Abstract
Background
Lung cancer has become the most common malignant tumor worldwide, with the highest rates of morbidity and mortality. The detection of circulating tumor cells (CTCs) can be simple, rapid, and minimally invasive, thus endowing them with a high value in the diagnosis of malignant tumors. We aimed to explore the correlation between CTCs in peripheral blood and benign or malignant solitary pulmonary nodules (SPNs).
Methods
A total of 223 patients with SPNs from January 2018 to May 2020 were recruited. During the same period, 20 healthy volunteers were recruited as controls. Venous blood samples were collected from participants for detecting CTCs using a folate receptor (FR)-positive cell detection kit, as well as tumor biomarkers.
Results
A significant difference in the level of CTCs were observed between the malignant SPNs group, the benign SPNs group, and the control group, which was markedly higher in the malignant SPNs group (10.48±3.49 FU/3 mL) than both the benign SPNs and control groups (6.38±0.53 and 4.45±1.21 FU/3 mL, respectively) (P<0.001). In addition, the level of CTCs was significantly higher in the benign SPNs group than in the control group (P=0.023). In particular, in the malignant SPNs group, patients older than 60 years (11.45±3.92 FU/3 mL) presented a notably higher level of CTCs than other patients (9.55±2.74 FU/3 mL). The patients were then classified according to the pathological subtypes of lung cancer. There was a significant difference in level of CTCs among patients with squamous cell carcinoma (9.10±1.94 FU/3 mL), adenocarcinoma (10.77±3.71 FU/3 mL), and adenosquamous cell carcinoma (11.78±2.61 FU/3 mL). Binary logistic regression analysis suggested that CTCs were an independent risk factor of malignant SPN (OR =3.698, 95% CI: 1.136–11.035, P=0.030). The sensitivity and specificity of CTCs in diagnosing malignant SPNs was significantly higher than tumor biomarkers (single or combined) [sensitivity =89.1%; specificity =92.3%; area under curve (AUC) (95% CI) =0.907 (0.861–0.942)].
Conclusions
Peripheral blood CTCs can be used in the diagnosis of malignant SPNs and are recommended for clinical application.
Keywords: Circulating tumor cells, solitary pulmonary nodules, lung cancer
Introduction
Lung cancer has become the most common malignant tumor worldwide, with the highest rates of morbidity and mortality. In 2018, there were approximately 2.1 million new cases and 1.8 million deaths (1). In China, the morbidity and mortality rates of lung cancer rank are higher than all others. More than 80% of lung cancer cases are non-small cell lung cancers (NSCLCs). With the extensive application of low-dose spiral CT (LDCT) in China, and the popularity of physical examination, the rate of detection of solitary pulmonary nodules (SPNs) has increased. SPNs are either benign or malignant, and malignant SPNs are an early manifestation of lung cancer. Clinical evidence has shown that early screening and treatment of malignant SPNs can significantly decrease the mortality of lung cancer (2,3). Therefore, sensitive biomarkers should be identified to develop efficient tools for the diagnosis of SPNs.
As a subtype of tumor cells, circulating tumor cells (CTCs) shed into the vasculature from primary or metastatic solid tumors naturally or as a result of therapeutic procedures, and give rise to tumor development, metastasis, or recurrence (4-6). The detection of CTCs can be simple, rapid, and minimally invasive, thus endowing them with a high value in the diagnosis of malignant tumors, assessment of tumor staging, prediction of prognosis, evaluation of chemotherapy efficacy, and guidance of targeted medication. In the present study, we aimed to explore the correlation between peripheral blood CTCs with benign or malignant SPNs, thereby determining the potential of CTCs as a specific biomarker of lung cancer in early-stage screening of SPNs. We present the following article in accordance with the STARD reporting checklist (available at http://dx.doi.org/10.21037/atm-21-889).
Methods
This study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University. All participants signed the informed consent. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013) .
Baseline characteristics
In total, 223 patients with SPNs treated in the inpatient and outpatient departments of thoracic surgery at the First Affiliated Hospital of Soochow University from January 2018 to May 2020 were recruited into this retrospective analysis. During the same period, 20 healthy volunteers were recruited as controls. Venous blood samples were collected from participants for detecting CTCs using a folate receptor (FR)-positive cell detection kit [negative screening of immunomagnetic beads + ligand-targeted polymerase chain reaction (LT-PCR); National Machinery Registration Standard: 20163400061; Genosaber, Nantong, China]. Relative levels of carcinoembryonic antigen (CEA), carbohydrate antigen 125 (CA125), carbohydrate antigen 724 (CA724), cytokeratin fragment 21-1 (CYFRA21-1), and neuron-specific enolase (NSE) were detected using an automatic electrochemiluminescence immunoassay analyzer (Roche Cobas e601). Recruited patients with SPNs were surgically treated and pathologically diagnosed. Lung cancer was assessed according to the TNM Classification of Malignant Tumors (the 8th edition, 2018) published by the Union for International Cancer Control (UICC).
The inclusion criteria were as follows: (I) patients with chest CT images showing SPNs and a definite indication of surgery determined by experienced chief or associate chief physicians; (II) patients that had not undergone preoperative treatments, including anti-infection, anti-tuberculosis, chemotherapy, targeted medication, or combination treatment; (III) patients that did not have other types of malignant tumors; (IV) patients that showed normal functions in other vital organs; (V) patients who were willing to cooperate and provide informed consent; and (VI) healthy volunteers.
The exclusion criteria were as follows: (I) patients with clotted, hemolyzed, or contaminated blood samples; (II) the volume of the blood sample was less than 3 mL; (III) patients that had undergone nonstandard procedures in the collection, preservation, and analysis of blood samples; (IV) patients or their families were poorly compliant, did not cooperate with treatment, or refused to sign the informed consent; (V) clinical data were incomplete; (VI) patients diagnosed as small cell lung cancer; and (VII) patients with stage IV tumor.
Reference values
The reference value of CTCs was 8.70 FU according to the recommendation of the FR-positive cell detection kit. The reference values of serum tumor biomarkers were as follows: CEA <5 ng/mL, CA125 <35 U/mL, CA724 <6 U/mL, CYFRA21-1 <3.07 ng/mL, and NSE <7 ng/mL. A higher level than the reference value was considered as positive, and a lower level as negative. Any serum tumor biomarker higher than the reference value in combination treatment was considered as a positive result.
Indicators
The observed indicators included: (I) peripheral blood CTC level; (II) receiver operating characteristic (ROC) curves, sensitivity, and specificity of CTCs and serum tumor biomarkers in diagnosing malignant or benign SPNs.
Statistical analysis
Data were first subjected to the normality test. Normally distributed data were expressed as mean ± standard derivation; otherwise, they were expressed as medians. Differences of normally distributed data between groups were analyzed by the independent Student’s t-test, and those among groups were analyzed by one-way analysis of variance (ANOVA). In addition, non-normally distributed data between groups were compared using the Mann-Whitney U test, and those among groups were compared by Kruskal-Wallis H test. Enumeration data were expressed as n (%), and were compared using the Chi-square test. Independent risk factors of malignant SPNs were assessed by binary logistic regression analysis. ROC curves were constructed for assessing diagnostic potentials, with sensitivity (%) as the Y-axis and 100-specificity (%) as the X-axis. The area under curve (AUC), sensitivity [sensitivity = true positive rate/(true positive rate + false negative rate) ×100%], and specificity [specificity = true negative rate/(true negative rate + false positive rate) ×100%] were calculated. SPSS 24.0, MedCalc_v12.3 and GraphPadPrism7 were used for statistical analyses. P<0.05 considered statistically significant.
Results
A total of 223 patients with SPNs were recruited. During the same period, 20 healthy volunteers were recruited as controls. Among the 184 (82.51%) patients with malignant SPNs were 79 males and 105 females, with an average age of 57.69±11.96 years. Of these, 34 (18.48%) were squamous cell carcinoma cases, 147 (79.89%) were adenocarcinoma cases, and three (1.63%) were adenosquamous cell carcinoma cases. Among the 39 (17.49%) patients with benign SPNs were 11 males and 28 females, with an average age of 60.12±9.75 years, including 10 (25.64%) sclerosing hemangioma cases, 15 (38.46%) hamartoma cases, 13 (33.33%) intrapulmonary lymph node cases, and one (2.56%) tuberculosis case.
CTC levels were subjected to the normality test. After the Kolmogorov-Smirnov test, Z values in the benign SPNs group, malignant SPNs group, and control group were 0.771 (P=0.593), 0.525 (P=0.946), and 0.833 (P=0.491), respectively, suggesting that CTC levels among the three groups were normally distributed, and were therefore expressed as mean ± standard derivation.
Level of CTCs in peripheral blood
Peripheral blood CTC levels in the malignant SPNs group, benign SPNs group, and control group were 10.48±3.49, 6.38±0.53, and 4.45±1.21 FU/3 mL, respectively. One-way ANOVA analysis showed that the CTC level was significantly different among the three groups (F=55.965, P<0.001). The subsequent post-hoc test suggested that peripheral blood CTC levels in the malignant SPNs group were significantly higher than those in both the benign SPNs group and the control group (both P<0.001). Moreover, a higher peripheral blood CTC level was detected in the benign SPNs group compared to the control group (P=0.023) (Figure 1).
Figure 1.
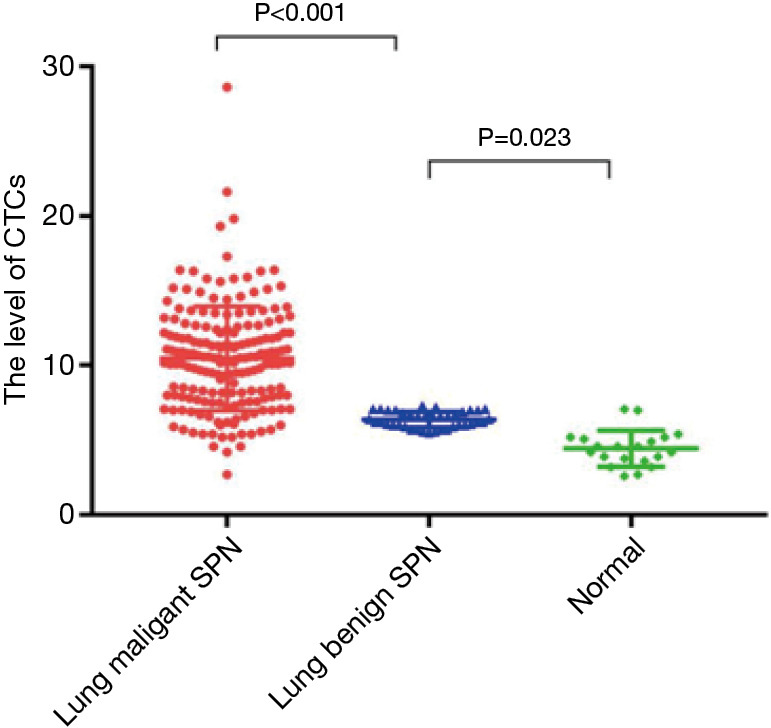
Comparison of peripheral CTC levels in the malignant SPNs group, benign SPNs group, and control group. CTC, circulating tumor cell; SPN, solitary pulmonary nodule.
As shown in Table 1, we recorded clinical data of patients in the malignant or benign SPNs groups, including CTCs (malignant/benign), age, pathological subtype, differentiation grade, location of SPNs (left/right lobe, upper/middle/lower lobe), largest diameter of SPNs, T stage, N stage, TNM stage, smoking history, and tumor biomarkers (negative/positive).
Table 1. Clinical data of patients in the malignant or benign SPNs groups.
| Clinical data | Malignant SPNs (n=184) | Benign SPNs (n=39) |
|---|---|---|
| CTCs level (FU/3 mL) | 10.48±3.49 | 6.38±0.53 |
| CTCs (malignant/benign) | 164/20 | 3/36 |
| Age (years) | ||
| ≤60 | 94 | 21 |
| >60 | 90 | 18 |
| Sex | ||
| Male | 79 | 11 |
| Female | 105 | 28 |
| Pathological subtypes | ||
| Squamous cell carcinoma | 34 | – |
| Adenocarcinoma | 147 | – |
| Adenosquamous cell carcinoma | 3 | – |
| Differentiation level | ||
| G1 | 99 | – |
| G2 | 67 | – |
| G3 | 18 | – |
| Location of SPNs | ||
| Left lobe | 91 | 19 |
| Right lobe | 93 | 20 |
| Location of SPNs | ||
| Upper lobe | 121 | 20 |
| Middle lobe | 8 | 4 |
| Lower lobe | 55 | 15 |
| Largest diameter of SPNs (cm) | ||
| ≤1 | 66 | 23 |
| >1 | 118 | 16 |
| T stage | ||
| T1 | 126 | – |
| T2 | 42 | – |
| T3 | 16 | – |
| N stage | ||
| N0 | 153 | – |
| N1 | 25 | – |
| N2 | 6 | – |
| TNM stage | ||
| Stage I | 137 | – |
| Stage II | 41 | – |
| Stage III | 6 | – |
| Smoking history | ||
| N/A | 160 | 30 |
| I/A | 24 | 9 |
| Tumor biomarkers (positive/negative) | ||
| CEA | 51/133 | 8/31 |
| CA125 | 55/129 | 6/33 |
| CA724 | 53/131 | 8/31 |
| CYFRA21-1 | 56/128 | 7/32 |
| NSE | 76/108 | 5/34 |
| CEA + CA125 + CA724 + CYFRA21-1 + NSE | 109/75 | 9/30 |
SPN, solitary pulmonary nodule; CTCs, circulating tumor cell; N/A, not available; I/A, is available; CEA, carcinoembryonic antigen; CA125, carbohydrate antigen 125; CA724, carbohydrate antigen 724; CYFRA21-1, cytokeratin fragment 21-1; NSE, neuron-specific enolase.
We subsequently performed subgroup analyses on peripheral blood CTC levels in malignant SPN patients classified by age, sex, pathological subtype, differentiation grade, location of SPN, largest diameter of SPN, T stage, N stage, TNM stage, and smoking history. The levels of CTCs were significantly higher in malignant SPN patients older than 60 years than other patients (11.45±3.92 vs. 9.55±2.74 FU/3 mL) (P<0.001). Classified by pathological subtypes, there was a notable difference in the level of CTCs among patients with squamous cell carcinoma, adenocarcinoma, and adenosquamous cell carcinoma (9.10±1.94 vs. 10.77±3.71 vs. 11.78±2.61 FU/3 mL) (F=3.436, P=0.034). Peripheral blood CTC levels in patients with malignant SPNs classified by sex, differentiation grade, location of SPN, largest diameter of SPN, T stage, N stage, TNM stage, and smoking history were comparable (Table 2).
Table 2. Clinical data of patients with malignant SPNs.
| Clinical data | Case number (n=184) | CTCs level (FU/3 mL) | F/χ2 | P |
|---|---|---|---|---|
| Age (years) | ||||
| ≤60 | 94 | 9.55±2.74 | 4.671 | <0.001 |
| >60 | 90 | 11.45±3.92 | ||
| Sex | ||||
| Male | 79 | 10.45±3.94 | 0.984 | 0.923 |
| Female | 105 | 10.50±3.13 | ||
| Pathological subtypes | ||||
| Squamous cell carcinoma | 34 | 9.10±1.94 | 3.436 | 0.034 |
| Adenocarcinoma | 147 | 10.77±3.71 | ||
| Adenosquamous cell carcinoma | 3 | 11.78±2.61 | ||
| Differentiation grade | ||||
| G1 | 99 | 10.39±3.74 | 0.899 | 0.409 |
| G2 | 57 | 10.25±3.37 | ||
| G3 | 18 | 11.29±2.75 | ||
| Location of SPN | ||||
| Left lobe | 91 | 10.49±4.02 | 3.731 | 0.968 |
| Right lobe | 93 | 10.47±2.91 | ||
| Location of SPN | ||||
| Upper lobe | 121 | 10.51±3.38 | 0.943 | 0.391 |
| Middle lobe | 8 | 8.85±1.84 | ||
| Lower lobe | 55 | 10.65±3.89 | ||
| Largest diameter of SPN (cm) | ||||
| ≤1 | 66 | 10.22±3.53 | 0.512 | 0.475 |
| >1 | 118 | 10.63±3.48 | ||
| T staging | ||||
| T1 | 126 | 10.57±3.71 | 0.964 | 0.383 |
| T2 | 42 | 9.92±3.11 | ||
| T3 | 16 | 11.25±2.43 | ||
| N staging | ||||
| N0 | 153 | 10.44±3.40 | 0.344 | 0.709 |
| N1 | 25 | 10.43±4.11 | ||
| N2 | 6 | 11.65±3.38 | ||
| TNM staging | ||||
| Stage I | 137 | 10.35±3.50 | 0.553 | 0.576 |
| Stage II | 41 | 10.75±3.53 | ||
| Stage III | 6 | 11.65±3.38 | ||
| Smoking history | ||||
| N/A | 160 | 10.43±3.25 | 1.914 | 0.168 |
| I/A | 24 | 10.82±4.89 |
SPN, solitary pulmonary nodule; CTC, circulating tumor cell; N/A, not available; I/A, is available.
To assess the value of CTCs and other serum tumor biomarkers in diagnosing benign or malignant SPNs, binary logistic regression analysis was performed. Patients with benign or malignant SPNs were enrolled as dependent variables (patients with benign SPNs =0; patients with malignant SPNs =1), and CTCs, CEA, CA125, CA724, CYFRA21-1, and NSE were independent variables (negative result =0, positive result =1).
The omnibus tests of model coefficients (χ2=39.127, P<0.001), likelihood ratio test (maximum likelihood estimation =64.128), Cox & Snell R2 (0.201), Nagelkerke R2 (0.471), and Hosmer and Lemeshow test (χ2=8.027, P=0.117) all suggested that the current model was well fitted with a significant difference. They suggested that peripheral blood CTC level was an independent risk factor of diagnosing malignant SPNs (OR =3.698, 95% CI: 1.136–11.035, P=0.030) (Table 3).
Table 3. Logistic regression analysis of independent risk factors for malignant SPNs.
| Variables | B | SE | Wald | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| CTCs | 1.318 | 0.702 | 4.817 | 0.030 | 3.698 | 1.136–11.035 |
| CEA | 0.518 | 0.626 | 0.725 | 0.415 | 1.661 | 0.526–5.336 |
| CA125 | 0.221 | 0.359 | 0.624 | 0.234 | 1.567 | 0.367–4.267 |
| CA724 | 0.456 | 0.547 | 0.636 | 0.365 | 1.778 | 0.412–3.612 |
| CYFRA21-1 | 0.697 | 0.627 | 0.784 | 0.657 | 3.364 | 0.524–6.147 |
| NSE | 0.239 | 0.412 | 0.125 | 0.362 | 2.263 | 0.127–5.236 |
SPN, solitary pulmonary nodule; CTC, circulating tumor cell; CEA, carcinoembryonic antigen; CA125, carbohydrate antigen 125; CA724, carbohydrate antigen 724; CYFRA21-1, cytokeratin fragment 21-1; NSE, neuron-specific enolase.
Sensitivity and specificity of CTCs and serum tumor biomarkers in diagnosing benign or malignant SPNs
To assess the value of CTCs in diagnosing benign or malignant SPNs, patients with benign or malignant SPNs were recruited as a control and experimental group, respectively. ROC curves were constructed, with sensitivity (%) as the Y-axis, and 100-specificity (%) as the X-axis, and the AUC was calculated. For comparison, ROC curves were constructed based on serum tumor biomarkers in diagnosing benign or malignant SPNs.
It was shown that the sensitivity and specificity of CTCs in diagnosing malignant SPNs were 89.1% and 92.3%, respectively [AUC (95% CI) =0.907 (0.861–0.942)], which were both superior to those of a single serum tumor biomarker or their combination (Table 4 and Figures 2,3).
Table 4. Diagnostic values for malignant SPNs.
| Indicators | Cut-off value | Sensitivity% | Specificity % | AUC (95% CI) |
|---|---|---|---|---|
| CTCs | 8.7 | 89.1 | 92.3 | 0.907 (0.861–0.942) |
| CEA | 5 | 27.7 | 78.9 | 0.533 (0.435–0.632) |
| CA125 | 35 | 29.9 | 84.2 | 0.571 (0.476–0.665) |
| CA724 | 6 | 28.8 | 78.9 | 0.539 (0.441–0.637) |
| CYFRA21-1 | 3.07 | 30.4 | 81.6 | 0.560 (0.464–0.656) |
| NSE | 7 | 41.3 | 86.8 | 0.641 (0.553–0.728) |
| CEA + CA125 + CA724 + CYFRA21-1 + NSE | – | 59.2 | 76.3 | 0.678 (0.588–0.768) |
SPN, solitary pulmonary nodule; CTC, circulating tumor cell; CEA, carcinoembryonic antigen; CA125, carbohydrate antigen 125; CA724, carbohydrate antigen 724; CYFRA21-1, cytokeratin fragment 21-1; NSE, neuron-specific enolase.
Figure 2.
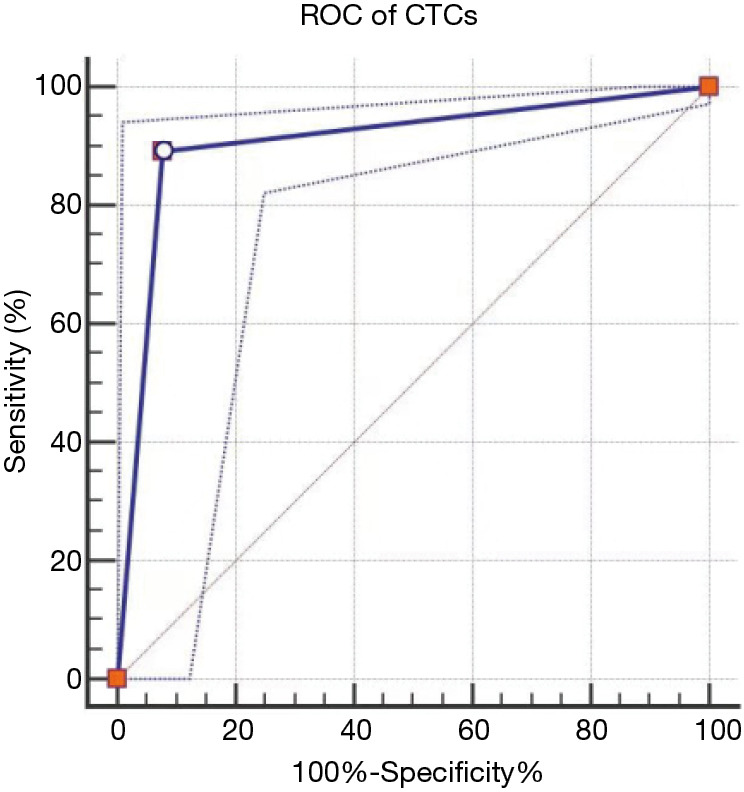
ROC curves of CTCs in diagnosing benign or malignant SPNs. ROC, receiver operating characteristic; CTC, circulating tumor cell; SPN, solitary pulmonary nodule.
Figure 3.
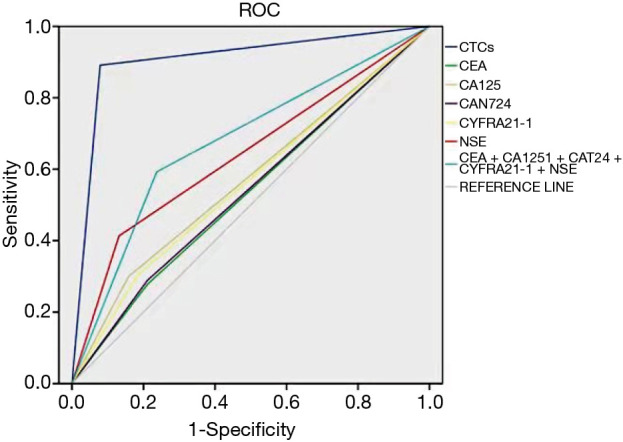
ROC curves of serum tumor biomarkers in diagnosing benign or malignant SPNs. ROC, receiver operating characteristic; CTC, circulating tumor cell; CEA, carcinoembryonic antigen; CA125, carbohydrate antigen 125; CA724, carbohydrate antigen 724; CYFRA21-1, cytokeratin fragment 21-1; NSE, neuron-specific enolase.
Discussion
At present, lung cancer can be screened mainly through cexfoliative cell examination of sputum (low sensitivity), chest X-ray examination (high false negative rate), LDCT (high false positive rate and high radiation exposure), and lung biopsy (invasive procedure, and the accuracy may be influenced by sample insufficiency) (7-12). In 2013, liquid biopsy was defined as a non-invasive procedure for early-stage cancer screening. It is also an auxiliary approach to develop therapeutic decisions for advanced cancer, featuring high accuracy, low sampling-related risk, less restriction by sample size, and simple preparation of samples (13-15). Blood samples are most commonly used in liquid biopsies. A wide range of biomarkers can be detected by liquid biopsy, including CTCs, circulating tumor DNAs (ctDNAs), exosomes, circulating tumor RNAs (ctRNAs), etc. Currently, only CTCs and ctDNAs detected by liquid biopsy are approved for clinical application (16).
The migration of CTCs into the bloodstream is affected by various factors, including anoikis, shear stress of blood flow, and immune elimination. As a result, CTCs remain at a low level (about 1 cell/mL of peripheral blood), which makes their detection challenging (17).
FR is a glycoprotein with a strong specificity to tissue and tumor, which is mainly distributed on the surface of cell membrane. It is highly expressed on tumor cell surfaces, but is barely or lowly expressed on normal cell surfaces (18,19). In 2013, Yue et al. (20) proposed LT-PCR, which is a novel method for detecting CTCs by specifically recognizing FR on the cell membrane. LT-PCR is being widely applied in clinical practice.
Some studies have pointed out the clinical value of CTCS in the treatment of lung cancer (21,22). In the present study, we detected significantly different CTC levels in patients with benign or malignant SPNs and healthy volunteers. The level of CTCs was markedly higher in the malignant SPNs group (10.48±3.49 FU/3 mL) than in the benign SPNs group (6.38±0.53 FU/3 mL) and the control group (4.45±1.21 FU/3 mL) (P<0.001). Moreover, the level of CTCs was significantly higher in the benign SPNs group than in the control group (P=0.023). In particular, in the malignant SPNs group, patients older than 60 years (11.45±3.92 FU/3 mL) presented a significantly higher CTC level than other patients (9.55±2.74 FU/3 mL). Classified by pathological subtypes of lung cancer, there was a significant difference in the CTC level among patients with squamous cell carcinoma (9.10±1.94 FU/3 mL), adenocarcinoma (10.77±3.71 FU/3 mL), and adenosquamous cell carcinoma (11.78±2.61 FU/3 mL). Binary logistic regression analysis suggested that CTCs were an independent risk factor of malignant SPNs (OR =3.698, 95% CI: 1.136–11.035, P=0.030). Compared with single or combined serum tumor biomarkers, the sensitivity and specificity of CTCs in the diagnosis of malignant SPNs were significantly higher [sensitivity =89.1%, specificity =92.3%, AUC (95% CI) =0.907 (0.861–0.942)]. Taken together, our results were consistent with previously reported findings (23,24).
There were several limitations in this study that should be noted. Firstly, this was a single-center study with a small sample size. Secondly, the bias in selecting participants may have influenced the results. A multi-center study with a large sample size is required in the future to validate our findings. Finally, this study is relatively limited. In the future, we will carry out researches on the prognosis evaluation, surgical effect evaluation, chemotherapy efficacy evaluation and targeted drug use guidance of CTCs in lung cancer to prove the clinical application value of CTCs.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by the Natural Science Foundation of Jiangsu Province (grant BK20191174), the National Natural Science Foundation of China (grant 81672281), and the National Key R&D Program of China (grant 2017YFC0114303).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University. All participants signed the informed consent. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013).
Footnotes
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at http://dx.doi.org/10.21037/atm-21-889
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-21-889
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-21-889). The authors have no conflicts of interest to declare.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.National Lung Screening Trial Research Team , Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pastorino U, Bellomi M, Landoni C, et al. Early lung-cancer detection with spiral CT and positron emission tomography in heavy smokers: 2-year results. Lancet 2003;362:593-7. 10.1016/S0140-6736(03)14188-8 [DOI] [PubMed] [Google Scholar]
- 4.Plaks V, Koopman CD, Werb Z. Circulating Tumor Cells. Science 2013;341:1186-8. 10.1126/science.1235226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiang AC, Massagué J. Molecular basis of metastasis. N Engl J Med 2008;359:2814-23. 10.1056/NEJMra0805239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2002;2:563-72. 10.1038/nrc865 [DOI] [PubMed] [Google Scholar]
- 7.Goebel C, Louden CL, Mckenna R, Jr, et al. Diagnosis of non-small cell lung cancer for early stage asymptomatic patients. Cancer Genomics Proteomics 2019;16:229-44. 10.21873/cgp.20128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calabrese F, Lunardi F, Pezzuto F, et al. Are there new biomarkers in tissue and liquid biopsies for the early detection of non-small cell lung cancer? J Clin Med 2019;8:414. 10.3390/jcm8030414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Memoli JS, Nietert PJ, Silvestri GA, et al. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest 2012;142:385-93. 10.1378/chest.11-1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradley SH, Kennedy MPT, Neal RD. Recognising lung cancer in primary care. Adv Ther 2019;36:19-30. 10.1007/s12325-018-0843-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu GX, Raz DJ. Lung Cancer Screening. Cancer Treat Res 2016;170:1-23. 10.1007/978-3-319-40389-2_1 [DOI] [PubMed] [Google Scholar]
- 12.Chabon JJ, Simmons AD, Lovejoy AF, et al. Circulating tumour DNA profiling reveals heterogeneity of EGFR inhibitor resistance mechanisms in lung cancer patients. Nat Commun 2016;7:11815. 10.1038/ncomms11815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alix-Panabières C, Pantel K. Circulating tumor cells: liquid biopsy of cancer. Clin Chem 2013;59:110-8. 10.1373/clinchem.2012.194258 [DOI] [PubMed] [Google Scholar]
- 14.Jiang P, Chan KCA, Lo YMD. Liver-derived cell-free nucleic acids in plasma: Biology and applications in liquid biopsies. J Hepatol 2019;71:409-21. 10.1016/j.jhep.2019.04.003 [DOI] [PubMed] [Google Scholar]
- 15.De Rubis G, Rajeev Krishnan S, Bebawy M. Liquid Biopsies in Cancer Diagnosis, Monitoring, and Prognosis. Trends Pharmacol Sci 2019;40:172-86. 10.1016/j.tips.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 16.Geeurickx E, Hendrix A. Targets, pitfalls and reference materials for liquid biopsy tests in cancer diagnostics. Mol Aspects Med 2020;72:100828. 10.1016/j.mam.2019.10.005 [DOI] [PubMed] [Google Scholar]
- 17.Kim HS, Lee KS, Ohno Y, et al. PET/CT versus MRI for diagnosis, staging, and follow-up of lung cancer. J Magn Reson Imaging 2015;42:247-60. 10.1002/jmri.24776 [DOI] [PubMed] [Google Scholar]
- 18.Parker N, Turk MJ, Westrick E, et al. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem 2005;338:284-93. 10.1016/j.ab.2004.12.026 [DOI] [PubMed] [Google Scholar]
- 19.He W, Kularatne SA, Kalli KR, et al. Quantitation of circulating tumor cells in blood samples from ovarian and prostate cancer patients using tumor-specific fluorescent ligands. Int J Cancer 2008;123:1968-73. 10.1002/ijc.23717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu Y, Chen Z, Dong J, et al. Folate receptor-positive circulating tumor cells as a novel diagnostic biomarker in non-small cell lung cancer. Transl Oncol 2013;6:697-702. 10.1593/tlo.13535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ulivi P. Non-Invasive methods to monitor mechanisms of resistance to tyrosine kinase inhibitors in Non-Small-Cell lung cancer: where do we stand? Int J Mol Sci 2016;17:1186. 10.3390/ijms17071186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Messaritakis I, Politaki E, Plataki M, et al. Heterogeneity of circulating tumor cells (CTCs) in patients with recurrent small cell lung cancer (SCLC) treated with pazopanib. Lung Cancer 2017;104:16-23. 10.1016/j.lungcan.2016.12.008 [DOI] [PubMed] [Google Scholar]
- 23.Xu Y, Qin T, Li J, et al. Detection of circulating tumor cells using negative enrichment immunofluorescence and an in situ hybridization system in pancreatic cancer. Int J Mol Sci 2017;18:622-30. 10.3390/ijms18040622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dorsey JF, Kao GD, Macarthur KM, et al. Tracking viable circulating tumor cells (CTCs) in the peripheral blood of non-small cell lung cancer (NSCLC) patients undergoing definitive radiation therapy: Pilot study results. Cancer 2015;121:139-49. 10.1002/cncr.28975 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


