Abstract
Background
The identification of the important elements that control hepatic stellate cell (HSC) activation will expand our understanding of the mechanism of liver fibrosis induced by hypoxia and affect the outcome of clinical treatment. A previous research demonstrated that N-Myc downstream-regulated gene 2 (NDRG2) is a potential regulator of fibrosis and a downstream target gene of hypoxia-inducible factor 1 (HIF-1). In this research, we studied the expression and function of NDRG2 in liver fibrosis induced by hypoxia.
Methods
LX-2 cells/NF-κB-silenced LX-2 cells were exposed to hypoxic conditions (1% O2) to activate HSCs in vitro. The protein and mRNA expression levels of NDRG2, α-SMA and transforming growth factor beta 1 (TGF-β1) were evaluated by western blotting and real-time polymerase chain reaction (RT-PCR), respectively. Functional studies were performed using adenovirus-mediated gene upregulation.
Results
The NDRG2 mRNA and protein levels were reduced under hypoxic conditions in LX-2 cells and overexpression of NDRG2 resulted in a decrease in the expression of TGF-β1 and α-SMA. Interestingly, no relationship was observed between NDRG2 and TGF-β1 when the NF-κB pathway was blocked, which indicates that NDRG2 can regulate the expression of TGF-β1 in LX-2 cells via the NF-κB pathway under hypoxic conditions.
Conclusions
NDRG2 may regulate the expression of TGF-β1 via the NF-κB pathway and may be a novel therapeutic target for liver fibrosis induced by hypoxia.
Keywords: Liver fibrosis, N-Myc downstream-regulated gene 2 (NDRG2), hypoxia, therapeutic target
Introduction
Liver fibrosis is the liver’s reaction to chronic injury and a major cause of fatal liver disease worldwide, especially in Asian countries. There are many causes of liver fibrosis, including metabolic disorders, cholestatic diseases, chronic hepatitis, and others (1,2). A hypoxia response system may contribute to the pathophysiology of liver fibrosis (3), and hypoxia may be a prominent determinant of liver fibrosis in pathological situations, Cellular hypoxia is an important feature of liver injury (4). Studies have shown that hypoxia regulates liver fibrosis by activating hepatic stellate cells (HSCs), and that HSC activation may be a key event in extracellular matrix (ECM) production (5), the biosynthetic changes of ECM production present as interstitial and perivascular fibrosis of liver. Despite advances in the characterization of liver fibrosis, the exact molecular mechanisms underlying hypoxia-induced liver fibrosis are still poorly understood. Therefore, clarification of these mechanisms and identification of potential therapies that could reduce the progression of hypoxia-induced fibrosis are urgently needed.
Transforming growth factor beta 1 (TGF-β1) is an important molecule involved in the progress of liver fibrosis through the activation of its downstream Smad signaling pathway (6,7). The signaling pathway of TGF-β1/Smad is integrally involved in the activation of HSCs, but the connection between hypoxia and the TGF-β1 signaling pathway is still unclear.
N-Myc downstream-regulated gene 2 (NDRG2) participates in the differentiation and growth of the liver cell and in hormonal responses (8,9). It has been demonstrated that NDRG2 is involved in liver histogenesis; the expression levels of NDRG2 mRNA and protein are markedly low in the early phases of histogenesis and are significantly higher in the later phases of histogenesis in the fetal livers of human (10,11). One study has shown that NDRG2 may be involved in the activation of LX-2 cells, which are HSCs (12). NDRG2 is a target of hypoxia-inducible factor 1 (HIF-1), which is a key mediator of the signaling pathway of hypoxia (13); however, the degree to which NDRG2 participates in the liver fibrotic process under hypoxic conditions is still unclear. This study tried to analyse the role of NDRG2 in hypoxia-induced liver fibrosis.
We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/atm-21-1298).
Methods
Cell culture
LX-2 cells were kindly provided by Dr. Scott Friedman (The Icahn School of Medicine at Mount Sinai, New York City, NY, USA). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; PeproTech, Rocky Hill, NJ, USA) supplemented with 10% fetal bovine serum (FBS; PeproTech,) at 37 °C in an incubator (normoxic conditions 21% O2, 5% CO2, and 74% N2).
TGF-β1 or hypoxia treatment
The cells were plated in 60 mm dishes and cultured in DMEM containing 10% FBS and 0.2% bovine serum albumin (BSA) and serum starved for 48 h after they reached 70% confluence. Cells were then incubated with TGF-β1 (2.5 ng/mL, Invitrogen Life Technologies, Carlsbad, CA, USA) for 0 or 24 h. Cells were placed in a humidified atmosphere normoxic conditions or in a hypoxic condition (37 °C, containing 1% O2, 5% CO2, and 94% N2).
Adenovirus transduction
Adenoviral vectors expressing human NDRG2 (Ad-NDRG2), β-galactosidase [Ad-LacZ, negative control (NC)], and enhanced green fluorescent protein (Ad-EGFP, NC) were purchased from Zhengyang Benyuan (Beijing, China). The cells were seeded in the 100 mm dishes and then incubated with Ad-NDRG2, Ad-LacZ, or Ad-EGFP in the serum-free DMEM for 2 h. The medium was then replaced with the fresh DMEM containing 10% FBS and cells were incubated for 48 h. The multiplicity of infection (MOI) was set at 40.
Production of lentiviral vector
NF-κB small-interfering RNAs (siRNAs) or control siRNAs (scrambled sequence) were subcloned in lentiviral vectors, as previously reported (14,15). The NF-κB siRNA sequence was 5'-GGACCTACGAGACCTTCAA-3'. The NC siRNA was 5'-TTCTCCGAACGTGTCACGT-3'. The final titer of the recombinant virus was 5×108 TU/mL.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA of LX-2 cells was isolated by the TRIzol reagent (Invitrogen) and quantified. Complementary DNA (cDNA) was synthesized with the TaqMan Reverse Transcriptase Reagent Kit (Invitrogen) primed with oligo(dT) from 5 µg RNA and then analyzed using qRT-PCR. Then the mRNAs were detected with SYBR Green PCR Master Mix and an ABI PRISM 7500 Sequence Detection System (Thermo Fisher Scientific, Waltham, MA, USA), and relative quantification was performed with the comparative threshold cycle method. The PCR consisted of 10 pmol of the forward and reverse primers, 12.5 µL SYBR Green PCR Master Mix, and 5 µL of template cDNA in a total volume of 25 µL. The conditions of thermal cycling comprised a step of initial denaturation at 95 °C for 10 s, followed by 45 cycles at 95 °C for 5 s and 60 °C for 34 s.
The following primers were used for the human genes:
NDRG2-F: 5'-GAGATATGCTCTTAACCACCCG-3', NDRG2-R: 5'-GCTGCCCAATCCATCCAA-3';
α-SMA-F: 5'-GACAATGGCTCTGGGCTCTGTAA-3', α-SMA-R: 5'-CTGTGCTTCGTCACCCACGTA-3';
TGF-β1-F: 5'-CAATTCCTGGCGATACCTCAG-3', TGF-β1-R: 5'-GCACAACTCCGGTGACATCAA-3';
β-actin-F: 5'-AGCGAGCATCCCCCAAAGTT-3', β-actin-R: 5'-GGGCACGAAGGCTCATCATT-3'.
Analysis of Western blot
The cells were harvested from the 60 mm culture dishes, and then lysed in 200 µL RIPA buffer [0.15 M NaCl, 0.05 M Tris-HCl (pH 7.4), 0.25% deoxycholic acid, 1% Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM EDTA, 10 µg/mL leupeptin, and 10 µg/mL aprotinin]. Protein concentrations were measured by the bicinchoninic acid protein assay (Pierce, Rockford, IL, USA). The proteins were separated using SDS-PAGE and transferred to the nitrocellulose membranes (Amersham Biosciences, Piscataway, NJ, USA). The membranes were saturated using Tris-buffered saline mixed with 3% TBST-BSA and 0.1% Tween 20 and then probed using the appropriate antibodies for the following targets: NDRG2 (1:2,000, Cell Signaling Technology, Danvers, MA, USA), β-actin (1:2,000, Cell Signaling Technology), α-SMA (1:1,000, Sigma-Aldrich, St. Louis, MO, USA), and TGF-β1 (1:1,000 Cell Signaling Technology). The membranes were then incubated using the species-matched secondary antibodies. The bands were detected with enhanced chemiluminescence (Pierce). The band intensities were quantified using Kodak Digital Science 1D software (version 3.0; Eastman Kodak, New Haven, CT, USA).
Statistical analysis
Statistical analyses were performed with the SPSS software (version 16.0; IBM Corp., Armonk, NY, USA). The t-test method was used to compare the differences between the two groups, and the variance method analysis was used to compare the differences of the groups. Statistical significance was based on a P value <0.05.
Result
NDRG2 was involved in liver hypoxia-induced fibrosis
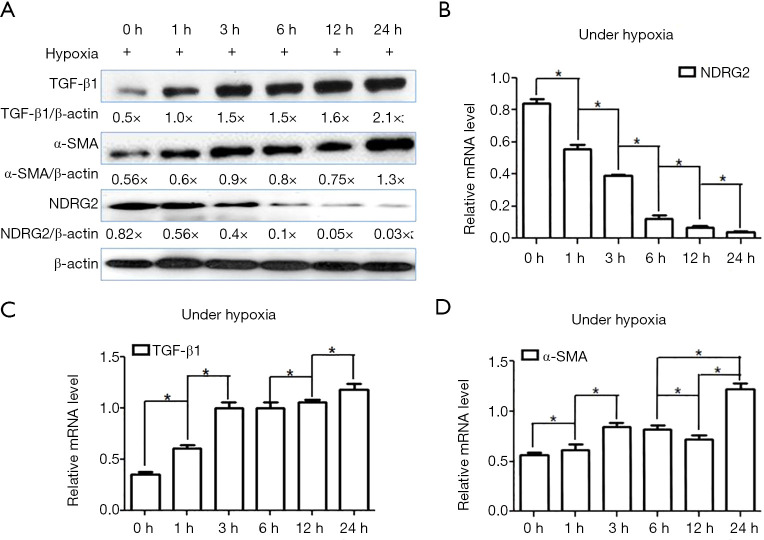
qRT-PCR and western blotting analysis revealed that the protein and mRNA levels of NDRG2 significantly decreased under hypoxia condition in LX-2 cells in a time-dependent manner (P<0.05) (Figure 1A,B).
Figure 1.
NDRG2 expression was downregulated by hypoxia in HSCs (LX-2). (A) The protein levels of NDRG2, TGF-β1, and α-SMA in LX-2 cells at various time points (from 0 to 24 h) after hypoxic exposure were analysed by western blot. (B,C,D) The expression levels of the mRNAs encoding NDRG2, α-SMA, and TGF-β1 in LX-2 cells at various time points (from 0 to 24 h) after hypoxic exposure were analysed by qRT-PCR, and β-actin served as a control to ensure equal loading. qRT-PCR and western blot analysis showed that the mRNA and protein levels of α-SMA and TGF-β1 were significantly enhanced as the duration of hypoxic exposure increased in LX-2 cells (P<0.05); however, the protein and mRNAs levels of NDRG2 decreased significantly as the duration of hypoxic exposure increased (P<0.05). *, P<0.05.
Hypoxia-induced liver fibrosis
The spontaneously immortalized human HSC cell line, LX-2, was grown to confluence and exposed to hypoxia (1% O2). The protein and mRNA expression levels of TGF-β1 and α-SMA increased as the duration of hypoxic exposure increased (P<0.05) (Figure 1A,C,D). The analyses of western blot and qRT-PCR showed that the mRNA and protein levels of TGF-β1 and α-SMA were increased in a time-dependent manner following incubation of LX-2 cells under the hypoxic (1% O2) conditions for 6 and 24 h (Figure 2A,B,C,D). These results demonstrated that HSC activation could be induced by hypoxia within 24 h.
Figure 2.
NDRG2 could reverse fibrosis though TGF-β1 under hypoxic conditions. (A,B,C,D) The results of western blot and qRT-PCR showed that the increase in the protein and mRNA levels of TGF-β1 and α-SMA in LX-2 cells under hypoxic conditions (1% O2) for 24 h was higher than that in the cells exposed to these conditions only for 6 h. qRT-PCR and western blot also revealed that the decrease in the mRNA and protein expression of α-SMA and TGF-β1 in LX-2 cells after treatment with Ad-NDRG2 (10 µM) under hypoxic conditions (1% O2) for 24 h or 6 h was larger than that in cells only exposed to hypoxic conditions (1% O2) (P<0.05). These results showed that the mRNA and protein expression levels of α-SMA and TGF-β1 were increased as the duration of hypoxia increased, and that the overexpression of NDRG2 could inhibit the mRNA and protein expression levels of α-SMA and TGF-β1. *, P<0.05.
NDRG2 inhibited the expression of TGF-β1 in hypoxia-induced liver fibrosis
To determine whether TGF-β1 can be regulated by NDRG2, LX-2 cells were infected with Ad-NDRG2 (10 µM) under hypoxic conditions (1% O2) for 24 h, and their effects were evaluated using qRT-PCR and western blot analysis. The increase in the mRNA and protein expression of α-SMA and TGF-β1 in LX-2 cells incubated under hypoxic (1% O2) conditions for 24 h was greater than that in cells incubated with hypoxic (1% O2) conditions for 6 h (Figure 2A,B,C,D). However, incubation of LX-2 cells treated with Ad-NDRG2 (10 µM) under hypoxic (1% O2) conditions resulted in a time-dependent decrease in the levels of mRNA and protein expression of TGF-β1 and α-SMA (P<0.05) (Figure 2A,B,C,D). Therefore, the overexpression of NDRG2 can reduce the protein and mRNA expression levels of α-SMA and TGF-β1 in LX-2 cells subjected to hypoxic conditions, which indicates that under hypoxic conditions, NDRG2 can reverse fibrosis through TGF-β1.
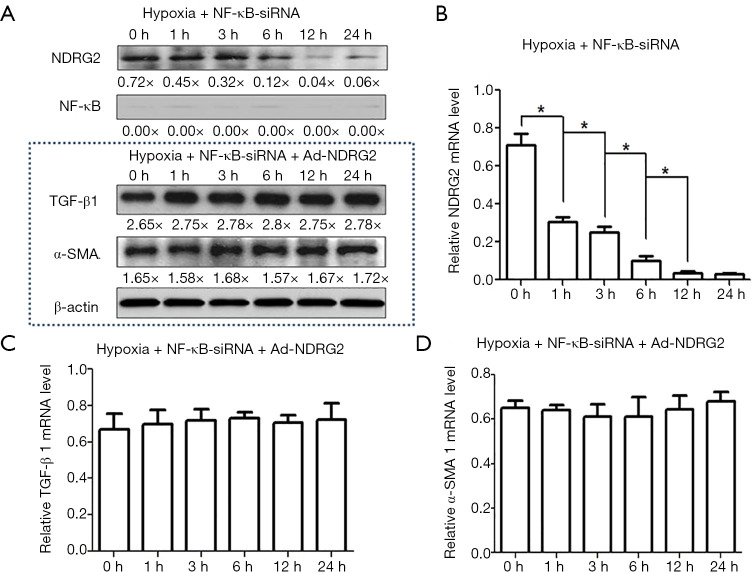
NDRG2 could not inhibit the expression of TGF-β1 in the absence of NF-κB
To examine the role of NF-κB, hypoxic NF-κB-silenced LX-2 cells were treated with Ad-NDRG2 (10 µM). The analyses of western blotting and qRT-PCR revealed that the mRNA and protein expression of NDRG2 decreased as the hypoxia exposure time increased (from 0 to 24 h) in NF-κB-silenced cells (Figure 3A,B). However, the protein and mRNA levels of TGF-β1 and α-SMA expression did not significantly change under Ad-NDRG2 treatment conditions (10 µM) as the hypoxia exposure time increased in these NF-κB-silenced LX-2 cells (Figure 3A,C,D), which indicated treatment with Ad-NDRG2 results in overexpression and the hypoxic conditions result in NDRG2 decrease had no effect on the mRNA and protein expression of α-SMA and TGF-β1 in hypoxia-induced LX-2 cells when NF-κB expression was blocked. Therefore, NDRG2 could not inhibit the expression of TGF-β1 in the absence of NF-κB.
Figure 3.
NDRG2 could not inhibit the mRNA and protein expression of TGF-β1 and α-SMA without NF-κB. (A,B,C,D) NF-κB protein expression was barely detected, indicating successful knockdown of the NF-κB gene. The results of western blot and qRT-PCR showed that the protein and mRNA expression of NDRG2 were decreased as the hypoxia exposure time increased (from 0 to 24 h) in NF-κB-silenced cells; however, the level of TGF-β1 and α-SMA expression did not significantly change when the cells were treated with Ad-NDRG2 (10 µM) as the hypoxia exposure time increased in these NF-Κb-silenced cells (LX-2HSCs). *, P<0.05.
Discussion
Hypoxia is an important factor in cell damage, especially in acute and chronic liver injury, and may thus play an important role in the pathogenesis of liver fibrosis. The chronic injury may be related to the activation of HSCs in the hepatic fibrotic process (16). When stimulated, HSCs transition from a quiescent state to an activated state (17), and the levels of α-SMA can be regarded as a marker of HSC activation. In this study, we investigated whether HSCs are activated by hypoxia in vitro. The results showed that the protein expression of α-SMA increased, reaching a peak at 24 h after the induction of hypoxia, indicating that HSC activation occurred within the first 24 h of exposure to hypoxic conditions. NDRG2 has been found to be a downstream molecular target of HIF-1 (18), HIF-1 is thought to facilitate fibrosis through interaction with TGF-β1 (19). In this study, a decrease in the levels of the protein and mRNA expression of NDRG2 was found following exposure of LX-2 cells to hypoxic conditions (1% O2), which indicates that NDRG2 might be involved in hypoxia-induced liver fibrosis.
TGF-β1 may be the most potent fibrogenic cytokine in the liver (20,21), and a biologically inactive form of TGF-β1 can convert into an active form of that from in response to injury. When activated, TGF-β1 can regulate the transcription of profibrotic target genes via its cognate TGF-β1 receptor in the nucleus (22). The signaling pathway of TGF-β1 can influence aspects of the fibrogenic process, including activation of HSC, and the subsequent production of ECM during the process of liver injury (23,24), which present as the fibrosis of liver. We examined whether NDRG2 links hypoxia with the TGF-β1 signaling pathway in the fibrogenic process. In the present study, the analysis of qRT-PCR and western blot revealed an increased mRNA and protein expression of TGF-β1 in LX-2 cells under hypoxic conditions (1% O2), and compared to the control, treatment of LX-2 cells with Ad-NDRG2 (10 µM) under hypoxic conditions (1% O2) for 24 h reduced TGF-β1 and α-SMA expression, indicating that NDRG2 can reverse fibrosis through TGF-β1. Western blotting and qRT-PCR also demonstrated that the protein and mRNA expression of TGF-β1 and α-SMA did not significantly change after treatment with Ad-NDRG2 (10 µM) in NF-κB-silenced LX-2 cells under hypoxic conditions (1% O2), indicating that NDRG2 cannot inhibit the expression of TGF-β1 in the absence of NF-κB. Activation of NF-κB has been found to attenuate TGF-β1-induced cell death in liver cells (25). In our study, NDRG2 did not affect the activation of TGF-β1 signaling pathways in NF-κB-silenced LX-2 cells under hypoxic conditions (1% O2). These data suggest that NDRG2 can inhibit the expression of TGF-β1 in hypoxia-induced liver fibrosis via the NF-κB pathway.
So, we inferred that TGF-β1 could be regulated by NDRG2 via the NF-κB pathway in hypoxia-induced liver fibrosis. Furthermore, adenovirus-mediated NDRG2 overexpression may attenuate hypoxia-induced liver fibrosis.
Conclusions
Our findings demonstrate that NDRG2 participates in the regulation of hypoxia-induced liver fibrosis. Overexpression of NDRG2 can decrease the expression of TGF-β1 via the NF-κB pathway in LX-2 cells under hypoxic conditions. Therefore, NDRG2 may be a novel target for the development of treatments for patients with hypoxia-induced liver fibrosis.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors appreciate the academic support provided by the AME Liver Fibrosis Collaborative Group.
Funding: This study was supported in part by the Natural Science Foundation of Shaanxi Province (No. 2016SF-108) and the National Natural Science Foundation of China (No. 81170400). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/atm-21-1298
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-21-1298
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-21-1298). The authors have no conflicts of interest to declare.
References
- 1.Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer 2006;6:674-87. 10.1038/nrc1934 [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Boix L, Sala M, et al. Focus on hepatocellular carcinoma. Cancer Cell 2004;5:215-9. 10.1016/S1535-6108(04)00058-3 [DOI] [PubMed] [Google Scholar]
- 3.Kietzmann T, Dimova EY, Flügel D, et al. Oxygen: modulator of physiological and pathophysiological processes in the liver. Z Gastroenterol 2006;44:67-76. 10.1055/s-2005-858987 [DOI] [PubMed] [Google Scholar]
- 4.Siegmund SV, Brenner DA. Molecular pathogenesis of alcohol-induced hepatic fibrosis. Alcohol Clin Exp Res 2005;29:102S-9S. 10.1097/01.alc.0000189275.97419.58 [DOI] [PubMed] [Google Scholar]
- 5.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology 2008;134:1655-69. 10.1053/j.gastro.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003;425:577-84. 10.1038/nature02006 [DOI] [PubMed] [Google Scholar]
- 7.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J 2004;18:816-27. 10.1096/fj.03-1273rev [DOI] [PubMed] [Google Scholar]
- 8.Boulkroun S, Fay M, Zennaro MC, et al. Characterization of rat NDRG2 (N-Myc downstream regulated gene 2), a novel early mineralocorticoid-specific induced gene. J Biol Chem 2002;277:31506-15. 10.1074/jbc.M200272200 [DOI] [PubMed] [Google Scholar]
- 9.Shen L, Zhao ZY, Wang YZ, et al. Immunohistochemical detection of Ndrg2 in the mouse nervous system. Neuroreport 2008;19:927-31. 10.1097/WNR.0b013e32830163d0 [DOI] [PubMed] [Google Scholar]
- 10.Hu XL, Liu XP, Deng YC, et al. Expression analysis of the NDRG2 gene in mouse embryonic and adult tissues. Cell Tissue Res 2006;325:67-76. 10.1007/s00441-005-0137-5 [DOI] [PubMed] [Google Scholar]
- 11.Hu XL, Yao LB, Zhang YQ, et al. Distribution characteristic of NDRG2 expression in human fetal tissues. Sheng Li Xue Bao. 2006;58:331-6. [PubMed] [Google Scholar]
- 12.Yang J, Zheng J, Wu L, et al. NDRG2 ameliorates hepatic fibrosis by inhibiting the TGF-β1/Smad pathway and altering the MMP2/TIMP2 ratio in rats. PLoS One 2011;6:e27710. 10.1371/journal.pone.0027710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams JM, Difazio LT, Rolandelli RH, et al. HIF-1: a key mediator in hypoxia. Acta Physiol Hung 2009;96:19-28. 10.1556/APhysiol.96.2009.1.2 [DOI] [PubMed] [Google Scholar]
- 14.Coleman JE, Huentelman MJ, Kasparov S, et al. Efficient large-scale production and concentration of HIV-1-based lentiviral vectors for use in vivo. Physiol Genomics 2003;12:221-8. 10.1152/physiolgenomics.00135.2002 [DOI] [PubMed] [Google Scholar]
- 15.Sun T, Luo J, Jia M, et al. Small interfering RNA-mediated knockdown of NF-κBp65 attenuates neuropathic pain following peripheral nerve injury in rats. Eur J Pharmacol 2012;682:79-85. 10.1016/j.ejphar.2012.02.017 [DOI] [PubMed] [Google Scholar]
- 16.Senoo H. Structure and function of hepatic stellate cells. Med Electron Microsc 2004;37:3-15. 10.1007/s00795-003-0230-3 [DOI] [PubMed] [Google Scholar]
- 17.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest 2005;115:209-18. Erratum in: J Clin Invest 2005;115:1100. 10.1172/JCI24282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L, Liu N, Yao L, et al. NDRG2 is a new HIF-1 target gene necessary for hypoxia-induced apoptosis in A549 cells. Cell Physiol Biochem 2008;21:239-50. 10.1159/000113765 [DOI] [PubMed] [Google Scholar]
- 19.Kellenberger T, Marcussen N, Nyengaard JR, et al. Expression of hypoxia-inducible factor-1α and hepatocyte growth factor in development of fibrosis in the transplanted kidney. Transpl Int 2015;28:180-90. 10.1111/tri.12475 [DOI] [PubMed] [Google Scholar]
- 20.Breitkopf K, Godoy P, Ciuclan L, et al. TGF-beta/Smad signaling in the injured liver. Z Gastroenterol 2006;44:57-66. 10.1055/s-2005-858989 [DOI] [PubMed] [Google Scholar]
- 21.Inagaki Y, Okazaki I. Emerging insights into Transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut 2007;56:284-92. 10.1136/gut.2005.088690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 2008;214:199-210. 10.1002/path.2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gressner AM, Weiskirchen R, Breitkopf K, et al. Roles of TGF-beta in hepatic fibrosis. Front Biosci 2002;7:d793-807. 10.2741/A812 [DOI] [PubMed] [Google Scholar]
- 24.Dooley S, ten Dijke P. TGF-β in progression of liver disease. Cell Tissue Res 2012;347:245-56. 10.1007/s00441-011-1246-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang F, Kaur S, Cavin LG, et al. Nuclear-factor-kappaB (NF-kappaB) and radical oxygen species play contrary roles in transforming growth factor-beta1 (TGF-beta1)-induced apoptosis in hepatocellular carcinoma (HCC) cells. Biochem Biophys Res Commun 2008;377:1107-12. 10.1016/j.bbrc.2008.10.130 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as