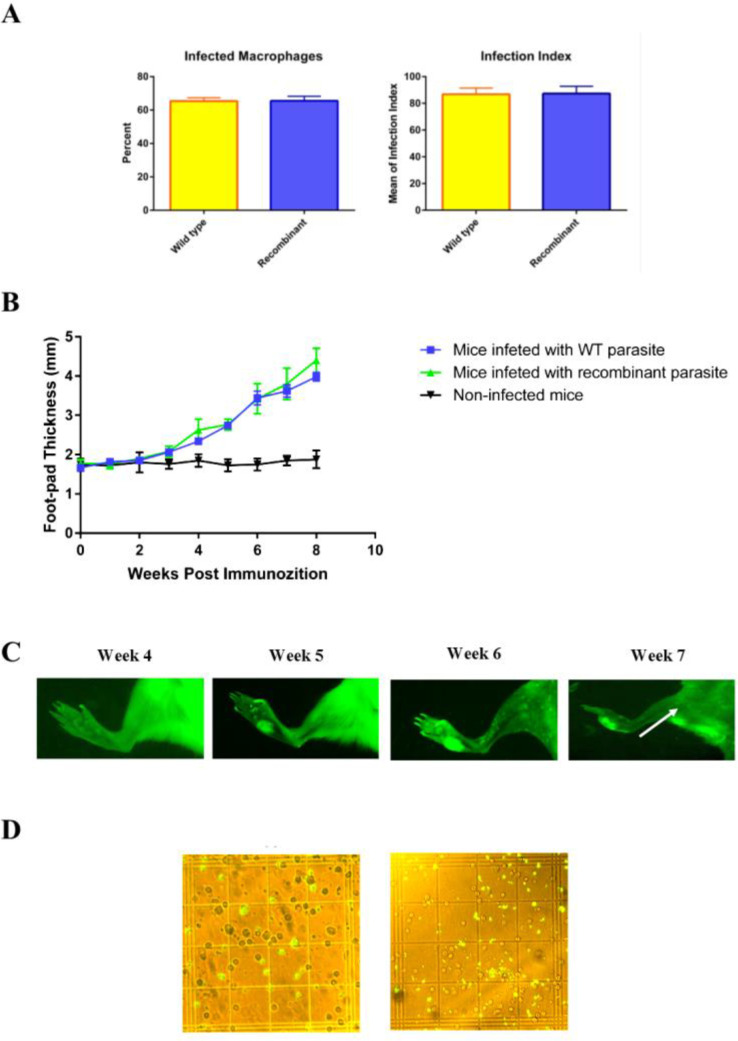
The WT and recombinant promastigotes were used to infect the mouse peritoneal macrophages. As expected, between WT and L. majorEGFP, there was no significant difference in the mean percentage of the infected macrophages (respectively: 65.33% ± 2.02 vs. 65.5% ± 2.75, P>0.05) (Fig. 3A), as well as, in the average number of the parasites per macrophage (respectively: 8.16 ± 0.38 vs. 8.16 ± 0.49, P>0.05). In addition, the infection index was similar in the macrophages infected by either WT or L. majorEGFP promastigotes (respectively: 86.8 ± 2.67 vs. 87.27 ± 5.45, P>0.05; Fig. 3A).
Fig. 3:
Evaluation the infectivity of L. majorEGFP. (A) The mean percent of in vitro infected macrophages and the average number of parasites per each macrophage was counted, and infection index was calculated by multiplying these two variables. (B) Assessment of foot-pad swelling in infected mice. Foot-pad swelling of infected mice with L. majorEGFP or wild type promastigote was not statistically different. (C) In vivo imaging of left footpad of BALB/c mouse at different time intervals after inoculation with 2 × 105 L. majorEGFP promastigotes. The popliteal lymph node infected with L. majorEGFP at week 7 is visible and is shown by arrow. Four mice were used in this experiment and one mouse was represented in all photographs as a sample. (D) Evaluation of EGFP expression by amastigotes in cells isolated from the lymph nodes of infected mice, eight weeks after infection with L. majorEGFP. The left image represents low and the right image represents high parasitic load (400× magnification). Results are shown as mean ± S.E
L. majorEGFP were inoculated to the foot-pad of BALB/c mice, then foot-pad swelling (Fig. 3B) and EGFP expression was measured at different time intervals (Fig. 3C). The mice were monitored for eight weeks. The parasite continued to express EGFP in vivo and, green fluorescent was observed in lesions and also in lymph nodes. The fluorescence intensity increased proportionally to the size of the lesion on the foot-pad. The visible and measurable lesion appeared two weeks before the measurable fluorescence signals. The results of in vivo imaging revealed that until five weeks’ post-challenge, no measurable fluorescence signal was detectable in the infected tissues. The results of DFM showed that the EGFP-expressing amastigotes were observed clearly in the cell suspension of lymph nodes of infected mice (Fig. 3D).
Correlation between DFM, real-time PCR, and LDA for parasite burden assay in infected lymph nodes
We quantified the parasite burden in popliteal lymph nodes of L. majorEGFP infected mice using three methods including DFM, LDA, and real-time PCR. The SODB1-based real-time PCR assay showed a dynamic range (the smallest and largest amounts of a quantified DNA that produce a linear standard curve) of at least 5 orders of magnitude (5 × 106 to 5 × 101 parasite DNA equivalents/reaction) with an R-value of >0.994, and slopes of −3.485 ± 0.25. The correlation between LDA and real-time PCR was significant (r = 0.857, P<0.0001). Generally, the parasite burden counted in each lymph node using real-time PCR was higher than the count of L. major enumerated by using the DFM method. A significant correlation was found between the LDA and DFM results, with a correlation coefficient of 0.976 (P<0.0001). Comparisons between the parasite counts obtained from DFM, LDA and real-time PCR results suggested that the real-time PCR assay tended to overestimate the parasite burden in comparison to DFM and LDA.
Discussion
In the present study, we developed a recombinant L. major expressing EGFP to compare a simple method i.e. DFM with LDA and with real-time PCR, to estimate the parasite burden in the animal experimental model of leishmaniasis. The results of DFM showed a strong significant correlation with the attained data by the LDA; as well, the same outcome was observed between DFM and real-time PCR methods. To the best of our knowledge, this is the first study comparing the correlation coefficient between the DFM, LDA and real-time PCR methods.
As a reporter gene, EGFP has many advantages over the other reporter genes for parasite burden assay; such as, greater simplicity for transfection, as well as for assay, and also easier kinetic monitoring (17). Preliminary results of the current study demonstrated that pLEXSY-EGFP cassette was ligated into the chromosomal ssu locus through homologous recombination, and did not affect the infection rate in mouse macrophages. As well, L. majorEGFP did not require any antibiotic pressure for the continuous and stable expression of EGFP.
In the recent years, the parasites expressing GFP were used by many researchers to scan the entire body fluorescence to analyze the efficacy of the vaccines or anti-parasite candidates in animal models (18). Although in vivo fluorescence measurements is useful to pursu the Leishmania infection; this method suffers from low sensitivity (6). We observed that the non-infected sterile lesions had green fluorescent emission, which may interfere with the emitted signals from the lesions formed by L. majorEGFP. Therefore, the formation of lesions at the site of inoculation could cause false-positive results or lead to an extremely overestimated parasite burden. Moreover, unlike the other methods, the in vivo imaging study cannot be used, when the parasite burden is low. However, in vivo imaging is a valuable approach to investigate the kinetics of infection in the site of inoculation and the subcutaneous draining lymph nodes. Nonetheless, this method cannot be used to determine the infection in other organs such as spleen and bone marrow.
Now, LDA and real-time PCR are among the most frequent techniques used for evaluating the parasite burden (3). LDA is a useful technique for measuring parasite burden; nonetheless, it is costly in terms of time; requires at least one week for growing the parasites. However, real-time PCR method allows the parasitic load to be determined in a short time, approximately 2–4 hours. Additionally, real-time PCR allows evaluating a large number of samples in a short time (19). So far, many researchers have developed various real-time PCR assay with high specificity and sensitivity based on the SYBR-Green or TaqMan chemistry (19). The pioneering work described a real-time PCR-based method to quantify Leishmania parasites using the multi-copy kDNA as a target (20). The heterogeneity of copy number of kDNA could be an issue for accurate quantification (21, 22). In order to remove the impact of this heterogeneity, previously, we developed an SYBR-Green real-time PCR assay, by targeting the conserved region of the SODB. This real-time PCR showed a significant correlation coefficient with LDA method for assessing the parasite burden in infected mice (6).
Although the results of the current study showed that the parasite burdens measured by DFM and SODB1-based real-time PCR were comparable to LDA method, in comparison to LDA and DFM, the real-time PCR approach remains expensive and requires technological expertise. On the other hand, DFM allowed easy interpretation of the results by using fluorescent microscopy, which is a common instrument in most laboratories. As well, DFM required quite a short time completing. The DFM was a more feasible method than the other methods to determine the half inhibitory concentration (IC50) values for candidate compounds with leishmanicidal activity (23). Additionally, for the primary screening and potency follow-up of the candidate drug or vaccine libraries, some researchers have developed a high throughput DFM-based assay (96- or 384-well), with sufficient sensitivity (24). Overall, DFM allows more practical, sensitive, faster and straightforward assay than other conventional methods for screening the drug candidates (25). However, further studies should be conducted to characterize the detection limit of DFM method.
Conclusion
Conclusively, each of the mentioned assays has benefits and disadvantages. The most straightforward way to measure the parasite burden is by using direct fluorescent microscopy. By using DFM, the parasite burden assessment could be done in a quicker, simpler, and less expensive manner, than LDA and real-time PCR.
Acknowledgements
This study was supported financially by grant from the deputy of research, Shahid Beheshti University of Medical Sciences (Grant No 7328).
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Aguilar Torrentera F, Lambot MA, et al. Parasitic load and histopathology of cutaneous lesions, lymph node, spleen, and liver from BALB/c and C57BL/6 mice infected with Leishmania mexicana. Am J Trop Med Hyg. 2002;66(3):273–9. [DOI] [PubMed] [Google Scholar]
- 2.Berman JD, Lee LS. Activity of antileishmanial agents against amastigotes in human monocyte-derived macrophages and in mouse peritoneal macrophages. J Parasitol. 1984;70(2):220–5. [PubMed] [Google Scholar]
- 3.Sacks DL, Melby PC. Animal models for the analysis of immune responses to leishmaniasis. Curr Protoc Immunol. 2001;Chapter 19:Unit 19.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Titus RG, Marchand M, Boon T, et al. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985;7(5):545–55. [DOI] [PubMed] [Google Scholar]
- 5.Taswell C. Limiting dilution assays for the separation, characterization, and quantitation of biologically active particles and their clonal progeny. Cell Separation.1987;4:109–45. [Google Scholar]
- 6.Ghotloo S, Haji Mollahoseini M, Najafi A, et al. Comparison of Parasite Burden Using Real-Time Polymerase Chain Reaction Assay and Limiting Dilution Assay in Leishmania major Infected Mouse. Iran J Parasitol. 2015;10(4):571–6. [PMC free article] [PubMed] [Google Scholar]
- 7.Mohammadiha A, Mohebali M, Haghighi A, et al. Comparison of real-time PCR and conventional PCR with two DNA targets for detection of Leishmania (Leishmania) infantum infection in human and dog blood samples. Exp Parasitol. 2013;133(1):89–94. [DOI] [PubMed] [Google Scholar]
- 8.Mohammadiha A, Haghighi A, Mohebali M, et al. Canine visceral leishmaniasis: a comparative study of real-time PCR, conventional PCR, and direct agglutination on sera for the detection of Leishmania infantum infection. Vet Parasitol. 2013;192(1–3):83–90. [DOI] [PubMed] [Google Scholar]
- 9.Dube A, Gupta R, Singh N. Reporter genes facilitating discovery of drugs targeting protozoan parasites. Trends Parasitol. 2009;25(9):432–9. [DOI] [PubMed] [Google Scholar]
- 10.Stiner L, Halverson LJ. Development and characterization of a green fluorescent protein-based bacterial biosensor for bioavailable toluene and related compounds. Appl Environ Microbiol. 2002;68(4):1962–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei T, Dai H. Quantification of GFP signals by fluorescent microscopy and flow cytometry. Methods Mol Biol. 2014;1163:23–31. [DOI] [PubMed] [Google Scholar]
- 12.Chudakov DM, Matz MV, Lukyanov S, et al. Fluorescent proteins and their applications in imaging living cells and tissues. Physiol Rev. 2010;90(3):1103–63. [DOI] [PubMed] [Google Scholar]
- 13.Misslitz A, Mottram JC, Overath P, et al. Targeted integration into a rRNA locus results in uniform and high level expression of transgenes in Leishmania amastigotes. Mol Biochem Parasitol. 2000;107(2):251–61. [DOI] [PubMed] [Google Scholar]
- 14.Dehghani F, Haji Molla Hoseini M, Memarnejadian A, et al. Immunomodulatory activities of chitin microparticles on Leishmania major-infected murine macrophages. Arch Med Res. 2011;42(7):572–6. [DOI] [PubMed] [Google Scholar]
- 15.Kropf P, Kadolsky UD, Rogers M, et al. The Leishmaniasis Model. Methods in Microbiology. 2010; 37: 307–28. [Google Scholar]
- 16.Nicolas L, Prina E, Lang T, et al. Real-time PCR for detection and quantitation of Leishmania in mouse tissues. J Clin Microbiol. 2002;40(5):1666–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh N, Gupta R, Jaiswal AK, et al. Transgenic Leishmania donovani clinical isolates expressing green fluorescent protein constitutively for rapid and reliable ex vivo drug screening. J Antimicrob Chemother. 2009;64(2):370–4. [DOI] [PubMed] [Google Scholar]
- 18.Mehta SR, Huang R, Yang M, et al. Real-time in vivo green fluorescent protein imaging of a murine leishmaniasis model as a new tool for Leishmania vaccine and drug discovery. Clin Vaccine Immunol. 2008;15(12):1764–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reithinger R, Dujardin JC. Molecular diagnosis of leishmaniasis: current status and future applications. J Clin Microbiol. 2007;45(1):21–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodgers MR, Popper SJ, Wirth DF. Amplification of kinetoplast DNA as a tool in the detection and diagnosis of Leishmania. Exp Parasitol. 1990;71(3):267–75. [DOI] [PubMed] [Google Scholar]
- 21.Jara M, Adaui V, Valencia BM, et al. Real-time PCR assay for detection and quantification of Leishmania (Viannia) organisms in skin and mucosal lesions: exploratory study of parasite load and clinical parameters. J Clin Microbiol. 2013;51(6):1826–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mary C, Faraut F, Lascombe L, et al. Quantification of Leishmania infantum DNA by a real-time PCR assay with high sensitivity. J Clin Microbiol. 2004;42(11):5249–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rocha MN, Correa CM, Melo MN, et al. An alternative in vitro drug screening test using Leishmania amazonensis transfected with red fluorescent protein. Diagn Microbiol Infect Dis. 2013;75(3):282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siqueira-Neto JL, Moon S, Jang J, et al. An image-based high-content screening assay for compounds targeting intracellular Leishmania donovani amastigotes in human macrophages. PLoS Negl Trop Dis. 2012;6(6):e1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bastos MS, Souza LA, Onofre TS, et al. Achievement of constitutive fluorescent pLEXSY-egfp Leishmania braziliensis and its application as an alternative method for drug screening in vitro. Mem Inst Oswaldo Cruz. 2017;112(2):155–9. [DOI] [PMC free article] [PubMed] [Google Scholar]