Abstract
In this review of Y chromosome microdeletions, azoospermia factor (AZF) deletion subtypes, histological features and microTESE sperm retrieval rates are summarized after a systematic literature review. PubMed was searched and papers were identified using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Approximately half of infertile couples have a male factor contributing to their infertility. One of the most common genetic etiologies are Y chromosome microdeletions. Men with Y chromosome microdeletions may have rare sperm available in the ejaculate or undergo surgical sperm retrieval and subsequent intracytoplasmic sperm injection to produce offspring. Azoospermia or severe oligozoospermia are the most common semen analysis findings found in men with Y chromosome microdeletions, associated with impaired spermatogenesis. Men with complete deletions of azoospermia factor a, b, or a combination of any loci have severely impaired spermatogenesis and are nearly always azoospermic with no sperm retrievable from the testis. Deletions of the azoospermia factor c or d often have sperm production and the highest likelihood of a successful sperm retrieval. In men with AZFc deletions, histologically, 46% of men demonstrate Sertoli cell only syndrome on biopsy, whereas 38.2% have maturation arrest and 15.7% have hypospermatogenesis. The microTESE sperm retrieval rates in AZFc-deleted men range from 13-100% based on the 32 studies analyzed, with a mean sperm retrieval rate of 47%.
Keywords: Y chromosome microdeletion, microTESE, sperm retrieval, testis biopsy, male infertility, azoospermia factor, azoospermia
Introduction
Fifteen to twenty percent of couples globally report infertility issues and 20% to 70% of these cases have male factors contributing (1). The most severe form of male infertility is termed azoospermia, where no sperm are identified in semen. Azoospermia can be further divided into obstructive azoospermia (OA), as a result of an obstruction in the ejaculatory pathway or non-obstructive azoospermia (NOA), as a result of defective spermatogenesis (2). Genetic causes of NOA include sex chromosomal abnormalities, Y chromosome microdeletions (YCM), gene copy number variations (CNVs) and mutations in a variety of different genes (3,4).
The Y chromosome is one of the smallest chromosomes in the human genome (5). Structurally, the Y chromosome is composed of a short (Yp) and a long arm (Yq) with a rich assortment of repetitive elements that render it highly unstable and prone to internal recombination with subsequent segmental deletions (5,6). Functionally, genes on the Y chromosome have been recognized to drive gonadal differentiation and testicular development to create the male phenotype (7).
The Y chromosome was first suspected to be involved in azoospermia in the 1970s, when Tiepolo & Zuffardi (8) identified deletions in Yq of patients with an otherwise normal karyotype. Vogt (9) reviews the work that continued into the 1990s, summarizing that researchers using technology that ranged from fluorescent tagging to polymerase chain reaction (PCR), found deletions that commonly spanned regions within the long arm of the Y chromosome. This region was later termed the azoospermic factor (AZF) locus. Vollrath et al. (10) and Vogt et al. (11) separately used sequence tagged sites (STS) and PCR analyses to assemble a map of the AZF locus (10,11). The AZF locus was then classified into four gene regions (AZFa, AZFb, AZFc, AZFd) that were believed to contain spermatogenesis genes involved in male infertility (12). It has subsequently been proposed by some investigators that deletions of the AZFd region (resulting from gr/gr recombination) are within the AZFc region and may not be clinically relevant (13). Clinically, screening for YCM is conducted using multiplex PCR to search for the presence of STS in the AZF locus (9,14). YCM normally results in men being severely oligozoospermic or azoospermic.
Depending on the AZF-deleted region, biopsies of the testes generally reveal different histological features. These include: Sertoli Cell Only syndrome (SCO), maturation arrest (MA), and hypospermatogenesis (HS) (15). The severity of infertility is greatest among men with SCO, followed by MA and then HS. It is common for different regions of the testes of these men to have variable histologic patterns, so a simple biopsy of the testis that samples very little testicular tissue, may not reflect the overall function of the testis and fail to capture spermatogenic heterogeneity.
Treatment for men with YCM and severe oligozoospermia often relies on in vitro fertilization coupled with intracytoplasmic sperm injection (ICSI). However, among men with azoospermia, surgical sperm retrieval is necessary through testicular extraction of sperm (TESE), or microdissection testicular sperm retrieval (microTESE), first reported in 1999 (16).
In this review, Y chromosome microdeletion subtypes and its associated histology and effects on testicular sperm extraction in men with YCM are summarized.
Methods
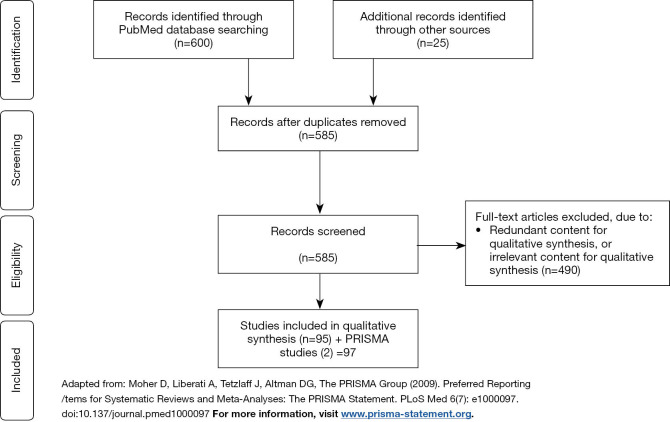
The search engine PubMed was used to identify publications published between January 1997 – May 21, 2019 addressing Y chromosome microdeletions and surgical sperm retrieval. Search terms included: “y chromosome microdeletion”, “male infertility”, “micro tese”, “microdissection testicular sperm extraction”, “microtese”, “micro-tese”, “hormone therapy”, “gr/gr”, “AZFa”, “AZFb” and “AZFc”. The search term “y chromosome microdeletion” and the Boolean operator “AND” were used to search for articles with the following search terms: “hormone therapy”, “fertilization”, “offspring”, “sperm retrieval”, “therapy”. Search restrictions included the English language and full text availability. A total of 600 articles were identified, and 560 articles remained after removing duplications. Abstracts were screened for pertinent information. An additional 25 articles were identified outside of the search. Overall, 585 papers were screened, and 97 articles were included in this review. Inclusion and exclusion processes are represented graphically below using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) in Figure 1 (17,18).
Figure 1.
PRISMA flow diagram describing inclusion and exclusion process.
Y chromosome microdeletions
Depending on geographic and ethnic background and selection criteria for patients in studies, the reported prevalence of Y chromosome microdeletions in azoospermic and oligozoospermic men vary from 3.2% to 29.4% (Table 1), with the global prevalence reported as 7% among azoospermic and severely oligozoospermic men (4). Within the AZF regions, patients may have partial or complete deletions that can result in a range of different clinical phenotypes. In general, YCM men present with significantly higher FSH levels than fertile men and exhibit lower, though not statistically significant, LH levels, testosterone levels and testicular volume (19-22,50). Semen analysis and testicular histology differ depending on the AZF region that is deleted and whether the deletion is a complete or partial deletion. Table 1 outlines the proportion of deletion of each region, sperm profile and histological distribution.
Table 1. Y chromosome microdeletion types, the proportion of each deletion type and their respective sperm count and histology profiles.
| Deletion (complete or partial) | Mean frequency of AZF deletions (%) (from references 12,19-26) | Sperm count (from references 12,19-26) | Histology, n [%] | Total n | Reference | ||
|---|---|---|---|---|---|---|---|
| SCO | MA | HS | |||||
| AZFa | 5.3 | Azoospermia—severe oligozoospermia | 35 [69] | 15 [29] | 1 [2] | 51 | (12,21,24,27-35) |
| AZFb | 10.8 | Azoospermia—severe oligozoospermia | 20 [38] | 25 [48] | 7 [13] | 52 | (12,21,23,28,29,31,33,36-39) |
| AZFc | 57.2 | Azoospermia—oligozoospermia | 82 [46] | 68 [38] | 28 [16] | 178 | (12,21-23,28-36,39-44) |
| AZFd | N/A | Azoospermia—normozoospermia | 4 [50] | 4 [50] | – | 8 | (12,37,43,45) |
| AZFab | 1.2¶ | N/A | 1 [100] | – | – | 1 | (29) |
| AZFbc (&AZFbdc) | 13.5 | Azoospermia—severe oligozoospermia | 26 [72] | 9 [25] | 1 [3] | 36 | (21,23,28,29,31,33,36,37,39,40,43) |
| AZFbd | N/A | N/A | – | 2 [100] | – | 2 | (37) |
| AZFcd | N/A | N/A | – | 2 [67] | 1 [33] | 3 | (37,45) |
| AZFabc | 7.0 | Azoospermia | 11 [79] | 3 [21] | – | 14 | (21,23,29,30,32-34,36,40,43) |
| Partial AZFc deletions | Azoospermia—normozoospermia | (44,46-49) | |||||
| Gr/gr | 11 [61] | 7 [39] | – | 18 | |||
| B1/b3 | 8 [73] | 3 [27] | – | 11 | |||
| B2/b3 | 2 [100] | – | – | 2 | |||
¶From (12) Sequence Tagged Sites (STSs). SCO, Sertoli cell only syndrome; MA, maturation arrest (did not differentiate between subtype of maturation arrest); HS, hypospermatogenesis.
Complete vs. partial deletions
Since men with partial deletions may have a variable pattern of genetic profiles, it is clinically important for clinicians to remember that the most reliable data on clinical value for YCM deletions comes from those patients with complete deletions of the AZFa, b, or c regions. Data from men with complete deletions cannot be used to predict the clinical activity or testicular function of men with partial deletions. Hence, careful genetic characterization is critical of YCM-deleted men clinically.
AZFa
The frequency of complete or partial AZFa deletions among YCM men with oligozoospermia and azoospermia has been reported to range from 1.7% to 15.4%, with the mean frequency calculated to be 5.3% (Table 1). The AZFa region spans more than 1 megabyte (mb) on the Y chromosome and has a nonrepetitive structure with a low deletion frequency (51,52). This locus contains the three genes USP9Y (or DFFRY), DDX3Y and UTY, though only USP9Y and DDX3Y are believed to be involved in spermatogenesis (51,53,54).
Studies have shown that men with complete AZFa deletions typically have a pure SCO histology on testis biopsy (Table 1). Based on twelve articles with a combined n of 51, 68.6% (35/51) are SCO, 29.4% (15/51) are MA and 2.0% (1/51) are HS (Table 1). Blagosklonova et al. (27) conducted a retrospective study using archived testicular biopsies and found that AZFa-deleted specimens exhibited a combination of reduced tubular diameter, normal to thickened tunica propria, normal to increased intertubular space, hyperplastic Leydig cells and spermatogenic arrest or SCO. Later studies revealed that complete AZFa deletions, involving both USP9Y and DDX3Y, typically lead to the SCO phenotype whilst partial deletions, involving one of USP9Y or DDX3Y, lead to hypospermatogenesis (HS) or maturation arrest (MA) (21,27,45,55). Furthermore, AZFa-deleted men typically present with azoospermia (23,24,56). Indeed, it is likely that an AZFa-deleted man with sperm reported in the ejaculate may have only a partial AZFa deletions, since it is these men who can present with cryptozoospermia or oligozoospermia (21,24).
AZFb
AZFb microdeletions are more common than AZFa microdeletions, appearing in 3.5% to 20% of YCM patients, with a mean frequency of 11% (Table 1). Structurally, the AZFb region spans approximately 3.2 mb (57). Although the region is organized as a single copy sequence, it contains numerous palindromic sequences of large direct and inverted repeats, leading to different interpretations of where AZFb ends and AZFc begins (52,57). The region contains several different families of genes: 6 copies of RBMY1 and RBMY2; single copies of EIF1AY, RPS4Y2, KDM5D, HSFY, PRY1, and PRY2 (27,46,58,59). The RBMY1 and 2 proteins are related to RNA splicing (46,60) and it is believed that RBMY1 is important in RNA processing during spermatogenesis because the protein localizes in the nuclei of testicular germ cells and Sertoli cells (61). Plotton et al. (58) demonstrated that co-conservation of at least two RBMY1 and DAZ (AZFc gene) is sufficient for preserving spermatogenesis. Krausz & Casamonti (46) summarize that EIF1AY, RPS4Y2, KDM5D are involved in post-transcriptional or epigenetic control. Next, HSFY encodes for a heat shock protein that was implicated in male infertility because two of the three transcripts are testis-specific and the gene is typically deleted in YCM (46,62,63). Stahl et al. (64) discovered that HSFY mRNA expression is elevated in NOA patients with successful microTESE and is reduced in AZFb-deleted patients (64,65). However, another group suggests HSFY’s impact on infertility is not as deleterious and is simply a time-dependent effect that only manifests in older men (66). Kichine et al. (66), with a sample size of four patients, discovered the HSFY-deleted Y chromosome had been transmitted through generations and thus, HSFY’s contribution to infertility likely to be minimal. Lastly, the AZFb locus contains 2 copies of PRY, of which two copies also exist in the AZFc locus (59). Patients with deleted PRY1 and PRY2 genes present with azoospermia in testis biopsies (59).
Complete AZFb deletions result in azoospermia. Histologically, MA and SCO phenotypes are most commonly observed (12,36). Based on eleven articles, with an n of 52, 38.5% (20/52) are SCO, 48.1% (25/52) are MA and 13.5% (7/52) are HS (Table 1). As a result, microTESE to date has not been successful in classic complete AZFb deletions. However, there are reported cases where patients that had a partial or non-classical AZFb microdeletion were conducive to spermatogenesis and fertilization were successful (28,67).
Again, it is critical to remember that the clinical relevance of AZFb deletions refers only to those men with complete deletions of AZFb (or deletions of AZFb & c regions); men with partial deletions of AZFb may present with cryptozoospermia (12,21,23,56).
AZFc
The AZFc locus is the most commonly deleted, clinically relevant, region of the Y chromosome that is deleted in YCM, with a mean frequency of 57% (Table 1). The region spans 4.5 mb, with six distinct families of amplicons, containing at least seven gene families, and palindromic sequences (52,68). Since the AZFc locus is rich in amplicons and palindromic sequences, it is particularly susceptible to structural rearrangements like deletions and duplications via non-homologous recombination (46,69), explaining why AZFc deletions are so frequent. The gene families present in this locus include DAZ, BPY2, CDY1, CSPG4LY, GOLGA2LY, TTTY3 and TTTY4 (68). AZFc is also suspected to overlap with a limited segment of AZFb deletions and thus contains RBMY genes as well (52,68). It is believed that these genes play a role in fertility. The AZFc locus contains four copies of DAZ that are involved in spermatogenesis. The DAZ gene family encodes for RNA-binding proteins and is involved in meiosis (70). It appears that combinations of deletions of DAZ does not always preclude spermatogenesis. Fernandes et al. (71) report DAZ1 and DAZ2 co-deletion was the cause of five cases of severe oligozoospermia and they later report that partial deletions of AZFc involving DAZ3 and DAZ4 co-deletions are found in fertile normozoospermic men (72,73). This observation supports our prior comments that partial deletions of AZFc cannot be compared to clinical observations for complete AZFc deletions. Complete AZFc deletions are proposed to be a result of homologous recombination between b2 and b4 within the Y chromosome (68).
The phenotype for AZFc YCM varies significantly, depending on which genes are deleted. The sperm profile ranges from azoospermia to oligozoospermia (Table 1). In most studies, it has been reported that azoospermia is proportionately more common than severe oligozoospermia in AZFc-deleted men and that azoospermia did not necessarily preclude the presence of sperm during biopsy or testicular sperm extraction (23,40,56). Certainly, studies may inherently select for more severe cases of male infertility, skewing the proportion of azoospermic men to oligozoospermic men. Liu & Jiang (25) proposed that patients initially are oligozoospermic, then gradually progress to azoospermia because they found that younger patients tend to have significantly greater sperm counts than older patients. However, this is not clear considering that Oates et al. (41) did not find that sperm producing capability declined over time.
Men with AZFc microdeletions typically have a combination of SCO, maturation arrest and hypospermatogenesis regions within the testis (Table 1). From nineteen studies, with a total n of 178, 46% (82/178) had a most advanced histologic pattern of SCO, 38% (68/178) had MA and 16% (28/178) had HS (Table 1). Based on the patient cohort, the distribution of histological features can differ significantly. For instance, Ferlin et al. (21) reported that 72% of the patients in their study had some foci of hypospermatogenesis; however, based on the literature consulted for Table 1, there are more reported cases of SCO and maturation arrest than hypospermatogenesis. Again, in our experience, these men often had mixed histologic patterns of activity in different regions of the same testis, despite the observation that the cause of low sperm production was a uniform genetic abnormality. It is worthwhile to remember that a predominant pattern of SCO histology does not reflect an absence of sperm in other regions of the testis. Oates et al. (41) found that 6/14 men with predominantly SCO histology had sperm present and 6/9 with purely SCO histology had sperm present. Furthermore, patients may present with a combination of histological features. Silber et al. (42) report 2/7 AZFc-deleted patients who had a combination of MA and SCO while Brandell et al. (36) report two patients who had SCO in one testis and MA or HS in the other testis.
It has been suggested that AZFc microdeletions are associated with partial AZFc deletions and Y haplogroups. In a Chinese study, Zhang et al. (47) discovered several haplogroups (N*, N1 and Q1) had a high proportion of AZFc microdeletions. Furthermore, participants in these haplogroups were more likely to have partial AZFc deletions and in one pedigree, a complete AZFc deletion descended from gr/gr deletions, suggesting partial deletions may predispose to complete broader deletions in later generations, although documentation of such expanded deletions is rare and anecdotal at this time (47). This study, however, had a limited number of participants within each deletion subtype binned into haplogroups. Another, more recent study found no significant effect of haplogroups on YCM (26). Further studies are warranted to determine if haplogroups are predictive of YCM.
AZFbc
The prevalence of AZFbc microdeletions range from 2.3% to 20% of infertile men, with a mean frequency of 13% (Table 1). All patients with AZFbc microdeletions present with azoospermia (23,56). Histological presentation varies depending on the size of the deletion and which genes are deleted. Most frequently, patients present with SCO (75%), MA (21%) and HS (3.6%) (21,23,28,29,36,37,40,43).
AZFabc
AZFabc microdeletion is the most extensive region of YCM. Researchers have reported that AZFabc-deleted patients are all azoospermic, and exhibit SCO histologically (21,23,36,56). Prognosis for surgical retrieval of sperm is zero to date.
MicroTESE and histology
The complete deletion of a specific Y chromosome region has important value in predicting the sperm retrieval rate (SRR) in men with azoospermia. Prior to proceeding with surgical sperm extraction, it is critically important to perform a detailed semen analysis, with extended analysis of the centrifuged semen sample (74). As discussed, studies of men with AZFc deletions have reported an SRR ranging from 13% to 100% (Table 2). Of the reported testicular histopathologies amongst AZFc-deleted men (n=178), 28 (16%), 68 (38%), and 82 (46%) men had HS, MA, and SCO, respectively. Among AZFa, AZFb, and AZFc loci, hypospermatogenesis was the most common amongst the AZFc microdeletion (16%), followed by AZFb (13%) and AZFa (2%) (Table 1). However, these numbers should be interpreted with caution because older studies used PCR and STS techniques to characterize AZF deletions, and therefore did not discriminate between complete and partial AZF deletions; thus, these histology results likely reflect a mixture of complete and partial AZF deletions.
Table 2. Sperm retrieval rates stratified by AZF deletion subtypes, collected from 32 studies.
| Author | Year | n | AZF subtype | Histology | Sperm profile (AZOY†, SOLGY‡) | Adjuvant therapy | Sperm retrieval fraction [%] | Procedure |
|---|---|---|---|---|---|---|---|---|
| Silber et al. (42) | 1998 | 10 | AZFc | N/A | AZOY | N/A | 5/10 [50] | TESE |
| Kleiman et al. (75) | 1999 | 1 | AZFc | MA and Leydig cell hyperplasia | AZOY | N/A | 2/2 [100] | Multiple sample TESE |
| Page et al. (76) | 1999 | 1 | AZFc | N/A | AZOY | N/A | 1/1 [100] | TESE |
| Peterlin et al. (77) | 2002 | 1 | AZFa | SCOS | AZOY | N/A | 0/1 [0] | TESE |
| 3 | AZFc | 2 MA, germinal hypoplasia (successful sperm retrievals); 1 SCOS (unsuccessful sperm retrieval) | AZOY | N/A | 2/3 [67] | TESE | ||
| 2 | AZFabc | SCOS | AZOY | N/A | 0/2 [0] | TESE | ||
| Oates et al. (41) | 2002 | 42 | AZFc | SCOS +/− MA | 16 SOLGY; 14 AZOY with sperm in testis; 7 AZOY w/no sperm; 5 AZOY w/no TESE | N/A | 14/21 [67] | TESE & mTESE mix |
| Hopps et al. (56) | 2003 | 3 | AZFa | N/A | AZOY | N/A | 0/1 [0] mTESE; 0/2 [0] biopsy | mTESE or TESE |
| 9 | AZFb | N/A | AZOY | N/A | 0/6 [0] mTESE; 0/3 [0] biopsy | |||
| 32 | AZFc | N/A | SOLGY to AZOY | N/A | 9/12 [75] mTESE; 9/20 [45] biopsy | |||
| 10 | AZFbc | N/A | AZOY | N/A | 0/6 [0] mTESE; 0/4 [0] biopsy | |||
| 4 | AZFabc | N/A | AZOY | N/A | 0/3 [0] mTESE; 0/1 [0] biopsy | |||
| Kihaile et al. (30) | 2004 | 1 | AZFa | SCOS | AZOY | N/A | 0/1 [0] | TESE |
| 2 | Partial AZFc (ΔsY202, ΔsY243) | 1 SCOS; 2 SCOS | AZOY | N/A | 0/2 [0] | |||
| 2 | AZFc (except sY158 and sY159 | MA | AZOY | N/A | 2/2 [100] | |||
| 1 | AZFabc | SCOS | AZOY | N/A | 0/1 [0] | |||
| Tsujimura et al. (43) | 2004 | 1 | AZFabc | SCOS | AZOY | N/A | 0/1 [0] | mTESE |
| 1 | AZFbc | SCOS | AZOY | N/A | 0/1 [0] | |||
| 1 | AZFc | SCOS | AZOY | N/A | 0/1 [0] | |||
| 3 | AZFd | SCOS or MA | AZOY | N/A | 2/3 [67] | |||
| Schlegel et al. (78) | 2004 | 1 | AZFb | N/A | AZOY | N/A | 0/1 [0] | mTESE |
| 1 | AZFabc | N/A | AZOY | N/A | 0/1 [0] | |||
| 1 | AZFbc** | N/A | AZOY | N/A | 0/1 [0] | |||
| 3 | AZFc¶ | N/A | AZOY | N/A | 2/3 [67] | |||
| Choi et al. (31) | 2004 | 1 | AZFa | SCOS | N/A | N/A | 0/1 [0] | mTESE |
| 1 | AZFb | MA | N/A | N/A | 0/1 [0] | |||
| 1 | AZFb (partial) | HS | N/A | N/A | 1/1 [100] | |||
| 2 | AZFbc | SCOS or MA | N/A | N/A | 0/2 [0] | |||
| 7 | AZFc | SCOS, SCOS/MA or MA | N/A | N/A | 6/7 [86] | |||
| Kihaile et al. (32) | 2005 | 1 | AZFa | SCOS | AZOY | N/A | 0/1 [0] | TESE |
| 2 | AZFc | MA | AZOY | N/A | 2/2 [100] | |||
| 1 | AZFabc | SCOS | AZOY | N/A | 0/1 [0] | |||
| Stouffs et al. (33) | 2005 | 1 | AZFa | SCOS | AZOY | N/A | 0/1 [0] | TESE |
| 3 | AZFb | MA | 2 AZOY; 1 SOLGY | N/A | 0/3 [0] | |||
| 17 | AZFc | HS to MA and SCOS | N/A | N/A | 10/17 [59] | |||
| 5 | AZFbc | 3 SCOS; 1 MA | AZOY | N/A | 0/5 [0] | |||
| 2 | AZFabc | SCOS | AZOY | N/A | 0/2 [0] | |||
| Stahl et al. (79) | 2010 | 2 | AZFa | N/A | AZOY | N/A | 0/2 [0] | mTESE |
| 7 | AZFb | N/A | AZOY | N/A | 0/7 [0] | |||
| 7 | AZFbc | N/A | AZOY | N/A | 0/7 [0] | |||
| 4 | AZFc | N/A | SOLGY to AZOY | N/A | 15/21 [71] | |||
| 21 | AZFabc | N/A | AZOY | N/A | 0/4 [0] | |||
| Gambera et al. (80) | 2010 | 1 | AZFc | HS | AZOY | N/A | 1/1 [100] | mTESE |
| Kilic et al. (81) | 2010 | 1 | ΔsY127, ΔsY134 (partial AZFb); Mosaicsm 45X (5%)/46XY (95%) by cytogenetic analysis | SCOS, MA | AZOY | N/A | 1/1 [100] | TESE |
| 1 | ΔsY127, ΔsY134 (partial AZFb); ΔsY254, ΔsY255 (partial AZFc); 45,X(45%)/46,XY(55%) mosaicism | N/A | AZOY | N/A | 0/1 [0] | |||
| Stahl et al. (13) | 2011 | 22 | Partial AZFc—gr/gr | N/A | AZOY to SOLGY | N/A | 14/22 [64] | mTESE |
| Kalsi et al. (82) | 2012 | 1 | AZFc | MA | AZOY | N/A | 1/1 [100] | mTESE |
| Zhang et al. (23) | 2013 | 8 (only 8/12 received TESA) | AZFb | MA | AZOY | N/A | 0/8 [0] | TESA |
| 11 | AZFb | MA | AZOY | N/A | 3/11 [27] | mTESE | ||
| 16 | AZFc | SCOS or MA or HS | 26% SOLGY; 74% AZOY | N/A | 0/16 [0] | TESA | ||
| 50 | AZFc | SCOS or MA or HS | 26% SOLGY; 74% AZOY | N/A | 11/40 [28] | mTESE | ||
| Choi et al. (83) | 2013 | 21 | AZFc | N/A | AZOY | N/A | 8/21 [38] | TESE |
| 9 | AZFbc | N/A | AZOY | N/A | 0/9 [0] | |||
| Ando et al. (84) | 2013 | 4 | N/A | N/A | AZOY | N/A | 1/4 [25] | mTESE |
| Bonarriba et al. (85) | 2013 | 2 | AZFab | N/A | AZOY | N/A | 0/2 [0] | mTESE |
| 3 | AZFc | N/A | AZOY | N/A | 1/3 [33] | |||
| Gallego et al. (34) | 2014 | 2 | AZFa | 1 SCOS; biopsy not performed on rest | AZOY | N/A | 0/1 [0] | TESE |
| 5 | AZFc | 1 SCOS; biopsy not performed on rest | 4 AZOY; 1 SOLGY | N/A | 0/1 [0] | |||
| 1 | AZFbc | N/A | AZOY | N/A | Not performed | N/A | ||
| 2 | AZFabc | 1 MA; biopsy not performed on rest | AZOY | N/A | 0/1 [0] | TESE | ||
| Lo Giacco et al. (35) | 2014 | 1 | AZFa | SCOS | AZOY | N/A | 0/1 [0] | TESE |
| 18 | AZFc | 4 complete SCOS, 2 80-90% SCOS, 1 L testicle only; 1 HS, one 10% HS; one 10% sclero hialynosis; one with R mixed atrophy with no mature spermatids | 11 AZOY; 7 SOLGY | N/A | 1/8 [13] | |||
| 2 | AZFc (terminal deletion) | 1 HS; 1 R 50% sclero hialynosis, 50% SCOS; L SCOS | AZOY | N/A | 0/2 [0] | |||
| 2 | AZFbc | N/A | AZOY | N/A | Not performed | N/A | ||
| 4 | AZFabc | N/A | N/A | N/A | Not performed | |||
| Cetinkaya et al. (86) | 2015 | 1 | AZFb | N/A | AZOY | N/A | 0/1 [0] | mTESE |
| 5 | AZFc | N/A | AZOY | N/A | 1/5 [20] | |||
| 1 | AZFac | N/A | AZOY | N/A | 0/1 [0] | |||
| 2 | AZFbc | N/A | AZOY | N/A | 0/2 [0] | |||
| 1 | AZFabc | N/A | AZOY | N/A | 0/1 [0] | |||
| Schwarzer et al. (39) | 2016 | 1 | AZFb | 1 MA | N/A | N/A | 0/1 [0] | Conventional multilocular TESE or mTESE (not stated) |
| 20 | AZFc | 11 SCOS; 5 MA; 4 mixed atrophy | N/A | N/A | 2/8 [25] conventional multilocular TESE; 8/12 [67] mTESE | conventional multilocular TESE; mTESE | ||
| 2 | AZFbc | 1 SCOS; 1 MA; 4 mixed atrophy | N/A | N/A | 0/2 [0] | Conventional multilocular TESE or mTESE (not stated) | ||
| 2 | AZFc + other chromosomal disorder | 2 SCOS | N/A | N/A | 0/2 [0] | Conventional multilocular TESE or mTESE (not stated) | ||
| Mascarenhas et al. (87) | 2016 | 5 | AZFb | N/A | AZOY | N/A | 0/4 [0] | TESA |
| 5 | AZFc | N/A | 2 SOLGY; 3 AZOY | N/A | 1/1 AZOY [100] TESA; 1/1 AZOY [100] mTESE | TESA; mTESE | ||
| 2 | AZFbc | N/A | AZOY | N/A | 0/2 [0] | TESA | ||
| 1 | AZFabc | N/A | AZOY | N/A | 0/1 [0] | TESA | ||
| Ko et al. (88) | 2016 | 2 | ΔAZFbc; (46XY/45X (33:1) and 45,X/46,X,idic(Y)(q11.2) (12:18) | N/A | AZOY | N/A | 0/2 [0] | TESE or mTESE (not stated) |
| 1 | ΔAZFabc; 46,X,der(Y).ish i(Y)(p10)(pter++,SRY++) | N/A | AZOY | N/A | 0/1 [0] | TESE or mTESE | ||
| 6 | AZFc | N/A | AZOY | N/A | 3/6 [50] | TESE or mTESE | ||
| Bahmanimehr et al. (20) | 2018 | 1 | AZFabc | N/A | AZOY | N/A | 1/1 [100] | TESE |
| Sabbaghian et al. (89) | 2018 | 96 (# who attempted mTESE) | AZFc | Of 103 patients (some did not attempt mTESE): 69/103 (67) SCOS, 27/103 (26) MA, remaining histopathology not stated | AZOY or SOLGY | N/A | 42/96 [44] | mTESE |
| Klami et al. (90) | 2018 | 7 | AZFc | N/A | AZOY | Men with low testosterone were treated with aromatase inhibitor (n=26), clomiphene citrate (n=5), tamoxifen (n=11) or human chorionic gonadotropin (n=6) for four to six months prior to the operation in order to reach normal serum testosterone levels | 4/7 [57] | mTESE |
| Miraghazadeh et al. (91) | 2019 | 11 | gr/gr (partial AZFc) | N/A | AZOY | N/A | 7/11 [64] | mTESE |
| 5 | b2/b3 (partial AZFc) | N/A | AZOY | N/A | 2/5 [40] | |||
| Johnson et al. (19) | 2019 | 1 | AZFa | N/A | AZOY | N/A | N/A | mTESE |
| 1 | AZFa (partial) | N/A | AZOY | N/A | N/A | |||
| 4 | AZFb | MA | AZOY | N/A | 0/3 [0] | |||
| 44 | AZFc | N/A | 12 SOLGY; 32 AZOY | N/A | 7/21 [33] | |||
| 8 | AZFbc | N/A | AZOY | N/A | N/A |
†azoospermia with a Y-chromosome microdeletion (0 sperm/cc); ‡severe oligozoospermia with a Y-chromosome microdeletion (sperm concentration >0 – <5×106 sperm/cc); **One patient from this cohort received varicocele repair; ¶One patient from this cohort received varicocele repair.
Sperm retrieval
Thirty-two studies involving men with Y chromosome microdeletions who underwent any surgical sperm retrieval procedure are summarized in Table 2.
AZFa
Men with complete and pure AZFa deletions are solely azoospermic and sperm has not been retrieved by any method (Tables 1,2). Surprisingly, a case report describing a man with a complete AZFa deletion, partial AZFb and AZFc deletions had sperm in his ejaculate (92). This is remarkable as no other comparable cases were identified in our literature search. The presence of outlier studies that report finding sperm in men with genetic abnormalities that are not verified by other laboratories may reflect a distortion of published literature and should be interpreted with caution. In all other studies of those undergoing sperm retrieval attempts, all failed (Table 2).
AZFb
Men with complete AZFb microdeletions have been azoospermic (Tables 1,2). For the exceptional AZFb-deleted men with a successful sperm extraction procedure, SR via mTESE was higher than conventional TESE or TESA (testicular sperm aspiration). The overall SRR for men with complete AZFb deletions was 0/30 (0%) (Table 2). This extremely poor prognosis is fitting with published studies that recommends men with complete AZFb deletions should not be offered mTESE (56,93-95). One group, Zhang et al. (23) report a SRR of 3/11 (27%) using mTESE in men with AZFb deletions, with two resulting in pregnancy; however, it is difficult to determine whether study participants had a complete or partial AZFb deletion. Previous literature reports that the AZFb locus is proximally defined by sY108 and distally characterized by sY134 or sY135 (57) however Zhang et al. (23) defined the AZFb deletions using sY127 and sY134 marker. Thus, the reported SRR of 27% was more likely conducted in men with partial AZFb deletions. In men with partial AZFb deletions, sperm extraction attempts were successful (Table 2). The discrepancy in sperm retrieval success in complete vs. partial AZFb deletions underscore the importance of correct identification of the size of deletion in the AZFb locus in order to more precisely approximate the chances of a successful sperm retrieval.
AZFc
Studies of men with YCM, when based on patients who require surgical sperm, may overemphasize the prevalence of azoospermia (vs. oligozoospermia). According to Table 1 and 2, men with either partial or complete AZFc microdeletions are typically azoospermic, though severe oligozoospermia is also common. Based on the 32 studies in Table 2, the proportion of men with partial or complete AZFc microdeletions that are azoospermic and oligozoospermic were 67% and 33%, respectively. When considering men referred for genetic testing, independent of semen parameters, we have found that more men had sperm in the ejaculate than not (70% oligozoospermic vs. 30% azoospermic). As demonstrated in Table 2, men with AZFc microdeletions have the most favourable chances of successful sperm retrieval compared to men with any other type of deletion in the AZF region of the Yq. The SRR for AZFc-deleted men are reported to be between 13% to 100%, with a mean of 47% across the 32 studies reviewed, though some studies also report failure to retrieve any sperm at all (Table 2). Concordant with published literature, mTESE had a higher SRR than conventional TESE or TESA in men with YCM (Table 2).
It is critically important to remember that the results of microTESE, and other sperm retrieval procedures, are dependent on the extent of tissue searched for sperm. Even microTESE procedures may vary substantially from center to center or amongst surgeons. The more extensive the procedure, the higher the chance of finding rare sites of sperm production.
Multiple AZF Deletions (AZFabc, AZFab, AZFbc)
Men with multiple AZF deletions that attempted any sperm retrieval procedure are listed in Table 2. Apart from one azoospermic man with an AZFabc deletion (20), all surgical sperm retrieval attempts failed (Table 2). Given these findings, we conclude that testicular sperm extraction attempts in men with combination deletions is very unlikely to be successful.
Adjuvant therapy
Men with severe infertility often attempt empiric medical therapy (EMT) in order to improve sperm production through enhancing endogenous testosterone production, and therefore supporting spermatogenesis. Empiric medical therapy may include the use of hormone altering agents such as human chorionic gonadotropin (hCG), aromatase inhibitors (testolactone, letrozole and anastrozole), selective estrogen receptor modifiers (clomiphene, tamoxifen), and antioxidant supplementation. A number of studies have attempted to optimize sperm production in men who are expected to undergo mTESE. Unfortunately, Level I evidence has not been produced to determine the value, if any, of medical therapy prior to attempted sperm retrieval.
In Table 2, only 7 men were treated prior to their operation. According to the retrospective study, men with low testosterone were treated with aromatase inhibitor, clomiphene citrate, tamoxifen, or hCG for four to six months prior to their operations to restore their serum testosterone concentration (90). However, the SRR was no different between the medically treated versus the untreated men, though the participants were not limited to men with YCM.
Data on the effects of EMT on SRR among men with YCM was absent in our literature search. However, upon an additional search, we identified a case report of a normogonadotropic azoospermic man with an AZFc deletion (96). Upon semen analyses at 2-week intervals, complete absence of sperm was concluded. A diagnostic testicular biopsy displayed maturation arrest at the spermatocyte stage. The patient underwent recombinant follicle-stimulating hormone (FSH) treatment for 6 months and subsequent semen analyses at 15-day intervals were performed. In the ejaculate, 0.001×106 and 0.002×106 were found in the entire first and second semen samples, respectively. The patient then underwent successful ICSI resulting in the delivery of two healthy girls. Unfortunately, it is not clear if a repeat semen analysis with a more thorough evaluation of the centrifuged specimen prior to receiving recombinant FSH treatment would have also demonstrated rare sperm, as reported by Ron-El et al., given this study discovered occasional sperm cells after meticulous microscopic investigation amongst patients set for TESE (74). Studies on the effects of EMT on SRR among men with NOA who have failed prior sperm retrieval attempts is more extensive, though the results of the data are not clear, as these studies have not evaluated the relative effects of repeat sperm retrieval alone vs. sperm retrieval repeat with EMT (97). According to this review of preoperative patient optimization for mTESE, adjuvant therapy prior to mTESE for men with YCM and NOA may be used but insufficient data exists to determine if a positive effect on spermatogenesis occurs.
Conclusion
In this review, YCM and its subtypes, microTESE results, ICSI results and ART sequelae in offspring were summarized. In conclusion, YCM typically reflect deletion of discrete, predictable gene regions of the Y chromosome that severely impact male fertility. Deletions can be single- or multi-locus deletions and lead to distinct clinical and histological phenotypes. AZFa, AZFb and multi-locus deletions have the most dramatic adverse effects on spermatogenesis. As a result, sperm are rarely, if ever, retrievable. AZFc deletions are the most benign, resulting in a combination of histologic testicular abnormalities resulting in severe oligospermia or azoospermia. Careful analysis for rare sperm in the ejaculate is critical for men with AZFc deletions. The sperm retrieval rate for AZFc ranges between 13% to 100%; for azoospermic AZFc-deleted men, a careful and detailed microTESE procedure is critical to obtain optimal sperm retrieval results.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editors (Keith Jarvi and Jared Bieniek) for the series “Genetic Causes and Management of Male Infertility” published in Translational Andrology and Urology. The article has undergone external peer review.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau.2020.03.35). The series “Genetic Causes and Management of Male Infertility” was commissioned by the editorial office without any funding or sponsorship. RF reports grants from American Society of Reproductive Medicine, grants from Canadian Institute of Health Research, grants from Canadian Urologic Association Scholarship Foundation, grants from New Frontiers Research Fund, grants from Vancouver Coastal Health Research Institute, personal fees from Paladin Labs, personal fees from Boston Scientific, outside the submitted work. The authors have no other conflicts of interest to declare.
References
- 1.Agarwal A, Mulgund A, Hamada A, et al. A unique view on male infertility around the globe. Reprod Biol Endocrinol 2015;13:37. 10.1186/s12958-015-0032-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schlegel PN. Causes of azoospermia and their management. Reprod Fertil Dev 2004;16:561-72. 10.1071/RD03087 [DOI] [PubMed] [Google Scholar]
- 3.Miyamoto T, Minase G, Shin T, et al. Human male infertility and its genetic causes. Reprod Med Biol 2017;16:81-8. 10.1002/rmb2.12017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colaco S, Modi D. Genetics of the human Y chromosome and its association with male infertility. Reprod Biol Endocrinol 2018;16:14. 10.1186/s12958-018-0330-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quintana-Murci L, Fellous M. The human Y chromosome: The biological role of a “functional wasteland.” J Biomed Biotechnol 2001;1:18-24. 10.1155/S1110724301000080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girardi SK, Mielnik A, Schlegel PN. Submicroscopic deletions in the Y chromosome of infertile men. Hum Reprod 1997;12:1635-41. 10.1093/humrep/12.8.1635 [DOI] [PubMed] [Google Scholar]
- 7.Schlegel PN. The Y chromosome. Reprod Biomed Online 2002;5:22-5. 10.1016/S1472-6483(10)61592-1 [DOI] [PubMed] [Google Scholar]
- 8.Tiepolo L, Zuffardi O. Localization of factors controlling spermatogenesis in the nonfluorescent portion of the human y chromosome long arm. Hum Genet 1976;34:119-24. 10.1007/BF00278879 [DOI] [PubMed] [Google Scholar]
- 9.Vogt PH. Human chromosome deletions in Yq11, AZF candidate genes and male infertility: history and update. Mol Hum Reprod 1998;4:739-44. 10.1093/molehr/4.8.739 [DOI] [PubMed] [Google Scholar]
- 10.Vollrath D, Foote S, Hilton A, et al. The human Y chromosome: A 43-interval map based on naturally occurring deletions. Science 1992;258:52-9. 10.1126/science.1439769 [DOI] [PubMed] [Google Scholar]
- 11.Vogt PH, Edelmann A, Kirsch S, et al. Human Y chromosome azoospermia factors (AZF) mapped to different subregions in Yq11. Hum Mol Genet 1996;5:933-43. 10.1093/hmg/5.7.933 [DOI] [PubMed] [Google Scholar]
- 12.Kent-First M, Muallem A, Shultz J, et al. Defining regions of the Y-chromosome responsible for male infertility and identification of a fourth AZF region (AZFd) by Y-chromosome microdeletion detection. Mol Reprod Dev 1999;53:27-41. [DOI] [PubMed] [Google Scholar]
- 13.Stahl PJ, Mielnik A, Margreiter M, et al. Diagnosis of the gr/gr Y chromosome microdeletion does not help in the treatment of infertile American men. J Urol 2011;185:233-7. 10.1016/j.juro.2010.09.016 [DOI] [PubMed] [Google Scholar]
- 14.Shirakawa T, Fujisawa M, Kanzaki M, et al. Y chromosome (Yq11) microdeletions in idiopathic azoospermia. Int J Urol 1997;4:198-201. 10.1111/j.1442-2042.1997.tb00170.x [DOI] [PubMed] [Google Scholar]
- 15.Abdel Raheem A, Rushwan N, Garaffa G, et al. Factors influencing intracytoplasmic sperm injection (ICSI) outcome in men with azoospermia. BJU Int 2013;112:258-64. 10.1111/j.1464-410X.2012.11714.x [DOI] [PubMed] [Google Scholar]
- 16.Schlegel PN. Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Hum Reprod 1999;14:131-5. 10.1093/humrep/14.1.131 [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med 2009;6:e1000100. 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson M, Raheem A, De Luca F, et al. An analysis of the frequency of Y-chromosome microdeletions and the determination of a threshold sperm concentration for genetic testing in infertile men. BJU Int 2019;123:367-72. 10.1111/bju.14521 [DOI] [PubMed] [Google Scholar]
- 20.Bahmanimehr A, Zeighami S, Namavar Jahromi B, et al. Detection of Y Chromosome Microdeletions and Hormonal Profile Analysis of Infertile Men undergoing Assisted Reproductive Technologies. Int J Fertil Steril 2018;12:173-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferlin A, Arredi B, Speltra E, et al. Molecular and clinical characterization of Y chromosome microdeletions in infertile men: a 10-year experience in Italy. J Clin Endocrinol Metab 2007;92:762-70. 10.1210/jc.2006-1981 [DOI] [PubMed] [Google Scholar]
- 22.Ambulkar PS, Sigh R, Reddy M, et al. Genetic Risk of Azoospermia Factor (AZF) Microdeletions in Idiopathic Cases of Azoospermia and Oligozoospermia in Central Indian Population. J Clin Diagn Res 2014;8:88-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang F, Li L, Wang L, et al. Clinical characteristics and treatment of azoospermia and severe oligospermia patients with Y-chromosome microdeletions. Mol Reprod Dev 2013;80:908-15. 10.1002/mrd.22226 [DOI] [PubMed] [Google Scholar]
- 24.Liu X-Y, Zhang H-Y, Pang D-X, et al. AZFa Microdeletions: Occurrence in Chinese Infertile Men and Novel Deletions Revealed by Semiconductor Sequencing. Urology 2017;107:76-81. 10.1016/j.urology.2017.04.024 [DOI] [PubMed] [Google Scholar]
- 25.Liu T, Song Y-X, Jiang Y-M. Early detection of Y chromosome microdeletions in infertile men is helpful to guide clinical reproductive treatments in southwest of China. Medicine (Baltimore) 2019;98:e14350. 10.1097/MD.0000000000014350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rani DS, Rajender S, Pavani K, et al. High frequencies of Non Allelic Homologous Recombination (NAHR) events at the AZF loci and male infertility risk in Indian men. Sci Rep 2019;9:6276. 10.1038/s41598-019-42690-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blagosklonova O, Fellmann F, Clavequin MC, et al. AZFa deletions in Sertoli cell-only syndrome: a retrospective study. Mol Hum Reprod 2000;6:795-9. 10.1093/molehr/6.9.795 [DOI] [PubMed] [Google Scholar]
- 28.Kim MJ, Choi HW, Park SY, et al. Molecular and cytogenetic studies of 101 infertile men with microdeletions of Y chromosome in 1,306 infertile Korean men. J Assist Reprod Genet 2012;29:539-46. 10.1007/s10815-012-9748-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujisawa M, Shirakawa T, Kanzaki M, et al. Y-chromosome microdeletion and phenotype in cytogenetically normal men with idiopathic azoospermia. Fertil Steril 2001;76:491-5. 10.1016/S0015-0282(01)01955-0 [DOI] [PubMed] [Google Scholar]
- 30.Kihaile PE, Kisanga RE, Aoki K, et al. Embryo outcome in Y-chromosome microdeleted infertile males after ICSI. Mol Reprod Dev 2004;68:176-81. 10.1002/mrd.20074 [DOI] [PubMed] [Google Scholar]
- 31.Choi JM, Chung P, Veeck L, et al. AZF microdeletions of the Y chromosome and in vitro fertilization outcome. Fertil Steril 2004;81:337-41. 10.1016/j.fertnstert.2003.06.030 [DOI] [PubMed] [Google Scholar]
- 32.Kihaile PE, Yasui A, Shuto Y. Prospective assessment of Y-chromosome microdeletions and reproductive outcomes among infertile couples of Japanese and African origin. J Exp Clin Assist Reprod 2005;2:9. 10.1186/1743-1050-2-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stouffs K, Lissens W, Tournaye H, et al. The choice and outcome of the fertility treatment of 38 couples in whom the male partner has a Yq microdeletion. Hum Reprod 2005;20:1887-96. 10.1093/humrep/deh847 [DOI] [PubMed] [Google Scholar]
- 34.Gallego A, Rogel R, Luján S, et al. AZF gene microdeletions: Case series and literature review. Actas Urol Esp 2014;38:698-702. 10.1016/j.acuro.2014.04.003 [DOI] [PubMed] [Google Scholar]
- 35.Lo Giacco D, Chianese C, Sanchez-Curbelo J, et al. Clinical relevance of Y-linked CNV screening in male infertility: new insights based on the 8-year experience of a diagnostic genetic laboratory. Eur J Hum Genet 2014;22:754-61. 10.1038/ejhg.2013.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brandell RA, Mielnik A, Liotta D, et al. AZFb deletions predict the absence of spermatozoa with testicular sperm extraction: preliminary report of a prognostic genetic test. Hum Reprod 1998;13:2812-5. 10.1093/humrep/13.10.2812 [DOI] [PubMed] [Google Scholar]
- 37.Müslümanoglu MH, Turgut M, Cilingir O, et al. Role of the AZFd locus in spermatogenesis. Fertil Steril 2005;84:519-22. 10.1016/j.fertnstert.2005.02.024 [DOI] [PubMed] [Google Scholar]
- 38.Yang Y, Ma MY, Xiao CY, et al. Massive deletion in AZFb/b+c and azoospermia with Sertoli cell only and/or maturation arrest. Int J Androl 2008;31:573-8. 10.1111/j.1365-2605.2007.00808.x [DOI] [PubMed] [Google Scholar]
- 39.Schwarzer JU, Steinfatt H, Schleyer M, et al. Microdissection TESE is superior to conventional TESE in patients with nonobstructive azoospermia caused by Y chromosome microdeletions. Andrologia 2016;48:402-5. 10.1111/and.12460 [DOI] [PubMed] [Google Scholar]
- 40.Yamada K, Fujita K, Quan J, et al. Increased apoptosis of germ cells in patients with AZFc deletions. J Assist Reprod Genet 2010;27:293-7. 10.1007/s10815-010-9400-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oates RD, Silber S, Brown LG, et al. Clinical characterization of 42 oligospermic or azoospermic men with microdeletion of the AZFc region of the Y chromosome, and of 18 children conceived via ICSI. Hum Reprod 2002;17:2813-24. 10.1093/humrep/17.11.2813 [DOI] [PubMed] [Google Scholar]
- 42.Silber SJ, Alagappan R, Brown LG, et al. Y chromosome deletions in azoospermic and severely oligozoospermic men undergoing intracytoplasmic sperm injection after testicular sperm extraction. Hum Reprod 1998;13:3332-7. 10.1093/humrep/13.12.3332 [DOI] [PubMed] [Google Scholar]
- 43.Tsujimura A, Matsumiya K, Takao T, et al. Clinical analysis of patients with azoospermia factor deletions by microdissection testicular sperm extraction. Int J Androl 2004;27:76-81. 10.1046/j.0105-6263.2003.00450.x [DOI] [PubMed] [Google Scholar]
- 44.Li Q, Song N-H, Cao W-Z, et al. Relationship between AZFc deletions and testicular histology in infertile South Chinese men with azoospermia and severe oligospermia. Springerplus 2016;5:1805. 10.1186/s40064-016-3512-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao G, Chen G, Pan T. Study of microdeletions in the Y chromosome of infertile men with idiopathic oligo- or azoospermia. J Assist Reprod Genet 2001;18:612-6. 10.1023/A:1013117123244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krausz C, Casamonti E. Spermatogenic failure and the Y chromosome. Hum Genet 2017;136:637-55. 10.1007/s00439-017-1793-8 [DOI] [PubMed] [Google Scholar]
- 47.Zhang F, Lu C, Li Z, et al. Partial deletions are associated with an increased risk of complete deletion in AZFc: a new insight into the role of partial AZFc deletions in male infertility. J Med Genet 2007;44:437-44. 10.1136/jmg.2007.049056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Machev N, Saut N, Longepied G, et al. Sequence family variant loss from the AZFc interval of the human Y chromosome, but not gene copy loss, is strongly associated with male infertility. J Med Genet 2004;41:814-25. 10.1136/jmg.2004.022111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shahid M, Dhillon VS, Khalil HS, et al. Associations of Y-chromosome subdeletion gr/gr with the prevalence of Y-chromosome haplogroups in infertile patients. Eur J Hum Genet 2011;19:23-9. 10.1038/ejhg.2010.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tomasi PA, Oates R, Brown L, et al. The pituitary-testicular axis in Klinefelter’s syndrome and in oligo-azoospermic patients with and without deletions of the Y chromosome long arm. Clin Endocrinol (Oxf) 2003;59:214-22. 10.1046/j.1365-2265.2003.01828.x [DOI] [PubMed] [Google Scholar]
- 51.Foresta C, Moro E, Rossi A, et al. Role of the AZFa candidate genes in male infertility. J Endocrinol Invest 2000;23:646-51. 10.1007/BF03343788 [DOI] [PubMed] [Google Scholar]
- 52.Silber SJ. The y chromosome in the era of intracytoplasmic sperm injection: A personal review. Fertil Steril 2011;95:2439-2448.e1. 10.1016/j.fertnstert.2011.05.070 [DOI] [PubMed] [Google Scholar]
- 53.Sargent CA, Boucher CA, Kirsch S, et al. The critical region of overlap defining the AZFa male infertility interval of proximal Yq contains three transcribed sequences. J Med Genet 1999;36:670-7. [PMC free article] [PubMed] [Google Scholar]
- 54.Matsumura T, Endo T, Isotani A, et al. An azoospermic factor gene, Ddx3y and its paralog, Ddx3x are dispensable in germ cells for male fertility. J Reprod Dev 2019;65:121-8. 10.1262/jrd.2018-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kamp C, Huellen K, Fernandes S, et al. High deletion frequency of the complete AZFa sequence in men with Sertoli-cell-only syndrome. Mol Hum Reprod 2001;7:987-94. 10.1093/molehr/7.10.987 [DOI] [PubMed] [Google Scholar]
- 56.Hopps C V, Mielnik A, Goldstein M, et al. Detection of sperm in men with Y chromosome microdeletions of the AZFa, AZFb and AZFc regions. Hum Reprod 2003;18:1660-5. 10.1093/humrep/deg348 [DOI] [PubMed] [Google Scholar]
- 57.Ferlin A, Moro E, Rossi A, et al. The human Y chromosome’s azoospermia factor b (AZFb) region: sequence, structure, and deletion analysis in infertile men. J Med Genet 2003;40:18-24. 10.1136/jmg.40.1.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Plotton I, Ducros C, Pugeat M, et al. Transmissible microdeletion of the Y-chromosome encompassing two DAZ copies, four RBMY1 copies, and both PRY copies. Fertil Steril 2010;94:2770.e11-2770.e16. 10.1016/j.fertnstert.2010.04.038 [DOI] [PubMed] [Google Scholar]
- 59.Stouffs K, Lissens W, Van Landuyt L, et al. Characterization of the genomic organization, localization and expression of four PRY genes (PRY1, PRY2, PRY3 and PRY4). Mol Hum Reprod 2001;7:603-10. 10.1093/molehr/7.7.603 [DOI] [PubMed] [Google Scholar]
- 60.Elliott DJ. RBMY genes and AZFb deletions. J Endocrinol Invest 2000;23:652-8. 10.1007/BF03343789 [DOI] [PubMed] [Google Scholar]
- 61.Osterlund C, Stabi B, Bhasin S, et al. Specific localization of RBM1a in the nuclei of all cell types except elongated spermatids within seminiferous tubules of the human. Int J Androl 2001;24:272-7. 10.1046/j.1365-2605.2001.00299.x [DOI] [PubMed] [Google Scholar]
- 62.Tessari A, Salata E, Ferlin A, et al. Characterization of HSFY, a novel AZFb gene on the Y chromosome with a possible role in human spermatogenesis. Mol Hum Reprod 2004;10:253-8. 10.1093/molehr/gah036 [DOI] [PubMed] [Google Scholar]
- 63.Vinci G, Raicu F, Popa L, et al. A deletion of a novel heat shock gene on the Y chromosome associated with azoospermia. Mol Hum Reprod 2005;11:295-8. 10.1093/molehr/gah153 [DOI] [PubMed] [Google Scholar]
- 64.Stahl PJ, Mielnik A, Schlegel PN, et al. Heat shock factor Y chromosome (HSFY) mRNA level predicts the presence of retrievable testicular sperm in men with nonobstructive azoospermia. Fertil Steril 2011;96:303-8. 10.1016/j.fertnstert.2011.05.055 [DOI] [PubMed] [Google Scholar]
- 65.Stahl PJ, Mielnik AN, Barbieri CE, et al. Deletion or underexpression of the Y-chromosome genes CDY2 and HSFY is associated with maturation arrest in American men with nonobstructive azoospermia. Asian J Androl 2012;14:676-82. 10.1038/aja.2012.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kichine E, Roze V, Di Cristofaro J, et al. HSFY genes and the P4 palindrome in the AZFb interval of the human Y chromosome are not required for spermatocyte maturation. Hum Reprod 2012;27:615-24. 10.1093/humrep/der421 [DOI] [PubMed] [Google Scholar]
- 67.Stouffs K, Vloeberghs V, Gheldof A, et al. Are AZFb deletions always incompatible with sperm production? Andrology 2017;5:691-4. 10.1111/andr.12350 [DOI] [PubMed] [Google Scholar]
- 68.Kuroda-Kawaguchi T, Skaletsky H, Brown LG, et al. The AZFc region of the Y chromosome features massive palindromes and uniform recurrent deletions in infertile men. Nat Genet 2001;29:279-86. 10.1038/ng757 [DOI] [PubMed] [Google Scholar]
- 69.Noordam MJ, van Daalen SKM, Hovingh SE, et al. A novel partial deletion of the Y chromosome azoospermia factor c region is caused by non-homologous recombination between palindromes and may be associated with increased sperm counts. Hum Reprod 2011;26:713-23. 10.1093/humrep/deq386 [DOI] [PubMed] [Google Scholar]
- 70.Reynolds N, Cooke HJ. Role of the DAZ genes in male fertility. Reprod Biomed Online 2005;10:72-80. 10.1016/S1472-6483(10)60806-1 [DOI] [PubMed] [Google Scholar]
- 71.Fernandes S, Huellen K, Goncalves J, et al. High frequency of DAZ1/DAZ2 gene deletions in patients with severe oligozoospermia. Mol Hum Reprod. 2002;8:286-98. 10.1093/molehr/8.3.286 [DOI] [PubMed] [Google Scholar]
- 72.Fernandes S, Paracchini S, Meyer LH, et al. A large AZFc deletion removes DAZ3/DAZ4 and nearby genes from men in Y haplogroup N. Am J Hum Genet 2004;74:180-7. 10.1086/381132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ghorbel M, Baklouti-Gargouri S, Keskes R, et al. Combined deletion of DAZ2 and DAZ4 copies of Y chromosome DAZ gene is associated with male infertility in Tunisian men. Gene 2014;547:191-4. 10.1016/j.gene.2014.05.061 [DOI] [PubMed] [Google Scholar]
- 74.Ron-El R, Strassburger D, Friedler S, et al. Extended sperm preparation: An alternative to testicular sperm extraction in non-obstructive azoospermia. Hum Reprod 1997;12:1222-6. 10.1093/humrep/12.6.1222 [DOI] [PubMed] [Google Scholar]
- 75.Kleiman SE, Yogev L, Gamzu R, et al. Three-generation evaluation of Y-chromosome microdeletion. J Androl 1999;20:394-8. [PubMed] [Google Scholar]
- 76.Page DC, Silber S, Brown LG. Men with infertility caused by AZFc deletion can produce sons by intracytoplasmic sperm injection, but are likely to transmit the deletion and infertility. Hum Reprod 1999;14:1722-6. 10.1093/humrep/14.7.1722 [DOI] [PubMed] [Google Scholar]
- 77.Peterlin B, Kunej T, Sinkovec J, et al. Screening for Y chromosome microdeletions in 226 Slovenian subfertile men. Hum Reprod 2002;17:17-24. 10.1093/humrep/17.1.17 [DOI] [PubMed] [Google Scholar]
- 78.Schlegel PN, Kaufmann J. Role of varicocelectomy in men with nonobstructive azoospermia. Fertil Steril 2004;81:1585-8. 10.1016/j.fertnstert.2003.10.036 [DOI] [PubMed] [Google Scholar]
- 79.Stahl PJ, Masson P, Mielnik A, et al. A decade of experience emphasizes that testing for Y microdeletions is essential in American men with azoospermia and severe oligozoospermia. Fertil Steril 2010;94:1753-6. 10.1016/j.fertnstert.2009.09.006 [DOI] [PubMed] [Google Scholar]
- 80.Gambera L, Governini L, De Leo V, et al. Successful multiple pregnancy achieved after transfer of frozen embryos obtained via intracytoplasmic sperm injection with testicular sperm from an AZFc-deleted man. Fertil Steril 2010;94:2330.e1-3. 10.1016/j.fertnstert.2010.03.069 [DOI] [PubMed] [Google Scholar]
- 81.Kilic S, Yukse B, Tasdemir N, et al. Assisted reproductive treatment applications in men with normal phenotype but 45,X/46,Xy mosaic karyotype: Clinical and genetic perspectives. Taiwan J Obstet Gynecol 2010;49:199-202. 10.1016/S1028-4559(10)60042-3 [DOI] [PubMed] [Google Scholar]
- 82.Kalsi J, Thum M-Y, Muneer A, et al. In the era of micro-dissection sperm retrieval (m-TESE) is an isolated testicular biopsy necessary in the management of men with non-obstructive azoospermia? BJU Int 2012;109:418-24. 10.1111/j.1464-410X.2011.10399.x [DOI] [PubMed] [Google Scholar]
- 83.Choi DK, Gong IH, Hwang JH, et al. Detection of Y chromosome microdeletion is valuable in the treatment of patients with nonobstructive azoospermia and oligoasthenoteratozoospermia: Sperm retrieval rate and birth rate. Korean J Urol 2013;54:111-6. 10.4111/kju.2013.54.2.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ando M, Yamaguchi K, Chiba K, et al. Outcome of microdissection testicular sperm extraction in azoospermic patients with Klinefelter syndrome and other sex-chromosomal anomalies. Syst Biol Reprod Med 2013;59:210-3. 10.3109/19396368.2012.733059 [DOI] [PubMed] [Google Scholar]
- 85.Bonarriba CR, Burgues JP, Vidana V, et al. Predictive factors of successful sperm retrieval in azoospermia. Actas Urol Esp 2013;37:266-72. 10.1016/j.acuro.2012.06.002 [DOI] [PubMed] [Google Scholar]
- 86.Cetinkaya M, Onem K, Zorba OU, et al. Evaluation of Microdissection Testicular Sperm Extraction Results in Patients with Non-Obstructive Azoospermia: Independent Predictive Factors and Best Cutoff Values for Sperm Retrieval. Urol J 2015;12:2436-43. [PubMed] [Google Scholar]
- 87.Mascarenhas M, Thomas S, Kamath MS, et al. Prevalence of chromosomal abnormalities and Y chromosome microdeletion among men with severe semen abnormalities and its correlation with successful sperm retrieval. J Hum Reprod Sci 2016;9:187-93. 10.4103/0974-1208.192065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ko JK, Chai J, Lee VC, et al. Sperm retrieval rate and pregnancy rate in infertile couples undergoing in-vitro fertilisation and testicular sperm extraction for non-obstructive azoospermia in Hong Kong. Hong Kong Med J 2016;22:556-62. 10.12809/hkmj154710 [DOI] [PubMed] [Google Scholar]
- 89.Sabbaghian M, Mohseni Meybodi A, Rafaee A, et al. Sperm retrieval rate and reproductive outcome of infertile men with azoospermia factor c deletion. Andrologia 2018;50:e13052. 10.1111/and.13052 [DOI] [PubMed] [Google Scholar]
- 90.Klami R, Mankonen H, Perheentupa A. Microdissection testicular sperm extraction in Finland - results of the first 100 patients. Acta Obstet Gynecol Scand 2018;97:53-8. 10.1111/aogs.13243 [DOI] [PubMed] [Google Scholar]
- 91.Miraghazadeh A, Sadighi Gilani MA, Reihani-Sabet F, et al. Detection of Partial AZFc Microdeletions in Azoospermic Infertile Men Is Not Informative of MicroTESE Outcome. Int J Fertil Steril 2019;12:298-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rossato M, Ferlin A, Garolla A, et al. Case report: high fertilization rate in conventional in-vitro fertilization utilizing spermatozoa from an oligozoospermic subject presenting microdeletions of the Y chromosome long arm. Mol Hum Reprod 1998;4:473-6. 10.1093/molehr/4.5.473 [DOI] [PubMed] [Google Scholar]
- 93.Krausz C, Hoefsloot L, Simoni M, et al. EAA/EMQN best practice guidelines for molecular diagnosis of Y-chromosomal microdeletions: state-of-the-art 2013. Andrology 2014;2:5-19. 10.1111/j.2047-2927.2013.00173.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jungwirth A, Diemer T, Dohle GR, et al. Male Infertility EAU Guidelines on Male Infertility. 2016; Available online: https://uroweb.org/wp-content/uploads/EAU-Guidelines-Male-Infertility-2016-2.pdf
- 95.Kleiman SE, Yogev L, Lehavi O, et al. The likelihood of finding mature sperm cells in men with AZFb or AZFb-c deletions: six new cases and a review of the literature (1994-2010). Fertil Steril 2011;95:2005-12, 2012.e1-4. [DOI] [PubMed]
- 96.Selman HA, Cipollone G, Stuppia L, et al. Gonadotropin treatment of an azoospermic patient with a Y-chromosome microdeletion. Fertil Steril 2004;82:218-9. 10.1016/j.fertnstert.2003.11.055 [DOI] [PubMed] [Google Scholar]
- 97.Flannigan RK, Schlegel PN. Microdissection testicular sperm extraction: preoperative patient optimization, surgical technique, and tissue processing. Fertil Steril 2019;111:420-6. 10.1016/j.fertnstert.2019.01.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as