Abstract
The management of SARS-CoV-2 has not yet been clearly determined and is based on potential therapies evaluated during the SARS-CoV and MERS-CoV outbreaks. An emerging potential therapeutic approach currently being evaluated in numerous clinical trials is the remdesivir agent, which acts on COVID-19 by interfering with key steps in the virus replication cycle. It is considered a therapeutic option to be evaluated against COVID-19, based on data on its in vitro and in vivo activity against MERS-CoV and SARS-CoV coronaviruses.
In this work, we provide an overview of remdesivir’s discovery, mechanism of action, and the current studies exploring its clinical effectiveness. Recommendations for its use against COVID-19 infection are also summarized.
Keywords: COVID-19, Remdesivir, Clinical tests
Introduction
Viral infections of the respiratory tract are one of the leading causes of human morbidity and mortality worldwide [1,2]. Among these respiratory viruses, coronaviruses, susceptible to infect humans and animals, are ubiquitous. Since the late 1960s, human coronaviruses have been recognized as upper respiratory tract pathogens associated with benign conditions, such as the common cold and the respiratory tract [[3], [4], [5], [6]].
More recently, three types of coronavirus have been identified responsible for serious pneumonitis: SARS-CoV, pathogen of severe acute respiratory syndrome (SARS), MERS-CoV, that of the Middle East respiratory syndrome [[7], [8], [9], [10], [11]] and COVID-19 caused by a virus called SARS-CoV-2. It appears to have first emerged in Wuhan, China, in late 2019. The outbreak has since spread across China to other countries around the world. By the end of January, the new coronavirus had been declared a public health emergency of international concern by the WHO and it is believed that the disease has jumped from bats to humans [12,13]. Insufficient human resources, porous borders, as well as the lack of hygiene knowledge of the population, are factors favouring the spread of the disease. Despite the significant progress made since the beginning of the epidemic, the control of COVID-19 remains a major challenge for governments and members of the international community.
Indeed, it appears very urgent to develop new molecular tools that will make it possible to verify the suspected medical implication of COVID-19, then to envisage prevention and treatment. As of July 29, 2020, more than 16 558 289 confirmed cases have been identified internationally of which 738 344 cases in Africa, 8 840 524 cases in Americas, 1 507 734 cases Eastern Mediterranean, 3 283 277cases in Europe, 1 892 056 cases in South-East Asia, and 295 613 cases in Western Pacific [14]. More than 528 204 deaths associated with COVID-19 have been reported, making COVID-19 a major health emergency [14]. Through great efforts, mainly based on strict containment measures, China managed to emerge from the first wave of the epidemic at the beginning of April 2020, when social order slowly returned to normality. While different precautionary measures have been taken at the national level in the EU to limit and monitor the entry of potential cases of COVID-19 from China, before the end of February the first symptomatic cases were reported in a number of EU countries, such as Italy, Spain, Germany and, some weeks later, in the UK.
Currently, antiviral drugs are being evaluated as potential treatments for COVID-19. Indeed, repositioning substances with historical antiviral activity is a promising avenue for finding an effective treatment for COVID-19 infection. In this context, studies published in the literature have emphasized the effectiveness of many drugs, in particular: ribavirin, penciclovir, nitazoxanide, nafamostat, chloroquine, remdesivir (RDV, GS-5734) and favipiravir (T-705) [15]. In addition, WHO has called for more complex clinical studies on the effect of molecules with apparent antiviral properties such as RDV [16]. It has been suggested that RDV may be an option of choice to inhibit COVID-19 [17].
In this study, we will give an overview of remdesivir (RDV, GS-5734) and its antiviral activities against Ebola virus. Recommendations for its use against COVID-19 infection are also summarized.
Structure and pharmacokinetics of remdesivir
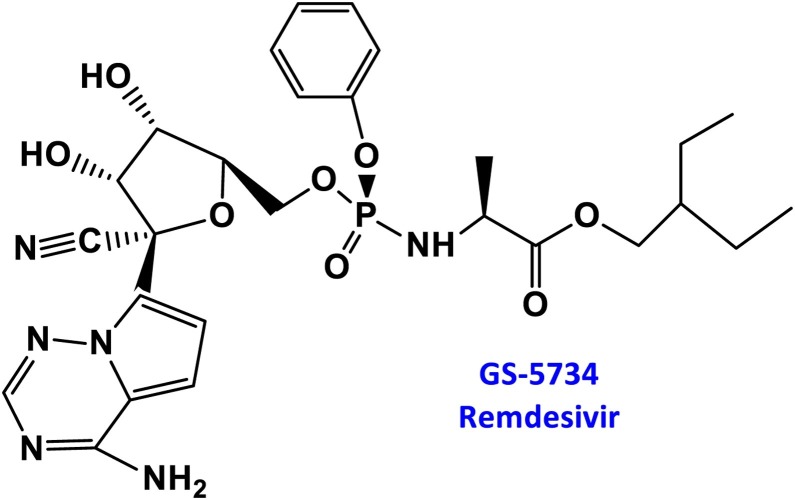
RDV is a prodrug of nucleoside analogs, which can be metabolized in cells to adenosine triphosphate analogs that inhibit viral RNA polymerase. Remdesivir has broad-spectrum activity against members of several families of viruses, including filoviruses (e.g. Ebola virus) and coronaviruses (e.g. SARS-CoV and Middle East Respiratory Syndrome Coronavirus [MERSCoV]). It is essentially a modified version of the natural component of adenosine which is essential for DNA and RNA. The active form of RDV contains three phosphate groups; it is this form that is recognized by the virus’s RNA polymerase enzyme. Once RDV is incorporated into the RNA growth chain, the presence of carbon-nitrogen (CN) groups can cause the sugar to bend, which in turn distorts the shape of the RNA chain. Therefore, only three additional nucleotides can be added. This stops the production of RNA strands and eventually interrupts virus replication. The main structural feature that distinguishes RDV from adenosine is the modification of specific chemical bonds on the molecule. Instead of joining a carbon and a nitrogen atom, the chemist replaces the nitrogen with another carbon to form a carbon-carbon bond. This is essential to the success of this drug because the coronavirus has a special enzyme that can recognize and eliminate artificial nucleosides. However, by modifying the chemical bond, the RDV cannot be removed by the enzyme, so it remains in the growing chain and prevents replication (Scheme 1 ).
Scheme 1.
Chemical structure of remdesivir.
In 2017, it was synthesized by the team of Dustin Siegel et al. for the treatment of the Ebola virus during the 2013–2016 epidemic in West Africa [18]. Antiviral activity was also subsequently demonstrated against other single-stranded RNA viruses: Marburg virus, SARS-CoV, MERS-CoV, and paramyxoviruses (e.g. respiratory syncytial virus, Nipah virus, and Hendra virus) [11,[18], [19], [20], [21], [22]].
To use RDV, a loading dose (200 mg for adults, adjusted for child weight) should be injected intravenously on the first day, followed by a daily maintenance dose (100 mg for adults) for up to 10 days. In non-human primates, daily administration of 10 mg/kg RDV results in a shorter plasma half-life of the prodrug (t1/2 = 0.39 h), but intracellular levels of the triphosphate form persist [23].
The effectiveness of RDV against SARS-CoV-2 and related coronaviruses was confirmed in vitro and preclinical in vivo animal models. These include a recent in vitro study evaluating antiviral activity against SARS-CoV-2. An IC50 of 770 nM and an IC90 of 1760 nM (with a cytotoxic concentration >100 mM) were demonstrated in this study [15]. In addition, Sheahan et al. and Wit et al. have demonstrated the in vivo efficacy of remdesivir in inhibiting viral replication and reducing virus-related pathology against related coronaviruses [24,25]. These findings, along with the safety profile of remdesivir in the clinical trial assessment against EBOV [26], support the evaluation of remdesivir as a potential therapeutic drug for repurposing against the SARS-CoV-2 pandemic.
In a non-fatal rhesus monkey model of SARS-CoV-2 infection, early administration of RDVs was shown to result in significant antiviral and clinical effects (reduced pulmonary infiltrates and virus titers in bronchoalveolar washings compared to vehicle alone) [27].
A study by Wang et al. showed that RDV was well tolerated and no new safety issues were identified. The overall proportion of patients with serious adverse events tended to be lower in patients receiving remdesivir than in placebo recipients [28]. However, the researchers found that a greater proportion of patients who received RDV than those who received placebo were discontinued prematurely by the investigators due to adverse events, including gastrointestinal symptoms (anorexia, nausea, and vomiting), increased aminotransferases or bilirubin, and deterioration of cardiopulmonary status.
It is currently in clinical development for the treatment of Ebola virus infection [26]. Indeed, it can reach persistent levels of its form in the bloodstream concomitantly [21].
Research studies, published in the literature, have demonstrated the antiviral activity of RDV on various viruses of the coronavirus family. They confirmed in vitro activity, including epithelial cell cultures in human respiratory tract. In addition, these results extend the spectrum of in vitro antiviral activity of RDV to infectious coronaviruses [22]. Furthermore, RDV can reduce the viral load in the lungs and improve the clinical signs of the disease as well as respiratory function. These data encourage the use of this drug against serious human coronavirus infections [21].
RDV has shown better results in controlling SARS-CoV-2 infection (EC50 = 0.77 μM) compared to other antivirals such as; ribavirin (EC50 = 109.5 μM), penciclovir (EC50 = 95.96 μM), nitazoxanide (EC50 = 2.12 μM), chloroquine (EC50 = 1.13 μM), hydroxychloroquine (EC50 = 0.77 μM) and favipiravir (EC50 = 61.9 μM) [15]. These results are encouraging to evaluate RDV for patients infected with COVID-19. Although there is no approved antiviral treatment for the treatment of coronavirus infections, the preclinical data obtained from the use of RDV are promising [25]. These antiviral effects in vitro have raised much hope to use this drug against many viral infections including COVID-19 [[29], [30], [31]].
Mechanism of action of remdesivir
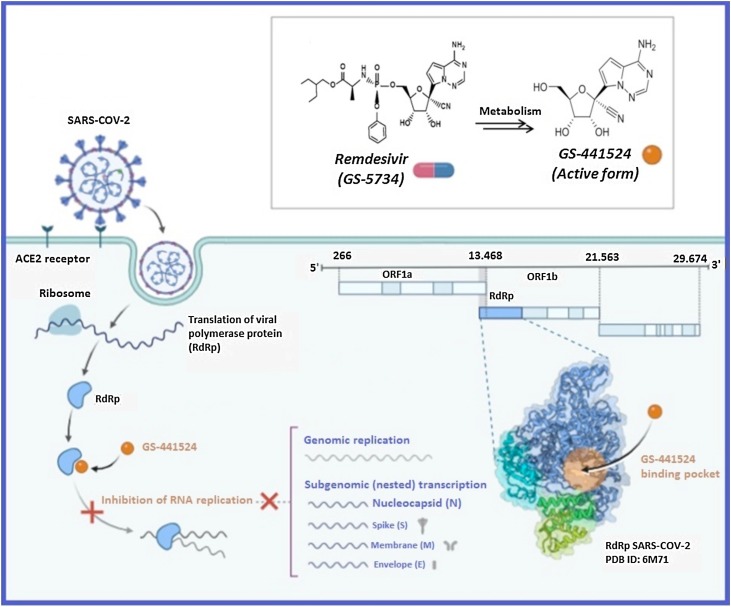
Recently, Calvin Gordon et al. [32] using polymerase enzymes from the coronavirus that causes MERS, found that the enzymes can incorporate RDV, which resembles an RNA building block, into new RNA strands. Very shortly after the addition of RDV, the enzyme ceases to be able to add other RNA subunits resulting in a halt in the replication of the genome [32].
Another work [33] reported that the efficacy of the viral RNA-dependent RNA polymerase has been shown by enzyme kinetics to effectively incorporate the active triphosphate form of RDV (RDV-TP) into the RNA. The scientists hypothesize that this might happen because RNA containing RDV takes on a strange shape that doesn’t fit into the enzyme. To find out for certain, structural data on the enzyme and the newly synthesized RNA should be collected. This data could also help researchers design future drugs with even greater polymerase activity [32]. Based on studies conducted on the Ebola virus, RDV was found to inhibit the action of the RNA-dependent RNA polymerase, causing the lengthening of the synthesized chain (Fig. 1 ).
Fig. 1.
SARS-CoV-2 genome and RNA-dependent RNA polymerase structure and mechanism of action of remdesivir according to [34]. (Created with “BioRender.com”).
Remdesivir in treating COVID-19
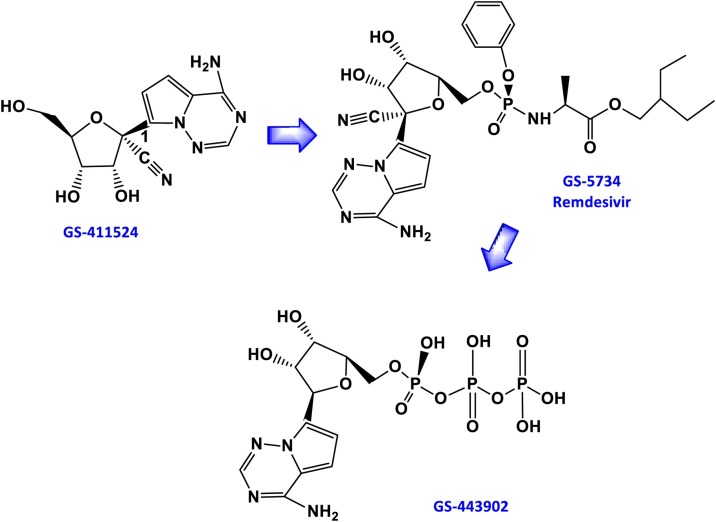
There is no recognized treatment for COVID-19. However, management of severe cases consists of intensive palliative care to maintain kidney function and electrolyte balance while limiting bleeding and shock [[29], [30], [31]]. Recently, remdesivir (RDV, GS-5734) has been shown to have very positive activity against COVID-19 infection, as evidenced by clinical trials in infected patients [15,35,36]. Note that RDV is 74% eliminated in the urine and 18% eliminated in the feces [37]. 49% of the recovered dose is in the form of the metabolite GS-441524 (Scheme 2 ), and 10% is recovered as the unmetabolized parent compound [37]. GS-441524, predominant metabolite of RDV and superior to Remdesivir against Covid-19, shows comparable efficacy in cell-based models of primary human lung and cat cells infected with coronavirus. In vitro study in simian or human cells with the coronavirus strain SARS-CoV-2 (COVID-19) indicates that RDV potentially blocks viral infection after the virus enters the cell.
Scheme 2.
Structure of remdesivir (B) and its precursors (A) and metabolites (C) derived from Drugbank.
RDV has a higher selectivity index (SI) > 129.87 in simian cells when compared to other nucleoside analogs including ribavirin SI > 3.65), penciclovir SI > 4.17) and favipiravir SI > 6.46) [15].
Publications for inpatients with COVID-19 who received RDV via compassionate access devices have been published [[29], [30], [31]]. Indeed, Grein et al. showed that for a group of 53 patients hospitalized for COVID-19 and who were treated with RDV for compassionate use, clinical improvement was observed in 36 patients for the most part over a period of 10 days. Approval of the efficacy of this drug will require continuous randomization, site-controlled trials of treatment with RDV [29].
A publication by Holshue et al. reports on a case that was treated by the RDV on the 11th day of his illness and then improved on the 12th day (oxygenation stopped and oxygen saturation 96%) [30]. These results, therefore, require in-depth studies and clinical tests to achieve definite results. Furthermore, Lescure et al. prescribe indications for using RDV as a treatment for COVID-19 depending on the worsening of the infection [31]. This study relates to a series of 5 cases, 3 of which received at least one dose of RDV. In two patients, treatment took place at the time of the worsening of the disease. In one of them, the RDV was stopped after 5 days. In the third patient, RDV was discontinued after a single dose due to renal replacement therapy to prevent accumulation of cyclodextrin. This research group considered that RDV may be the best potential drug for the treatment of COVID-19 infection. The loading dose of 200 mg has been indicated for patients with severe intravenous infection.
Another study carried out by the Hillaker team, tracks the treatment of a single patient on day 13 of his illness [38]. At the time of RDV administration, the patient was in the intensive care unit, under intubation, and treated with hydroxychloroquine. Forty-eight hours after treatment, the patient’s condition had improved. The patient was incubated 60 h after treatment and was able to breathe in ambient air 24 h later.
On the other hand, Manli Wang et al. suggesting that it’s working concentration will probably be reached in non-human models primate (PSN) [15]. In addition, the assay showed that this drug works at a later stage of entry of the virus, which is consistent with its putative antiviral mechanism as a nucleotide analog. This research team has thus shown that RDV effectively inhibits viral infection in a human cell line which is sensitive to COVID-19 [39]. In general, the remedy indicated for compassionate use has been based on the worsening of the clinical condition of the patient infected with COVID-19. Controlled clinical trials are required to determine the safety and efficacy of RDV and any other investigational agents for the treatment of patients with COVID-19 infection [40].
Recently, the efficacy of RDV has been the subject of scientific debate. In fact, in the United States, the preliminary results of a clinical trial for a thousand patients showed that RDV had helped hospital patients to recover 31% faster [[41], [42], [43]]. The mortality in the RDV group was 8% compared to 11.6% in the control group. The Food and Drug Administration (FAD) has given permission to hospitals in the United States to provide resuscitation to patients in intensive care [44].
On the other hand, Chinese researchers are also conducting a study on the efficacy of RDV. The data obtained is not encouraging. RDV has no significant effect on patients infected with COVID-19 since this study is conducted on a small number of patients [28]. In the majority of the published research, the authors indicated that the results of studies on the use of RDV in the treatment of COVID-19 are limited by the small size of the group, the short duration of follow-up, the potentially missing data due to the nature of the program, the lack of information on patients treated at baseline and the absence of a randomized control group.
Other potential candidates are the subject of clinical studies for COVID-19 disease, including chloroquine, hydroxychloroquine, and the combination lopinavir/ritonavir. However, since the pharmacodynamics rational is more limited, it is not possible to draw up a formal opinion pending the results of clinical studies. Table 1 summarize published primary studies on the use of antiviral RDV against coronaviruses
Table 1.
Published primary studies on the use of remdesivir against coronaviruses: case series and cohort series.
| Author name | study settings | Type of study | Infection classes | Study design | Concluding remarks | References |
|---|---|---|---|---|---|---|
| Brown et al. | United States | Cohort series | human endemic and zoonotic deltacoronaviruses | understand the spectrum of RDV efficacy among human and zoonotic Coronavirus | -RDV inhibits endemic human coronavirus 229E and OC43 and a member of the deltacoronavirus genus, PDCoV, which have the most divergent RdRp of known coronaviruses compared to SARS and MERS CoVs. -RDV as a potential antiviral for current endemic and epidemic coronavirus as well as future emerging coronavirus | [22] |
| Sheahan et al. | United States | Cohort series | epidemic and zoonotic coronaviruses | Evaluate the antiviral potency and extent of activity of GS-5734 (RDV) against a diverse panel of human and zoonotic coronaviruses. | -RDV may prove effective against endemic MERS coronavirus in the Middle East, circulating human CoV, and, possibly most importantly, emerging CoV of the future | [11] |
| Grein et al. | United States | – | MERS-CoV | compare the prophylactic and therapeutic efficacy of RDV with the combination of LPV/RTV and IFNb | -RDV may improve disease outcomes in coronavirus -infected patients, serve to protect health care workers in areas with endemic MERS-CoV and prove valuable in preventing future epidemics in the event of novel coronavirus emergence in the future. | [25] |
| Gordon et al. | Canada | Cohort series | COVID-19 | compassionate-use basis to patients hospitalized with Covid-19, the illness caused by infection with SARS-CoV-2. Patients were those with confirmed SARS-CoV-2 infection who had an oxygen saturation of 94% or less while they were breathing ambient air or who were receiving oxygen support | - RDV may have clinical benefit in patients with severe Covid-19. - The type of supportive care (e.g., concomitant medications or variations in ventilatory practices) and differences in institutional treatment protocols and hospitalization thresholds may impact on outcomes.- | [29] |
| Agostini et al. | United states | Cohort series | human CoV | Asses the efficacy of the nucleotide prodrug remdesivir (GS-5734) to include a group β-2a CoV | - RDV is highly active against coronaviruses and that there is a high genetic barrier to achieve resistance. - potential novel determinants of polymerase function and nucleotide selectivity or fidelity that will guide future structure-function and biochemical studies of the polymerase and RDV mechanism are identified. - RDV demonstrate its potential utility in the broad-spectrum treatment of coronavirus infections. | [21] |
| Zhong et al. | Beijing, China | – | 2019-nCoVs | Measurement of the effects of these compounds on the cytotoxicity, virus yield and infection rates of 2019-nCoVs. | -RDV is highly effective in the control of 2019-nCoV infection in vitro | [15] |
Abbreviations: PDCoV, Porcine deltacoronavirus; LPV, Lopinavir; RTV, Ritonavir; IFNb, Inc. Recombinant human interferon beta.
Conclusion
RDV is beneficial for COVID-19 treatment but uncertain on very limited preclinical data (degree of low certainty), a priori good security profile. The majority of clinical practice trials report that there is currently insufficient evidence to recommend a specific treatment for subjects with COVID-19. In fact, any treatment should be administered as part of a randomized controlled trial. In the context of health emergencies, the researchers recommend, however, to use different molecules alone or in combination. To this end, RDV, which presents a broad spectrum of activities against SARS-CoV2, and MERS-CoV, is now recommended as a potential drug for COVID-19.
Funding
No funding sources.
Competing interests
None declared.
Ethical approval
Not required.
References
- 1.Vareille M., Kieninger E., Edwards M.R., Regamey N. The airway epithelium: soldier in the fight against respiratory viruses. Clin Microbiol Rev. 2011;24(1):210–229. doi: 10.1128/CMR.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jartti T., Jartti L., Ruuskanen O., Söderlund-Venermo M. New respiratory viral infections. Curr Opin Pulm Med. 2012;18(3):271–278. doi: 10.1097/MCP.0b013e328351f8d4. [DOI] [PubMed] [Google Scholar]
- 3.Desforges M., Favreau D.J., Brison É., Desjardins J., Meessen-Pinard M., Jacomy H., et al. In: Singh S.K., editor. Vol. 2013. CRC Press/Taylor and Francis; Boca Raton: 2013. Human coronaviruses: respiratory pathogens revisited as infectious neuroinvasive, neurotropic, and neurovirulent agents; pp. 93–121. (Neuroviral infections RNAViruses and retroviruses). [Google Scholar]
- 4.Buchmeier M.J., Lane T.E. Viral-induced neurodegenerative disease. Curr Opin Microbiol. 1999;2(4):398–402. doi: 10.1016/S1369-5274(99)80070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavanagh D. Coronaviruses in poultry and other birds. Avian Pathol. 2005;34(6):439–448. doi: 10.1080/03079450500367682. [DOI] [PubMed] [Google Scholar]
- 6.Talbot P.J., Desforges M., Brison E., Jacomy H., Tkachev S. Coronaviruses as encephalitis-inducing infectious agents. Non-flavirus Encephalitis. In-Tech. 2011;185–202:185–202. [Google Scholar]
- 7.Vabret A., Dina J., Brison E., Brouard J., Freymuth F. Coronavirus humains (HCoV) Pathol Biol. 2009;57(2):149–160. doi: 10.1016/j.patbio.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talbot P.J., Jacomy H., Desforges M. Nidoviruses. American Society of Microbiology; 2008. Pathogenesis of human coronaviruses other than severe acute respiratory syndrome coronavirus; pp. 313–324. [Google Scholar]
- 9.de Groot R.J., Baker S.C., Baric R.S., Brown C.S., Drosten C., Enjuanes L., et al. Commentary: Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the coronavirus study group. J Virol. 2013;87(14):7790–7792. doi: 10.1128/JVI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization, Middle East respiratory syndrome coronavirus (MERS-CoV) – update. http://www.who.int/csr/don/2013_11_10/fr/index.html, 10 November 2013.
- 11.Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B., et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9(396) doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coronavirus N. Situation report-1 21 January 2020. World Health. 2019:251. [Google Scholar]
- 13.Saqrane S., El Mhammedi M.A. Review on the global epidemiological situation and the efficacy of chloroquine and hydroxychloroquine for the treatment of COVID-19. New Microbes New Infect. 2020 doi: 10.1016/j.nmni.2020.100680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization . 2020. Coronavirus disease 2019 (COVID-19): situation report; p. 167. [Google Scholar]
- 15.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 27 March 2020. https://www.who.int/dg/speeches/detail/whodirector-general-s-opening-remarks-at-the-media-briefing-on-covid-19---27-march-2020. (March 27, 2020), [Accessed 27 March 2020].
- 17.Liu W., Morse J.S., Lalonde T., Xu S. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019‐nCoV. Chembiochem. 2020;21(5):730–738. doi: 10.1002/cbic.202000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siegel Dustin, Hui Hon C., Doerffler Edward, Clarke Michael O., Chun Kwon, Zhang Lijun, et al. Discovery and synthesis of a phosphoramidate pro drug of a pyrrolo[2,1-f][triazin-4-amino] adenine C-Nucleoside (GS-5734) for the treatment of Ebola and emerging viruses. J Med Chem. 2017;60(5):1648–1661. doi: 10.1021/acs.jmedchem.6b01594. [DOI] [PubMed] [Google Scholar]
- 19.Lo M.K., Jordan R., Arvey A., Sudhamsu J., Shrivastava-Ranjan P., Hotard A.L., et al. GS-5734 and its parent nucleoside analog inhibit filo-, pneumo-, and paramyxoviruses. Sci Rep. 2017;7:43395. doi: 10.1038/srep43395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warren T.K., Jordan R., Lo M.K., Ray A.S., Mackman R.L., Soloveva V., et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531(7594):381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X., et al. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio. 2018;9(2) doi: 10.1128/mBio.00221-18. e00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown A.J., Won J.J., Graham R.L., Dinnon K.H., III, Sims A.C., Feng J.Y., et al. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res. 2019;169 doi: 10.1016/j.antiviral.2019.104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green N., Ott R.D., Isaacs R.J., Fang H. Cell-based assays to identify inhibitors of viral disease. Expert Opin Drug Discovery. 2008;3(6):671–676. doi: 10.1517/17460441.3.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Wit E., Feldmann F., Cronin J., Jordan R., Okumura A., Thomas T., et al. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci. 2020;117(12):6771–6776. doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J., et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11(1):1–14. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mulangu S., Dodd L.E., Davey R.T., Jr., Tshiani Mbaya O., Proschan M., Mukadi D., et al. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381(24):2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williamson B.N., Feldmann F., Schwarz B., Meade-White K., Porter D.P., Schulz J., et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature. 2020;585(7824):273–276. doi: 10.1038/s41586-020-2423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., et al. Compassionate use of remdesivir for patients with severe covid-19. N Engl J Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lescure F.X., Bouadma L., Nguyen D., Parisey M., Wicky P.H., Behillil S., et al. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis. 2020;20(6):697–706. doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gordon C.J., Tchesnokov E.P., Feng J.Y., Porter D.P., Götte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem. 2020;295(15):4773–4779. doi: 10.1074/jbc.AC120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordon C.J., Tchesnokov E.P., Woolner E., Perry J.K., Feng J.Y., Porter D.P., et al. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J Biol Chem. 2020;295(20):6785–6797. doi: 10.1074/jbc.RA120.013679. jbc-RA120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eastman R.T., Roth J.S., Brimacombe K.R., Simeonov A., Shen M., Patnaik S., et al. Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent Sci. 2020;6(5):672–683. doi: 10.1021/acscentsci.0c00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ko W.C., Rolain J.M., Lee N.Y., Chen P.L., Huang C.T., Lee P.I., et al. Arguments in favour of remdesivir for treating SARS-CoV-2 infections. Int J Antimicrob Agents. 2020;55(4) doi: 10.1016/j.ijantimicag.2020.105933. 105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.FDA: fact sheet for health care providers EUA of remdesivir.
- 38.Hillaker E., Belfer J.J., Bondici A., Murad H., Dumkow L.E. Delayed initiation of remdesivir in a COVID-19 positive patient. Pharmacotherapy. 2020;40(6):592–598. doi: 10.1002/phar.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.NIH clinical trial shows remdesivir accelerates recovery from advanced COVID-19. https://www.niaid.nih.gov/news-events/nih-clinical-trial-shows-remdesivir-accelerates-recovery-advanced-covid-19.
- 42.Coronavirus: le médicament “remdesivir” vanté aux Etats-Unis est-il vraiment efficace? RTBF Info, April 30, 2020. https://www.rtbf.be/info/dossier/epidemie-de-coronavirus/detail_coronavirus-le-medicament-remdesivir-vante-aux-etats-unis-est-il-vraiment-efficace?id=10492627.
- 43.Covid-19: les Etats-Unis autorisent les traitements à base de remdesivir. Le monde, April 30, 2020. https://www.lemonde.fr/sciences/article/2020/04/30/covid-19-resultats-contradictoires-pour-l-antiviral-remdesivir_6038304_1650684.html.
- 44.Coronavirus dans le monde: déconfinement à des rythmes variés selon les pays, sous la menace d’une pandémie loin d’être endiguée. Le Monde.fr, May 2, 2020. https://www.lemonde.fr/planete/article/2020/05/02/coronavirus-dans-le-monde-deconfinement-a-des-rythmes-varies-aux-etats-unis-qui-misent-sur-le-remdesivir_6038412_3244.html.