Abstract
Background
Pain is a predominant symptom in rheumatoid arthritis (RA) patients that results from joint inflammation and is augmented by central sensitization. Regulator of G-protein signaling 12 (RGS12) is the largest protein in the RGS protein family and plays a key role in the development of inflammation. This study investigated the regulation of RGS12 in inflammatory pain and explored the underlying mechanisms and potential RA pain targets.
Methods
Macrophage-specific RGS12-deficient (LysM-Cre+;RGS12fl/fl) mice were generated by mating RGS12fl/fl mice with LysM-Cre+ transgenic mice. Collagen antibody-induced arthritis (CAIA) models were induced in LysM-Cre+;RGS12fl/fl mice by the administration of a cocktail of five monoclonal antibodies and LPS. Mouse nociception was examined using the von Frey and heat plate tests. Primary macrophages and RAW264.7 cells were used to analyze the regulatory function and mechanism of RGS12 in vitro. The expression and function of RGS12 and COX2 (cyclooxygenase 2) were determined by real-time PCR, ELISA, and luciferase assays.
Results
Ablation of RGS12 in macrophages decreased pain-related phenotypes, such as paw swelling, the clinical score, and the inflammatory score, in the CAIA model. LysM-Cre+;RGS12fl/fl mice displayed increased resistance to thermal and mechanical stimulation from day 3 to day 9 during CAIA, indicating the inhibition of inflammatory pain. Overexpression of COX2 and PGE2 in macrophages enhanced RGS12 expression, and PGE2 regulated RGS12 expression through the G-protein-coupled receptors EP2 and EP4. Furthermore, RGS12 or the RGS12 PTB domain strengthened the transcriptional regulation of COX2 by NF-κB, whereas inhibiting NF-κB suppressed RGS12-mediated regulation of COX2 in macrophages.
Conclusions
Our results demonstrate that the deletion of RGS12 in macrophages attenuates inflammatory pain, which is likely due to impaired regulation of the COX2/PGE2 signaling pathway.
Keywords: Regulator of G-protein signaling, macrophage, inflammatory pain, hypersensitivity
Introduction
Rheumatoid arthritis (RA) pain is one the most prevalent symptoms in RA patients and can further cause psychological distress and fatigue (1). RA pain is associated with local inflammation in the peripheral tissue or central nervous system. Recent studies suggest that immune cells such as T lymphocytes, monocytes, and macrophages play active roles in the pathogenesis and resolution of pain (2). Moreover, inflammatory mediators released by macrophages, such as cytokines or enzymes, may provoke central pain sensitization through both local and systemic inflammation (3). This study aimed to identify a novel molecular mechanism by which macrophages regulate pain sensation (4).
During RA, cyclooxygenase (COX) enzymes (COX1 and COX2) may be the most important molecular players. These enzymes are membrane-bound glycoproteins that are predominantly located in the endoplasmic reticulum and produce prostanoids in macrophages (5). COX1 and COX2 share a 61% amino acid sequence homology and have molecular weights of 72 kD (6). However, COX2 contains multiple transcriptional regulatory sequences in the promoter region, including an enhancer box (E-box), TATA box, nuclear factor interleukin-6 (NF-IL6) motif, nuclear factor kappa B (NF-κB) motif, Apetala 2 and specificity protein 1 sites (7). COX2 gene expression can be regulated by multiple transcription factors, cytokines, and growth factors (8). Numerous prostanoids are produced by COX enzymes, including prostaglandin E2 (PGE2), prostaglandin D2 (PGD2), and prostaglandin I2 (PGI2). PGE2 is the major contributor to inflammatory pain in arthritic conditions and exerts its effects via a variety of E prostanoid (EP) receptors (EP1-EP4), which is a family of G protein-coupled receptors (GPCRs), in various cells, such as neurons and macrophages (9). EP1 associates with the Gq pathway, EP2 and EP4 couple with Gs and regulate the cAMP pathway, and EP3 inhibits cAMP generation via a Gi-coupled mechanism (10). Four subtypes of PGE2 receptors (EP1-4) have been cloned and are seven hydrophobic transmembrane-segment GPCRs (11,12).
Regulator of G protein signaling (RGS) proteins are negative modulators of GPCR signaling and play important roles in regulating cellular responses. Therefore, RGS proteins have a variety of functions and are critically involved in clinical diseases, including cancer, inflammation, and cardiovascular processes (13). Recently, several RGS proteins have been shown to regulate pain symptoms. For example, RGS4 maintains chronic pain, and inhibiting RGS4 promotes recovery from sensory hypersensitivity symptoms (14). RGS9 in the spinal cord also contributes to sensory and affective components of pain (15).
RGS12 regulates multiple signaling pathways, such as GPCRs, receptor tyrosine kinases (RTKs) and mitogen-activated protein kinases (MAPKs) (16,17). RGS12 was recently shown to play a critical role in macrophages and is involved in the regulation of inflammation and immunity (17). RGS12 also regulates the downstream kappa opioid receptor (KOR) via both G protein-dependent and G protein-independent signaling mechanisms, which could affect neuropathic pain (18). However, the exact function and mechanism of RGS12 in regulating inflammatory pain are still unknown. Based on its potent modulatory effect on inflammatory signal transduction (16,17), we expect that changes in RGS12 activity in macrophages will affect the functional responses of GPCR signaling and inflammatory pain.
In this study, we used monocyte/macrophage-specific RGS12-knockout (cKO) (C57/BL6 background) mice to examine the role of RGS12 in the induction and maintenance of chronic pain symptoms in an RA model. Our data demonstrate that macrophage RGS12 is an activator of the COX2/PGE2 signaling pathway, which results in enhanced inflammatory pain during RA. Thus, targeting RGS12 is a new promising therapeutic strategy for the management of RA or other diseases related to pain.
We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-5729).
Methods
RGS12 conditional knockout mice
To generate monocyte/macrophage-specific RGS12-knockout (cKO) mice, RGS12fl/fl mice were crossed with mice expressing Cre recombinase under the control of the LysM promoter (LysM-Cre+) (19). Cre control (females, 8 weeks old, n=5) and cKO mice (females, 8 weeks old, n=5) were littermates derived from the breeding of heterozygous animals. The animals were randomly assigned to cages (5 animals/cage) by a computer-generated randomized list and maintained under specific pathogen-free conditions. The number of animals used per experiment is stated in the figure legends as described in previous studies (20,21), and three independent replicate experiments were performed. The mice were fed a chow diet and raised in a clean-grade room with 12-h light and 12-h dark cycles. Experiments were performed under a project license (No. 806005) granted by the board of the American Association for Laboratory Animal Science (IACUC) in compliance with the University of Pennsylvania guidelines for the care and use of animals.
Collagen antibody-induced arthritis (CAIA)
CAIA was induced in LysM-Cre+ and LysM-Cre+;RGS12fl/fl mice (C57BL/6 background, females, 8 weeks old, n=5/cage) using a mixture of 5 mg of mAbs (Chondrex #53100) according to the manufacturer’s instructions. Mice were randomly selected from both groups and intraperitoneally injected with 5 mg of monoclonal antibodies (mAbs) on day 0 and 50 µg of lipopolysaccharide (LPS) (Chondrex #9028) on day 3 to synchronize the development of arthritis. The mice began to develop arthritis on day 4 and were euthanized with carbon dioxide (CO2) on day 9.
Clinical scoring of the mouse RA model
Mice were scored on a scale of 0–3 per hind paw based on the following score evaluation criteria as previously described (22): Score 1, the emergence of ankle swelling, which is the earliest visible sign of arthritis; Score 2, moderate to severe redness and swelling of the ankle/wrist/pad; and Score 3, redness and swelling of the entire paw, including digits, and inflamed limbs with the involvement of multiple joints. The thickness of each hind paw was measured using a thickness gauge and is expressed in millimeters (mm).
Histology and inflammation scores
Hind paws were fixed in 10% formalin, decalcified, and embedded in paraffin. Tissues were cut into 6 µm sections and stained with hematoxylin and eosin to assess joint pathology. The degree of inflammation was evaluated on a scale from 0 to 4 (0= no inflammation, 1= minimal inflammation, 2= mild inflammation, 3= moderate inflammation, and 4= severe inflammation) by three different experts as described previously (23).
Hotplate test
The analgesic effects of morphine were examined in female mice (8 weeks old, n=5) with CAIA using a hotplate set at 52 °C as described with slight modifications (24). Briefly, CAIA-control LysM-Cre and CAIA-LysM-Cre;RGS12fl/fl mice were individually placed on the hotplate, any physical signs of sensitivity to heat (i.e., licking paw) were observed, after which the mice were quickly removed to avoid burns. The data were analyzed by two-way ANOVA with Dunnett’s post hoc test.
von Frey microfilament test
Female control LysM-Cre and LysM-Cre;RGS12fl/fl mice with CAIA (8 weeks old, n=5) were subjected to manual von Frey microfilament tests to evaluate mechanical allodynia and hyperalgesia. A series of von Frey fibers (0.41–3.63 g) were applied to the plantar surface of both hind paws beginning with the finest fiber as previously described (20,21,25). Once the mice showed brisk paw withdrawal, licking or shaking/flicking of the paw, the stimulus was removed to avoid harm. The time at which monofilament was applied to the hind paw until the time of the withdrawal response was recorded. All animals were tested 5 times with a 10-second interval. The data were analyzed by two-way ANOVA with Dunnett’s post hoc test.
Real-time PCR
Total RNA was extracted from cells using a QIAsymphony RNA kit (Qiagen, USA). Then, the RNA was reverse transcribed into complementary DNA (cDNA) using a Prime Script RT reagent kit (Takara Bio, USA) according to the manufacturer’s instructions. Reverse transcription quantitative polymerase chain reaction (RT-qPCR) was then conducted using a SYBR Green PCR kit (Bio-Rad, USA). The relative gene expression levels were calculated by the 2−ΔΔCT method. GAPDH was used as the internal reference. The primer sequences were as follows: COX2-F: AGAAGGAAATGGCTGCAGAA; COX2-R: GCTCGGCTTCCAGTATTGAG; RGS12-F: TGGTGAGTTGACTGGA GCTG; RGS12-R: ATCCTGAAGGAGACGCTCAA; GAPDH-F: AGGTCGGTGT GAAC GGATTTG; and GAPDH-R: TGTAGACCATGTAGTTGAGGTCA.
Pull-down assay
A flag pull-down assay was used to identify the interactions between the RGS12 PTB domain and NF-κB p65. In brief, Flag-fused beads (L00432, GeneScript, USA) were prepared in a rotating incubator at room temperature for 1 hour, and the resins were washed 3 times. Input proteins (1 mg/mL) were dissolved in the reaction buffer and incubated with the resins overnight at 4 °C. After removing the supernatant, the beads were washed three times. The target proteins were washed with 10% sodium dodecyl sulfate (SDS). The eluates were then analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) and Western blotting.
Western blotting
In brief, protein lysate was extracted from macrophages and synovium by radioimmunoprecipitation assay buffer. Protein extracts were boiled and subjected to SDS-PAGE and then transferred and incubated as previously described (26). Anti-β-Actin (sc-8432, Santa Cruz, USA) at a dilution of 1:2,000 was used as the internal control. Anti-RGS12 (1:1,000 dilution, GW21317, Sigma-Aldrich, USA) anti-COX2 (1:1,000 dilution, 4842, CST, USA), anti-NF-κB P65 (1:1,000 dilution, 10745-1-AP, Proteintech, USA), and anti-Flag (1:2,000 dilution, F3165, Sigma-Aldrich, USA) were used. Protein bands were visualized on a ChemiDoc™ touch imaging system (Bio-Rad, Hercules, CA, USA) using a Clarity™ Western enhanced chemiluminescence (ECL) detection kit (Bio-Rad, Hercules, CA, USA). The analysis was performed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
ELISA
Synovial tissues or BMM homogenates were collected according to the commercial kit instructions (KGE004B, R&D Systems, USA). Standard ELISA kits were used to measure PGE2 (KGE004B, R&D Systems, USA), COX2 (DYC4198-2, R&D Systems, USA), and RGS12 (MBS9326858, MYBIOSOURCE, USA). The plate wells were measured at 450 nm, and the OD readings for the duplicate wells were averaged.
Plasmid construction
Plasmids were constructed by subcloning the mouse RGS12 gene open‐reading frame (ORF) or the RGS12 PTB domain into p3xFLAG-Myc-CMV-26 (pCMV) and subcloning the COX2 gene (ORF) into pcDNA3.1+C-HA. The COX2 reporter plasmid (COX2‐luc), containing the 5'‐flanking sequence (2 kilobase) of the COX2 gene (NM_011198.4), was constructed into the pGL3-basic vector (Promega, Madison, WI, USA). pcDNA3.1-NF-κBp65 (#20012), pSicoR-shNF-κBp65-1 (#22507) and pSicoR-shNF-κBp65-2 (#22508) were obtained from Addgene.
Luciferase assay
The luciferase assay was performed as previously described (27). The macrophage cell line RAW264.7 was transiently transfected with 1 µg of pGL3‐COX2 plasmid, 1 µg of pRL‐TK plasmid, and 1 µg of pCMV-RGS12 or pCMV-RGS12 PTB domain and pcDNA3.1-NF-κB plasmids using Lipofectamine 3000 (Thermo Fisher, USA). Cells were harvested 24 hours after transfection for firefly and Renilla luciferase activity analysis using the Dual-Luciferase® Reporter Assay System (Promega, Madison, WI, USA). Firefly luciferase activity was normalized to Renilla luciferase activity.
Cell culture and transfection
RAW264.7 cells were maintained in 5% CO2 at 37 °C in Eagle’s minimal essential medium supplemented with L-glutamine, penicillin/streptomycin, nonessential amino acids and 10% fetal bovine serum. For cell transfection, RAW264.7 cells or BMMs were grown in 6-well plates and transfected with 3 µg of the indicated plasmid for 24 hours using Lipofectamine 3000 reagent (Thermo Fisher, USA) according to the instructions. Then, the cells were used for cell biology and molecular biology analyses.
Statistical analysis
The data are presented as the mean ± SEM, and significance was determined by using GraphPad Prism software (GraphPad Software 7.0). A two‐tailed Student’s t-test was performed to determine the significance of any difference between two groups. One-way or repeated two-way ANOVA with a post hoc multiple comparison test was used when more than two groups were compared. Any P values <0.05 were considered significant.
Results
Generation of macrophage-specific RGS12-cKO mice
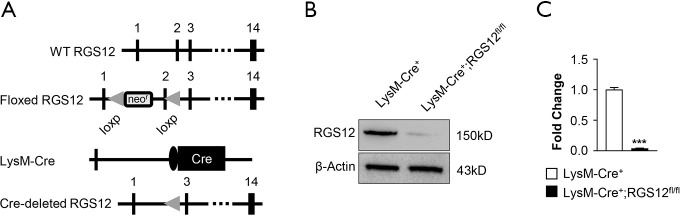
To determine the role of RGS12 in inflammatory diseases, macrophage-specific RGS12-cKO mice were generated by mating mice with a floxed RGS12 gene with LysM‐Cre+ mice expressing Cre‐recombinase under the control of the endogenous LysM promoter. Therefore, the Cre‐recombinase removes exon 2 of the floxed RGS12 gene (Figure 1A) specifically in macrophages. Cre‐recombinase-negative mice, with or without the floxed RGS12 gene, exhibited a WT phenotype and were distinguished from cKO mice harboring the Cre and floxed RGS12 alleles. Each phenotype of mouse was fertile. The loss of RGS12 protein from bone marrow macrophages in cKO animals was confirmed by immunoblotting. Only LysM-Cre+ control BMM samples showed a clear signal for the RGS12 protein, whereas the RGS12 protein was nearly absent in LysM-Cre+;RGS12fl/fl BMMs (Figure 1B,C).
Figure 1.
Generation of macrophage‐specific RGS12-KO mice. (A) Organization of the wild-type RGS12 allele, the floxed RGS12 targeting construct, the LysM-Cre+ transgene, and the recombinant RGS12 allele. Exons are shown as black boxes and are numbered. Cre: Cre recombinase coding sequence; neo: neomycin resistance gene; and loxP: loxP sites. (B) Immunoblot analysis of bone marrow macrophage (BMM) lysates isolated from the long bone showing the absence of RGS12 protein in RGS12-cKO mice. (C) Quantitative analysis of the results in (B). Note that an immunoreactive band for RGS12 (150 kD) is detectable in LysM-Cre+ mice, whereas LysM-Cre+;RGS12fl/fl mice almost completely lack the RGS12 protein. ***, P<0.001, n=5.
RGS12 deficiency in macrophages inhibits the development of CAIA
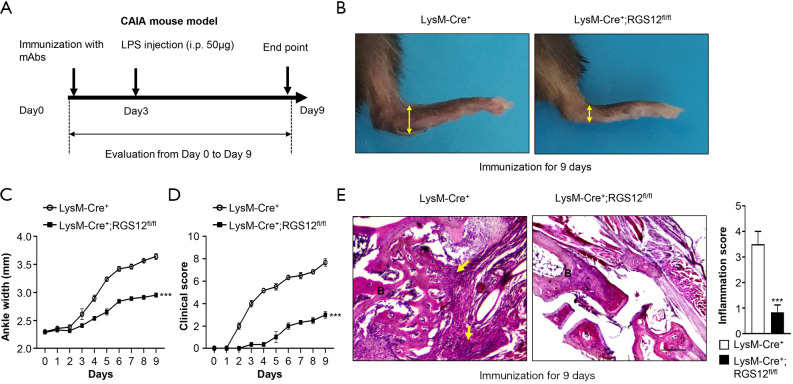
Macrophages play key roles in the induction and resolution of inflammation. During inflammation, intracellular events lead to the formation of prostaglandins (PEGs) in macrophages and the perception of pain (28). Here, to determine the role of RGS12 in regulating inflammation and pain via macrophages, we deleted RGS12 in macrophages by using the Cre/loxP system. CAIA model mice were inducted by i.p. injection of collagen type II antibodies on day 0 and LPS on day 3 according to the kit instructions (Arthritogenic Monoclonal Antibody Cocktails, 53100, Chondrex, US), and all mice were sacrificed on day 9 (Figure 2A). The paws in the LysM-Cre+;RGS12fl/fl mouse group showed much less severe swelling and hypertrophy than those in the LysM-Cre+ group at 9 days after immunization (Figure 2B). The ankle widths and of scores of LysM-Cre+;RGS12fl/fl mice were also significantly less than those in LysM-Cre+ control mice (Figure 2C,D). LysM-Cre+;RGS12fl/fl mice showed a decrease in ankle width and clinical score beginning on day 2. On day 9, LysM-Cre+;RGS12fl/fl mice showed a 23.3% (P<0.01) decrease in ankle width and a 156% (P<0.01) decrease in clinical score (Figure 2C,D). Moreover, LysM-Cre+;RGS12fl/fl mice showed a 1.13-fold reduction in inflammatory cell infiltration in the synovium (P<0.001) by HE staining compared with LysM-Cre+ mice at 9 days after immunization (Figure 2E). These findings suggest that the loss of RGS12 in macrophages can inhibit the development of CAIA.
Figure 2.
Conditional RGS12 KO in mice inhibits the development of CAIA. (A) CAIA was induced in LysM-Cre+ and LysM-Cre+;RGS12fl/fl mice by injection 5 mg of a mAb mixture (Chondrex #53100) suspended in sterile PBS and subsequent challenge with 50 μg of LPS on day 3. At 9 days after immunization, the mice were sacrificed. (B) The paws of LysM-Cre+ and LysM-Cre+;RGS12fl/fl mice were immunized with 5 mg of a mAb mixture for 9 days as described in (A). Note that LysM-Cre+;RGS12fl/fl mice showed less swelling in the ankles (yellow double arrow) than LysM-Cre+ mice. (C) Ankle widths and (D) arthritis clinical scores of LysM-Cre+ and LysM-Cre+;RGS12fl/fl mice were measured and evaluated every day for 9 days. Statistically significant differences (***, P<0.001) were observed between LysM-Cre+ and LysM-Cre+;RGS12fl/fl mice. The data are expressed as the means ± SEM. (E) Hematoxylin and eosin (H&E) staining of RA synovial tissue samples from mice as described in (A) and (B). H&E staining showed a decrease in local inflammatory cell infiltration (yellow arrows) within synovial tissue in the LysM-Cre+;RGS12fl/fl group (B, bone area). ***, P<0.001, n=5. Scale bar: 500 mm.
Loss of RGS12 in macrophages attenuates RA pain
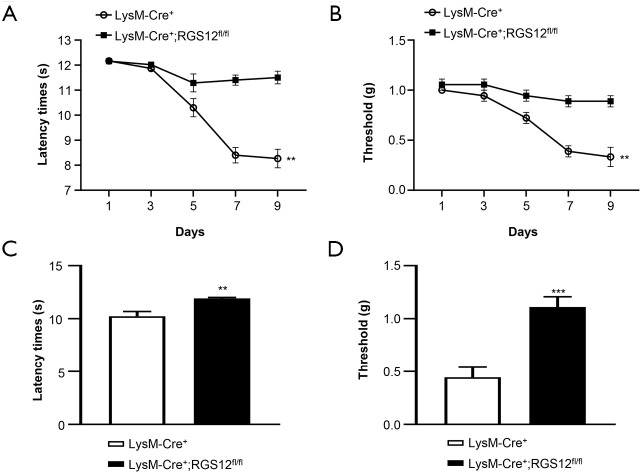
To examine whether the loss of RGS12 in macrophages affects pain behavior during RA, we first measured the responses evoked by heat in LysM-Cre+ and LysM-Cre+;RGS12fl/fl mice. LysM-Cre+;RGS12fl/fl mice showed 9.4% (P<0.05), 35.7% (P<0.01) and 139.2% (P<0.01) increases in reaction latencies on days 5, 7 and 9, respectively, compared to those of LysM-Cre+ mice (Figure 3A). Moreover, the results of the von Frey microfilament test showed that LysM-Cre+;RGS12fl/fl mice had lower sensitivity to pressure-induced pain than LysM-Cre+ mice. The pressure-induced pain thresholds in the LysM-Cre+;RGS12fl/fl groups were increased by 30.7% (P<0.05), 128.6% (P<0.01) and 166.7% (P<0.01) in the LysM-Cre+ group on days 5, 7 and 9, respectively, compared to those of the control group (Figure 3B). These results indicate that the loss of RGS12 decreased physiological pain sensitivity. To further determine whether the deletion of RGS12 affects pain during CAIA independent of disease severity, we analyzed the pain threshold and latency time in LysM-Cre+ and LysM-Cre+;RGS12fl/fl mice, which had the same clinical scores (score =2). The results showed that the deletion of RGS12 led to a 16.5% (P<0.01) increase in latency time in the LysM-Cre+;RGS12fl/fl group compared to the LysM-Cre+ group (Figure 3C). Additionally, the pain threshold was increased 1.5-fold (P<0.001) in LysM-Cre+;RGS12fl/fl mice compared to LysM-Cre+ mice (Figure 3D) These results demonstrated that, under the same pathological conditions, the loss of RGS12 in macrophages reduces pain sensitivity.
Figure 3.
The loss of RGS12 in macrophages attenuates RA pain. (A) Effect of RGS12 deletion in macrophages on pain thresholds as measured by the von Frey pain test in arthritic mice. A significant decrease in the pain thresholds of LysM-Cre+;RGS12fl/fl mice compared to LysM-Cre+ mice was observed in arthritic animals beginning on day 5 after induction with mAbs. The data are expressed as the means ± SEM. n=5, **, P<0.01. (B) Effect of RGS12 deficiency in macrophages on the latency of paw withdrawal from the thermal stimulus in arthritic mice. Each value in the line graph represents the mean ± SEM. A significant decrease in the latency time of LysM-Cre+;RGS12fl/fl mice compared to LysM-Cre+ mice was observed in arthritic animals beginning on day 5 after induction with mAbs. (C,D) Behavioral responses of LysM-Cre+ and LysM-Cre+;RGS12fl/fl mice with the same clinical score (score level 2) to mechanical and heat stimulation in the context of RA. (C) Mechanical paw withdrawal thresholds of naive LysM-Cre+ and LysM-Cre+;RGS12fl/fl mice obtained by von Frey monofilament stimulation. (D) Heat-induced paw withdrawal thresholds of LysM-Cre+ and LysM-Cre+;RGS12fl/fl mice obtained by the plantar heat test. **, P<0.01; ***, P<0.001.
COX2 and PGE2 regulate RGS12 expression via EP2 and EP4
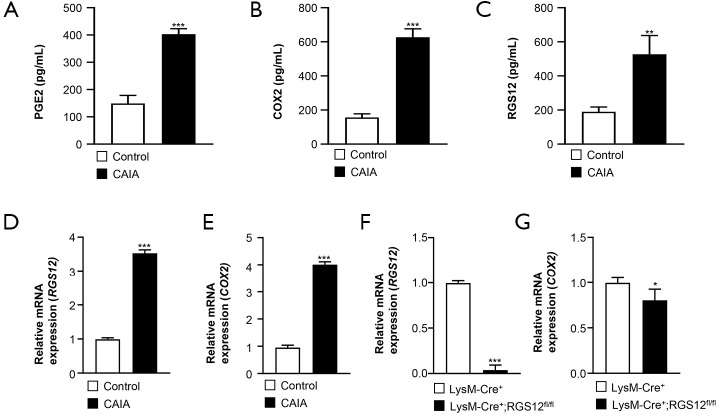
The inflammatory enzyme COX2 is expressed in macrophages in response to various stimuli (9). The lipid mediator PGE2 is produced by COX2 during inflammation and is considered a major PGE species in RA due to an increased level of PGE2 in the synovial tissues of RA patients (29). PGE2 exhibits multiple biological effects, such as mediating pain and inflammatory responses (29). We first measured the protein expression levels of PGE2 and COX2 in synovial macrophages from control and CAIA mice by ELISA (Figure 4A,B). We found that the expression levels of PGE2 (402.9±18 pg/mL) (Figure 4A) and COX2 (627.2±44.2 pg/mL) (Figure 4B) in macrophages were significantly increased in CAIA mice compared to control mice [COX2 (156±16.3 pg/mL) and PGE2 (149±26.4 pg/mL)]. Moreover, we found that the RGS12 expression level (527±98.1 pg/mL) was increased 1.78-fold (P<0.01) in CAIA mice compared with control mice (198.4±25.5 pg/mL) (Figure 4C). We then measured the gene expression levels of RGS12 and COX2 in control and CAIA mice on day 9. The results showed that the expression of RGS12 and COX2 was significantly increased in mice with CAIA (Figure 4D,E). To determine how RGS12 regulates COX2 expression in macrophages, we extracted BMMs from LysM-Cre+ and LysM-Cre+;RGS12fl/fl mice. We found that the mRNA expression of COX2 was decreased in LysM-Cre+;RGS12fl/fl BMMs (Figure 4F,G).
Figure 4.
The protein levels of PGE2, COX2, and RGS12 in the synovium is increased in CAIA model mice. (A,B,C) The levels of PGE2, COX2, and RGS12 in the synovium of control and CAIA model mice were analyzed by ELISA. ***, P<0.001, **, P<0.01 compared with the control group, n=5. The values are the mean ± SEM. (D,E) Analysis of RGS12 and COX2 mRNA levels in the synovium from control and CAIA mouse models. ***, P<0.001 compared with those in the control group, n=5. The values are the mean ± SEM. (F,G) Gene expression levels of RGS12 and COX2 in BMMs from LysM-Cre+ and LysM-Cre+;RGS12fl/fl mice. The data are presented as the mean ± SEM, n=5. *, P<0.05, and ***, P<0.001.
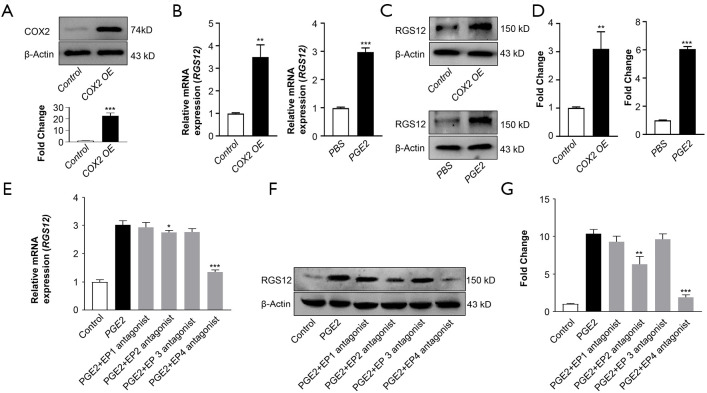
To further determine whether there was a correlation between COX2 and RGS12, COX2 was overexpressed in primary BMMs by transfection with pcDNA3.1-COX2. The COX2 protein level was confirmed by immunoblotting (Figure 5A). Interestingly, real-time PCR results showed that COX2 overexpression in primary BMMs increased RGS12 mRNA levels (Figure 5B). Since COX is the major enzyme that catalyzes arachidonic acid conversion into prostaglandins (PGs) and PGE2 facilitates both inflammation and immune regulation (9), we further determined whether increased PGE2 could regulate RGS12 expression in macrophages. Indeed, PGE2 upregulated RGS12 mRNA levels by threefold-in primary BMMs compared to PBS-treated cells (Figure 5B). Consistent with the gene expression level, immunoblotting showed that RGS12 protein expression was also increased by exogenous enhancement of COX2 and PGE2 (Figure 5C,D).
Figure 5.
COX2/PGE2 enhances RGS12 expression through EP2 and EP4. (A) Western blot analysis of COX2 overexpression (OE) in BMMs. Primary BMMs were isolated from WT mice and transfected with pCMV-empty vector (control) and pCMV-COX2 (COX2 OE) plasmids by Lipofectamine 3000 for 24 hours. Immunoblot quantification shows significantly increased expression of COX2. ***, P<0.001 versus the control. n=5. (B) Analysis of RGS12 levels after COX2 overexpression (OE) and PGE2 induction. RGS12 levels were determined by strand-specific RT-qPCR analysis of total RNA. The data are presented as the mean ± SEM, n=5. **, P<0.01; ***, P<0.001. (C,D) Western blot analysis of RGS12 expression after COX2 overexpression (OE) and PGE2 induction in BMMs (C). RGS12 levels were measured by semiquantitative Western blotting (D). The data are presented as the mean ± SEM, n=5. **, P<0.01 and ***, P<0.001. (E) RGS12 mRNA levels were determined by real-time PCR. Primary BMMs were treated with PGE2 (Cayman) or PGE2 plus individual PGE2 receptor antagonists (EP1 (ONO-8711, Cayman) 10 µM; EP2 (TG11-77, Cayman) 10 µM; EP3 (Sulprostone, Cayman) 10 µM; or EP4 (GW 627368X, Cayman) 10 µM). The data were normalized to GAPDH levels and are presented as the mean ± SEM, n=5. *, P<0.05 and ***, P<0.001. (F,G) RGS12 protein levels were quantitatively analyzed. Primary BMMs were treated as described in (E). **, P<0.01 and ***, P<0.001, n=5. The data are presented as the mean ± SEM.
Significant progress has been made in elucidating the function of PGE2 and EP receptors in health and diseases (30,31). The biological effects of PGE2 are regulated through four EP receptors (EP1-EP4), which are GPCRs (29,30). EP1 is coupled to the Gαq protein and activates phosphoinositide‐PLC. EP3 exists as alternatively spliced variants. EP2 and EP4 are linked to G‐stimulatory (Gαs) proteins, which are responsible for the activation of PKA (29). Interestingly, we found that both RGS12 gene and protein levels were increased by PGE2 induction, which was inhibited by EP2 and EP4 antagonists but not EP1 or EP3 antagonists (Figure 5E,F,G). These results suggest that COX2/PGE2 regulates RGS12 expression mainly through EP2- and EP4-mediated signaling pathways.
RGS12 or the RGS12 PTB domain promotes COX2 expression through NF-κB
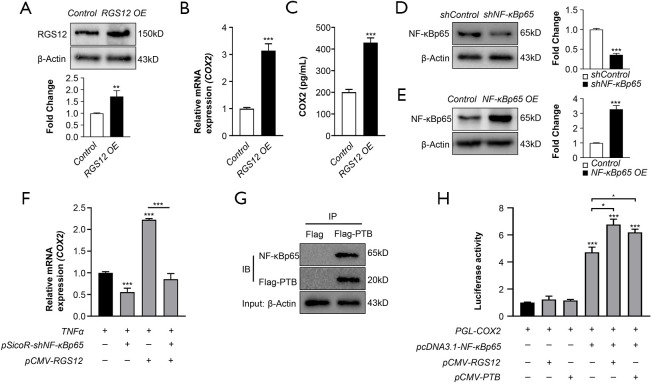
COX2 is a critical pain mediator that can be transcriptionally regulated by NF-κB in acute and chronic pain states (32). Recently, RGS12 was shown to have a strong relationship with NF-κB activity (17). However, whether RGS12 regulates pain signaling through the NF-κB/COX2 pathway is still unclear. To elucidate whether RGS12 regulates COX2 levels, we first overexpressed RGS12 in primary BMMs using the pCMV-RGS12 vector and confirmed the RGS12 protein level by Western blotting (Figure 6A). Interestingly, we found that ectopic expression of RGS12 upregulated the mRNA and protein levels of COX2 (Figure 6B,C). To further examine whether RGS12 regulates COX2 levels through NF-κB activation, we verified the efficiency of NF-κB knockdown (Figure 6D) or overexpression (Figure 6E) in BMMs by immunoblotting. BMMs were then treated with TNFα and transfected with shNF-κB and/or pcDNA-RGS12 plasmids for 48 hours. We found that RGS12 significantly increased COX2 mRNA levels, which were blocked by NF-κB knockdown (Figure 6F). A previous report showed that RGS12 or the RGS12 PTB domain regulates NF-κB activity by enhancing its nuclear translocation (17). To further determine whether the RGS12 PTB domain could promote the transcriptional activity of NF-κB, we performed a protein pulldown assay. The results showed that the RGS12 PTB domain was associated with NF-κB p65 in macrophages (Figure 6G). Furthermore, we found that NF-κB overexpression promoted COX2 luciferase activity and that overexpression of RGS12 or the RGS12 PTB domain significantly enhanced NF-κB overexpression-induced COX2 production (Figure 6H). Thus, our results show that RGS12 or the RGS12 PTB domain regulates COX2 expression by regulating NF-κB activity.
Figure 6.
RGS12 can strengthen the transcriptional regulation of COX2 via NF-κB. (A) Western blot analysis of RGS12 expression. Primary BMMs were transfected with pCMV-empty (control) or pCMV-RGS12 (RGS12 OE) plasmids by Lipofectamine 3000 for 24 hours, and the expression levels of RGS12 were analyzed. **, P<0.01 vs. the control group, n=3. (B) Relative mRNA expression of COX2 was analyzed by real-time PCR. Primary BMMs were treated as described in (A), and the expression levels of COX2 were analyzed. **, P<0.01, ***, P<0.001 compared with the control group. The values are the mean ± SEM (n=5). (C) COX2 expression levels in BMMs were analyzed by ELISA. Primary BMMs were treated as described in (A), and COX2 expression levels were analyzed. Note that RGS12 OE enhanced the expression level of COX2. **, P<0.01, ***, P<0.001 compared with the control group. (D) Relative protein levels of NF-κBp65. RAW264.7 cells were transfected with shControl (pSicoR-empty) or shNF-κB plasmids (pSicoR-shNFκBp65-1 and pSicoR-shNFκBp65-2) for 24 hours. β-Actin was used as an internal reference. The data are presented as the means ± SEM. ***, P<0.001 vs. shControl group, n=3. (E) Western blot analysis of NF-κBp65. RAW264.7 cells were transfected with pcDNA3.1-empty (control) or pcDNA3.1-NF-κBp65 (NF-κBp65 OE) plasmids for 24 hours. β-Actin was used as an internal reference. The data are presented as the means ± SEM. ***, P<0.001 vs. the control group, n=3. (F) RAW 264.7 cells were transfected with pUC-shNF-κBp65 and/or pCMV-RGS12 for 24 hours and then induced with TNFα for 24 hours. Relative mRNA expression was measured by real-time PCR. Note that RGS12 could not increase COX2 expression when NF-κB expression was decreased. The values are the mean ± SEM. ***, P<0.001, n=5. (G) Analysis of the interactions between RGS12-PTB and NF-κBp65 by pulldown assay. RAW264.7 cells were transfected with pCMV-Flag or pCMV-PTB-Flag plasmids, and cell lysates were precipitated with anti-Flag affinity gel and immunoblotted (IB) with anti-NF-κB p65 and anti-Flag antibodies. (H) RGS12 promotes the transcriptional activity of NF-κB and COX2 expression (luciferase assay). Stable knockdown of NF-κB in RAW264.7 cells was achieved via the transfection of pSicoR-shNFκBp65-1 and pSicoR-shNFκBp65-2 vectors. The stably transfected RAW264.7 cells were treated with COX2-Luc, pcDNA3.1-NF-κBp65, pCMV-RGS12 and/or pCMV-PTB for 48 hours. *, P<0.05, ***, P<0.001 versus the control. The data are presented as the means ± SEM. Note that RGS12 or PTB could increase the transcriptional activity of NF-κB.
Discussion
Chronic pain is a disease process that includes neuropathic pain and inflammatory pain (33). Neuropathic pain is caused by damage to neurons in the peripheral and central nervous systems (34). Inflammatory pain is associated with tissue damage and the subsequent inflammatory process, which elicits physiological responses to promote healing (35). A previous report showed that RGS12 was expressed in the ventral striatum (vSTR) of the mesolimbic dopaminergic system and regulates vSTR dopamine (DA) homeostasis via KOR signaling (18), suggesting that RGS12 is involved in the regulation of pain-related signaling by the nervous system. Interestingly, RGS12 was also shown to play a key role in regulating the phosphorylation and activation of NF-κB, which serves as a pivotal mediator of inflammatory responses (17). Moreover, we found that RGS12 was involved in inflammatory pain in CAIA model mice by regulating the pain-specific molecule COX2, which forms a positive feedback loop to regulate the expression of downstream pain factors such as PGE2 and pain-related signaling pathways via EP2 and EP4. Thus, our results provide evidence that RGS12 regulates pain through the NF-κB-COX2-PGE2-EP2/4 axis. Although we found that RGS12 regulates inflammatory pain, it is still not clear whether RGS12 is involved in the crosstalk and balance between inflammatory pain and neuropathic pain during RA, which will be examined in future studies.
There is wide variation in RA prevalence and incidence (36). The female-to-male ratio of RA prevalence has been indicated to be approximately 3:1 (37). Thus, most studies use female mice for RA-related research because of the increased susceptibility (38,39). Additionally, population-based studies indicated a higher pain prevalence in females than males (40). Studies have shown that women are at higher risk for many common pain conditions, including fibromyalgia, RA, and osteoarthritis (41,42). It has been suggested that interactions between biological and psychological factors lead to these differences (43). Based on these reports, female mice were used in the present study. Future studies will examine whether RGS12 is also involved in sex differences in the prevalence of RA.
Macrophages play critical roles in many conditions associated with chronic pain by releasing proinflammatory mediators that induce pain via the activation of multiple nociceptors (44). Local depletion of macrophages after complete Freund’s adjuvant (CFA)-induced paw inflammation inhibits the development of inflammatory pain (45). In our study, we found that conditional deletion of RGS12 in macrophages could attenuate pain sensitivity. Moreover, we found that RGS12 overexpression in macrophages could enhance the expression of proinflammatory mediators such as COX2. Consistently, macrophages expressing high levels of COX2 were shown to exacerbate inflammation-driven pathology in arthritis (46). COX2 is a crucial mediator of pain in inflammatory diseases such as RA and OA (osteoarthritis), making it an attractive therapeutic target (47). COX2 deletion considerably suppresses synovial inflammation and joint destruction and suppresses inflammatory pain (9). We found that RGS12 or the RGS12 PTB domain regulated COX2 expression at the transcriptional level, which was dependent on NF-κB. Moreover, RGS12 enhanced the transcriptional activity of NF-κB, which was consistent with previous studies showing that RGS12 or the PTB domain activates NF-κB via phosphorylation (17). Thus, RGS12 controls COX2 expression through NF-κB activation in macrophages.
Macrophages express a large number of GPCRs that are critical for the migration and accumulation of inflammatory cells. Here, we found that RGS12 plays a role in regulating inflammation through specific GPCR-dependent pathways. PGE2 controls the immune response through four GPCR subtypes (EP1-4) (48). In this study, RGS12 was regulated by PGE2-mediated EP2 and EP 4 but not EP1 or EP3 signaling. RGS12 is involved in regulating the activity of several Gα subunits, such as Gαi and Gα12/13 (49). Huang et al. showed that RGS12 inactivates Gαi to abrogate the inhibition of cAMP formation (50). RGS12 inhibits the activity of Gα12 and Gα13 but not Gαs in NIH3T3 cells (49). Therefore, it is believed that RGS12 is activated by specific and functional GPCRs. In addition to regulating GPCRs, RGS12 shares features with MAPK scaffolds, which regulate nucleotide release and endosomal targeting (51). RGS12 also interacts with multiple components of the Ras/Raf/MEK signaling cascade via PDZ and PTB domains to mediate neuronal differentiation (52). However, there are still numerous GPCRs and signaling pathways that can trigger RGS12 due to its multiple functional domains (53,54). The RGS domain and the GoLoco motif display high-affinity for Gαi, while the PTB domain associates with a large number of proteins (53,55). Thus, our future studies will identify the functional GPCRs and related signaling pathways during arthritis pathogenesis.
PGE2 is a crucial mediator of inflammatory pain sensitization that is produced in peripheral inflamed tissues and the spinal cord (56). PGE2 activates prostaglandin E receptors and leads to different cellular outcomes (57). Here, we found that PGE2 could enhance RGS12 expression in macrophages mainly through EP2 and EP4, suggesting that PGE2 may act upstream of RGS12 signaling pathways. Interestingly, we also found that RGS12 overexpression promotes COX2 expression and PGE2 production. Thus, RGS12 and COX2/PGE2 form a positive feedback loop to regulate inflammatory pain. Previous studies indicated that RGS12 overexpression in inflamed macrophages could promote osteoclast differentiation, which has a similar effect as that of EP2/EP4 agonists (58). Hence, these results demonstrate that RGS12 is the key factor and therapeutic target in regulating bone remodeling and inflammatory pain.
In summary, the findings of the current study provide new evidence that RGS12 in macrophages contributes to inflammatory pain hypersensitivity via the COX2/PGE2/EP2 and EP4 pathways. Our study provides a new therapeutic target that may facilitate the design of clinical treatments for inflammatory pain during RA.
Supplementary
The article’s supplementary files as
Acknowledgments
We thank Springer Nature for language editing.
Funding: This work was supported by grants from the National Institute on Aging (NIA) (AG048388); National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) (AR066101). This work was supported by grants from the Penn Center for Musculoskeletal Disorders (PCMD), NIH/NIAMS P30-AR069619.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No. 806005) granted by the board of the American Association for Laboratory Animal Science (IACUC), in compliance with the University of Pennsylvania guidelines for the care and use of animals.
Footnotes
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-5729
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-5729
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-5729). The authors have no conflicts of interest to declare.
References
- 1.Walsh DA, McWilliams DF. Pain in rheumatoid arthritis. Curr Pain Headache Rep 2012;16:509-17. 10.1007/s11916-012-0303-x [DOI] [PubMed] [Google Scholar]
- 2.Ji RR, Chamessian A, Zhang YQ. Pain regulation by non-neuronal cells and inflammation. Science 2016;354:572-7. 10.1126/science.aaf8924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ji RR, Nackley A, Huh Y, et al. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018;129:343-66. 10.1097/ALN.0000000000002130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McWilliams DF, Walsh DA. Pain mechanisms in rheumatoid arthritis. Clin Exp Rheumatol 2017;35 Suppl 107:94-101. [PubMed] [Google Scholar]
- 5.Zarghi A, Arfaei S. Selective COX-2 Inhibitors: A Review of Their Structure-Activity Relationships. Iran J Pharm Res 2011;10:655-83. [PMC free article] [PubMed] [Google Scholar]
- 6.Appleby SB, Ristimaki A, Neilson K, et al. Structure of the human cyclo-oxygenase-2 gene. Biochem J. 1994;302:723-7. 10.1042/bj3020723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Dross RT, Hong X, Essengue S, et al. Modulation of UVB-induced and basal cyclooxygenase-2 (COX-2) expression by apigenin in mouse keratinocytes: role of USF transcription factors. Mol Carcinog 2007;46:303-14. 10.1002/mc.20281 [DOI] [PubMed] [Google Scholar]
- 8.Kang YJ, Mbonye UR, DeLong CJ, et al. Regulation of intracellular cyclooxygenase levels by gene transcription and protein degradation. Prog Lipid Res 2007;46:108-25. 10.1016/j.plipres.2007.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 2011;31:986-1000. 10.1161/ATVBAHA.110.207449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Wei Y, Zheng F, et al. Prostaglandin E2 in the Regulation of Water Transport in Renal Collecting Ducts. Int J Mol Sci 2017;18:2539. 10.3390/ijms18122539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev 1999;79:1193-226. 10.1152/physrev.1999.79.4.1193 [DOI] [PubMed] [Google Scholar]
- 12.Breyer RM, Bagdassarian CK, Myers SA, et al. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol 2001;41:661-90. 10.1146/annurev.pharmtox.41.1.661 [DOI] [PubMed] [Google Scholar]
- 13.Steury MD, McCabe LR, Parameswaran N. G. Protein-Coupled Receptor Kinases in the Inflammatory Response and Signaling. Adv Immunol 2017;136:227-77. 10.1016/bs.ai.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avrampou K, Pryce KD, Ramakrishnan A, et al. RGS4 Maintains Chronic Pain Symptoms in Rodent Models. J Neurosci 2019;39:8291-304. 10.1523/JNEUROSCI.3154-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terzi D, Gaspari S, Manouras L, et al. RGS9-2 modulates sensory and mood related symptoms of neuropathic pain. Neurobiol Learn Mem 2014;115:43-8. 10.1016/j.nlm.2014.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin-McCaffrey L, Hains MD, Pritchard GA, et al. Differential expression of regulator of G-protein signaling R12 subfamily members during mouse development. Dev Dyn 2005;234:438-44. 10.1002/dvdy.20555 [DOI] [PubMed] [Google Scholar]
- 17.Yuan G, Yang S, Ng A, et al. RGS12 Is a Novel Critical NF-kappaB Activator in Inflammatory Arthritis. iScience 2020;23:101172. 10.1016/j.isci.2020.101172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gross JD, Kaski SW, Schmidt KT, et al. Role of RGS12 in the differential regulation of kappa opioid receptor-dependent signaling and behavior. Neuropsychopharmacology 2019;44:1728-41. 10.1038/s41386-019-0423-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang S, Li YP, Liu T, et al. Mx1-cre mediated Rgs12 conditional knockout mice exhibit increased bone mass phenotype. Genesis 2013;51:201-9. 10.1002/dvg.22373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo X, Huh Y, Bang S, et al. Macrophage Toll-like Receptor 9 Contributes to Chemotherapy-Induced Neuropathic Pain in Male Mice. J Neurosci 2019;39:6848-64. 10.1523/JNEUROSCI.3257-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Z, Doyle TM, Luongo L, et al. Sphingosine-1-phosphate receptor 1 activation in astrocytes contributes to neuropathic pain. Proc Natl Acad Sci U S A 2019;116:10557-62. 10.1073/pnas.1820466116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nieto FR, Clark AK, Grist J, et al. Neuron-immune mechanisms contribute to pain in early stages of arthritis. J Neuroinflammation 2016;13:96. 10.1186/s12974-016-0556-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trebino CE, Stock JL, Gibbons CP, et al. Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc Natl Acad Sci U S A 2003;100:9044-9. 10.1073/pnas.1332766100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romberg R, Sarton E, Teppema L, et al. Comparison of morphine-6-glucuronide and morphine on respiratory depressant and antinociceptive responses in wild type and mu-opioid receptor deficient mice. Br J Anaesth 2003;91:862-70. 10.1093/bja/aeg279 [DOI] [PubMed] [Google Scholar]
- 25.Chen CC, Zimmer A, Sun WH, et al. A role for ASIC3 in the modulation of high-intensity pain stimuli. Proc Natl Acad Sci U S A 2002;99:8992-7. 10.1073/pnas.122245999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan G, Xu L, Cai T, et al. Clock mutant promotes osteoarthritis by inhibiting the acetylation of NFκB. Osteoarthritis Cartilage 2019;27:922-31. 10.1016/j.joca.2019.01.012 [DOI] [PubMed] [Google Scholar]
- 27.Yuan G, Hua B, Cai T, et al. Clock mediates liver senescence by controlling ER stress. Aging 2017;9:2647-65. 10.18632/aging.101353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jakobsson PJ. Pain: how macrophages mediate inflammatory pain via ATP signaling. Nat Rev Rheumatol 2010;6:679-81. 10.1038/nrrheum.2010.175 [DOI] [PubMed] [Google Scholar]
- 29.Fattahi MJ, Mirshafiey A. Prostaglandins and rheumatoid arthritis. Arthritis 2012;2012:239310. 10.1155/2012/239310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Callaghan G, Houston A. Prostaglandin E2 and the EP receptors in malignancy: possible therapeutic targets? Br J Pharmacol 2015;172:5239-50. 10.1111/bph.13331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Markovič T, Jakopin Z, Dolenc MS, et al. Structural features of subtype-selective EP receptor modulators. Drug Discov Today 2017;22:57-71. 10.1016/j.drudis.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 32.Lee KM, Kang BS, Lee HL, et al. Spinal NF-kB activation induces COX-2 upregulation and contributes to inflammatory pain hypersensitivity. Eur J Neurosci 2004;19:3375-81. 10.1111/j.0953-816X.2004.03441.x [DOI] [PubMed] [Google Scholar]
- 33.Vardeh D, Mannion RJ, Woolf CJ. Toward a Mechanism-Based Approach to Pain Diagnosis. J Pain 2016;17:T50-69. 10.1016/j.jpain.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci 2009;32:1-32. 10.1146/annurev.neuro.051508.135531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skaper SD, Facci L, Zusso M, et al. An Inflammation-Centric View of Neurological Disease: Beyond the Neuron. Front Cell Neurosci 2018;12:72. 10.3389/fncel.2018.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gabriel SE, Michaud K. Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis Res Ther 2009;11:229. 10.1186/ar2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Vollenhoven RF. Sex differences in rheumatoid arthritis: more than meets the eye. BMC Med 2009;7:12. 10.1186/1741-7015-7-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taneja V, Behrens M, Mangalam A, et al. New humanized HLA-DR4-transgenic mice that mimic the sex bias of rheumatoid arthritis. Arthritis Rheum 2007;56:69-78. 10.1002/art.22213 [DOI] [PubMed] [Google Scholar]
- 39.Tang W, Lu Y, Tian QY, et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science 2011;332:478-84. 10.1126/science.1199214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fillingim RB, King CD, Ribeiro-Dasilva MC, et al. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain 2009;10:447-85. 10.1016/j.jpain.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Theis KA, Helmick CG, Hootman JM. Arthritis burden and impact are greater among U.S. women than men: intervention opportunities. J Womens Health (Larchmt) 2007;16:441-53. 10.1089/jwh.2007.371 [DOI] [PubMed] [Google Scholar]
- 42.Murphy SL, Lyden AK, Phillips K, et al. Association between pain, radiographic severity, and centrally-mediated symptoms in women with knee osteoarthritis. Arthritis Care Res (Hoboken) 2011;63:1543-9. 10.1002/acr.20583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fillingim RB. Individual differences in pain: understanding the mosaic that makes pain personal. Pain 2017;158 Suppl 1:S11-S18. 10.1097/j.pain.0000000000000775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen O, Donnelly CR, Ji RR. Regulation of pain by neuro-immune interactions between macrophages and nociceptor sensory neurons. Curr Opin Neurobiol 2020;62:17-25. 10.1016/j.conb.2019.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Godai K, Hasegawa-Moriyama M, Kurimoto T, et al. Peripheral administration of morphine attenuates postincisional pain by regulating macrophage polarization through COX-2-dependent pathway. Mol Pain 2014;10:36. 10.1186/1744-8069-10-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakurai Y, Fujita M, Kawasaki S, et al. Contribution of synovial macrophages to rat advanced osteoarthritis pain resistant to cyclooxygenase inhibitors. Pain 2019;160:895-907. 10.1097/j.pain.0000000000001466 [DOI] [PubMed] [Google Scholar]
- 47.Withrow J, Murphy C, Liu Y, et al. Extracellular vesicles in the pathogenesis of rheumatoid arthritis and osteoarthritis. Arthritis Res Ther 2016;18:286. 10.1186/s13075-016-1178-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Keijzer S, Meddens MB, Torensma R, et al. The multiple faces of prostaglandin E2 G-protein coupled receptor signaling during the dendritic cell life cycle. Int J Mol Sci 2013;14:6542-55. 10.3390/ijms14046542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mao J, Yuan H, Xie W, et al. Guanine nucleotide exchange factor GEF115 specifically mediates activation of Rho and serum response factor by the G protein alpha subunit Galpha13. Proc Natl Acad Sci U S A. 1998;95:12973-6. 10.1073/pnas.95.22.12973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang J, Nalli AD, Mahavadi S, et al. Inhibition of Galphai activity by Gbetagamma is mediated by PI 3-kinase-gamma- and cSrc-dependent tyrosine phosphorylation of Galphai and recruitment of RGS12. Am J Physiol Gastrointest Liver Physiol 2014;306:G802-10. 10.1152/ajpgi.00440.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sambi BS, Hains MD, Waters CM, et al. The effect of RGS12 on PDGFbeta receptor signalling to p42/p44 mitogen activated protein kinase in mammalian cells. Cell Signal 2006;18:971-81. 10.1016/j.cellsig.2005.08.003 [DOI] [PubMed] [Google Scholar]
- 52.Willard MD, Willard FS, Li X, et al. Selective role for RGS12 as a Ras/Raf/MEK scaffold in nerve growth factor-mediated differentiation. EMBO J 2007;26:2029-40. 10.1038/sj.emboj.7601659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Richman RW, Strock J, Hains MD, et al. RGS12 interacts with the SNARE-binding region of the Cav2.2 calcium channel. J Biol Chem 2005;280:1521-8. 10.1074/jbc.M406607200 [DOI] [PubMed] [Google Scholar]
- 54.Yuan G, Yang S, Liu M, et al. RGS12 is required for the maintenance of mitochondrial function during skeletal development. Cell Discov 2020;6:59. 10.1038/s41421-020-00190-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kimple RJ, De Vries L, Tronchere H, et al. RGS12 and RGS14 GoLoco motifs are G alpha(i) interaction sites with guanine nucleotide dissociation inhibitor Activity. J Biol Chem 2001;276:29275-81. 10.1074/jbc.M103208200 [DOI] [PubMed] [Google Scholar]
- 56.Zeilhofer HU. The glycinergic control of spinal pain processing. Cell Mol Life Sci 2005;62:2027-35. 10.1007/s00018-005-5107-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andreasson K. Emerging roles of PGE2 receptors in models of neurological disease. Prostaglandins Other Lipid Mediat 2010;91:104-12. 10.1016/j.prostaglandins.2009.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yuan X, Cao J, Liu T, et al. Regulators of G protein signaling 12 promotes osteoclastogenesis in bone remodeling and pathological bone loss. Cell Death Differ 2015;22:2046-57. 10.1038/cdd.2015.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as