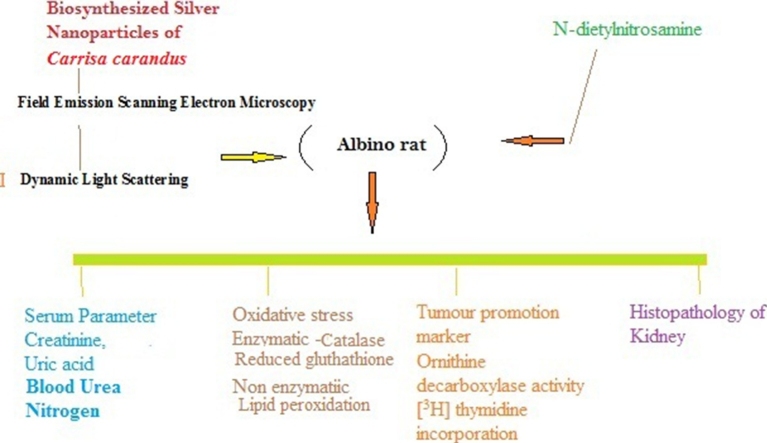
Graphical abstract
Abbreviations: NPs, nanoparticles; CCAgNPs, silver nanoparticles of carissa carandas aqueous extract; DEN, diethylnitrosamine; DMN, dimethylnitrosamine; AgNPs, silver nanoparticles; CC, carissa carandas; AgNO3, silver nitrate; ABTS, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid; DMSO, dimethyl sulphoxide; IAEC, institutional animal ethical committee; GSH, glutathione; CDNB, 1-chloro-2,4-dinitrobenzene; FE-SEM, field emission scanning electron microscopy; DLS, dynamic light scattering; GGT, γ-glutamyl transpeptidase activity; SOD, superoxide dismutase; GPx, glutathione peroxidase; GST, glutathione –S- Transferase; GR, glutathione reductase activity; H2O2, hydrogen peroxide; GGT, gamma glutamyl transpeptidase; XO, xanthine oxidase; LDH, lactate dehydrogenase; BUN, blood urea nitrogen; ODC, ornithine decarboxylase; ROS, reactive oxygen species; MDA, malondialdehyde
Keywords: Carissa carandas embedded silver nanoparticles, NF-κB pathway, Renal carcinoma
Highlights
-
•
Phytofabricated silver nanoparticles (CCAgNPs) were biosynthesized and characterized.
-
•
CCAgNPs were evaluated against diethylnitrosamine induced renal cancer.
-
•
Silver nanoparticles have an antioxidant property.
-
•
Silver nanoparticles unveiled a therapeutic effect against renal cancer in vivo.
Abstract
Introduction
Inflammation and oxidative stress are the main factors ascribed with interruption in the process of renal tissue impairment. The toxicity of different types of nitrosamine is well recognized in animals and humans. Administration of the smallest quantities of diethylnitrosamine or dimethylnitrosamine either orally or parenterally results into renal damage. Therapeutic effects of phytofabricated silver nanoparticles of Carissa carandas aqueous extract has been scrutinised in current study for the assessment of renal cancer activity in animal model.
Methodology
Phytofabricated silver nanoparticles were characterized by using different instrumentation. Nephroprotective activity of silver nanoparticles at different doses was evaluated against N-diethylnitrosamine (200 mg/kg b.w., intraperitoneal) in animal model. Serum and renal homogenate were taken to evaluate the renal toxicity markers, oxidative stress, and antioxidant parameter, proinflammatory cytokines and histopathological study.
Result
Significant outcomes of silver nanoparticles in dose dependent manner down regulated the elevated serum marker, tumour marker enzymes and histopathology observation of repaired tissue assured the renal cancer activity in animals. In addition, profile of enzymatic and non-enzymatic antioxidant, proinflammatory cytokines and tumour promotion marker also favours the anticancer property of silver nanoparticles.
Conclusion
The data of current study reveals silver nanoparticles ameliorates renal oxidative stress and carcinogenesis which was induced by N-diethylnitrosamine and accredited to antioxidant and anticancer activities of phytofabricated nanoparticles by biological approach.
1. Introduction
Over a decade, metallic nanoparticles such as iron, gold, manganese, silver, zinc and copper have been used as an antimicrobial agent [1]. Various researcher shown great interest in the biosynthesis of silver nanoparticles du to use as biocide and in biomedical applications such as anticancer, anti-inflammatory, antimicrobial, analgesic and etc. Among all metal, silver contains highest thermal and electrical conductivity and low contact resistance in pure forms [2,3]. Literature cited that nano-silver show adverse effects on environment as well as on living beings [4]. Therefore green synthesis provides toxic free chemical and environment-friendly fabrication of silver nanoparticles [5]. It is approved as a safe by the U.S. Food and Drug administration due to its small shape and size, eco-friendly, cost effective and higher chemical stability. Silver nanoparticles of herbal drugs does not require any capping or reducing agent as used in other chemical method [6]. Additionally, secondary metabolites present in plants have reduction properties which are ascribed to higher capability of plant extracts to fabricate nanoparticles with superior quality. During fabrication of silver nanoparticles, secondary metabolites in plant extracts acts as a reducing agent for the reduction of Ag+ to Ag0 and prevents the agglomeration of the nanoparticles via acting as capping or stabilizing agents [7,8].
Horta-Fraijo et al. reported antibacterial and thermochromic applications of Ag clusters in zeolite A4. They used a Density Functional Theory (DFT) to measure the electronic, structural and vibrational properties of the planar silver clusters and prepared using the ion exchange method. TEM was used to explore the morphology of silver clusters. It was correlated experimentally and theoretically [9]. In other study, Horta-Piñeres et al. reported the biosynthesis of the silver nanowires embedded with silver nanoparticles using leaf extract of Mangifera indica. The prepared nanocomposite was characterized UV–vis spectroscopy, transmission electron microscopy, X-ray energy dispersive spectrometry and Fourier transform infrared spectroscopy. The adsorption energy was investigated in the presence of glycine molecule when Ag2 clusters interact with cross section of silver nanowires (different sizes) using the Hartree Fock and DFT theories. The prepared silver nanowires possess better antibacterial properties. In short, it was concluded that the it would be the first report to prepare this type of nanocomposite with silver nanoparticles via green synthesis [10].Calderón-Ayala et al. proposed an ecological approach to exfoliate few layer graphene and its functionalization with silver nanoparticles using Jatropha Cordata without adding any toxic substances. Identification of the crystalline plane on silver nanostructure and graphene were performed using Fast Fourier Transform. It also reveals a strong affinity at the edge of the graphene layers [11]. Docea et al. proposed and effect of two types of silver nanoparticles (EG-AgNPs and PVP-EG-AgNPs) on antioxidant/pro-oxidant balance in rats (murine model) [12] Sayed et al. reported the hepatoprotective activity of the silver nanoparticles and silver nitrate on African catfish (Clarias garepinus) via evaluated the biochemical chanes, histochemical and histopathology changes and effects of silver ions on the liver tissue of the fish [13].
Oxidative stress and free radical induced damages to various tissues due to increased production of reactive oxygen species. Increased generation of free radical develops a various diseases i.e. mutagenesis, atherosclerosis, arthritis and carcinogenesis [14]. Various nitrosamines i.e. N-diethylnitrosamine and dimethylnitrosamine is well recognized inducer of renal cancer and present in the air, alcohol, smoke, plastic, processed meats and smoked food. It is mutagenic in nature and cancer causing agent in rodents and humans [15]. As reported earlier in literature, cytochrome P450-dependent mono-oxidase systems metabolise the diethylnitrosamine chemical and this chemical initiated the mutagenicity, carcinogenicity and cytotoxicity by inducing oxidative stress. [16]. Therefore, use of nano-approach of plant extract could suggest nephroprotective effect against DEN induced renal injury.
Carissa Carandas, folklore, dietary and an evergreen deciduous shrub belongs to Apocynaceae family, indigenous to subtropical and topical regions. This plant grows abundantly in the Himalayas, especially in Siwalik Hills, the Western Ghats, in India, and neighbouring countries of Pakistan, Srilanka and Nepal. The different types of bioactive compounds convey the medicinal importance to the shrub, are cardiac glycosides, phenolic, tannins, alkaloids, saponins, triterpenoids and flavonoids compounds. Ethnopharmacological importance of C. carandas has been attributed because of anti-oxidant, antimicrobial, cytotoxic activity, antidiabetic, epilepsy, neuroprotective activity, DNA damage inhibition and anticancer property [17]. The current study envisioned to biofabricate, characterize and biologically evaluate the anticancer property of silver nanoparticles of Carissa carandas aqueous extract (CCAgNPs) in animal model with the analysis of its antioxidant, biochemical parameter, tumour promotion enzymes and pro-inflammatory cytokines evaluation.
2. Material and methods
2.1. Plant collection and its authentication
Fresh leaves material of Carissa carandas was collected from the rural region of Mirzapur district, Uttar Pradesh, India. Leaves of C. carandas were scientifically authenticated and the voucher specimen was submitted to concern department.
2.2. Plant extracts preparation
10 g of shade dried leaves material (RT) were transferred to Erlenmeyer flask (500 mL) with the addition of 250 mL distilled water. This mixture was heated at 85 °C for 60 min at 200 rpm with continuous stirring. The resultant solution was allowed to cool, and whatman filter paper no. 1 was used to filter the solution and obtained aqueous extract was kept in refrigerator for experimental studies [18].
2.3. Phytofabrication of CCAgNPs
Phytofabrication of CCAgNPs was started by the addition of 80 mL of 1 mM AgNO3 into the 20 mL of plant extract and kept on stirrer for 2 min. Resultant mixture was incubated for 2 h. Colour change of solution from orange to brown confirmed the formation of silver nanoparticles. Precipitates were separated by using centrifuge machine at 12,000 rpm for 15 min. The pellets of CCAgNPs was collected and dried in hot air oven and stored in air-tight bottle for further experiment [19].
3. Instrumental characterization of the silver nanoparticles
3.1. Field emission scanning electron microscopy (FE-SEM)
The FE-SEM system (FESEM Carl Zeiss SMT AG, Germany) was utilized to disclose information about the shape and morphological character of materials. A suspension of nanoparticles i.e. drop was kept on the 200 mesh grip which have a carbon support film. Finally the product was rinsed with ethanol, dried and placed on FESEM holder and pictures of samples were taken at accelerated voltage of 20 KV [20].
3.2. Dynamic light scattering (DLS)
DLS was utilize to know the average sizes or size distribution of phytofabricated silver nanoparticles which was synthesized from plant extract in the form of suspension [21].
4. In vitro antioxidant study
4.1. ABTS radical scavenging assay
The free radical scavenging activity of CCAgNPs was measured by ABTS·+ cation radical decolourization test as per the method described by Re et al. ABTS·+ cation radical was developed by the reaction between 5 mL (2.45 mM) potassium persulfate (K2S2O8) solution and 5 mL of ABTS stock solution. The various concentrations of CCAgNPs were diluted with DMSO to obtain a stock solution. The sample about 5 μL was added to the 195 μL ABTS •+ solution and incubated for 6 min at RT and absorbance noted at 734 nm. It was measured by using the formula and expressed as IC50 (μg/mL) [22].
ABTS·+ scavenging effect (%) = ((AB –AA)/AB) ×100
Where AB is the absorbance of control and AA is the absorbance of the sample
5. In vivo anticancer study
5.1. Animals
Animal study was conducted as per the institutional guidelines of ethical conduct for purpose of use and care of research animals. The protocol for experiment was acceptable by the Institutional Animal Ethical Committee (IAEC) and under the approval number IAEC/SHIATS/PA16III/SDSAV08. Twenty eight healthy albino wistar rats were utilized for the present in vivo study. All the experimental rats were exposed to adapt the standard laboratory condition at RT (25 ± 2 °C) and RH (45–55 %). All the rats were lodged in cages made up of polypropylene and permitted to access water ad libitum and standard diet throughout the research work.
5.1.1. Induction of carcinogen
The tumourgenesis was induced by the single intraperitoneal injection of diethylnitrosamine (DEN) at a dose level (200 mg/kg bw). Cancer was induced in all rats except normal control group.
5.2. Treatment regimen
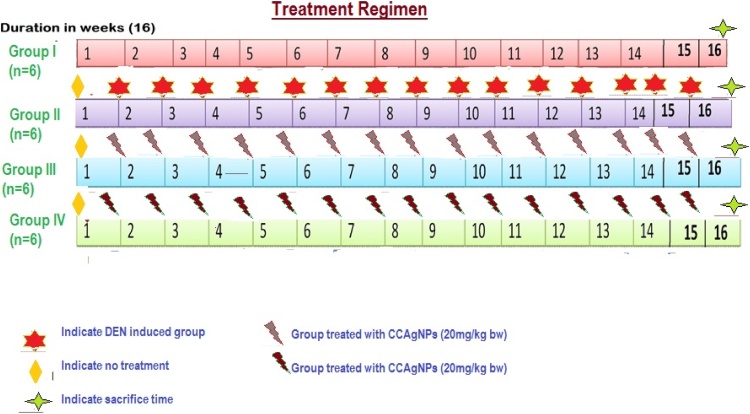
All the rats were categorized into four groups and each group contains six animals as depicted in Fig. 1.
Fig. 1.
Experimental protocol for Carissa carandas embedded silver nanoparticles.
Group I animals received physiologic solution (saline water) and consider as normal control
Group II animals received intraperitoneal injection of DEN (200 mg/kg)
Group III animals received intraperitoneal injection of DEN + CCAgNPs (20 mg/Kg BW)
Group IV animals received intraperitoneal injection of DEN + CCAgNPs (30 mg/Kg BW)
All the animals were euthanized 12 h after the completion of experimental work. Blood from the animals was collected and serum was separated by using centrifuge machine for the determination of urea, creatinine and blood urea nitrogen.
Kidneys were isolated from the animals and washed in isotonic saline (ice cold) solution. It was homogenized in buffer solution (0.1 M Tris−HCl) at the pH range of 7.4 in homogenizer machine for 5 min at 600 rpm.
5.3. Measurement of serum marker enzymes
Kidney homogenate was used to assay the serum marker enzyme such as urea [23], and blood urea nitrogen [24] and creatinine [25].
5.4. Determination of tumour marker enzymes
Kidney homogenate were used to assay the tumour marker enzyme i.e. gamma glutamyl transpeptidase [26], xanthine oxidase [27], and lactate dehydrogenase [28]
5.5. Determination of antioxidant enzymes
5.5.1. Lipid peroxidation
The level of malondialdehyde was determined by the reaction between thiobarbituric acid and acetic acid to produce a pink colour chromophoric product and optical activity read at 532 [29].
5.5.2. Catalase
The activity of catalase was detected by the method as reported by sinha et al. In the presence of hydrogen peroxide, reduction of dichromate in acetic acid to chromic acetate takes place and colorimetrically the chromic acetate was recorded at 610 nm [30].
5.5.3. Glutathione peroxidase
The unreacted GSH amount was used to measure the Glutathione Peroxidase by Ellman’s method. In the presence of GSH, definite amount of enzyme preparation was reacted with hydrogen peroxide for a definite time period [31].
5.5.4. Glutathione S transferase
The method of Habig et al. was used to assay the Glutathione S transferase activity. It is depend on the rate on which conjugate takes place between GSH and 1-chloro-2,4-dinitrobenzene (CDNB). The activity of enzyme was recorded at 340 nm [32].
5.5.5. Reduced glutathione
The method of Ellman was used to assay the Reduced Glutathione activity. It is based on the rate of reaction between 5,5′-dithiobis-2-(nitrobenzoic acid) (DTNB) and free sulphydryl groups to produce chromophoric product and recorded at 412 nm [33].
5.5.6. H2O2 assay
The method of Pick was used to assay the hydrogen peroxide in renal homogenate. It is based on the reaction between horseradish peroxidase, dextrose (5.5 nM)), and phosphate buffer 2 mL of (10 % w/v). The absorbance was read at 610 nm against a blank and was calculated as nmol H2O2/h/g tissue [34].
5.6. Evaluation of cytokines/inflammatory mediators
The levels of proinflammatory cytokines and inflammatory mediators (IL-6, IL-1b, TNF-a, and NF-κB) in the renal tissue of normal control and treated animals were analyzed by specific ELISA kits as per manufacturer instructions. Absorbance of proinflammatory cytokines was calculated at 450 nm spectrophotometrically.
5.7. Estimation of tumour promotion markers
5.7.1. Ornithine decarboxylase (ODC) activity& [3H] thymidine incorporation
ODC activity was done by the method of Byus et al. [35] and calculated as pmol 14CO2 released/h mg protein. The isolation of renal DNA and measurement of incorporation of [3H] thymidine into DNA was explained by Athar et al. [36] and the prepared mixture was placed in a liquid scintillation counter to count the [3H] counting.
5.8. Statistical evaluation
The experimental data were subjected to ANOVA and comparison of the groups is done by Dunnett’s method using Graph pad prism software as considered as statistically significant when (P < 0.001). All the data stated in the experiment are three replicate of means ± standard deviation.
5.9. Histopathological examination
The kidneys of rats were removed after euthanized and stored in a buffered formalin solution (10 %). The kidneys were fixed in paraffin and by the use microtome it was longitudinally sectioned for the histopathological processing. The section was stained by the hematoxylin and eosin and observed under light microscope at the magnification of 40 × .
6. Result
6.1. Structural characterization
6.1.1. FESEM
This study is used to determine the surface morphology, size and shape of the phytofabricated silver nanoparticles. Fig. 2 display the FESEM of silver nanoparticles of C.carandas extract. The image represent that AgNPs were distinct, cauliflower like look and spherical in shape. Biosynthesized AgNPs embedded Phoenix dactylifer extract shows the same result and obtained a spherical shape [37].
Fig. 2.
FESEM images of Carissa carandas embedded silver nanoparticles.
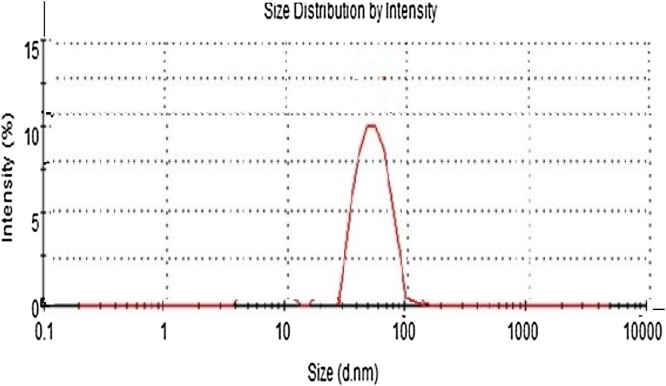
6.1.2. Dynamic light scattering (DLS)
Fig. 6 portrays the DLS graph of CCAgNPs. Dynamic light scattering (DLS) is a sophisticated instrument used to measure the size, size distribution, average diameter and poly dispersity index of particles in suspension. Poly disparity index (PDI) is a determination for distribution of synthesized silver nanoparticles from to 0.5. (PDI > 0.5 =aggregation of particles). This graph suggests that CCAgNPs does not show cluster over all (Fig. 3) [38].
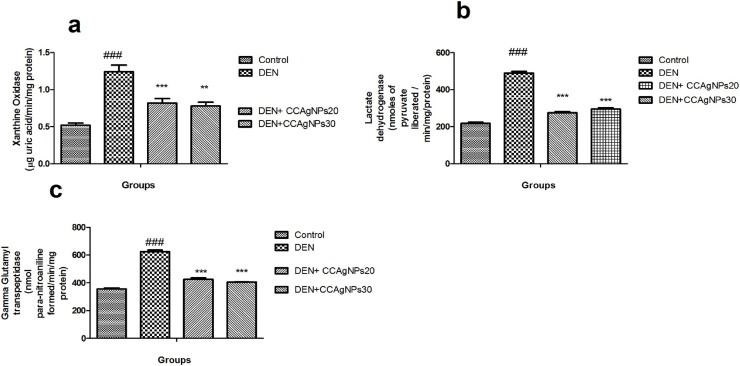
Fig. 6.
Effect of CCAgNPs on tumour marker enzymes.
Results were represented as mean ± SEM of six animals in each group. It was analysed statistically significantly (#p < 0.05, ##p < 0.01, ###p < 0.001) groups compared to normal control; (*p < 0.05, **p < 0.01, ***p < 0.001) groups compared to DEN control; ns -not significant.
Fig. 3.
FESEM images of Carissa carandas embedded silver nanoparticles.
6.2. In vitro antioxidant activity
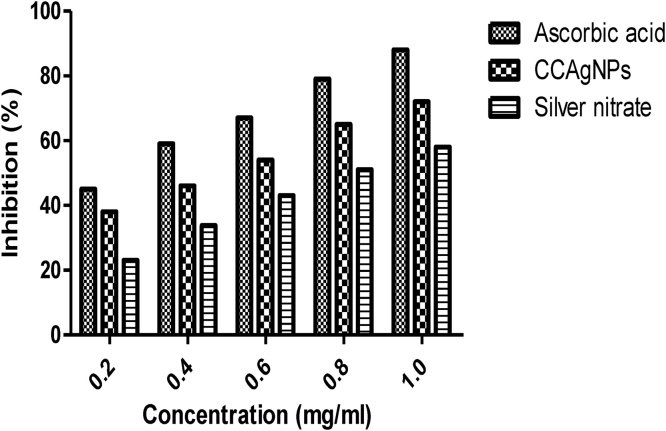
Fig. 4 show that CCAgNPs show antioxidant property, and effective in quenching the ABTS cation radical. ABTS radical was found to be concentration-dependent and supported by the percentage inhibition. The IC50 of CCAgNPs and ascorbic acid was 4.39 and 2.63 mg/mL respectively.
Fig. 4.
in vitro antioxidant study by ABTS scavenging activity of CCAgNPs.
ABTS assay is used as a parameter to determine the antioxidant profile of the drugs. ABTS radical scavenging property have a hydrogen–donating capability and tannins (high molecular weight phenolic) possess a more capacity to scavenge the ABTS•+cation radical [39]. CCAgNPs having a wide range of bioactive molecule which have a capability to quench the free radicals and inhibiting lipid oxidation through chain breaking reaction and signifying that it may be valuable therapeutic agent for the treatment of various free radical related pathological disorder.
6.3. In vivo anticancer activity
6.3.1. Variation in the level of serum marker enzymes of rats
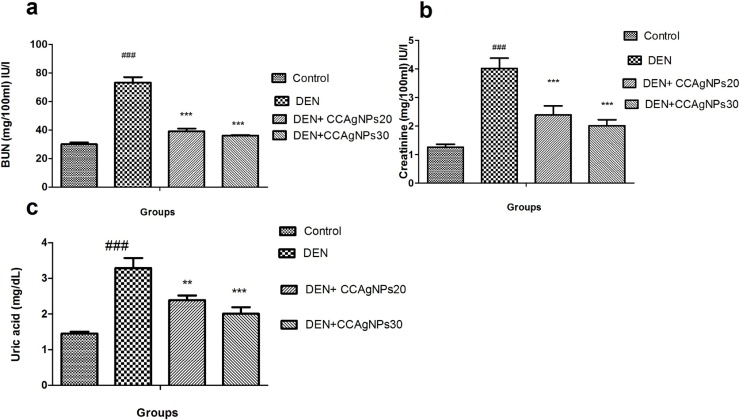
The result of anticancer treatment with CCAgNPs on DEN induced renal cancer in the level of urea, creatinine, and BUN is shown in Fig. 5. Treatment with DEN produced an increment in the levels of renal markers such as urea, creatinine, and BUN when related to the control group. On treatment with CCAgNPs at both doses, a prominent reclamation of the all serum marker enzyme levels was remarked as compared with the DEN treated group.
Fig. 5.
Effect of CCAgNPs on theserum marker enzymes of rats.
Results were represented as mean ± SEM of six animals in each group. It was analysed statistically significantly (#p < 0.05, ##p < 0.01, ###p < 0.001) groups compared to normal control; (*p < 0.05, **p < 0.01, ***p < 0.001) groups compared to DEN control; ns -not significant.
6.3.2. Variation in the level of tumour marker enzymes of rats
It is observe from Fig. 6 that the level of gamma glutamyl transpeptidase, xanthine oxidase, and lactate dehydrogenase were significantly upgraded in DEN induced group II animals when compared to the control rats. CCAgNPs showed a noticeable down regulation in the levels of all enzymes in a dose dependent manner.
6.3.3. Variation in the level of antioxidant enzymes of rats
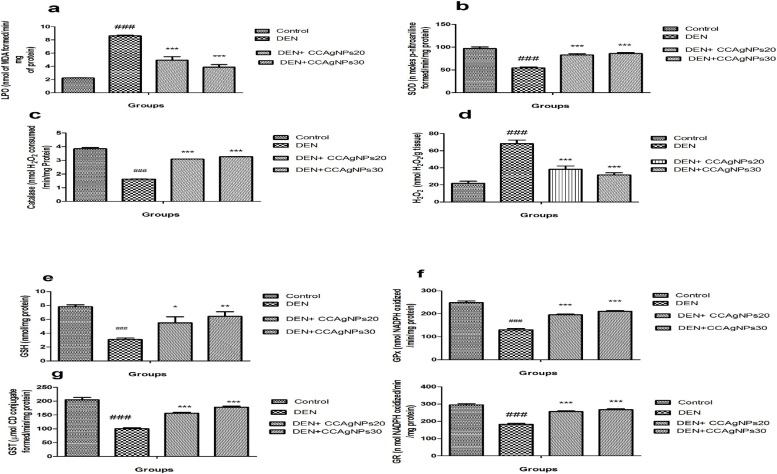
It is declared from Fig. 7 that the level of lipid peroxidation was raised significantly (P > 0.001) in DEN induced group II animals with respect to the control (group I) animals. Introduction of the CCAgNPs significantly ((P > 0.001)) declined the lipid peroxidation index.
Fig. 7.
Effect of CCAgNPs on the levels of endogenous antioxidant enzymes.
Results were represented as mean ± SEM of six animals in each group. It was analysed statistically significantly (#p < 0.05, ##p < 0.01, ###p < 0.001) groups compared to normal control; (*p < 0.05, **p < 0.01, ***p < 0.001) groups compared to DEN control; ns -not significant.
There was significant reduction found in the activities of antioxidant parameter namely, superoxide dismutase, catalase, glutathione peroxidase, and glutathione S transferase, and endogenous antioxidant enzymes was recorded in rats intoxicated with DEN when compared to control. However, rats treated with CCAgNPs with the dose levels of 20 mg and 30 mg/kg bw, the ranges of all these enzymes were significantly upregulated when contrast with normal control.
6.3.4. Proinflammatory cytokines and inflammatory mediators
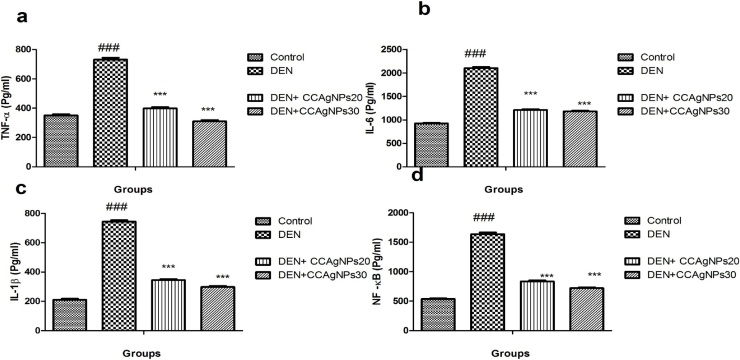
Alteration in the level of proinflammatory cytokines and inflammatory mediators, namely, TNF-, IL-6, NF-κB is enlightened in Fig. 8. DEN intoxication noticeably enhanced the proinflammatory cytokine and inflammatory mediators status of renal tissue when compare to normal control. The current study revealed that plant extract silver nanoparticles attenuated the noxious effect of DEN by reducing the activity of enhanced inflammatory mediators. Dose dependent downregulation was found in the activity, when treated with the different doses of silver nanoparticles.
Fig. 8.
Effect of CCAgNPs on the proinflammatory cytokines and inflammatory mediators.
Results were represented as mean ± SEM of six animals in each group. It was analysed statistically significantly (#p < 0.05, ##p < 0.01, ###p < 0.001) groups compared to normal control; (*p < 0.05, **p < 0.01, ***p < 0.001) groups compared to DEN control; ns -not significant.
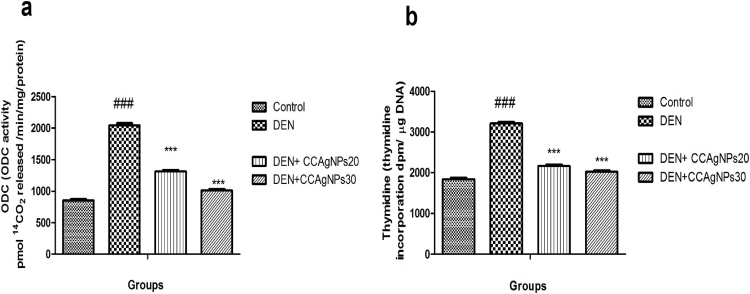
6.3.5. Tumour promotion markers
Wistar rats when subjected to DEN treatment show significant enhancement in thymidine activity as depicted in Fig. 9. Treatment with biofabricated silver nanoparticles of C. carandas leaf aqueous extract caused mark reduction in the raise level of ODC and thymidine in a dose dependent manner till the end of experiment. No significant differences were observed in the in ODC and thymidine of DEN + CCAgNPs (30 mg/kg BW) and normal control.
Fig. 9.
Effect of CCAgNPs on Tumour promotion enzymes.
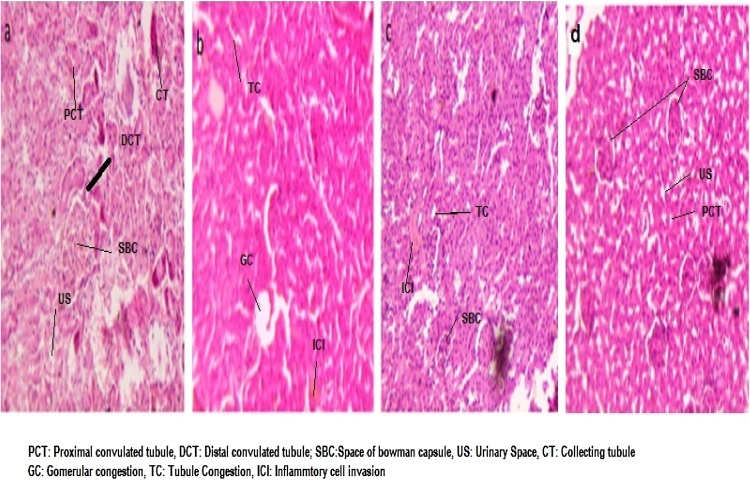
6.3.6. Histopathology of renal tissue
Fig. 10 represented the histopathological studies of renal tissue in DEN induced hepatic cancer. Control groups showed no sign of diseases in renal tissue with normal tubular architecture having normal convoluted tubules and presence of glomeruli within the cortex. DEN induced renal carcinoma group shows abnormal result in structure of renal tissue and has glomerular congestion, tubular congestion, and inflammatory cell invasion. Further treatment with CCAgNPs pointedly attenuated this histopathological changes by reduced inflammatory cell invasion, glomerular congestion and protectives changes in tubules in dose dependent manner. Sections of renal at a higher dose showed attenuated renal histological features which was somewhat similar to control group rats.
Fig. 10.
Photograph of eosin-haematoxylin stained histological renal section area for (a) Normal Control group, (b) DEN induced renal cancer group (c) DEN + CCAgNPs (20 mg/kg bw), (d) DEN + CCAgNPs (30 mg/kg bw).
7. Discussion
Diethylnitrosamine (DEN) is considered as highly toxic environmental carcinogen, which leads to generate reactive oxygen species (ROS) and resulting into cellular damage and oxidative stress. Cytochrome P450, metabolized the DEN in the body to produce highly reactive free radicals, that initiate the process of lipid peroxidation in other organelle of cell. Generated free radicals cause the oxidative injury to DNA, proteins and lipids in the cells [40]. So, the antioxidants agents present in the herbals drugs prevent the kidney against the oxidative stress and nephrotoxicity induced by diethylnitrosamine. In the present study, we investigated that biosynthesized silver nanoparticles could prevent against diethylnitrosamine (DEN) induced renal cancer through the antioxidant property which is retained by the chemical constituent of plant extract.
The current study revealed that the supplementation of diethylnitrosamine had initiated the renal cancer in animals and it was proved by the enhanced levels of the serum markers such as urea, creatinine and uric acids of DEN induced renal carcinogenesis in rats. Rise in the levels of the serum creatinine is an important indicator of renal failure and this range narrates the glomerular function [41]. Upgradation in the levels of uric acid in blood caused hyperuricemia and a sensitive marker of inflammation which takes places at various sites of the body [42]. Administration of biosynthesize silver nanoparticles significantly reduced the levels of urea, creatinine and uric acids and protected the diethylnitrosamine induced alterations in the renal tissue. Similar result was by reported Poornima et al., which extract of Tabernaemontana coronaria showed an anticancer activity against DEN induced clear cell renal cell carcinoma via reduction of urea and creatinine [43].
Significant upregulation was observed in the levels of lactodehydrogenase (LDH), Xanthine oxidase (XO) and Gamma-glutamyl transferase (GGT) which is an indicator for the nephrotoxicity and renal damage. Diethylnitrosamine induced kidney damage which is characterized by the elevations in the activity of LDH, XO and GGT. The rises in the levels of these serum enzymes might be owing to spillage of these cystolic enzymes into the blood stream and responsible for renal damage. This is an indicator for the beginning of kidney damage owing to renal dysfunction, disruption in the biosynthesis of these serum enzymes and change the permeability of the membrane [44]. Treatment with the Carissa carandas silver nanoparticles prevented DEN induced renal injury, by decrease in serum enzymes and maintaining the cellular integrity of the kidney.
Plenty of the long chain polyunsaturated fatty acids in the composition of lipids make the kidney susceptible to impairment caused by free radicals. The breakdown of nitrosamines has been advocated to produce reactive oxygen species. Generated reactive oxygen species initiates cellular damage, fragmentation to DNA, degrades the protein and lipid peroxidation and change the antioxidant defence mechanism [45]. We observed that the DEN induced renal cancer in animals unveiled a significant upregulation in the activity of MDA that shows a serious injury to renal tissue. Admistration of biosynthesized silver nanoparticles significantly attenuates the increased levels of maloaldehyde indicates that either therapy have a potent free radical quenching property. Prasad et al. reported that terminalia chebula extract reduced the level of maloaldehyde in kidney of wistar rats that was induced by DEN and FeNTA [46].
In our study, DEN administration resulted in significant elevation (p < 0.001) in the level of maloaldehyde with the reduction in the glutathione (free thiols), and its oxidized glutathione disulfide which suggest a major redox reaction within the cell. Glutathione (GSH) is a non-enzymatic antioxidant which react directly with thiols groups of reactive oxygen species or involved as a cofactor or coenzyme in the detoxification reaction for reactive oxygen species [47]. Reduction in GSH content may be accredited to the direct conjugation with DEN and its metabolites with free or protein bound-SH groups and significant reduction in the GPx activity with contrast to the control. It was suggested that DEN treatment caused inflammation and disturbed the redox cycle in renal [48]. Treatment with DEN also reduces the levels of other antioxidant enzymes such as GR and GST. Treatment with biosynthesized silver nanoparticles at both the dose levels increased the content of all renal antioxidant enzymes that close to normal range. Therefore we undertake that the nephroprotective activity of biosynthesized silver nanoparticles against DEN induced oxidative stress is partially intervened by protecting GSH reduction and potentiation of the enzymatic antioxidant defences. Siddqi et al. also suggested that Hesperidin reduced the lipid peroxidation and attenuates the antioxidant armory (GSH, GPx, GR, SOD, and catalase)of renal tissue [49].
Exposure to DEN induced the expression of proinflammatory cytokines (TNF-α, IL-6, IL-1β) under direct transcriptional directive of NF-κB. These cytokines plays a crucial role in the vascular permeability, cell multiplication and inflammation. Oxidative stress is also main reason that responsible for the mediation of inflammation because of various proinflammatory cytokines [50]. It also plays an important role in the induction of renal cancer. Redox system also affects the NF-κB regulation and several genes directly involved in the transformation, multiplication and angiogenesis of cell. There is a complex relationship between a reactive oxygen species and NF-κB. Inhibition of NF-κB is a good approach to regulate tumourgenesis and tumour advancement [51]. NF-κB is very delicate to reactive oxygen species and stimulated by strong carcinogens such as DEN. Biosynthesized silver nanoparticles inhibit the secretion of the cytokines and play a major role against DEN induced renal carcinogenesis. Therefore, the toxicity of DEN to a certain extent controlled by the inhibition of NF-κB.
Oxidative stress and inflammation are linked with the tumour promotion during renal nephropathy. ODC is considered as a first and rate limiting enzyme in the biosynthesis of polyamines, involved in DNA synthesis and multiplication of cell. Elevated levels of ODC was observed in transformed cell lines and is closely associated with tumour promotion and tumorigenesis [52]. ODC activity and 3[H] thymidine incorporation is an important biochemical marker tumour promotion and to evaluate the potential of any agent by targeting these [53]. Treatment with DEN in the current study directed to a significant elevation in the ODC activity and 3[H] thymidine incorporation and that was significantly improved by the silver nanoparticles administration. Inhibition of ODC activity and synthesis of DNA discloses that silver nanoparticles may interrupt tumour promotion and polyamine biosynthesis functions. In one study, Singh et al. discussed that the silver nanoparticles of Madhuca longifolia were found to be protective against DEN induced renal cancer via attenuates the ODC activity and 3[H] thymidine incorporation [54].
Morphological examination of renal tissue showed the presence of tumour necrosis and proteinous casts and leads to renal tubular damage [55]. Alteration in the histopathological study of kidneys was restored by higher doses of silver nanoparticles
This study reveals the chemoprotective effect of silver nanoparticles against DEN induced renal cancer. In short, our proposed study suggests that treatment with silver nanoparticles ameliorated DEN induced oxidative stress and inflammation in rats. We focuses on molecular pathways in future that involves in the modulatory mechanism of CCAgNPs on DEN induced renal tumourgenesis.
8. Conclusion
The present study reveals that the phytofabricated silver nanoparticles of Carissa carandas shows a strong nephroprotective effect against DEN induced renal cancer via reducing inflammation and oxidative stress due to presence of chemical constituents that acts as reducing and stabilizing agent.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgements
We are highly thankful to Dr. Abhinav Kumar Singh, Lucknow University and Mr. Ujendra Komal (IIT, Roorkee) for help during research work. The authors are sincerely thankful to Sophisticated Analytical Instrumentation Facilities (SAIF), Punjab University, and Chandigarh for analysis, Maratha Mandal Dental College, Belgaum for help rendered in cytotoxic activity and Shalom Institute of Health and Allied Sciences, Sam Higginbottom University of Agriculture, Technology and Sciences, Allahabad (U. P.) for providing research facilities.
Edited by: DR. A.M Tsatsaka
Contributor Information
Deepika Singh, Email: deepi.chhoti@gmail.com.
Amita Verma, Email: amitaverma.dr@gmail.com.
References
- 1.Shah M., Fawcett D., Sharma S., Tripathy S.K., Poinern G.E.J. Green synthesis of metallic nanoparticles via biological entities. Materials (Basel) 2015 doi: 10.3390/ma8115377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rai M., Yadav A., Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009;27:76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Schröfel A., Kratošová G., Šafařík I., Šafaříková M., Raška I., Shor L.M. Applications of biosynthesized metallic nanoparticles - a review. Acta Biomater. 2014 doi: 10.1016/j.actbio.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 4.Nelayah J., Kociak M., Stéphan O., De Abajo F.J.G., Tencé M., Henrard L., Taverna D., Pastoriza-Santos I., Liz-Marzán L.M., Colliex C. Mapping surface plasmons on a single metallic nanoparticle. Nat. Phys. 2007 doi: 10.1038/nphys575. [DOI] [Google Scholar]
- 5.Jain S., Mehata M.S. Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci. Rep. 2017 doi: 10.1038/s41598-017-15724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yi Z., Li X., Xu X., Luo B., Luo J., Wu W., Yi Y., Tang Y. Green, effective chemical route for the synthesis of silver nanoplates in tannic acid aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2011 doi: 10.1016/j.colsurfa.2011.09.045. [DOI] [Google Scholar]
- 7.Chompoo J., Upadhyay A., Fukuta M., Tawata S. Effect of Alpinia zerumbet components on antioxidant and skin diseases-related enzymes. BMC Complement. Altern. Med. 2012 doi: 10.1186/1472-6882-12-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed R.H., Mustafa D.E. Green synthesis of silver nanoparticles mediated by traditionally used medicinal plants in Sudan. Int. Nano Lett. 2020 doi: 10.1007/s40089-019-00291-9. [DOI] [Google Scholar]
- 9.Horta-Fraijo P., Cortez-Valadez M., Flores-Lopez N.S., Britto Hurtado R., Vargas-Ortiz R.A., Perez-Rodriguez A., Flores-Acosta M. Ultra-small Ag clusters in zeolite A4: antibacterial and thermochromic applications. Phys. E Low-Dimension. Syst. Nanostruct. 2018 doi: 10.1016/j.physe.2017.10.003. [DOI] [Google Scholar]
- 10.Horta-Piñeres S., Britto Hurtado R., Avila-Padilla D., Cortez-Valadez M., Flores-López N.S., Flores-Acosta M. Silver nanoparticle-decorated silver nanowires: a nanocomposite via green synthesis. Appl. Phys. A Mater. Sci. Process. 2020 doi: 10.1007/s00339-019-3178-4. [DOI] [Google Scholar]
- 11.Calderón-Ayala G., Cortez-Valadez M., Martínez-Núñez C.E., Flores-Acosta M. FLG/silver nanoparticles: nanocomposite by green synthesis. Diam. Relat. Mater. 2020;101 doi: 10.1016/j.diamond.2019.107618. [DOI] [Google Scholar]
- 12.Docea Anca Oana, Calina Daniela, Buga Ana Maria, Zlatian Ovidiu, Paoliello M.M.B., Mogosanu George Dan. The effect of silver nanoparticles on antioxidant/pro-oxidant balance in a murine model. Int. J. Mol. Sci. 2020;21:1233. doi: 10.3390/ijms21041233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naguib M., Mahmoud U.M., Mekkawy I.A., Sayed A.E.D.H. Hepatotoxic effects of silver nanoparticles on Clarias gariepinus; biochemical, histopathological, and histochemical studies. Toxicol. Rep. 2020 doi: 10.1016/j.toxrep.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alak G., Yeltekin A.Ç., Tas I.H., Ucar A., Parlak V., Topal A., Kocaman E.M., Atamanalp M. Corrigendum to’ Investigation of 8-OHdG, CYP1A, HSP70 and transcriptional analyses of antioxidant defence system in liver tissues of rainbow trout exposed to eprinomectin’. Fish Shellfish Immunol. 2017;65:136–144. doi: 10.1016/j.fsi.2018.03.032. (S1050464817302103) (10.1016/j.fsi., Fish Shellfish Immunol. (2018) [DOI] [PubMed] [Google Scholar]
- 15.Tolba R., Kraus T., Liedtke C., Schwarz M., Weiskirchen R. Diethylnitrosamine (DEN)-induced carcinogenic liver injury in mice. Lab. Anim. 2015;49:59–69. doi: 10.1177/0023677215570086. [DOI] [PubMed] [Google Scholar]
- 16.Hosohata K. Role of oxidative stress in drug-induced kidney injury. Int. J. Mol. Sci. 2016 doi: 10.3390/ijms17111826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehmood M.H., Anila N., Begum S., Syed S.A., Siddiqui B.S., Gilani A.H. Pharmacological basis for the medicinal use of Carissa carandas in constipation and diarrhea. J. Ethnopharmacol. 2014 doi: 10.1016/j.jep.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 18.Das R.K., Gogoi N., Bora U. Green synthesis of gold nanoparticles using Nyctanthes arbortristis flower extract. Bioprocess Biosyst. Eng. 2011 doi: 10.1007/s00449-010-0510-y. [DOI] [PubMed] [Google Scholar]
- 19.Roopan S.M., Rohit, Madhumitha G., Rahuman A.A., Kamaraj C., Bharathi A., Surendra T.V. Low-cost and eco-friendly phyto-synthesis of silver nanoparticles using Cocos nucifera coir extract and its larvicidal activity. Ind. Crops Prod. 2013 doi: 10.1016/j.indcrop.2012.08.013. [DOI] [Google Scholar]
- 20.Yadav E., Singh D., Yadav P., Verma A. Comparative evaluation of Prosopis cineraria (L.) druce and its ZnO nanoparticles on scopolamine induced amnesia. Front. Pharmacol. 2018 doi: 10.3389/fphar.2018.00549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erjaee H., Rajaian H., Nazifi S. Synthesis and characterization of novel silver nanoparticles using Chamaemelum nobile extract for antibacterial application. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017 doi: 10.1088/2043-6254/aa690b. [DOI] [Google Scholar]
- 22.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999 doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 23.Beale R.N., Croft D. A sensitive method for the colorimetric determination of urea. J. Clin. Pathol. 1961 doi: 10.1136/jcp.14.4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanter M. The Bobber Merrill Company Inc.; USA: 1975. Clinical Chemistry. [Google Scholar]
- 25.Toora B.D., Rajagopal G. Measurement of creatinine by Jaffe’s reaction - Determination of concentration of sodium hydroxide required for maximum color development in standard, urine and protein free filtrate of serum. Indian J. Exp. Biol. 2002 [PubMed] [Google Scholar]
- 26.Orlowski M., Meister A. Isolation of gamma-glutamyl transpeptidase from hog kidney. J. Biol. Chem. 1965 [PubMed] [Google Scholar]
- 27.Fried R., Fried L.W. Methods Enzym. Anal. 1974. Xanthine oxidase (xanthine dehydrogenase) [DOI] [Google Scholar]
- 28.Ec L., Nad L. Lactate dehydrogenase biochemistry and fwnction of lactate dehydrogenase. Biochemistry. 1984 doi: 10.1002/cbf.290020302. [DOI] [Google Scholar]
- 29.Devasagayam T.P.A., Boloor K.K., Ramasarma T. Methods for estimating lipid peroxidation: an analysis of merits and demerits. Indian J. Biochem. Biophys. 2003 [PubMed] [Google Scholar]
- 30.Sinha A.K. Colorimetric assay of catalase. Anal. Biochem. 1972 doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 31.Rotruck J.T., Pope A.L., Ganther H.E., Swanson A.B., Hafeman D.G., Hoekstra W.G. Selenium: biochemical role as a component of glatathione peroxidase. Science (80-.) 1973 doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 32.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974 doi: 10.14026/j.cnki.0253-9705.2010.23.013. [DOI] [PubMed] [Google Scholar]
- 33.Sedlak J., Lindsay R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968 doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 34.Pick E., Keisari Y. Superoxide anion and hydrogen peroxide production by chemically elicited peritoneal macrophages-induction by multiple non phagocytic stimuli. Cell. Immunol. 1981;59:301–318. doi: 10.1016/0008-8749(81)90411-1. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez G.G., Byus C.V. Effect of dietary arginine restriction upon ornithine and polyamine metabolism during two-stage epidermal carcinogenesis in the mouse. Cancer Res. 1991 [PubMed] [Google Scholar]
- 36.Athar M., Iqbal M. Ferric nitrilotriacetate promotes N-diethylnitrosamine-induced renal tumorigenesis in the rat: implications for the involvement of oxidative stress. Carcinogenesis. 1998;19:1133–1139. doi: 10.1093/carcin/19.6.1133. [DOI] [PubMed] [Google Scholar]
- 37.Aitenneite H., Abboud Y., Tanane O., Solhy A., Sebti S., El Bouari A. Rapid and green microwave-assisted synthesis of silver nanoparticles using aqueous Phoenix dactylifera L. (date palm) leaf extract and their catalytic activity for 4-Nitrophenol reduction. J. Mater. Environ. Sci. 2016 [Google Scholar]
- 38.Rashid M.I., Mujawar L.H., Rehan Z.A., Qari H., Zeb J., Almeelbi T., Ismail I.M.I. One-step synthesis of silver nanoparticles using Phoenix dactylifera leaves extract and their enhanced bactericidal activity. J. Mol. Liq. 2016 doi: 10.1016/j.molliq.2016.09.030. [DOI] [Google Scholar]
- 39.Miller N.J., Rice-Evans C.A. Factors influencing the antioxidant activity determined by the ABTS •+ radical cation assay. Free Radic. Res. 1997 doi: 10.3109/10715769709097799. [DOI] [PubMed] [Google Scholar]
- 40.El-Tohamy M.M. The mechanisms by which oxidative stress and free radical damage produces male infertility. Life Sci. J. 2012 [Google Scholar]
- 41.Perrone R.D., Madias N.E., Levey A.S. Serum creatinine as an index of renal function: new insights into old concepts. Clin. Chem. 1992 doi: 10.1590/S0101-28002011000200023. [DOI] [PubMed] [Google Scholar]
- 42.Ronco C., Inguaggiato P., Bordoni V., De Cal M., Bonello M., Andrikos E., Assuman Y., Rattanarat R., Bellomo R. Rasburicase therapy in acute hyperuricemia and renal dysfunction. Contrib. Nephrol. 2005 doi: 10.1159/000082549. [DOI] [PubMed] [Google Scholar]
- 43.Poornima K., Gopalakrishnan V.K. Anticancer activity of Tabernaemontana coronaria against carcinogen induced clear cell renal cell carcinoma. Chin. J. Biol. 2014 doi: 10.1155/2014/584074. [DOI] [Google Scholar]
- 44.Sadik N.A.H., El-maraghy S.A., Ismail M.F. Diethylnitrosamine-induced hepatocarcinogenesis in rats: possible chemoprevention by blueberries. Afr. J. Biochem. Res. 2008 [Google Scholar]
- 45.Greggi Antunes L.M., Darin J.D.A.C., Bianchi M.D.L.P. Effects of the antioxidants curcumin or selenium on cisplatin-induced nephrotoxicity and lipid peroxidation in rats. Pharmacol. Res. 2001 doi: 10.1006/phrs.2000.0724. [DOI] [PubMed] [Google Scholar]
- 46.Prasad L., Khan T.H., Jahangir T., Sultana S. Abrogation of DEN/Fe-NTA induced carcinogenic response, oxidative damage and subsequent cell proliferation response by Terminalia chebula in kidney of wistar rats. Pharmazie. 2007;62:790–797. doi: 10.1691/ph.2007.10.6092. [DOI] [PubMed] [Google Scholar]
- 47.Jahangir T., Sultana S. Modulatory effects of shape Pluchea Lanceolata against chemically induced oxidative damage, hyperproliferation and two-stage renal carcinogenesis in wistar rats. Mol. Cell. Biochem. 2006 doi: 10.1007/s11010-006-9213-8. [DOI] [PubMed] [Google Scholar]
- 48.Hayes J.D., McLellan L.I. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic. Res. 1999 doi: 10.1080/10715769900300851. [DOI] [PubMed] [Google Scholar]
- 49.Siddiqi A., Saidullah B., Sultana S. Anti-carcinogenic effect of hesperidin against renal cell carcinoma by targeting COX-2/PGE2 pathway in wistar rats. Environ. Toxicol. 2018;33:1069–1077. doi: 10.1002/tox.22626. [DOI] [PubMed] [Google Scholar]
- 50.Hübner G., Brauchle M., Smola H., Madlener M., Fässler R., Werner S. Differential regulation of pro-inflammatory cytokines during wound healing in normal and glucocorticoid-treated mice. Cytokine. 1996 doi: 10.1006/cyto.1996.0074. [DOI] [PubMed] [Google Scholar]
- 51.Hoesel B., Schmid J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer. 2013 doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kahana C. Regulation of cellular polyamine levels and cellular proliferation by antizyme and antizyme inhibitor. Essays Biochem. 2009 doi: 10.1042/bse0460004. [DOI] [PubMed] [Google Scholar]
- 53.Prasad L., Khan T.H., Jahangir T., Sultana S. Effect of luteolin on nickel chloride-induced renal hyperproliferation and biotransformation parameters in wistar rats. Pharm. Biol. 2007 doi: 10.1080/13880200601113057. [DOI] [Google Scholar]
- 54.Singh D., Yadav E., Kumar V., Verma A. Madhuca longifolia embedded silver nanoparticles attenuates diethylnitrosamine (DEN) -induced renal cancer via regulating oxidative stress. Curr. Drug Deliv. 2020;17 doi: 10.2174/1567201817666200910154301. [DOI] [PubMed] [Google Scholar]
- 55.Ahmed M.M., Ali S.E. Protective effect of pomegranate peel ethanol extract against ferric nitrilotriacetate induced renal oxidative damage in rats. J. Cell Mol. Biol. 2010 [Google Scholar]