Abstract
Background
Due to varying degrees of difficulty in obtaining different mesenchymal stem cells (MSCs), the distinct pain levels and treatment costs, and for providing concrete evidence for future clinical practice, a thorough comparison of all relevant MSCs remained critical. Hence, this study aimed to achieve this objective to compare the efficacy of MSCs obtained from different sources in clinical outcomes and cartilage repair of knee osteoarthritis (KOA).
Methods
The EmBase, PubMed and Cochrane Library databases were searched for eligible studies. Randomized controlled trials (RCTs) that compared MSCs from different sources with placebo or each other in KOA patients. Conventional meta-analysis and frequentist network meta-analysis (NMA) were conducted. The primary clinical outcome was pain relief. The frequentist NMA was conducted using Stata with the “network” command.
Results
Eight studies (seven trials) involving 203 KOA patients were included in this meta-analysis. The MSCs were considered superior over placebo for pain relief and improved function in KOA, but showed no statistically significant differences for cartilage regeneration. Among all the MSCs, the adipose tissue-derived MSCs (AD-MSCs) most effectively relieved pain.
Conclusion
These findings suggested that MSCs are effective in the treating of KOA. AD-MSCs might be the most effective for relieving pain, and Umbilical cord-derived mesenchymal stem cells (UC-MSCs) might be the most effective for improving function. However, the current evidence does not support the use of MSCs for improving cartilage repair in KOA patients.
Keywords: Relative efficacy, mesenchymal stem cells (MSCs), knee osteoarthritis (KOA), meta-analyses, randomized controlled trials
Introduction
Osteoarthritis (OA) is the most common degenerative disease, affecting a vast majority of patients aged over 65 years. The prevalence of OA is steadily rising, and these patients suffer from OA pain (1,2). According to the guidelines issued by the American College of Rheumatology, the treatment methods for OA are very limited. In addition to the preventive methods such as swimming or other aerobic exercises and weight loss, clinical treatments of OA are restricted to symptomatic managements such as the use of analgesics have limited efficacy and joint replacement after OA reaches mid-late stage of the patient (3). Currently, intra-articular injection has become an indispensable drug treatment strategy for OA. Compared with oral and intravenous administrations, intra-articular treatment involves topical administration with fewer side effects and higher drug concentrations (4).
Mesenchymal stem cells (MSCs) are pluripotent stem cells that have all the characteristics of stem cells, namely self-renewability and multi-directional differentiation. Several animal models and clinical studies have confirmed the therapeutic effects of MSCs in OA (5,6). However, these effects are not clearly defined. The overall quality of studies on MSCs in OA treatment has not been determined yet, and the risk of placebo effect when intra-articular injection is administered yielded controversial results with MSC treatment (7,8). Therefore, it is necessary to identify the efficacy of MSC injection in OA patients in order to provide evidence for clinical decision-making.
MSCs can be obtained from various sources, such as the bone marrow, adipose tissue, cord blood, etc. After in vitro amplification, the MSCs can be used for transplantation. However, the access methods used for acquiring MSCs from different sources are highly variable. Bone marrow derived mesenchymal stromal cells (BM-MSCs) are acquired by means of autologous and allogeneic transplantations. Allogenic BM-MSCs obtained from healthy donors are subjected to autologous MSC transplantation. However, autologous BM-MSC isolation requires bone marrow extraction surgery of the pelvic bone (iliac crest) prior to intra-articular injection of MSCs, which in turn increases the patient’s mental stress and financial burden. Isolation of adipose-derived mesenchymal stem cells (AD-MSCs) is considered to be relatively simple and safe. Adipose tissues of patients or healthy donors are obtained by liposuction from abdominal subcutaneous fat. Umbilical cord-derived mesenchymal stem cells (UC-MSCs) are the simplest to obtain, and require only healthy donors to provide postpartum placenta for isolation and amplification.
Several clinical trials have evaluated the efficacy and safety profiles of MSCs from different sources. MSCs are obtained from various sources, including the bone marrow, adipose tissue and cord blood. BM-MSCs are extracted from the bone marrow of the pelvic bone (iliac crest) of healthy donors. AD-MSCs are isolated from the adipose tissue after liposuction. UC-MSCs are easily accessible, and the whole umbilical cord might be a source of MSCs. Because the difficulty of obtaining these MSCs is inconsistent, and the pain suffered by patients is also inconsistent. Although all treatment methods are intra-articular injections, the additional economic burden and psychological burden are highly different. We indicate that MSC from different sources are different treatments, although they are all called “mesenchymal stem cells”. However, due to varying degrees of difficulty in obtaining different MSCs, the distinct pain levels and treatment costs, and for providing concrete evidence for future clinical practice, a thorough comparison of all relevant MSCs remained critical. Hence, this study aimed to achieve this objective by conducting conventional meta-analysis for verifying whether MSC is truly effective in the treatment of KOA and network meta-analysis (NMA) for finding the most effective one in KOA treatment from MSCs obtained from different sources.
We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/atm-20-5116).
Methods
Protocol and registration
The study is developed by the International Prospective Register of Systematic Reviews (PROSPERO) guidelines for reporting of systematic reviews. This systematic review with conventional and network meta-analyses is registered (CRD42019135946) with the PRISMA.
Literature search
The databases such as Embase, PubMed and Cochrane Library were searched for eligible studies from inception to April 5, 2019. A series of keywords and MeSH terms related to osteoarthritis, MSCs and randomized controlled trials were used to identify relevant RCTs that investigated the efficacy of MSCs in patients with KOA (Appendixes 1-7). The reference lists of all retrieved studies and reviews were also manually searched for any eligible articles.
Identification and selection of trials
The inclusion criteria were as follows: (I) studies with RCT design; (II) patients with KOA; (III) studies that compare MSCs from different sources with placebo or each other; and (IV) trials reporting pain, function or cartilage regeneration outcomes. The exclusion criteria were as follows: (I) studies that combined MSCs with other intra-articular injection; and (II) studies with only abstract available (insufficient data).
Two investigators (ZJ Wei and QQ Wang) independently screened all the identified studies according to the above mentioned inclusion and exclusion criteria. Any inconsistencies were resolved by reaching consensus.
Quality assessment
The Cochrane risk of bias assessment tool was used to determine the methodological quality of the identified RCTs. A total of six domains were evaluated, which included the random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias) and selective reporting (reporting bias). Each trial was attributed with a low risk of bias, unclear risk of bias or high risk of bias in each domain.
Endpoints
The prespecified primary outcome was pain relief. If multiple pain scales were reported, VAS score remained the first choice. The secondary choice was Western Ontario and McMaster Universities Osteoarthritis Index score-pain subscore (WOMAC-pain). The change-from-baseline score during the last follow-up period was used as the pain relief score.
The secondary outcome was physical function. Western Ontario and McMaster Universities Osteoarthritis Index score-physical function subscore (WOMAC-PF) was used for assessing functional improvement. If no WOMAC-PF scores were reported by studies, then Western Ontario and McMaster Universities Osteoarthritis Index score-general score (WOMAC-general), the Lequesne Index or another functional measurement scale were used. The change-from-baseline score during the last follow-up was used as the physical function score.
The third outcome was cartilage regeneration. The Whole-Organ Magnetic Resonance Imaging score (WORMS) was used for assessing cartilage regeneration. If no WORMS has been reported, then quantitative MRI or another structural assessment scale was used. The change-from-baseline score during the last follow-up period was used as the cartilage regeneration score.
For studies that involve multiple treatment groups with different doses of MSCs from the same source, the data were combined into one treatment group. The differences between two treatment arms were assessed. As different scales were used to evaluate the same outcome, the SMD was calculated.
Data extraction
Two investigators (ZJ Wei and QQ Wang) reviewed the full-texts of the eligible studies and extracted the data such as study design, trial size, inclusion/exclusion criteria, details of intervention (number of cells, source, delivery method and treatment duration), patient characteristics, clinical outcome measurement method (VAS score, WOMAC-pain, WOMAC-PF, WOMAC-general, WORMS and Quantitative MRI) and their scores (pre- vs. post-operative).
Statistical analysis
A conventional meta-analysis was used to compare the overall MSCs with placebo (in RCTs). Heterogeneity of the effect size across the studies was tested by Q statistics (P<0.05 was considered to be heterogeneous) and I-square statistics (I-square >50% was considered to be heterogeneous). Publication bias was evaluated by Begg’s test, along with funnel plot. In case of significant heterogeneity between studies, the random-effects model was used; otherwise, a fixed-effects model was employed. To determine the most effective MSCs, three subgroup analyses of different tissue sources, autologous or allogeneic, or combined were performed.
Subgroup analysis A included MSCs from different tissues (UC-MSC, BM-MSC and AD-MSC) versus placebo was conducted. Subgroup analysis B included MSCs from autologous or allogenic versus placebo was conducted. Subgroup analysis C included MSCs from different sources (UC-MSCs-allogenic, BM-MSCs-allogenic, BM-MSCs-autologous, AD-MSCs-allogenic and AD-MSCs-autologous) versus placebo was conducted.
The estimates were combined using frequentist NMAs. The frequentist effect size (ES) and associated 95% confidence intervals (CIs) were calculated. The frequentist NMA was conducted using Stata with the “network” command. The overall effect sizes (WMDs or SMDs) were calculated by dividing the differences in mean values between the treatment groups in a specific time window by the median pooled SD across all timepoints in a trial. When the SD values were not provided, the overall effect sizes were calculated from SEs or CIs. If the SDs remained unchanged from the given baseline value in an included study, they were calculated according to the Cochrane guidelines (9). In these imputations, we assumed a correlation coefficient of “r=0.5” between the baseline and follow-up periods. If only a sample size, median, range and/or IQR could be extracted from a trial, then the methodology from Wan et al. (10) was used to calculate the sample mean and SD. All effect sizes were pooled by Hedges’ g in order to adjust for trials with a small sample size (11). The goodness of fit model was assessed by calculating the consistency of the network (differences in the effect sizes derived from direct and indirect comparisons). To examine the data for small study effects, the comparison-adjusted funnel plots were generated. For trials containing three or more treatment arms, inconsistency was defined by the differences between direct and indirect effect estimates for the same comparison. The fit of the model with the data was measured by calculating the posterior mean residual deviance. The treatments were ranked based on efficacy, and the probabilities of the surface under the cumulative ranking curves (SUCRAs) were determined. The rankings of all the evaluated treatments were based on the effect levels according to posterior probabilities. SUCRAs could explain the outcome percentages relative to an ideal treatment, which always ranked first. Thus, the greater the SUCRA score, the more effective the MSCs were.
All statistical analyses were conducted using STATA software (V.15.1, Stata, College Station, Texas, USA) and Review Manager 5.3 software (RevMan 5.3, The Cochrane Collaboration, Oxford, UK).
Results
Characteristics of eligible studies
A total of 842 studies were retrieved from the databases, and 8 studies of these were considered eligible for inclusion in this meta-analysis (12-19). However, the only difference between the two studies (15,16) conducted by Lamo-Espinosa et al. was the follow-up time, and so we included them in the analysis as a single study. Moreover, the pain relief and functional improvement data from high dose BM-MSCs in Gupta et al. study could not be extracted. Finally, 7 studies including 203 KOA patients were enrolled in this meta-analysis. The results of literature search and reasons for exclusion are illustrated in Figure 1. All trials were published as full-text articles. Four trials compared BM-MSCs and placebo, one trial compared UC-MSCs and placebo, and two trials compared AD-MSCs and placebo. The characteristics of the included trials are presented in Table 1.
Figure 1.
Systematic review flow diagram.
Table 1. Characteristics of the included comparisons in RCTs and the results of conventional meta-analysis.
| First author/year/country | K-L grade |
No. of cases (MSC/CON) | Mean age (SD) |
n (%) females | Entity of cells |
No. of cells |
Source | Delivery method | Placebo | FU periods (months) | Pain scale | Function scale |
Structural assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vega, 2015, Spain | 2–4 | 30 (15/15) | MSC: 56.6 (9.6); CON: 57.3 (9.4) | MSC: 60%; CON: 67% | BM-MSCs | 4×107 | Allogenic | 1-stage injection | HA | 12 | VAS | WOMAC-global | NR |
| Gupta, 2016, India | 2–3 | 60 (40/20) | MSC: 57.7 (8.6); CON: 54.9 (8.3) | MSC: 75%; CON: 100% | BM-MSCs | (2.5–15)×107 | Allogenic | 1-stage injection | HA | 12 | VAS | WOMAC-PF | WORMS |
| Lamo-Espinosa, 2016, 2018, Spain | 2–4 | 30 (20/10) | MSC: 61.6 (8.3); CON: 58.7 (5.2) | MSC: 40%; CON: 30% | BM-MSCs | (1, 10)×107 | Autologous | 2-stage injection | HA | 12 | VAS | WOMAC-PF | WORMS |
| Emadedin, 2018, Iran | 2–4 | 43 (19/24) | MSC: 51.7 (9.2); CON: 54.7 (5.3) | MSC: 37.5%; CON: 36.8% | BM-MSCs | 4×107 | Autologous | 1-stage injection | NS | 6 | VAS | WOMAC-PF | NR |
| Kuah, 2018, Australia | 1–3 | 20 (16/4) | MSC: 52.9 (6.5); CON: 55.0 (10.4) | MSC: 31%; CON: 75% | AD-MSCs | (3.9, 6.7)×106 | Allogenic | 1-stage injection | NR | 12 | VAS | WOMAC-PF | cartilage defect in MRI |
| Matas, 2019, Chile | 1–3 | 26 (18/8) | MSC: 56.4 (5.5); CON: 54.8 (4.5) | MSC: 61%; CON: 63% | UC-MSCs | (2, 4)×107 | Allogenic | 1, 2-stage injection | HA | 12 | VAS | WOMAC-PF | WORMS |
| Lee, 2019, South Korea | 2–4 | 24 (12/12) | MSC: 62.2 (6.5); CON: 63.2 (4.2) | MSC: 75%; CON: 75% | AD-MSCs | 1×108 | Autologous | 1-stage injection | NS | 6 | VAS | WOMAC-PF | cartilage defect in MRI |
CON, control group; FU, follow-up; No. of cells, number of injected cells; No. pts, number of participants included; NR, not reported; RCTs, randomized controlled trials; SD, standard deviation; HA, hyaluronic acid; NS, normal saline; UC-MSC, umbilical cord-derived mesenchymal stromal cells; AD-MSCs, adipose tissue-derived mesenchymal stromal cells; BM-MSCs, bone marrow-derived mesenchymal stromal
Publication bias
Bias assessment was evaluated for all the included trials in Supplementary file (Appendixes 1-7). Begg’s test was performed to evaluate publication bias and revealed no statistical significance. The funnel plots are shown in Supplementary file (Appendixes 1-7).
Effects on pain relief
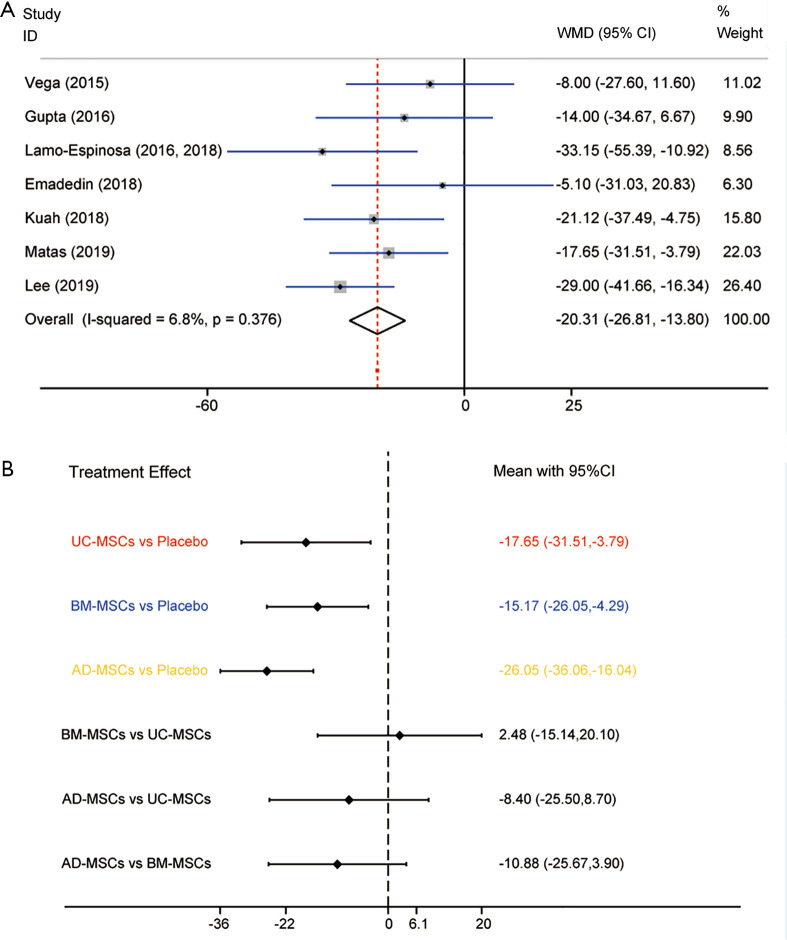
In conventional meta-analysis, the effects on pain relief in 7 RCTs involving the 3 MSC injections (UC-MSCs, BM-MSCs and AD-MSCs) were analyzed (Appendixes 1-7). Pain scale of all trials was assessed using VAS score. Comparison of MSCs from different tissues with placebo during the last follow-up period showed that the scores of overall MSCs were superior to placebo with regard to pain relief (WMD =−20.31, 95% CI: −26.81 to −13.80, P<0.001) in KOA patients (Figure 2A). Heterogeneity was analyzed by comparing the effects of pain relief (I-squared =6.8%, P=0.376), and so fixed-effects model was used. Obviously, there was no evidence showing heterogeneity in the pooled results for the direct comparison of efficacy.
Figure 2.
Meta-analysis of treatment effects on pain relief. (A) Conventional meta-analysis of treatment effects on pain relief for MSC from different sources overall compared with placebo in RCTs; (B) network meta-analysis of treatment effects on pain relief for MSC from different sources overall compared with placebo in RCTs. MSC, mesenchymal stem cell; RCT, randomized controlled trial.
Three subgroup analyses from different tissue sources were performed, autologous or allogeneic, or combined with them. Subgroup analysis A showed statistically significant differences in UC-MSCs (P=0.013), BM-MSCs (P=0.006) and AD-MSCs (P<0.001). Subgroup analysis B showed statistically significant differences in autologous MSCs (P<0.001) and allogeneic MSCs (P<0.001). Subgroup analysis C showed statistically significant differences in UC-MSCs-allogeneic (P=0.013), BM-MSCs-autologous (P=0.014), AD-MSCs-autologous (P=0.011) and AD-MSCs-allogeneic (P<0.001), except BM-MSCs-allogeneic.
In NMA, UC-MSCs, BM-MSCs and AD-MSCs were all highly effective when compared to placebo in achieving the treatment target. AD-MSCs were considered to be the most effective for pain relief (WMD =−26.05, 95% CI: −36.06 to −16.04, P<0.001). The WMDs from high to low were AD-MSCs, UC-MSCs, BM-MSCs and placebo (Figure 2B). The detailed results are illustrated in Table 2 and the rankings based on SUCRA are presented in Supplementary file (Appendixes 1-7). AD-MSCs have the largest probability of being the best treatment option for pain relief (SUCRA =92.0%). Structure of network formed by interventions was shown in Figure 3.
Table 2. Summary of the results of the effect sizes of MSC treatment on pain relief.
| First author/year | MSC | Source | Conventional meta-analysis | Network meta-analysis | Conventional meta-analysis | Network meta-analysis | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WMD (95% CI) |
Weight (%) | Subgroup analysis | WMD (95% CI) | P value | SUCRA [0-100] |
Subgroup analysis | WMD (95% CI) | P value | SUCRA [0-100] | ||||||||||
| WMD (95% CI) | P value | Weight (%) |
WMD (95% CI) | P value | Weight (%) | ||||||||||||||
| Vega/2015 | BM-MSCs | Allogenic | −8.00 (−27.60, 11.60) | 11.02 | −15.17 (−26.05, −4.29) | 0.006 | 35.77 | −15.17 (−26.05, −4.29) | 0.006 | 48.4 | −10.84 (−25.06, 3.38) | 0.135 | 20.92 | −10.88 (−27.01, 5.26) | 0.187 | 33.7 | |||
| Gupta/2016 | −14.00 (−34.67, 6.67) | 9.90 | |||||||||||||||||
| Lamo-Espinosa/2016, 2018 | Autologous | −33.15 (−55.39, −10.92) | 8.56 | −21.26 (−38.14, −4.39) | 0.014 | 14.85 | −20.91 (−39.78, −2.04) | 0.030 | 62.4 | ||||||||||
| Emadedin/2018 | −5.10 (−31.03, 20.83) | 6.30 | |||||||||||||||||
| Kuah/2018 | AD-MSCs | Allogenic | −21.12 (−37.49, −4.75) | 15.80 | −26.05 (−36.06, −16.04) | <0.001 | 42.20 | −26.05 (−36.06, −16.04) | <0.001 | 92.0 | −21.12 (−37.49, −4.75) | 0.014 | 15.80 | −21.12 (−40.70, −1.54) | 0.035 | 63.0 | |||
| Lee/2019 | Autologous | −29.0 (−41.66, −16.34) | 26.40 | -29.0 (−41.66, −16.34) | 0.011 | 26.40 | −29.0 (−45.61, −12.39) | 0.001 | 84.8 | ||||||||||
| Matas/2019 | UC-MSCs | Allogenic | −17.65 (−31.51, −3.79) | 22.03 | −17.65 (−31.51, −3.79) | 0.013 | 22.03 | −17.65 (−31.51, −3.79) | 0.013 | 59.4 | −17.65 (−31.51, −3.79) | 0.013 | 22.03 | −17.65 (−31.19, −0.11) | 0.049 | 53.1 | |||
Figure 3.
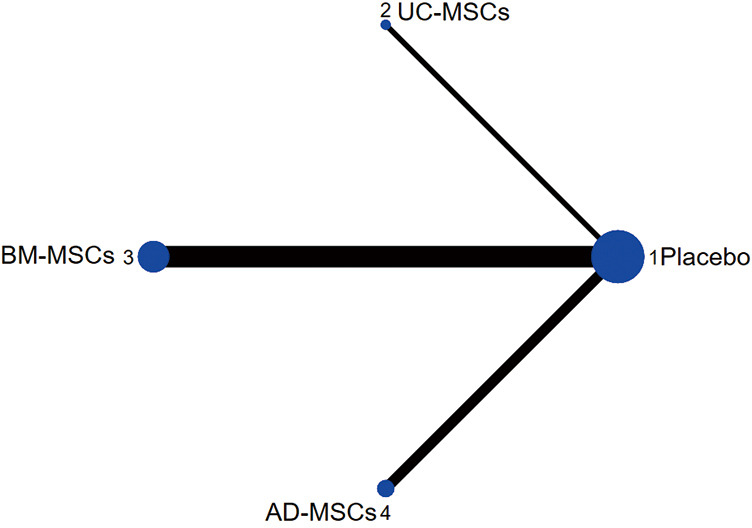
Structure of network formed by interventions.
Secondary NMA of MSCs from different tissue sources and combined with autologous or allogeneic was performed. This NMA further indicated statistically significant differences. AD-MSCs-autologous exhibited the largest effect for pain relief (WMD =−29.00, 95% CI: −45.61 to −12.39, P<0.001), and the WMDs from high to low were AD-MSCs-autologous, AD-MSCs-allogenic, BM-MSCs-autologous, UC-MSCs-allogenic, BM-MSCs-allogenic and placebo. AD-MSCs-autologous have the largest probability of being the best treatment option for pain relief (SUCRA =84.8%). The rankings based on SUCRA are shown in Supplementary file (Appendixes 1-7).
Effects on functional improvement
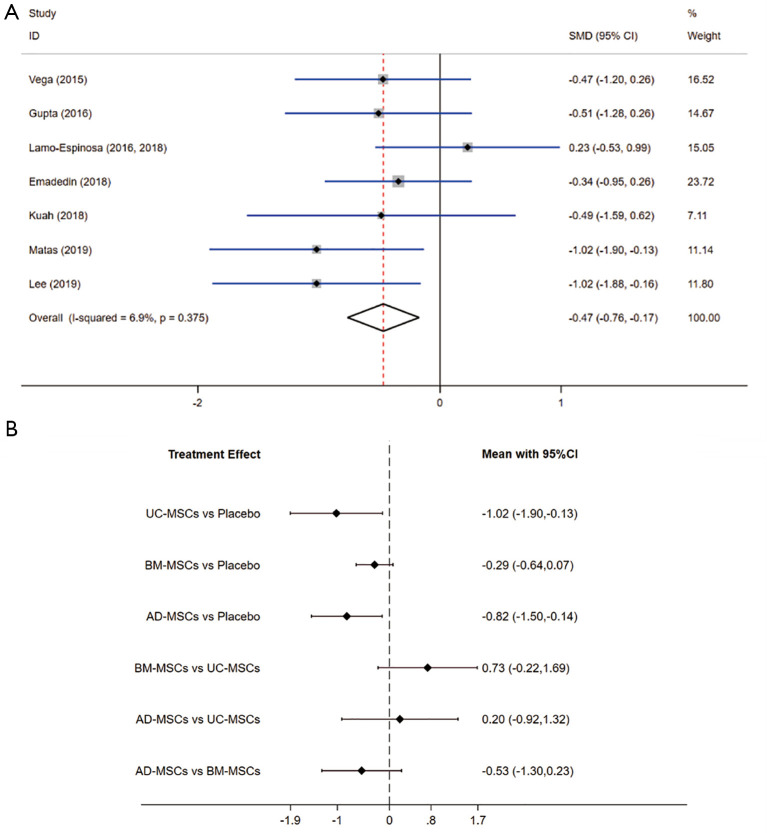
In conventional meta-analysis, the effects on functional improvement in 7 RCTs involving all the three MSC injections were analyzed. Function scale of all trials included WOMAC-PF, but Vega 2015 used WOMAC-global score. Comparison of MSCs from different tissues with placebo during the last follow-up period showed that the overall MSCs were superior to placebo for functional improvement (SMD =−0.47, 95% CI: −0.76 to −0.17, P=0.002) in KOA patients (Figure 4A). Heterogeneity was analyzed by comparing the effects on functional improvement (I-squared =6.9%, P=0.375), and so the fixed-effects model was used. The results showed no significant heterogeneity in the pooled results that directly compared efficacy.
Figure 4.
Meta-analysis of treatment effects on functional improvement. (A) Conventional meta-analysis of treatment effects on functional improvement for MSC from different sources overall compared with placebo in RCTs; (B) network meta-analysis of treatment effects on functional improvement for MSC from different sources overall compared with placebo in RCTs. MSC, mesenchymal stem cell; RCT, randomized controlled trial.
Subgroup analysis A showed statistically significant differences on UC-MSCs (P=0.024) and AD-MSCs (P=0.018). Subgroup analysis B showed statistically significant differences on allogeneic MSCs (P=0.005). Subgroup analysis C showed statistically significant differences on UC-MSCs-allogeneic (P=0.024), and AD-MSCs-autologous (P=0.020). The results of SMD (95% CI) were shown in Table 3.
Table 3. Summary of the results of the effect sizes of MSC treatment on functional improvement.
| First author/year | MSC | Source | Conventional meta-analysis | Network meta-analysis | Conventional meta-analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SMD (95% CI) | Weight (%) | Subgroup analysis | SMD (95% CI) | P value | SUCRA [0–100] | Subgroup analysis | |||||||
| SMD (95% CI) | P value | Weight (%) | WMD (95% CI) | ||||||||||
| Vega/2015 | BM-MSCs | Allogenic | −0.47 (−1.20 to 0.26) | 16.52 | −0.29 (−0.64, 0.07) | 0.113 | 69.96 | −0.29 (−0.64, 0.07) | 0.113 | 36.4 | −0.49 (−1.02, 0.04) | ||
| Gupta/2016 | −0.51 (−1.28 to 0.26) | 14.67 | |||||||||||
| Lamo-Espinosa/2016, 2018 | Autologous | 0.23 (−0.53 to 0.99) | 15.05 | −0.12 (−0.60, 0.35) | |||||||||
| Emadedin/2018 | −0.34 (−0.95 to 0.26) | 23.72 | |||||||||||
| Kuah/2018 | AD-MSCs | Allogenic | −0.49 (−1.59 to 0.62) | 7.11 | −0.82 (−1.50, −0.14) | 0.018 | 18.91 | −0.82 (−1.50, −0.14) | 0.018 | 75.6 | −0.49 (−1.59 to 0.62) | ||
| Lee/2019 | Autologous | −1.02 (−1.88 to −0.16) | 11.8 | −1.02 (−1.88 to −0.16) | |||||||||
| Matas/2019 | UC-MSCs | Allogenic | −1.02 (−1.90 to −0.13) | 11.14 | −1.02 (−1.90 to −0.13) | 0.024 | 11.14 | −1.02 (−1.90 to −0.13) | 0.024 | 85.3 | −1.02 (−1.90 to −0.13) | ||
The results showed in that UC-MSCs and AD-MSCs were highly effective when compared to placebo in achieving treatment target. UC-MSCs were considered to be the most effective cells for functional improvement (SMD =−1.02, 95% CI: −1.90 to −0.13, P=0.024). The SMD from high to low were UC-MSCs, AD-MSCs, BM-MSCs and placebo. The detailed results are illustrated in Table and the rankings based on SUCRA are shown in SA. UC-MSCs have the largest probability of being the best treatment option for pain relief (SUCRA =85.3%).
Secondary NMA of MSC from different tissue sources and combined with autologous or allogeneic was performed. This NMA further indicated statistically significant differences (Figure 4B). AD-MSCs-autologous exhibited the largest effect for functional improvement (SMD =−1.02, 95% CI: −1.88 to −0.16, P=0.024), the SMD from high to low were AD-MSCs-autologous, UC-MSCs-allogenic, BM-MSCs-allogenic, AD-MSCs-allogenic, BM-MSCs-autologous and placebo. AD-MSCs- autologous have the largest probability of being the best treatment option for pain relief (SUCRA =81.8%). The rankings based on SUCRA are shown in Supplementary file (Appendixes 1-7).
Comparisons between the effects of pain and function
The results from conventional and network meta-analyses are presented in Tables 2 and 3. The results were generally consistent, and showed no significant differences in WMDs and corresponding 95% CIs on pain relief. The node splitting method could not be performed due to the absence of closed loop in the data. Furthermore, AD-MSC and UC-MSC injections have demonstrated beneficial effects in pain relief and functional improvement. After autologous or allogeneic conditions were added, the autologous AD-MSCs became the most effective treatment option for MSC injection.
Effects on structural assessment by MRI
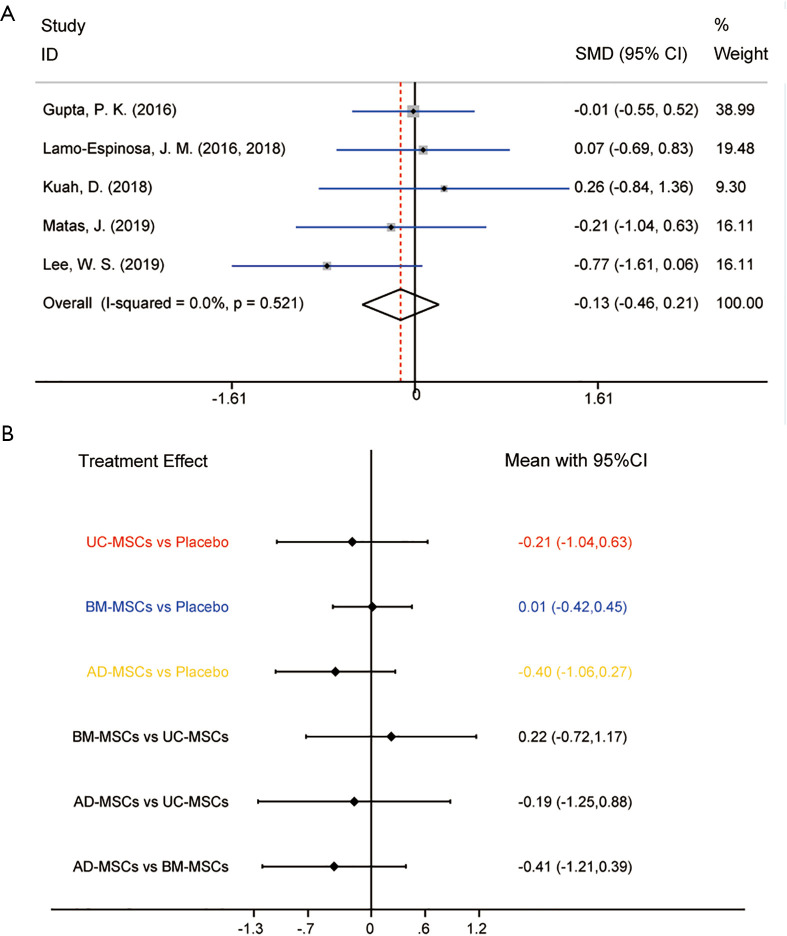
In conventional meta-analysis, the effects on cartilage regeneration in 5 RCTs involving all three MSC injections were analyzed (Appendixes 1-7). Regeneration scale of trials was evaluated by WORMS, but Kuah 2018 and Lee 2019 analyzed the changes in cartilage defects by MRI. Comparison of MSCs from different tissues with placebo during the last follow-up showed that the overall MSCs had fewer changes when compared with placebo for cartilage regeneration (SMD =-0.13, 95% CI: −0.46 to 0.21, P=0.4622) in KOA. Heterogeneity was analyzed by comparing the effects on cartilage regeneration (I-squared =0.0%, P=0.521), and so the fixed-effects model was used (Figure 5A). The results showed no evidence of heterogeneity in the pooled results for direct comparison of efficacy.
Figure 5.
Meta-analysis of treatment effects on structural assessment. (A) Conventional meta-analysis of treatment effects on structural assessment for MSC from different sources overall compared with placebo in RCTs; (B) network meta-analysis of treatment effects on structural assessment for MSC from different sources overall compared with placebo in RCTs. MSC, mesenchymal stem cell; RCT, randomized controlled trial.
Subgroup analyses A, B and C showed no statistically significant differences on all types of MSCs. Although the p value of AD-MSCs-autologous showed no statistically significant difference, the SMDs and corresponding 95% CIs of AD-MSCs-autologous were superior to placebo for cartilage repair.
Pooled SMDs and 95% CIs regarding the efficacy of MSC injections from different tissues in NMA are shown in Figure 5B. The SMDs from high to low were AD-MSCs, UC-MSCs, placebo and BM-MSCs. The detailed results and the rankings based on SUCRA are shown in Supplementary file (Appendixes 1-7). AD-MSCs have the largest probability of being the best treatment option for cartilage repair (SUCRA =78.2%).
Discussion
This was the first NMA conducted with all the available RCTs that directly compared MSCs from different sources in KOA patients, which increased the reliability of the findings. There were only 7 trials for direct comparison through NMA. The main findings were as follows: (I) 3 MSC types (UC-MSCs, BM-MSCs and AD-MSCs) significantly alleviated OA symptoms; (II) AD-MSCs were the most effective among all the MSCs for pain relief, whereas UC-MSCs were the most effective for functional improvement; (III) autologous MSCs were considered superior to allogeneic counterparts for pain relief, and allogeneic BM-MSCs were superior to autologous counterparts for functional improvement; (IV) autologous AD-MSCs showed more beneficial effects when compared to other MSCs for relieving pain and improving function among KOA patients; (V) intra-articular administration of MSCs showed insufficient clinical evidence for improving cartilage repair in KOA.
No direct or indirect (via NMA) evidence regarding the relative efficacy of MSCs from different sources has been previously published. Some previous meta-analyses studies indicated that MSC injection in KOA patients showed no significant pain relieving effect (20,21), while few other meta-analyses studies showed that treatment with MSCs significantly alleviated pain in OA patients (22-24). However, the present study showed that the MSCs were superior over placebo for pain relief (WMD =−20.31, 95% CI: −26.81 to −13.80, P<0.001), which might be explained by the recently published high quality RCTs. The novel findings of this study were that the AD-MSCs were used for effective pain relief; and WMDs from high to low were obtained for AD-MSCs, UC-MSCs, BM-MSCs and placebo. This may be due to that the AD-MSCs were less dependent on mitochondrial respiration for energy production. In addition, AD-MSCs have lower expression levels of human leukocyte antigen class I and higher immunosuppression capacity (25). Subgroup analysis indicated that autologous MSCs were superior over allogenic counterparts for pain relief, and this might be due to better biocompatibility and no possibility of immune rejection of autologous MSCs.
MSCs are obtained from various sources, including the bone marrow, adipose tissue and cord blood. BM-MSCs are extracted from the bone marrow of the pelvic bone (iliac crest) of healthy donors. This is an invasive procedure that requires anesthesia and involves the risk of nosocomial infection, increasing the patient’s mental stress as well as financial burden. AD-MSCs are isolated from the adipose tissue after liposuction. UC-MSCs are easily accessible, and the whole umbilical cord might be a source of MSCs. The objective of this study was to compare MSCs from different sources to identify those cells with the most pronounced effects for treating KOA patients. Nowadays, adipose tissue has been increasingly used as a source of MSCs, primarily because of its natural abundance of MSCs and requires less invasive surgical procedures for extraction, making it an ideal choice for clinical use. This study provided evidence that the adipose tissue is an ideal treatment option for KOA patients through conventional meta-analysis and NMAs of RCTs.
Nevertheless, this study has several limitations. Firstly, only 4 trials included multiple treatment groups with different doses of MSCs from the same source, and the data were combined as one treatment group. This might reduce the effects of heterogeneity, resulting in a minor bias in this analysis. Secondly, some estimated NMA data relied on indirect comparisons. However, our results were obtained only by direct comparisons, and inconsistency analysis could not be performed. Thirdly, this analysis included only 7 studies, in which all the RCTs with low bias, high quality and credibility were assessed, and provided sufficient evidence.
Conclusions
This NMA identified 7 RCTs that compared the effect of MSCs isolated from different sources. Overall, administration of MSCs resulted in reduced pain and improved function in KOA patients. AD-MSCs were considered as the most effective treatment option for pain relief, while UC-MSCs were the most effective option for improving function. Autologous AD-MSCs showed more beneficial effects when compared to other MSCs for relieving pain and improving function. Current evidence does not support the use of MSCs for improving cartilage repair in KOA patients. There are few high-quality RCTs in which MSCs are used for the treatment of KOA. Therefore, further RCTs are needed to assure optimal use of MSCs for OA treatment.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (Grant No. 81330043) and Beijing Municipal Health Commission (Grant No. BMHC-2019-9 and BMHC-2018-4).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-5116
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-5116). The authors have no conflicts of interest to declare.
References
- 1.Prieto-Alhambra D, Judge A, Javaid MK, et al. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann Rheum Dis 2014;73:1659-64. 10.1136/annrheumdis-2013-203355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbour KE, Helmick CG, Boring M, et al. Vital Signs: Prevalence of Doctor-Diagnosed Arthritis and Arthritis-Attributable Activity Limitation - United States, 2013-2015. MMWR Morb Mortal Wkly Rep 2017;66:246-53. 10.15585/mmwr.mm6609e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Childs BG, Gluscevic M, Baker DJ, et al. Senescent cells: an emerging target for diseases of ageing. Nat Rev Drug Discov 2017;16:718-35. 10.1038/nrd.2017.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McIntyre JA, Jones IA, Han B, et al. Intra-articular Mesenchymal Stem Cell Therapy for the Human Joint: A Systematic Review. Am J Sports Med 2018;46:3550-63. 10.1177/0363546517735844 [DOI] [PubMed] [Google Scholar]
- 5.Barry F, Murphy M. Mesenchymal stem cells in joint disease and repair. Nat Rev Rheumatol 2013;9:584-94. 10.1038/nrrheum.2013.109 [DOI] [PubMed] [Google Scholar]
- 6.Mamidi MK, Das AK, Zakaria Z, et al. Mesenchymal stromal cells for cartilage repair in osteoarthritis. Osteoarthritis Cartilage 2016;24:1307-16. 10.1016/j.joca.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 7.Orozco L, Munar A, Soler R, et al. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: two-year follow-up results. Transplantation 2014;97:e66-8. 10.1097/TP.0000000000000167 [DOI] [PubMed] [Google Scholar]
- 8.Chahla J, Piuzzi NS, Mitchell JJ, et al. Intra-Articular Cellular Therapy for Osteoarthritis and Focal Cartilage Defects of the Knee: A Systematic Review of the Literature and Study Quality Analysis. J Bone Joint Surg Am 2016;98:1511-21. 10.2106/JBJS.15.01495 [DOI] [PubMed] [Google Scholar]
- 9.Higgins JPT DJ, Altman DG. Cochrane handbook for systematic reviews of interventions. Chapter 16: special topics in statistics. Chichester, UK: John Wiley & Sons, 2008. [Google Scholar]
- 10.Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borenstein M. The handbook of research synthesis and meta-analysis. Effect sizes for continuous data. New York: Russell Sage Foundation, 2009. [Google Scholar]
- 12.Emadedin M, Labibzadeh N, Liastani MG, et al. Intra-articular implantation of autologous bone marrow-derived mesenchymal stromal cells to treat knee osteoarthritis: a randomized, triple-blind, placebo-controlled phase 1/2 clinical trial. Cytotherapy 2018;20:1238-46. 10.1016/j.jcyt.2018.08.005 [DOI] [PubMed] [Google Scholar]
- 13.Gupta PK, Chullikana A, Rengasamy M, et al. Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel(R)): preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res Ther 2016;18:301. 10.1186/s13075-016-1195-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuah D, Sivell S, Longworth T, et al. Safety, tolerability and efficacy of intra-articular Progenza in knee osteoarthritis: a randomized double-blind placebo-controlled single ascending dose study. J Transl Med 2018;16:49. 10.1186/s12967-018-1420-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamo-Espinosa JM, Mora G, Blanco JF, et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: long-term follow up of a multicenter randomized controlled clinical trial (phase I/II). J Transl Med 2018;16:213. 10.1186/s12967-018-1591-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamo-Espinosa JM, Mora G, Blanco JF, et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: multicenter randomized controlled clinical trial (phase I/II). J Transl Med 2016;14:246. 10.1186/s12967-016-0998-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee WS, Kim HJ, Kim KI, et al. Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl Med 2019;8:504-11. 10.1002/sctm.18-0122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matas J, Orrego M, Amenabar D, et al. Umbilical Cord-Derived Mesenchymal Stromal Cells (MSCs) for Knee Osteoarthritis: Repeated MSC Dosing Is Superior to a Single MSC Dose and to Hyaluronic Acid in a Controlled Randomized Phase I/II Trial. Stem Cells Transl Med 2019;8:215-24. 10.1002/sctm.18-0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vega A, Martin-Ferrero MA, Del Canto F, et al. Treatment of Knee Osteoarthritis With Allogeneic Bone Marrow Mesenchymal Stem Cells: A Randomized Controlled Trial. Transplantation 2015;99:1681-90. 10.1097/TP.0000000000000678 [DOI] [PubMed] [Google Scholar]
- 20.Xia P, Wang X, Lin Q, et al. Efficacy of mesenchymal stem cells injection for the management of knee osteoarthritis: a systematic review and meta-analysis. Int Orthop 2015;39:2363-72. 10.1007/s00264-015-2785-8 [DOI] [PubMed] [Google Scholar]
- 21.Xu S, Liu H, Xie Y, et al. Effect of mesenchymal stromal cells for articular cartilage degeneration treatment: a meta-analysis. Cytotherapy 2015;17:1342-52. 10.1016/j.jcyt.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 22.Cui GH, Wang YY, Li CJ, et al. Efficacy of mesenchymal stem cells in treating patients with osteoarthritis of the knee: A meta-analysis. Exp Ther Med 2016;12:3390-400. 10.3892/etm.2016.3791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yubo M, Yanyan L, Li L, et al. Clinical efficacy and safety of mesenchymal stem cell transplantation for osteoarthritis treatment: A meta-analysis. PLoS One 2017;12:e0175449. 10.1371/journal.pone.0175449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SH, Ha CW, Park YB, et al. Intra-articular injection of mesenchymal stem cells for clinical outcomes and cartilage repair in osteoarthritis of the knee: a meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg 2019;139:971-80. 10.1007/s00402-019-03140-8 [DOI] [PubMed] [Google Scholar]
- 25.Zhou W, Lin J, Zhao K, et al. Single-Cell Profiles and Clinically Useful Properties of Human Mesenchymal Stem Cells of Adipose and Bone Marrow Origin. Am J Sports Med 2019;47:1722-33. 10.1177/0363546519848678 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as