Highlights
-
•
During early- to mid-childhood, subfield development differs along the longitudinal axis of the hippocampus.
-
•
CA1 subfield volume increases between 4−5 years in hippocampal head.
-
•
CA2-4/DG and subiculum volume increases between 5−6 years in hippocampal body.
-
•
Changes in CA1 and subiculum between 4−5 years relate to memory between 4−5 years.
Keywords: Structural equation modeling, Longitudinal, Hippocampal subfields, Volumetric changes, Development
Abstract
The hippocampus has been suggested to show protracted postnatal developmental growth across childhood. Most previous studies during this developmental period have been cross-sectional in nature and have focused on age-related differences in either hippocampal subregions or subfields, but not both, potentially missing localized changes. This study capitalized on a latent structural equation modeling approach to examine the longitudinal development of hippocampal subfields (cornu ammonis (CA) 2-4/dentate gyrus (DG), CA1, subiculum) in both the head and the body of the hippocampus, separately, in 165 typically developing 4- to 8-year-old children. Our findings document differential development of subfields within hippocampal head and body. Specifically, within hippocampal head, CA1 volume increased between 4−5 years and within hippocampal body, CA2-4/DG and subiculum volume increased between 5−6 years. Additionally, changes in CA1 volume in the head and changes in subiculum in the body between 4−5 years related to improvements in memory between 4−5 years. These findings demonstrate the protracted development of subfields in vivo during early- to mid-childhood, illustrate the importance of considering subfields separately in the head and body of the hippocampus, document co-occurring development of brain and behavior, and highlight the strength of longitudinal data and latent modeling when examining brain development.
1. Introduction
Although much of brain development takes place in the first years of life (Gilmore et al., 2018), one structure that shows protracted growth across childhood is the hippocampus. This structure can be divided along its longitudinal axis into subregions (head, body, and tail; Poppenk et al., 2013) and into functional subunits (subfields; Lavenex and Banta Lavenex, 2013). Both divisions relate to behavior; however, most previous studies, particularly in early childhood, have focused on only one of these dimensions. The goal of this study is to begin to bridge the gap between these literatures by examining, longitudinally, age-related changes in hippocampal subfields in both the head and the body of the hippocampus via a latent approach and how these changes relate to memory performance during early childhood.
Extant research examining hippocampal subregion development in young children (e.g., 4- to 6-year-olds) has shown age-related increases in head volume (Canada et al., 2020; Riggins et al., 2018), increases in body volume (Canada et al., 2020), and mixed results in the tail (likely due to methodological differences and/or whether samples were cross-sectional or longitudinal; Canada et al., 2020; Gogtay et al., 2006; Riggins et al., 2018; Tamnes et al., 2018). These studies also suggest volume of hippocampal subregions relate to memory performance, perhaps due to differences in connectivity between subregions and the cortex (e.g., Poppenk et al., 2013). However, few of these studies considered the development of hippocampal subfields and utilized analytic approaches that are not able to characterize the amount of change occurring between each time point nor capitalize on the precision offered by latent modeling approaches.
Most studies examining hippocampal subfields (dentate gyrus, DG; cornu ammonis, CA1-CA4; subiculum) have included older children (i.e., > 6 years of age; Daugherty et al., 2016, 2017; Keresztes et al., 2017; Lee et al., 2014; Schlichting et al., 2017; Tamnes et al., 2018). These studies suggest age-related differences in the volume of CA1 and CA2-4/DG relate to performance on individual tasks examining episodic memory (i.e., Daugherty et al., 2017; Lee et al., 2014; Riggins et al., 2018; Schlichting et al., 2017). However, previous studies often restrict examination of subfields to the hippocampal body (or a subportion; Daugherty et al., 2016; Yushkevich et al., 2015a) or collapse across the head and body (Tamnes et al., 2018) because subfields are difficult to delineate in the head and tail due to greater variability (due to both inter- and intra-subject factors, reliably identifying subfields in tail is not yet possible, see Yushkevich et al., 2015a). This is potentially problematic since subfields of the hippocampus are disproportionally distributed along the anterior-posterior axis. Thus, these studies may miss localized developmental changes.
The few studies examining age-related differences of hippocampal subfields within head and body subregions separately have yielded mixed findings. For example, age-related differences in CA1 head volume have been characterized by a positive quadratic relation in 4- to- 8-year-olds (Riggins et al., 2018) and negative linear relation in 6- to 30-year-olds (Schlichting et al., 2017). These age-related differences in CA1 head volume were related to source memory (Riggins et al., 2018) and associative inference (Schlichting et al., 2017), although the direction of these effects varied.
Of the above-mentioned studies, none examined longitudinal changes in hippocampal subfield development during early childhood and most research examining development of the hippocampus during childhood has primarily used cross-sectional designs (e.g., Keresztes et al., 2017; Riggins et al., 2018; Schlichting et al., 2017). However, cross-sectional approaches are notoriously under-powered and inter-subject differences in the brain are known to be high. Moreover, the effects that can be observed at the level that MRI can detect are likely small, and cross-sectional samples require far more participants than longitudinal samples to detect small volumetric differences in the brain (Steen et al., 2007). Thus, such effects are likely under-reported. Last, although informative, cross-sectional studies do not address questions of developmental change, as longitudinal data is required for such questions (Haller et al., 2018).
Researchers have started to address the methodological concerns noted above (Mills and Tamnes, 2014; Sankar et al., 2017), by utilizing structural equation modeling (SEM) to model age-related differences in subfield volumes (Daugherty et al., 2017). Critically, SEM offers a flexible framework, the ability to query both intra- and inter-individual changes, and accommodation of both measured and latent variables (i.e., unobserved theoretical constructs), which allows for a hypothetically error-free underlying construct. For example, in a cross-sectional sample of 8- to 25-year-olds, SEM was utilized to study subfield development by using measures from left and right hemispheres in hippocampal body to indicate a latent construct of each subfield while accounting for measurement error (Daugherty et al., 2017). The SEM framework also affords flexibility in specifying error structures, including errors that are correlated between measurement occasions (Tisak and Tisak, 1996), making it especially well-suited to asking questions of change that require longitudinal data. Further, the use of latent modeling addresses questions of individuals differences in brain development as it can accommodate heterogeneity often observed and related outcomes (Becht and Mills, 2020; King et al., 2018).
The present study capitalized on the strength of SEM and latent variable modeling in order to characterize developmental changes in hippocampal subfields in both the head and body of the hippocampus during early childhood, a period in which protracted development of these functional subunits is thought to occur and in which subfields may show differential change along the longitudinal axis of the hippocampus. Given prior work examining the development of subfields in non-human primates (Lavenex and Banta Lavenex, 2013) and post-mortem humans (Seress, 2001) suggesting the earliest maturation of subiculum (Jabès et al., 2011; Lavenex et al., 2004), followed by CA1, and the most protracted development in DG and CA3, we predicted developmental increases in CA1 and CA2-4/DG volumes, but not subiculum, between the period of 4–8 years. In addition, we explored the extent to which developmental changes in subfield volumes relate to changes in memory. We predicted changes in CA1 and CA2-4/DG would relate, primarily between 4–6 years.
2. Method
2.1. Participants
The current study was part of a larger research project examining the development of the brain in relation to memory. Prior to data collection, all methods were approved by the University’s Institutional Review Board. Hippocampal subfields from this sample have been previously examined cross-sectionally (Riggins et al., 2018) and longitudinal analyses examining hippocampal subregions are reported in Canada et al. (2020). However, hippocampal subfield data have not yet been examined using the longitudinal sample nor using a latent approach allowing for greater measurement precision. Children were screened via caregiver self-report to ensure they were not born premature (via gestational age), had normal or corrected-to-normal vision, and had no diagnoses for any neurological conditions, developmental delays, or disabilities. Informed consent was obtained from parents, written assent was obtained for children aged 7 years or older than, and verbal assent was obtained for children younger than 7 years.
A total of 200 4- to 8-year-old children (100 reported females, 100 reported males) participated in the current study. A cohort-sequential (i.e., accelerated longitudinal) design was employed with cohorts overlapping at age 6 years in order to simulate a longer longitudinal trajectory. By simulating a traditional longitudinal design, the goal was to assess the developmental change that occurs during this period (Duncan et al., 1996). Of the 200 children who participated in the study at wave 1, 96 were invited to participate in subsequent visits and were included as the longitudinal cohorts. Children who were recruited at age 4 years or age 6 years were invited back at two subsequent waves and provided data maximally at 3 waves. Children in the cross-sectional cohorts (i.e., recruited at 5, 7, or 8 years of age) provided data only at one wave.
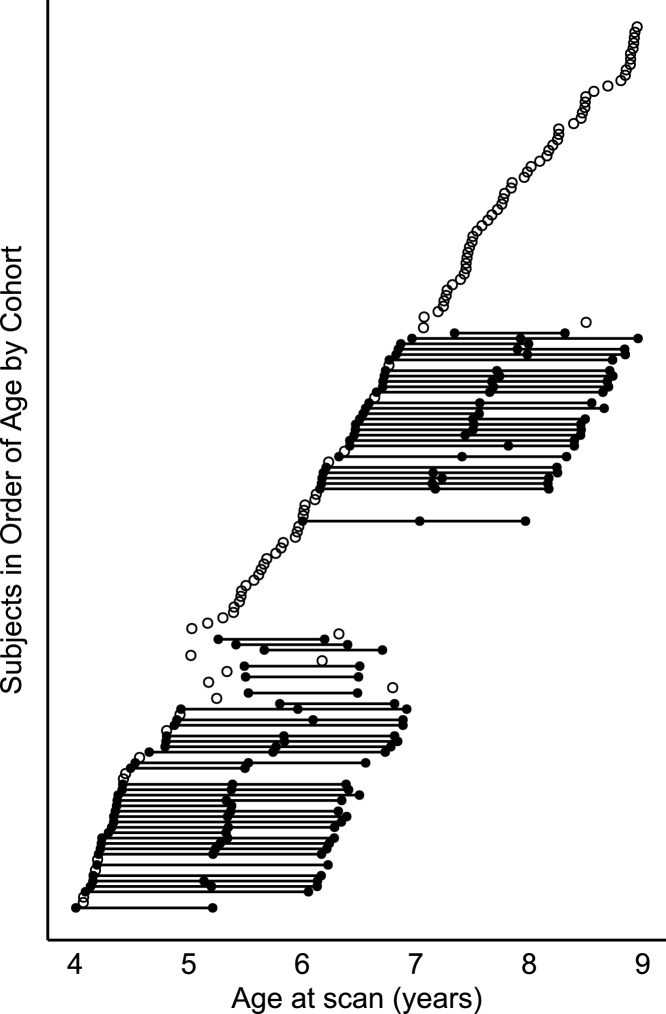
Younger age groups were oversampled to ensure enough usable data would be available and because participants were being followed longitudinally. Of the 200 subjects recruited, 165 participants provided useable neuroimaging data for this report and a total of 267 scans. Specifically, 102 participants provided data at only one time point, 24 children provided data at two time points, and 39 children provided data at all three time points. For the distribution of children’s age at each scan see Fig. 1. The final sample of 165 participants (88 reported females, 77 reported males) was approximately 58% Caucasian, 13% African American, 5% Asian, and 21% Multiracial from middle- to high-income households (median = >$105,000, range = <$15,000–>$105,000). An additional 3% of parents did not disclose their child's race and 4% did not disclose income. Eighty-eight percent of the sample had at least one parent who attended a 4-year college.
Fig. 1.
Age distribution of the sample. Each dot represents a single scan. Solid black dots connected by straight lines represent multiple scans from the same participant. Unfilled dots represent subjects who provided a single scan.
2.2. Materials and procedures
2.2.1. Magnetic resonance imaging
All participants completed training in a mock scanner before MR data acquisition in order to become acclimated to the scanner environment and receive motion feedback. Participants were scanned in a Siemens 3.0-T scanner (MAGNETOM Trio Tim System, Siemens Medical Solutions, Erlangen, Germany) using a 32-channel coil. An initial structural scan was acquired using a high-resolution T1 magnetization-prepared rapid gradient-echo (MPRAGE) sequence consisting of 176 contiguous sagittal slices (0.9 mm isotropic; 1900 ms TR; 2.32 ms TE; 900 ms inversion time; 9° flip angle; pixel matrix = 256 × 256). This was used to estimate intracranial volume (eICV) and isolate the hippocampus for a subsequent ultra-high-resolution structural scan using a T2-weighted fast spin echo sequence that was collected perpendicularly to the longitudinal axis of the hippocampus (TR = 4120 ms, TE = 41 ms, 24 slices, 149° flip angle, voxel size 0.4 mm × .4 mm × 2 mm). Intracranial volume was estimated (i.e., eICV) for each participant using Freesurfer (Version 5.1.0), which has been shown to be appropriate for use in children as young as 4 years of age (Ghosh et al., 2010).
Hippocampal subfield volumes were identified in the head and body of the hippocampus in both left and right hemispheres using an existing protocol (La Joie et al., 2010) based on Duvernoy (1998) and Harding et al. (1998). The protocol was selected after existing protocols for manual tracing of hippocampal subfields were reviewed (n = 21, see Yushkevich et al., 2015b). Protocols developed for T2-weighted images with resolution similar to data in this study and collected from 3 T scanners were compared. Although several exist, we selected a protocol (La Joie et al., 2010) that yielded the subfields of interest in both the head and body subregions of the hippocampus at the desired resolution (.4 mm × .4 mm × 2 mm) on a 3 T scanner (but see also Berron et al., 2017; Winterburn et al., 2013). This protocol was selected because previous research in children has suggested developmental effects may be present in both the hippocampal head and body (DeMaster et al., 2014; Riggins et al., 2015, 2018). Similar to La Joie et al. (2010), seven different slice types were identified from coronal slices and used for manual segmentation (see La Joie et al., 2010; Riggins et al., 2018 for details). Three subfields were identified: subiculum (including pre/para subiculum), CA1, and a combination region of CA2-4/dentate gyrus (CA2-4/DG). Details regarding identification of internal and external boundaries are reported in Riggins et al. (2018).
Two raters blinded to the age and sex of the subjects independently traced 10 cases (2 from each of the 5 age groups) bilaterally. Dice Similarity Coefficients (DSC) were calculated to determine overlap between raters and are as follows for each subfield: Subiculum (head) = .74, Subiculum (body) = .73, CA1 (head) = .70, CA1 (body) = .78, CA2-4/DG (head) = .81, CA2-4/DG (body) = .87. DSC values above 0.7 are typically considered acceptable for agreement (Zijdenbos et al., 1994), as such, overlap between the two raters indicated agreement. Intra-class correlations (ICC (2,1); Shrout and Fleiss, 1979) were also calculated to determine reliability of the volume measurement and are as follows for each subfield: Subiculum (head) = .94, Subiculum (body) = .56, CA1 (head) = .97, CA1 (body) = .74, CA2-4/DG (head) = .94, CA2-4/DG (body) = .90. ICC values above .90 are typically considered highly reliable, values between 0.75 and 0.9 indicate good reliability, and values between 0.5 and 0.75 indicate moderately reliable, indicating consistency in the volume measurements (Koo and Li, 2016). Given what is known from animal models (e.g., Lavenex and Banta Lavenex, 2013) and the relations documented between subfields and memory (Daugherty et al., 2017), our focus was on CA1 and CA2-4/DG more so than subiculum. Results regarding subiculum in hippocampal body should be considered with caution; although agreement between raters was acceptable, reliability was low, likely as a result of the inferior and medial boundary for the subiculum being less well characterized in the protocol. These values are comparable to those from a separate developmental sample examining subfields along the longitudinal axis (ICCs: .61–.92; Schlichting et al., 2017).
One rater then traced an additional 10 cases (again, 2 from each age group). These 10 segmentations were combined with the 10 cases used for manual reliability and the 20 total cases (with 4 subject per age group) input into ASHS (Yushkevich et al., 2015a) to create a study-specific template. This study-specific template was used to generate hippocampal subfield volumes for the entire sample. All resulting segmentations were checked visually for quality. No manual edits were made, and only data from subjects yielding high-quality segmentations were included in the present study. Segmentations were excluded due to failed segmentation (i.e., volumes only partially segmented) or boundary errors that clearly deviated from the manual protocol. Overall, 9 segmentations were excluded at wave 1, 6 segmentations at wave 2, and 6 segmentations at wave 3.
2.2.2. Source memory
To assess children’s cognitive development, a source memory task was used that is thought to assess episodic memory by testing children’s memory for novel items and the contextual details surrounding these items (Drummey and Newcombe, 2002; Riggins, 2014). At each wave of the study, the source memory task was administered across 2 visits in the lab that were separated by approximately 7 days. During the first visit, children watched digital videos in which they were taught 12 novel facts (e.g., “A group of rhinos is called a crash”), six each from one of two different sources: a person or a puppet. Children were instructed to remember the facts but were not told they needed to pay attention to the source. Children were not told they would be tested on the source of the facts. Before the answer to each fact question was given, children were asked if they knew the fact (e.g., “Do you know what a group of rhinos is called?”). If they answered correctly that fact was excluded at testing and an additional novel fact from the same list and source was presented.
Three lists of facts were created, consisting of unique facts that were similar across lists (e.g., “A group of kangaroos is called a mob” or “A group of goats is called a tribe”). Lists were randomly assigned across participants. For each list, sources had 8 possible facts. Consequently, if a child knew 3 or more facts from a source, the total number of facts the child was tested on was reduced (but this was a rare case, n = 16 across all waves). Presentation of facts was blocked by source, with 6 facts from the first source (e.g., person) followed by 6 facts from the second source (e.g., puppet). Order of the blocks was randomly assigned across participants. To ensure that longitudinal participants did not receive the same facts in subsequent years, participants who received facts from List 1 at wave 1 received List 2 at wave 2 and List 3 at wave 3, and so on. During the second visit, children were tested on their memory for both the novel facts and their source. Children were asked to answer 22 fact questions. Children were told that they had learned some of the questions the week before from the “puppet” or “person,” some they might have learned outside the laboratory (e.g., from a teacher or parent), and some they may not know. Each test list of 22 facts had two random presentation orders, with orders counterbalanced across participants. Six of the 22 facts had been presented by the person, six facts by the puppet, five were facts commonly known by children, and five were facts that children typically would not know. Each question was asked (e.g., “What is a group of rhinos called?”) and the child was given the opportunity to answer freely. If the child indicated they did not know the answer, they were given four pre-determined multiple-choice options (“hints”; e.g., Mob, Crash, Herd, or School). Once the child gave an answer, they were asked where or from whom they had learned that information. If children indicated they did not know the answer, they were given pre-determined multiple-choice options: parent, teacher, person in the video, puppet in the video, or just knew/guessed.
Source memory was calculated as the proportion of questions where the child provided an accurate answer for both the fact and the source of the fact.
2.3. Analytical framework: structural equation modeling
2.3.1. Latent growth models
A class of SEM, Latent Growth Modeling, is particularly well suited to analyze longitudinal data in the interest of examining developmental change over time (McArdle, 2009). Specifically, the goal of a latent growth model (LGM) is to describe the trajectory of change (Duncan and Duncan, 2009; Ghisletta and McArdle, 2001). To address questions of developmental change and individual differences in change over time, LGMs use both covariance and means structures. The covariance structure contains the information that can inform questions about individual differences in how children develop, whereas the means structure contains information at the group level (Kievit et al., 2018). The means of the latent growth factors (latent intercept and slopes) can be modeled by introducing a pseudovariable that assumes a constant score of 1 for all participants (Hancock et al., 2013). This variable has no variance, and consequently, does not impact the rest of the model (Thompson and Green, 2013). The main models in this study are second-order latent growth models (Hancock et al., 2001). As such, the growth factors capture information about change in the latent constructs of interest, hippocampal subfields. LGMs are capable of handling both unbalanced and incomplete data and are well suited for the analyses used in this study (Hancock et al., 2013). Although missingness of data was planned in the design of the current study (i.e., cohort-sequential; Duncan et al., 1996), additional data loss occurred due to attrition (e.g., families moving out of the area) and poor quality (e.g., due to motion). The 26 children recruited to the longitudinal sample who provided data at wave 1 but dropped out at wave 2 or wave 3 did not differ significantly in age, reported sex, or SES from participants who returned for subsequent visits. Consequently, data did not violate conditions to be considered missing at random and the current study utilized robust full information maximum likelihood (FIML) estimation in accommodating missing data.
Multi-group latent growth modeling (Ghisletta and McArdle, 2001; Hancock et al., 2013) was used in Mplus 8.2 (Muthén and Muthén, 1998-2017; Muthén and Muthén, 1998) in order to examine developmental changes in hippocampal subfields. Two cohorts, one starting at age 4 years and the other at age 6 years and overlapping at one time point, were measured at one-year intervals over a three-year period. Using this overlap, the longitudinal trajectory between age 4 to age 8 was estimated. In the current study, the ability to detect different rates of change between developmental time points was of interest. Consequently, piecewise (i.e., spline) latent growth models (Hancock et al., 2013; Meredith and Tisak, 1990) were used. Piecewise models can accommodate variations that best suit the developmental question at hand because of their sensitivity to detect different rates of change across time. For example, these models can include one transition point (e.g., age 6, where 4–6 and 6−8 are separate slopes) or transitions at each time point (e.g., 4−5, 5−6, 6−7, 7–8).
2.3.2. Modeling hippocampal subfield development
Subfields in the head and body were modeled separately based on work showing age-related differences in the contribution of subfields to each subregion and differences in the proposed functional significance between subfields in head and body. Each subject provided neuroimaging data, maximally, at three different waves for each cohort. As noted previously, planned missingness is present in the data.
Latent constructs of hippocampal subfields in the body were identified by left and right hemisphere measures. This model construction uses the commonality of the two measures to indicate the latent construct while removing measurement error and thereby produces error-free estimates of the hypothesized effects. At least two measures are required to indicate a latent construct apart from measurement error (i.e., left and right hemisphere volumes), and thus laterality (i.e., left versus right hemisphere) of effects could not be tested.
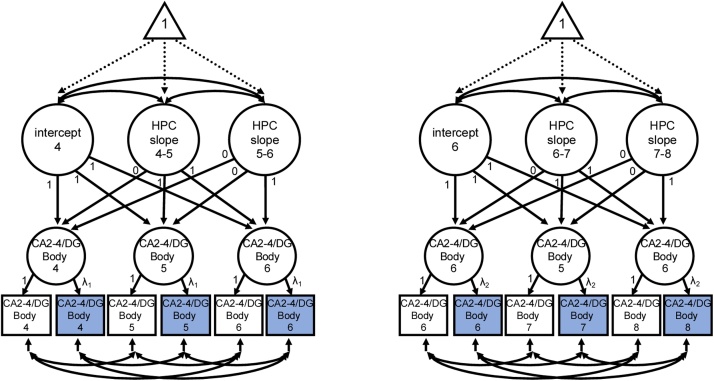
To indicate latent constructs of hippocampal subfields, loadings of right hemisphere indicators (e.g., CA2-4/DG in right body at age 4, 5, and 6) were constrained to 1 at each wave to scale the construct. For hippocampal head, loadings of left hemisphere indicators were constrained to be equal across waves within cohorts, with the exception of measurements at age 6 (e.g., CA2-4/DG in left body age 4, 5). For hippocampal body, loadings of left hemisphere indicators were constrained to be equal across waves (e.g., CA2-4/DG in left body age 4, 5, and 6) within cohorts. To estimate latent intercepts at 4- and 6-years, loadings of the first-order hippocampal subfield factors were constrained to 1. The loadings on the first slope factor (either between ages 4−5 or between ages 6−7) were constrained to 0 at wave 1, 1 at wave 2, and 1 at wave 3 to estimate the growth occurring between the initial measurement occasion and the change one year later. The loadings on the second slope factor (either between ages 4−5 or between ages 6−7) were constrained to 0 at wave 1, 0 at wave 2, and 1 at wave 3 to estimate the growth occurring between the second measurement occasion and the change one year later (the third measurement occasion). Intercepts of indicator variables were also constrained to be equal across time points in order to reflect that change in indicator variables should start at the same point (Hancock et al., 2001). Error variance of each measured variable (i.e., indicator) was freely estimated except in instances where constraints facilitated model convergence (i.e., subiculum body in the 4-year-old cohort). Error covariance parameters were estimated for each indicator across measurement waves, as it is likely that other aspects of the measurement tool (i.e., ASHS) not explained by the latent construct of hippocampal subfields will relate to each other.
Twelve models were tested, three per subfield (CA2-4/DG, CA1, subiculum) in both hippocampal head and body for each cohort (Fig. 2). Parameters of interest included means and variances for the intercept (i.e., differences in initial subfield volume) and slope between each age transition (e.g., change in subfield volume between age 4 and age 5). Statistical significance of model parameters was determined using Wald tests.
Fig. 2.
Conceptual diagram of second-order piecewise latent growth model for a selected hippocampal subfield (CA2-4/DG) in hippocampal body. Models in hippocampal head relaxed the loading and intercept constraint at age 6 in each cohort. Primary hypothesis models tested age-related changes in hippocampal subfield volume within each cohort separately with convergence tested at age 6 years between cohorts. Note. HPC = hippocampus.
To ensure that changes observed were not simply the result of changes in overall head size, raw hippocampal subfield volumes were adjusted for eICV. The adjustment was done using an analysis of covariance approach (Raz et al., 2005). Age and sex were used to estimate eICV values using the following formula (adjusted volume = raw volume – b * (eICV – predicted eICV, see Keresztes et al., 2017). Separate adjustments were performed for each wave of data collection. Additionally, because previous work has documented that different ICV adjustment methods can lead to spurious results (Perlaki et al., 2014; Pintzka et al., 2015), models were also run with raw volumes and results compared those from models of eICV-adjusted volumes. Given the high similarity between results for raw versus eICV-adjusted volumes, only model parameters for eICV-adjusted volumes are reported.
2.3.3. Modeling parallel development of hippocampal subfield and episodic memory
To assess the relation between cognition and hippocampal subfield volumes, piecewise models similar to those modeling hippocampal development were utilized. To facilitate identification of these models, composite measures of hippocampal subfields and performance on a source memory task were modeled across time by cohort. Specifically, measures of volume in left and right hemisphere were averaged for each subfield (i.e., CA2-4/DG, CA1, and subiculum). The source memory task was selected as a measure of cognition given its well-document use in the literature as a measure of children’s episodic memory ability (e.g., Drummey and Newcombe, 2002; Riggins, 2014; Riggins et al., 2018). Both cross-sectional (Riggins et al., 2018) and longitudinal (Riggins, 2014) studies employing this source memory task show age-related improvements in performance during early childhood. Although it has been associated with age-related differences in CA2-4/DG and CA1 hippocampal subfields cross-sectionally (Riggins et al., 2018), this task has not previously been examined in relation to hippocampal development over time during this developmental period.
Because all these models used single indicator variables, error variances of the indicator variables were necessarily constrained to 0 for the source memory measure given the lack of reliability information, and to a priori values determined using the ICC reliability estimates of segmented volumes ((1 – indicator ICC) * indicator variance). This adjustment allows for the potential diattenuation of structural relations due to measurement error while maintaining model identification (e.g., Mueller and Hancock, 2019). The resulting models were just-identified (i.e., 0 degrees of freedom). Consequently, fit indices for these models are perfect by default and specific hypothesis cannot be tested about the model as a whole. However, just-identified models still provide the ability to test hypotheses about relations within the model. Specifically, these models allowed for testing the extent to which development of hippocampal subfields relates to changes in episodic memory ability. Although these models provide cross-domain correlations for all intercept and slope factors, to address the hypotheses of whether the extent to which changes in the hippocampus and episodic memory co-occur, only parameters for cross-domain relations between intercepts and slopes, and slopes at corresponding time points, were examined (e.g., change in source memory between 4 to 5 years and change in volume between 4 to 5 years).
2.3.4. Trajectory convergence
To explore whether trajectories in each cohort converge at the 6-year knot, latent means and variances of the latent factor intercept (i.e., hippocampal subfields) were tested for equivalence. Specifically, intercept convergence between cohorts was tested by grouping subjects by cohort (i.e., 4-year and 6-year) and specifying model constraints to assess the average estimated intercept for both cohorts, the difference between the estimated intercept between cohorts, and the difference between the estimated variance of the intercept between cohorts.
2.3.5. Model fit
The following measures typical of the field were included: root mean square error of approximation (RMSEA ≤ 0.08 supports acceptable fit; Browne and Cudeck, 1993; MacCallum et al., 1996), and standardized root mean square residual (SRMR ≤ 0.08 supports good fit; Hu and Bentler, 1999). For a discussion on the subjective nature of recommended cutoff criteria for fit indices in latent variable analyses, see Browne and Cudeck (1993). For a discussion of the limitations and potential limitations when considering both fit indices and measurement quality, see McNeish et al. (2018). Parameters of interests (i.e., latent means, latent variances, latent covariances, latent and directional relations between intercept and slope factors) are reported for models with satisfactory fit.
3. Results
3.1. Preliminary analyses
Estimated latent volumes within hippocampal volume did not differ between participants in the 4- and 6-year-old cohorts at 6-years-old in terms of either means or variances in hippocampal head (CA2-4/DG ps > .80, CA1 ps > .80, subiculum ps > .37) nor body (CA2-4/DG ps > .28, CA1 ps > .46, subiculum ps > .08). This suggests the estimated trajectory applies across the entire age range examined.
Practice effects in the source memory task were important to consider in the current study because longitudinal cohorts (i.e., those enrolled at age 4 or 6 years) had repeated experience with the task, although the content varied at each measurement occasion. As a result, although the source memory task was incidental at wave 1, it was not incidental at the subsequent waves, and familiarity with the task was greater at subsequent waves. Consequently, practice effects could have modified performance as a result of repeated experience with the task or increased familiarity/knowledge of the task or both. To assess this possibility, pairwise comparisons were examined between performance of 4-year-olds enrolled in a longitudinal cohort at ages 5-years and 6-years to initial performance of the cross-sectional 5-year-old cohort, and the initial performance for the longitudinal 6-year-old cohort. Comparisons were also made for performance of 6-year-olds enrolled in a longitudinal cohort at ages 7-years and 8-years to initial performance of the cross-sectional 7-year-old cohort and cross-sectional 8-year-old cohort. Practice effects would be indicated if longitudinal subjects’ scores are higher than those of subjects completing the task for the first time. No practice effects were detected (Table S1).
3.2. Development of subfields in hippocampal head
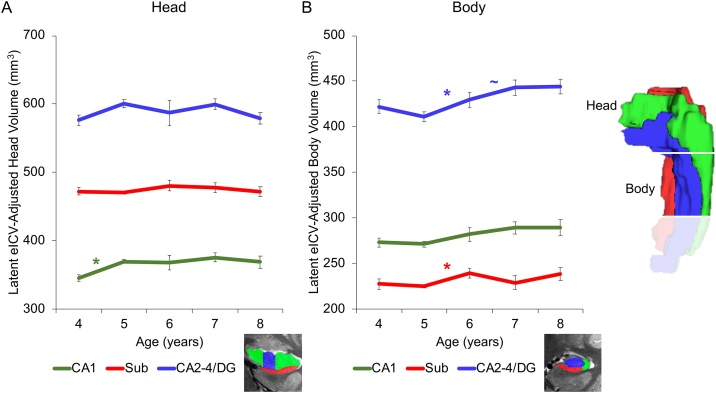
Fit indicators for the spline models for each subfield and cohort were acceptable (See Table 1). Critically, significant increases in CA1 hippocampal head subfield volume were observed between age 4–5 years (see path coefficients in Table 2, Fig. 3A). These results are consistent with the proposal that developmental trajectories of subfields differ between hippocampal head and body and that subfield development lasts into early childhood. In contrast, subiculum and CA2-4/DG volume did not show significant increases (Table 2). Although a negative error variance occurred, it was not statistically significantly below zero.
Table 1.
Fit Indices for Each Subfield Model (CA2-4/DG, CA1, and Subiculum) by Cohort.
| Cohort |
||||||||
|---|---|---|---|---|---|---|---|---|
| 4-year-old |
6-year-old |
|||||||
| Subfield | RMSEA (90 % CI) | SRMR | df | χ2 | RMSEA (90 % CI) | SRMR | df | χ2 |
| Head | ||||||||
| CA2-4/DG | .00 (.00−.156) | 0.017 | 2 | 0.527 | .157 (.034−.298) + | 0.048 | 2 | 6.613* |
| CA1 | .00 (.00−.166) | 0.032 | 2 | 0.635 | .086 (.00−.24) + | 0.038 | 2 | 3.366 |
| Subiculum | .00 (.00−.174) | 0.04 | 2 | 0.737 | .00 (.00−.052) | 0.014 | 2 | 0.132 |
| Body | ||||||||
| CA2-4/DG | .02 (.00−.057) | 0.03 | 4 | 0.892 | .00 (.00−.089) | 0.042 | 4 | 1.417 |
| CA1 | .00 (.00−.128) | 0.049 | 4 | 2.033 | .05 (.00−.17) | 0.043 | 4 | 4.944 |
| Subiculum | .00 (.00−.148) | 0.079 | 4 | 2.669 | .025 (.00−.162) | 0.07 | 4 | 4.241 |
Note. RMSEA = Root Mean Square Error of Approximation; SRMR = Standardized Root Mean Square Residual. df = degrees of freedom. χ2 = Satorra-Bentler scaled chi-square value. +: Although the fit falls above .08, the confidence interval contains .08, which is not uncommon in models with small degrees of freedom (Kenny et al., 2015). *p < .05.
Table 2.
Growth Parameters for Each Subfield Model (CA2-4/DG, CA1, and Subiculum) in Hippocampal Head by Cohort.
| Growth Parameter | Intercept | Slope 4−5 | Slope 5−6 | Slope 6−7 | Slope 7−8 |
|---|---|---|---|---|---|
| 4-year-old cohort | |||||
| CA2-4/DG | 576.43 (17.57)** | 24.08 (15.55) | −2.49 (18.63) | – | – |
| CA1 | 345.41 (7.56) ** | 24.29 (6.96) ** | −4.83 (7.12) | – | – |
| Subiculum | 472.00 (9.42)** | −1.81 (4.24) | −5.53 (7.54) | – | – |
| 6-year-old cohort | |||||
| CA2-4/DG | 576.32 (17.89)** | – | – | 23.45 (16.57) | −20.80 (20.67) |
| CA1 | 371.19 (8.00)** | – | – | 4.26 (8.20) | −6.71 (7.63) |
| Subiculum | 496.26 (13.13)** | – | – | −18.41 (12.80) | −6.24 (9.21) |
Note: Unstandardized path coefficients reflect eICV-adjusted volume in mm3. SE in parentheses. **p < .01,*p < .05, ∼ p = .10. Coefficients were estimated within each cohort separately (i.e., 4-year-old and 6-year-old cohorts).
Fig. 3.
Growth trajectories of hippocampal subfield eICV-adjusted volumes in A) hippocampal head and B) hippocampal body from 4 to 8 years of age. Note. Sub = subiculum. * p < .05, ∼ p = .10. Error bars represent the estimated standard error. Estimated volumes at age 6 years were averaged between cohorts for illustrative purposes. Analytically, volumes at age 6 years did not statistically significantly differ between cohorts for any subfield.
Results examining the variability of estimated latent intercepts (i.e., initial volume) and slopes (i.e., change in volume) revealed that the initial volume and growth of CA2-4/DG, CA1, and subiculum volumes in the head were similar across children over this period of time. Specifically, children did not statistically significantly differ from each other in their initial CA2-4/DG volume, subiculum volume, or CA1 volume (ps = .46–.73) at 4 years. Further, children did not statistically significantly differ from each other in their initial CA2-4/DG volume, subiculum volume, or CA1 volume (ps = .20–.73) at 6 years of age. Finally, children did not statistically significantly differ from each other in the change in volume between 4 to 5 or 5 to 6 years of age (ps > .56) or in change in volume between 6 to 7 or 7 to 8 years of age (ps > .45) for any subfield in hippocampal head.
3.3. Development of subfields in hippocampal body
Fit indicators for the spline models for each subfield and cohort were acceptable (See Table 1). Critically, significant increases in subiculum and CA2-4/DG hippocampal body subfield volumes were observed between age 5 to 6 years (see path coefficients in Table 3, Fig. 3B). These results are consistent with the proposal that there is significant protracted postnatal development of these subfields that lasts into early childhood. In contrast, CA1 volume did not show significant increases. Although a negative error variance occurred, it was not statistically significantly below zero.
Table 3.
Growth Parameters for Each Subfield Model (CA2-4/DG, CA1, and Subiculum) in Hippocampal Body by Cohort.
| Growth Parameter | Intercept | Slope 4−5 | Slope 5−6 | Slope 6−7 | Slope 7−8 |
|---|---|---|---|---|---|
| 4-year-old cohort | |||||
| CA2-4/DG | 422.19 (11.63)** | −10.78 (9.99) | 16.39 (7.65)* | – | – |
| CA1 | 273.01 (4.80) ** | −1.70 (2.88) | 5.49 (4.55) | – | – |
| Subiculum | 227.45 (5.73)** | −2.55 (6.59) | 20.27 (6.88)* | – | – |
| 6-year-old cohort | |||||
| CA2-4/DG | 431.13 (8.89)** | – | – | 11.77 (7.16)∼ | 1.61 (5.99) |
| CA1 | 287.41 (6.30)** | – | – | 1.98 (5.17) | .29 (1.72) |
| Subiculum | 233.33 (8.33)** | – | – | −4.29 (9.46) | 9.56 (7.05) |
Note: Unstandardized path coefficients reflect eICV-adjusted volume in mm3. **p < .01,*p < .05, ∼ p = .10. Coefficients were estimated within each cohort separately (i.e., 4-year-old and 6-year-old cohorts).
Results examining the variability of estimated latent intercepts (i.e., initial volume) and slopes (i.e., change in volume) revealed that the initial volume and growth of CA2-4/DG, CA1, and subiculum volumes in the body were similar across children over this period of time. Specifically, children did not statistically significantly differ from each other in their initial CA2-4/DG volume, subiculum volume, or CA1 volume (ps = .14–.91) at 4 years. Further, children did not statistically significantly differ from each other in their initial CA2-4/DG volume, subiculum volume, or CA1 volume (ps = .17–.73) at 6 years of age. Finally, children did not statistically significantly differ from each other in the change in volume between 4 to 5 or 5 to 6 years of age (ps > .12) or in change in volume between 6 to 7 or 7 to 8 years of age (ps > .32) for any subfield in hippocampal body.
3.4. Parallel development of hippocampal subfields and source memory
Composite measures of left and right hippocampal subfield volumes were modeled by cohort in relation to a source memory task and cross-domain relations between estimated intercepts and slopes were examined (e.g., change in source memory between 4 to 5 years and change in volume between 4 to 5 years). As reported elsewhere (Geng et al., 2021), source memory performance increased with age (See Fig. S1).
3.4.1. Relations in hippocampal head
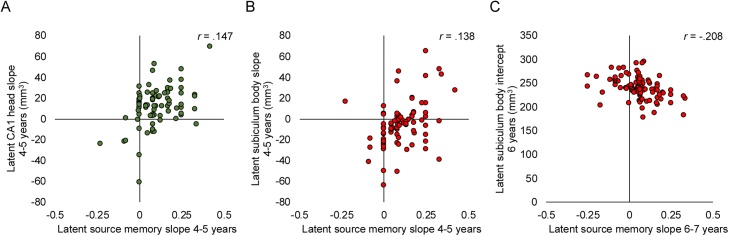
Within hippocampal head, in the 4-year-old cohort, changes in CA1 volume between 4 to 5 years positively related to change in source memory ability between 4 to 5 years (p = .042, Fig. 4A). Statistically significant relations were not observed between source memory and CA1 or subiculum volume (ps > .35). In the 6-year-old cohort, none of the examined relations between CA2-4/DG, CA1, or subiculum volume statistically significantly related to source memory (ps > .10).
Fig. 4.
Estimated correlations between hippocampal subfield volumes (mm3) and source memory performance (proportion correct). Values are latent estimates for each individual extracted from the respective model. A) Increases in CA1 head volume relate to increases in source memory performance between 4 to 5 years of age. B) Increases in subiculum body volume relate to increases in source memory performance between 4 to 5 years of age. C) Subiculum body volume at age 6 years relates to changes in source memory performance between 6 to 7 years of age.
3.4.2. Relations in hippocampal body
Within hippocampal body, in the 4-year-old cohort, changes in subiculum volume between 4 to 5 years positively related to change in source memory ability between 4 to 5 years (p = .014, Fig. 4B). Statistically significant relations were not observed between source memory and CA1 nor CA2-4/DG volume (ps > .07). In the 6-year-old cohort, smaller subiculum volume at age 6 related to improvements in source memory between 6 to 7 years (p = .011, Fig. 4C). Statistically significant relations were not observed between source memory and CA1 nor CA2-4/DG volume (ps > .10).
4. Discussion
This study characterized developmental trajectories of hippocampal subfields in a longitudinal sample of 4- to 8-year-old children using latent modeling. Results document the protracted development of this structure in early- to mid-childhood with CA1 in hippocampal head increasing in volume between 4- to 5-years and CA2-4/DG and subiculum in hippocampal body increasing in volume between 5- to 6-years. These findings are consistent with proposals of protracted development of the dentate gyrus of the hippocampus based in studies in non-human primates (Lavenex and Banta Lavenex, 2013) and postmortem humans (Seress, 2001). However, it is the first time this has been shown longitudinally in young human children. These data are important because they suggest a neurobiological mechanism that accompanies the great cognitive growth observed during this developmental period (Piaget and Inhelder, 1969).
Notably, the specifics of these findings vary somewhat from what has been documented in animals, as developmental changes were observed across all subfields during this period (as opposed to just the dentate gyrus). However, results showing increases in subiculum volume in hippocampal body should be noted with caution due to the lower reliability in segmentation. In addition, developmental changes in subfields varied by subregion (head versus body) suggesting that examining subfields along the longitudinal axis is an important dimension to consider in humans. This study also highlights the strength of using latent longitudinal modeling. Specifically, our methodological approach allowed us to characterize development in both the head of the hippocampus, which is known to be more variable in its morphology (Ding and Van Hoesen, 2015), as well as in the body using a hypothetically error-free measure of volume. Further, while the results were consistent with some aspects of the cross-sectional report from this sample (Riggins et al., 2018), our findings highlight the need to examine children’s brain development longitudinally, as the developmental variations observed in hippocampal body were not detected previously, possibly due to variability, power, and/or the statistical approach. We expand upon these findings below and discuss relations to previous developmental work focused on the development of the hippocampus. We also address the strengths and limitations of this study and suggest potential future directions. Finally, we note the implications of this work, which highlight early- to mid-childhood as a particularly important period for hippocampal development.
4.1. Advancing understanding of hippocampal development in early childhood
As noted above, subfield development differed between hippocampal head and hippocampal body, even after correcting for overall changes in eICV. This suggests these structures were growing in a disproportional manner compared to the brain overall. In the current study, within hippocampal head, developmental changes were observed between 4- to 5-years. Specifically, CA1 volume increased during this period. Changes in CA2-4/DG and subiculum volume were not observed. Within hippocampal body, developmental changes were observed between 5- to 6-years. Specifically, CA2-4/DG volume and subiculum volume increased during this period. Although the raw CA1 volume in body showed increases, this was proportional to the overall increase in eICV, suggesting that CA1 in this subregion was developing, but to the same extent as the rest of the brain.
Previous cross-sectional investigations have also examined developmental differences in subfield volume, by subregion, in children (Keresztes et al., 2017; Riggins et al., 2018; Schlichting et al., 2017). Two of these studies examined subfields separately in both hippocampal head and body (4–8 years, Riggins et al., 2018; 6–30 years, Schlichting et al., 2017), one focused on hippocampal body (e.g., 6–27 years, Keresztes et al., 2017). Our results align with the previous studies examining the hippocampal head and provide new information about the development of subfields in hippocampal body. Specifically, for subfields within hippocampal head, both Schlichting et al. (2017) and Riggins et al. (2018) documented age-related variations of CA1 volume. Critically, the cross-sectional study of 4- to 8-year-old in the current report (Riggins et al., 2018) also reported larger volumes in older children. The current study lends specificity to this finding by highlighting the period of 4- to 5-years as a time of robust increases in CA1 volume. Consistent with the current study, age-related variations were not observed in CA2-4/DG nor subiculum head volumes in previous cross-sectional reports (Riggins et al., 2018; Schlichting et al., 2017).
For subfields within hippocampal body, the present report adds new information to the literature. First, the current study detected developmental increases in both CA2-4/DG and subiculum, which were not observed in the cross-sectional subsample from the current study (Riggins et al., 2018). This may possibly be due to large inter-subject variability that can obscure individual change as other cross-sectional research investigating a wider age range has shown greater volumes in older children (Keresztes et al., 2017; Schlichting et al., 2017). For CA1 volume, the lack of age-related change observed in the current study is consistent with previous cross-sectional work (Riggins et al., 2018; Schlichting et al., 2017; c.f. Keresztes et al., 2017). As noted above, the youngest children in the Schlichting et al. (2017) and Keresztes et al. (2017) samples were 6-years-old. Thus, the current study is consistent with previous literature, but extends this work into early childhood.
Interestingly, the period of time in which significant changes were observed in all subfields (between 4 to 6 years) aligns with the period of time during which subfields are thought to be functionally mature based on work in non-human primates (Lavenex and Banta Lavenex, 2013) and postmortem humans (Seress, 2001). Although the volumetric changes observed are modest, it should be noted that this level of analysis is at the macro-level; it is likely substantial changes are occurring at the micro-level (e.g., number of cells, synapses, and connectivity; Lavenex and Banta Lavenex, 2013; Seress, 2001) facilitating the specific development observed in hippocampal subfields. Further, as children show impressive gains in their cognitive ability during the period of 4- to 6-years that have been proposed to coincide with the development of the hippocampus, it is likely that the subtle changes have great implications for additional aspects of development (Riggins et al., 2020).
4.2. Parallel hippocampal and memory development in early childhood
Our results suggest that the volumetric changes observed within a relatively small period of time have functional relevance. Changes in CA1 volume in hippocampal head between age 4 to 5 years related to changes in source memory performance between age 4 to 5 years. Changes in subiculum volume in hippocampal body between age 4 to 5 years related to changes in source memory performance between age 4 to 5 years, and subiculum volume in hippocampal body at 6 years related to changes in source memory performance between 6 to 7 years.
Our findings are consistent with previous cross-sectional work that noted a relation between CA1 volume and memory performance (Lee et al., 2014; Riggins et al., 2018; Schlichting et al., 2017; Tamnes et al., 2014). Cross-sectional work has also documented relations between subiculum volume and memory performance (Lee et al., 2014). Although the present study differs in its approach to examining change co-occurring at each time point, versus across a developmental period, results suggest CA1 and subiculum development support improvements in children’s cognitive ability during early- to- mid-childhood.
4.3. Limitations and directions for future research
Although this study breaks new ground, there are a few limitations worth noting that are important for future research to consider. First, although the protocol adapted and used to segment hippocampal subfields in the present study (La Joie et al., 2010; Riggins et al., 2018) was well-suited to the neuroimaging measures collected (e.g., T2 image) and allowed for the examination of subfields within body head and body, it combined DG subfield with other smaller subfields (CA2-4) that are not always considered together in the literature. Ongoing efforts by the Hippocampal Subfields Group (http://hippocampalsubfields.com) to create a reliable and harmonized subfield tracing protocol in both the head and the body of the hippocampus are underway (Wisse et al., 2017). Future work examining the development of these critically important subunits of the hippocampus should strive to adopt a protocol best suited to the questions at hand that also facilitates comparison with research conducted by other groups. However, given the differential development of subfields between head and body, future studies should strive to include examination of subfields in both of these subregions, separately, in order to further progress our understanding of the development of this important heterogeneous region. Additionally, given the variability in possible ways to control for ICV (e.g., Sankar et al., 2017), future research should strive to adopt similar methods in order to draw connections between samples.
Second, although the accelerated longitudinal design allowed for the assessment of change overtime, the current study was limited in only including one overlapping time point between cohorts. In order to better assess change over time, future research should consider implementing planned missingness that incorporates multiple overlapping time points, and potentially additional cohorts to expand the period of time under investigation.
Third, because of the modest number of subjects and our approach of modeling subfield development separately by subfield and cohort, there is the potential vulnerability to Type II errors.
Fourth, though structural variability is thought to relate to individual differences in function and memory ability (e.g., Carr et al., 2017), volumetric measures used to assess hippocampal maturity is not a direct measure of hippocampal function. Although relations were observed between changes in source memory and changes in CA1 and subiculum volume, the current work does not provide direct evidence of whether the observed volumetric changes in subfields have functional implications during this period of development. Function was not directly assessed in this study is due to the difficulty of collecting functional neuroimaging data within a population for whom staying still is a demanding task. However, with improvements in scanning methods (e.g., multiband or compressed sense acquisition), future work may be able to examine hippocampal subfield structure and function within the same developmental population.
4.4. Strengths of the current study
Despite these limitations, this study had several strengths. First, the focus of the current study on early childhood, a time in which hippocampal development is relatively underinvestigated compared to later periods, fills an important gap in the literature by characterizing the development of subfields in young children. Second, the use of an accelerated longitudinal design with a relatively large developmental sample including three time points allowed for the characterization of changes in subfield volume as opposed to age-related differences, as is the case with cross-sectional samples. Third, the current study used multi-group latent growth modeling to characterize the protracted development of hippocampal subfields. Specifically, developmental changes in hypothetically error-free estimates of hippocampal subfield volumes were examined by using of the commonality of the two hemispheric measures for each subfield to indicate the latent constructs while removing measurement error. Given the variability in hippocampal morphology, this approach is especially powerful in combination with reliable segmentations. Finally, the use of piecewise growth models allowed us to assess the possibility of different rates of change across early- to mid-childhood for each subfield, an important strength as our research question sought to characterize specificity of subfield development within both hippocampal head and body.
4.5. Conclusions
This study documents differential development of subfields along the longitudinal axis revealing, for the first time in young human children, the protracted development of these structures and relations with memory. These findings highlight the importance of longitudinal studies for detecting increases in hippocampal volume in early- to mid-childhood. Specifically CA1 in head increased between 4 to 5 years and CA2-4/DG and subiculum in body increased between 5 to 6 years.
Data and code availability statement
The data analyzed during the current study are available from the corresponding author on reasonable request and completion of a data sharing agreement. Sample Mplus code used to conduct analyses is available at https://osf.io/s8kuq.
CRediT authorship contribution statement
Kelsey L. Canada: Conceptualization, Investigation, Methodology, Formal analysis, Writing - original draft, Review, & editing, Visualization, Funding acquisition. Gregory R. Hancock: Methodology, Supervision, Writing - review & editing. Tracy Riggins: Project administration, Conceptualization, Investigation, Supervision, Writing - review & editing, Funding acquisition.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
This research was conducted as part of a doctoral dissertation by Kelsey L. Canada at the University of Maryland and was supported by the Jack Bartlett Memorial Award and a Janet Johnson Research Fellowship at the University of Maryland (K.L.C.), and the National Institutes of Health (grant HD079518, awarded to T.R.). The authors thank the members of the dissertation committee, the families who participated in this study, and the members of the Neurocognitive Development Lab, especially Morgan Botdorf, Marissa Clark, Lisa Cox, Elizabeth Mulligan, Jennifer Sloane, and Shane Wise for assistance with data collection.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2021.100947.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Becht A.I., Mills K.L. Modeling individual differences in brain development. Biol. Psychiatry. 2020;88(1):63–69. doi: 10.1016/j.biopsych.2020.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berron D., Vieweg P., Hochkeppler A., Pluta J.B., Ding S.L., Maass A. A protocol for manual segmentation of medial temporal lobe subregions in 7 Tesla MRI. Neuroimage Clin. 2017;15:466–482. doi: 10.1016/j.nicl.2017.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne M.W., Cudeck R. Alternative ways of assessing model fit. In: Bollen K.A., Long J.S., editors. Testing Structural Equation Models. Sage; Newbury Park, CA: 1993. pp. 136–162. [Google Scholar]
- Canada K.L., Botdorf M., Riggins T. Longitudinal development of hippocampal subregions from early‐to mid‐childhood. Hippocampus. 2020;30(10):1098–1111. doi: 10.1002/hipo.23218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr V.A., Bernstein J.D., Favila S.E., Rutt B.K., Kerchner G.A., Wagner A.D. Individual differences in associative memory among older adults explained by hippocampal subfield structure and function. Proc. Natl. Acad. Sci. 2017;114(45):12075–12080. doi: 10.1073/pnas.1713308114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty A.M., Bender A.R., Raz N., Ofen N. Age differences in hippocampal subfield volumes from childhood to late adulthood. Hippocampus. 2016;26(2):220–228. doi: 10.1002/hipo.22517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty A.M., Flinn R., Ofen N. Hippocampal CA3-dentate gyrus volume uniquely linked to improvement in associative memory from childhood to adulthood. NeuroImage. 2017;153:75–85. doi: 10.1016/j.neuroimage.2017.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaster D., Pathman T., Lee J.K., Ghetti S. Structural development of the hippocampus and episodic memory: developmental differences along the anterior/posterior axis. Cereb. Cortex. 2014;24(11):3036–3045. doi: 10.1093/cercor/bht160. [DOI] [PubMed] [Google Scholar]
- Ding S.L., Van Hoesen G.W. Organization and detailed parcellation of human hippocampal head and body regions based on a combined analysis of cyto‐and chemoarchitecture. J. Comp. Neurol. 2015;523(15):2233–2253. doi: 10.1002/cne.23786. [DOI] [PubMed] [Google Scholar]
- Drummey A.B., Newcombe N.S. Developmental changes in source memory. Dev. Sci. 2002;5(4):502–513. doi: 10.1111/1467-7687.00243. [DOI] [Google Scholar]
- Duncan T.E., Duncan S.C. The ABC’s of LGM: an introductory guide to latent variable growth curve modeling. Soc. Personal. Psychol. Compass. 2009;3(6):979–991. doi: 10.1111/j.1751-9004.2009.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan S.C., Duncan T.E., Hops H. Analysis of longitudinal data within accelerated longitudinal designs. Psychol. Methods. 1996;1(3):236–248. doi: 10.1037/1082-989X.1.3.236. [DOI] [Google Scholar]
- Duvernoy H. 2nd ed. Springer-Verlag; Berlin: 1998. The Hippocampus Book: Functional Anatomy, Vascularization and Serial Sections with MRI. [Google Scholar]
- Geng F., Botdorf M., Riggins T. How behavior shapes the brain and the brain shapes behavior: insights from memory development. J. Neurosci. 2021 doi: 10.1523/JNEUROSCI.2611-2619.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisletta P., McArdle J.J. Latent growth curve analyses of the development of height. Struct. Equ. Model. A Multidiscip. J. 2001;8(4):531–555. doi: 10.1207/S15328007SEM0804. [DOI] [Google Scholar]
- Ghosh S.S., Kakunoori S., Augustinack J., Nieto-Castanon A., Kovelman I., Gaab N. Evaluating the validity of volume-based and surface-based brain image registration for developmental cognitive neuroscience studies in children 4 to 11 years of age. Neuroimage. 2010;53(1):85–93. doi: 10.1016/j.neuroimage.2010.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore J.H., Knickmeyer R.C., Gao W. Imaging structural and functional brain development in early childhood. Nat. Rev. Neurosci. 2018;19(3):123. doi: 10.1038/nrn.2018.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Nugent T.F., Herman D.H., Ordonez A., Greenstein D., Hayashi K.M. Dynamic mapping of normal human hippocampal development. Hippocampus. 2006;16(8):664–672. doi: 10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
- Haller S.P., Mills K.L., Hartwright C.E., David A.S., Kadosh K.C. When change is the only constant: the promise of longitudinal neuroimaging in understanding social anxiety disorder. Dev. Cogn. Neurosci. 2018;33:73–82. doi: 10.1016/j.dcn.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock G.R., Kuo W., Lawrence F.R. An illustration of second-order latent growth models. Struct. Equ. Model. A Multidiscip. J. 2001;8(3):470–489. doi: 10.1207/S15328007SEM0803_7. [DOI] [Google Scholar]
- Hancock G.R., Harring J.R., Lawrence F.R. Using latent growth modeling to evaluate longitudinal change. In: Hancock G.R., Mueller R.O., editors. Structural Equation Modeling. A Second Course. 2nd ed. Information Age Publishing, Inc.; Charlotte, NC: 2013. pp. 309–341. [Google Scholar]
- Harding A.J., Halliday G.M., Kril J.J. Variation in hippocampal neuron number with age and brain volume. Cereb. Cortex. 1998;8(8):710–718. doi: 10.1093/cercor/8.8.710. [DOI] [PubMed] [Google Scholar]
- Hu L.T., Bentler P.M. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct. Equ. Model. A Multidiscip. J. 1999;6(1):1–55. doi: 10.1080/10705519909540118. [DOI] [Google Scholar]
- Jabès A., Banta Lavenex P., Amaral D.G., Lavenex P. Postnatal development of the hippocampal formation: a stereological study in macaque monkeys. J. Comp. Neurol. 2011;519(6):1051–1070. doi: 10.1002/cne.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny D.A., Kaniskan B., McCoach D.B. The performance of RMSEA in models with small degrees of freedom. Sociol. Methods Res. 2015;44(3):486–507. doi: 10.1177/0049124114543236. [DOI] [Google Scholar]
- Keresztes A., Bender A.R., Bodammer N.C., Lindenberger U., Shing Y.L., Werkle-Bergner M. Hippocampal maturity promotes memory distinctiveness in childhood and adolescence. Proc. Natl. Acad. Sci. 2017;114(34):9212–9217. doi: 10.1073/pnas.1710654114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kievit R.A., Brandmaier A.M., Ziegler G., van Harmelen A.L., de Mooij S.M.M., Moutoussis M. Developmental cognitive neuroscience using latent change score models: a tutorial and applications. Dev. Cogn. Neurosci. 2018;33:1–19. doi: 10.1016/j.dcn.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King K.M., Littlefield A.K., McCabe C.J., Mills K.L., Flournoy J., Chassin L. Longitudinal modeling in developmental neuroimaging research: common challenges, and solutions from developmental psychology. Dev. Cogn. Neurosci. 2018;33:54–72. doi: 10.1016/j.dcn.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo T.K., Li M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016;15(2):155–163. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Joie R., Fouquet M., Mézenge F., Landeau B., Villain N., Mevel K. Differential effect of age on hippocampal subfields assessed using a new high-resolution 3T MR sequence. Neuroimage. 2010;53(2):506–514. doi: 10.1016/j.neuroimage.2010.06.024. [DOI] [PubMed] [Google Scholar]
- Lavenex P., Banta Lavenex P. Building hippocampal circuits to learn and remember: insights into the development of human memory. Behav. Brain Res. 2013;254:8–21. doi: 10.1016/j.bbr.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Lavenex P., Banta Lavenex P., Amaral D.G. Nonphosphorylated high-molecular-weight neurofilament expression suggests early maturation of the monkey subiculum. Hippocampus. 2004;14(7):797–801. doi: 10.1002/hipo.20028. [DOI] [PubMed] [Google Scholar]
- Lee J.K., Ekstrom A.D., Ghetti S. Volume of hippocampal subfields and episodic memory in childhood and adolescence. Neuroimage. 2014;94:162–171. doi: 10.1016/j.neuroimage.2014.03.019. [DOI] [PubMed] [Google Scholar]
- MacCallum R.C., Browne M.W., Sugawara H.M. Power analysis and determination of sample size for covariance structure modeling. Psychol. Methods. 1996;1(2):130. doi: 10.1037/1082-989X.1.2.130. [DOI] [Google Scholar]
- McArdle J.J. Latent variable modeling of differences and changes with longitudinal data. Annu. Rev. Psychol. 2009;60(1):577–605. doi: 10.1146/annurev.psych.60.110707.163612. [DOI] [PubMed] [Google Scholar]
- McNeish D., An J., Hancock G.R. The thorny relation between measurement quality and fit index cutoffs in latent variable models. J. Pers. Assess. 2018;100(1):43–52. doi: 10.1080/00223891.2017.1281286. [DOI] [PubMed] [Google Scholar]
- Meredith W., Tisak J. Latent curve analysis. Psychometrika. 1990;55(1):107–122. doi: 10.1007/BF02294746https://doi.org/10.1007/BF02294746. [DOI] [Google Scholar]
- Mills K.L., Tamnes C.K. Methods and considerations for longitudinal structural brain imaging analysis across development. Dev. Cogn. Neurosci. 2014;9:172–190. doi: 10.1016/j.dcn.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller R.O., Hancock G.R. Structural equation modeling. In: Hancock G.R., Mueller R.O., editors. The Reviewer's Guide to Quantitative Methods in the Social Sciences. 2nd ed. Routledge; New York: 2019. pp. 445–456. [Google Scholar]
- Muthén L.K., Muthén B.O. 8th ed. Muthén & Muthén; Los Angeles, CA: 1998. Mplus User’s Guide. –2017. [Google Scholar]
- Perlaki G., Orsi G., Plozer E., Altbacker A., Darnai G., Nagy S.A. Are there any gender differences in the hippocampus volume after head-size correction? A volumetric and voxel-based morphometric study. Neurosci. Lett. 2014;570:119–123. doi: 10.1016/j.neulet.2014.04.013. [DOI] [PubMed] [Google Scholar]
- Piaget J., Inhelder B. Basic Books; New York, NY: 1969. The Psychology of the Child. [Google Scholar]
- Pintzka C.W.S., Hansen T.I., Evensmoen H.R., Håberg A.K. Marked effects of intracranial volume correction methods on sex differences in neuroanatomical structures: a HUNT MRI study. Front. Neurosci. 2015;9:238. doi: 10.3389/fnins.2015.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenk J., Evensmoen H.R., Moscovitch M., Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn. Sci. (Regul. Ed.) 2013;17(5):230–240. doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Raz N., Lindenberger U., Rodrigue K.M., Kennedy K.M., Head D., Williamson A. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb. Cortex. 2005;15(11):1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Riggins T. Longitudinal investigation of source memory reveals different developmental trajectories for item memory and binding. Dev. Psychol. 2014;50(2):449–459. doi: 10.1037/a0033622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggins T., Blankenship S.L., Mulligan E., Rice K., Redcay E. Developmental differences in relations between episodic memory and hippocampal subregion volume during early childhood. Child Dev. 2015;86(6):1710–1718. doi: 10.1111/cdev.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggins T., Geng F., Botdorf M., Canada K., Cox L., Hancock G.R. Protracted hippocampal development is associated with age-related improvements in memory during early childhood. NeuroImage. 2018;174:127–137. doi: 10.1016/j.neuroimage.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggins T., Canada K.L., Botdorf M. Empirical evidence supporting neural contributions to episodic memory development in early childhood: implications for childhood amnesia. Child Dev. Perspect. 2020;14(1):41–48. doi: 10.1111/cdep.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar T., Park M.T.M., Jawa T., Patel R., Bhagwat N., Voineskos A.N. Your algorithm might think the hippocampus grows in Alzheimer’s disease: caveats of longitudinal automated hippocampal volumetry. Hum. Brain Mapp. 2017;38(6):2875–2896. doi: 10.1002/hbm.23559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting M.L., Guarino K.F., Schapiro A.C., Turk-Browne N.B., Preston A.R. Hippocampal structure predicts statistical learning and associative inference abilities during development. J. Cogn. Neurosci. 2017;29(1):37–51. doi: 10.1162/jocn_a_01028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seress L. Morphological changes of the human hippocampal formation from midgestation to early childhood. In: Collins M.L., Nelson C.A., editors. Handbook of Developmental Cognitive Neuroscience. The MIT Press; Cambridge, MA: 2001. pp. 45–58. [Google Scholar]
- Shrout P.E., Fleiss J.L. Intraclass correlations: uses in assessing rater reliability. Psychol. Bull. 1979;86(2):420–428. doi: 10.1037/0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Steen R.G., Hamer R.M., Lieberman J.A. Measuring brain volume by MR imaging: impact of measurement precision and natural variation on sample size requirements. Am. J. Neuroradiol. 2007;28(6):1119–1125. doi: 10.3174/ajnr.A0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes C.K., Walhovd K.B., Engvig A., Grydeland H., Krogsrud S.K., Østby Y. Regional hippocampal volumes and development predict learning and memory. Dev. Neurosci. 2014;36(3-4):161–174. doi: 10.1159/00036244. [DOI] [PubMed] [Google Scholar]
- Tamnes C., Bos M., van de Kamp F., Peters S., Crone E. Longitudinal development of hippocampal subregions from childhood to adulthood. Dev. Cogn. Neurosci. 2018;30:212–222. doi: 10.1016/j.dcn.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson M.S., Green S.B. Evaluating between-group differences in latent variable means. In: Hancock G.R., Mueller R.O., editors. Structural Equation Modeling: A Second Course. 2nd ed. Information Age Publishing, Inc.; Charlotte, NC: 2013. pp. 309–341. [Google Scholar]
- Tisak J., Tisak M.S. Longitudinal models of reliability and validity: a latent curve approach. Appl. Psychol. Meas. 1996;20(3):275–288. doi: 10.1177/014662169602000307. [DOI] [Google Scholar]
- Winterburn J.L., Pruessner J.C., Chavez S., Schira M.M., Lobaugh N.J., Voineskos A.N., Chakravarty M.M. A novel in vivo atlas of human hippocampal subfields using high-resolution 3 T magnetic resonance imaging. Neuroimage. 2013;74:254–265. doi: 10.1016/j.neuroimage.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Wisse L.E., Daugherty A.M., Olsen R.K., Berron D., Carr V.A., Stark C.E.…Hippocampal Subfields Group A harmonized segmentation protocol for hippocampal and parahippocampal subregions: Why do we need one and what are the key goals? Hippocampus. 2017;27(1):3–11. doi: 10.1002/hipo.22671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich P.A., Pluta J.B., Wang H., Xie L., Ding S.L., Gertje E.C. Automated volumetry and regional thickness analysis of hippocampal subfields and medial temporal cortical structures in mild cognitive impairment. Hum. Brain Mapp. 2015;36(1):258–287. doi: 10.1002/hbm.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich P.A., Amaral R.S.C., Augustinack J.C., Bender A.R., Bernstein J.D., Boccardi M. Quantitative comparison of 21 protocols for labeling hippocampal subfields and parahippocampal subregions in in vivo MRI: Towards a harmonized segmentation protocol. NeuroImage. 2015;111:526–541. doi: 10.1016/j.neuroimage.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijdenbos A.P., Dawant B.M., Margolin R.A., Palmer A.C. Morphometric analysis of white matter lesions in MR images: method and validation. IEEE Trans. Med. Imaging. 1994;13(4):716–724. doi: 10.1109/42.363096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analyzed during the current study are available from the corresponding author on reasonable request and completion of a data sharing agreement. Sample Mplus code used to conduct analyses is available at https://osf.io/s8kuq.