Abstract
Hypoxemia is a frequent and potentially fatal complication occurring in patients during gastrointestinal endoscopy. The administration of propofol sedation increases the risk of most complications, especially hypoxemia. Nevertheless, propofol has been increasingly used in the United States, and the trend is likely to increase in the years to come. Patient satisfaction and endoscopist satisfaction along with rapid turnover are some of the touted reasons for this trend. However, propofol sedation generally implies deep sedation or general anesthesia. As a result, hypopnea and apnea frequently occur. Inadequate sedation and presence of irritable airway often cause coughing and laryngospasm, both leading to hypoxemia and potential cardiac arrest. Hence, prevention of hypoxemia is of paramount importance. Traditionally, standard nasal cannula is used to administer supplement oxygen. However, it cannot sufficiently provide continuous positive airway pressure (CPAP) or positive pressure ventilation. Device manufacturers have stepped in to fill this void and created many types of cannulas that provide apneic insufflation of oxygen and CPAP and eliminate dead space. Such measures decrease the incidence of hypoxemia. This review aimed to provide essential information of some of these devices.
Keywords: Airway, Devices, Gastrointestinal endoscopy, Sedation
INTRODUCTION
Hypoxemia remains a challenge in the practice of gastrointestinal (GI) endoscopy. Insufficient ventilation caused by either hypoventilation or airway obstruction is the common underlying mechanism of hypoxemia and contributes to morbidity and mortality [1,2]. As a result, patients are at risk for hemoglobin decompensation, hypoxic brain injury, dysrhythmia, and death [3]. Aspiration during endoscopy and colonoscopy is another mechanism that contribute to the development of hypoxemia, which is potentially fatal [4,5].
In the last few decades, there have advancements in the devices used for GI endoscopy, and the existing devices have been improvised, in order to reduce the risk of hypoxemia. The common goal is to maintain airway patency and eliminate dead space by providing high flow oxygen or positive pressure ventilation. Provision of any form of ventilation support while the endoscopist is performing the intervention can further add to the safety. Patients undergoing upper GI endoscopy are often sedated until apnea occurs, and the degree of sedation is frequently similar to that during general anesthesia. Nearly 50% of the time, the degree of propofol-induced sedation is similar to that during general anesthesia or deep general anesthesia [6]. As a result, apnea is inevitable, which further leads to oxygen desaturation. By appropriate preoxygenation, one can achieve a safe apnea time of up to 11 minutes, which is defined as the time it takes for pulse oximeter to register a desaturation to 90% despite the absence of ventilation. Apneic insufflation is another technique frequently employed by anesthesia providers to prolong safe apnea time. Devices that are used in endoscopy sedation delay desaturation and improve safety by one or more of the above mechanisms.
In the following paragraphs, we aimed to discuss the individual devices for endoscopy sedation and their merits and drawbacks. Particular effort is made to explain the physio-mechanical basis of their operation and their true clinical utility.
HIGH-FLOW NASAL CANNULA SUPPORTIVE OXYGEN THERAPY
This device (Fig. 1) utilizes the principle of prolonging safe apnea time by apneic insufflation. Its use is not new in the field of anesthesia and intensive care. Placement of a high-flow nasal cannula (HFNC), initially setting the oxygen flow rate to 4 L/min then increasing to 15 L/min to provide apneic oxygenation when the patient is sedated, is known to provide additional time during laryngoscopy and intubation [3]. Denitrogenation of the lungs to attain a 100% oxygen saturation before the administration of a sedative agent via a tight-fitting face mask is necessary to attain maximum benefit during the upper GI endoscopy.
Fig. 1.
High-flow nasal cannula Optiflow.
The appropriate administration of high-flow nasal oxygen is necessary to benefit from this technique. In a prospective randomized placebo-controlled trial involving 60 patients, the group who received nasal oxygen supplementation at 10 L/min during apnea associated with laryngoscopy did not experience any significant prolongation of apnea desaturation safety periods compared with those without nasal oxygen supplementation [7]. The time for pulse oximeter oxygen saturation (SpO2) to fall from 100% to 92% (desaturation safety time) was also measured. The use of appropriate device to administer high-flow nasal oxygen is important.
Although many devices are capable of providing high-flow nasal oxygen, only a few are used in GI endoscopy. Unlike noninvasive ventilation, HFNC reduces the dead space [8]. The HFNC system consists of an active heated humidifier, flow generator, single heated circuit, and nasal cannula. Such systems are used in critically ill patients to avoid invasive mechanical ventilation and after planned extubation [9]. In addition to reducing or eliminating dead space, these high-flow systems are known to provide positive end-expiratory pressure effect. By increasing nasopharyngeal airway pressure that peaks at the end of expiration, they potentially decrease the work of breathing and enhance oxygenation in patients with alveolar filling diseases such as congestive heart failure or acute respiratory distress syndrome. The mean nasopharyngeal pressure during nasal high-flow oxygen increases as flow increases [10]. Use of dry gas in these devices can lead to mucociliary malfunction, epithelial damage, mucus plugging, ulceration of mucosa, and lung injury; hence, the gas should be humidified.
Use of HFNC in upper GI endoscopy is a relatively new, but attractive concept. AIRVO 2 (Fisher & Paykel Healthcare Limited, Auckland, New Zealand) is a system employed in patients undergoing both routine and advanced upper GI endoscopic procedures. It is a humidifier with integrated flow generator that provides high-flow warmed and humidified gases to spontaneously breathing patients through a variety of patient interfaces. As mentioned, this system delivers oxygen nasally and generates flow-dependent positive airway pressure (PAP). This device was recently tried in a multicenter, prospective randomized single-blinded study of 1,994 outpatients undergoing routine gastroscopy with propofol sedation. Compared with nasal oxygen administered using the same cannula at a rate of 2 L/min, the HFNC group (O2 [30–60 L/min] had a lower incidence of hypoxia (75% ≤ SpO2 <90% for <60 sec) and severe hypoxia (SpO2 <75% for any duration or 75% ≤ SpO2 <90% for ≥60 sec) from 8.4% to 0% (p<0.001) and from 0.6% to 0% (p=0.03), respectively [11]. However, during this study, a number of ethical and practical issues had been raised. It is inconceivable that anesthesia providers allow severe hypoxia to develop for such durations without active aggressive interventions. Such interventions include increasing nasal oxygen flow, chin lift-jaw thrust maneuvers, and early endoscope withdrawal followed by face mask ventilation and artificial airway if necessary. Although such maneuvers were performed in this study, they were implemented late during the desaturation period. These maneuvers should be employed based on patients’ ventilatory efforts, well before desaturation. Considering the steep slope of oxyhemoglobin dissociation curve, employing these measures at an oxygen saturation of 95% could be too late in the process of airway management. Additionally, 2 L/min of oxygen in the non-high-flow group is not the standard practice of anesthesia providers in North America. A total of 1.5 to 2.5 mg/kg of propofol is considered Fig. 1. High-flow nasal cannula Optiflow. too high for a diagnostic upper endoscopy and predisposes patients’ to hypoventilation followed by hypoxemia. It seemed that the investigators triggered the development of hypoventilation and hypoxemia to determine whether high-flow nasal oxygen prevents such episodes. Consequently, it is not surprising that they could demonstrate striking differences between the two groups. Lastly, the average procedure duration was about 5 minutes. Bearing in mind that the safe apnea time after preoxygenation is higher than 5 minutes, the entire study should be called into question.
The adverse effects of high-flow nasal oxygen use include rhinalgia, pharyngalgia, xeromycteria, headache, and barotrauma (e.g., pneumothorax and subcutaneous emphysema).
The cost of this device is another important factor. With falling payments for such procedures and potentially changing insurance remuneration, the cost becomes an important factor. In selected patients such as those with obstructive sleep apnea, morbid obesity, chronic obstructive airway disease (COPD), and pulmonary fibrosis, and those who underwent long, complicated advanced endoscopic procedures, HFNC could be useful. It can avoid the need for endotracheal intubation and reduce the risks of hypoxemia.
MODIFIED BITE BLOCKS
Goudra’s bite block
The two primary mechanisms of HFNC are to administer 100% oxygen and provide some degree of positive end expiratory pressure in the pharynx. The same concept is employed in Goudra’s bite block (Fig. 2). Although the device is still not marketed, the functionality is worthy of further discussion.
Fig. 2.

Goudra’s bite block.
Anatomically, unlike many devices available in the market, this bite block incorporates the elements of a face mask and airway into the endoscopy bite block. The idea is to provide 100% oxygen at the laryngeal inlet with the likelihood of some degree of continuous PAP (CPAP) due to the high concentration of oxygen flowing into the pharyngeal area. It also reduces the anatomical dead space. The oxygen can leak only into the atmosphere through the mouth, which is often sealed, and nose, which is generally open. The device is connected to a Mapleson breathing system. By occluding all avenues of air leak aside from adjusting the pressure-limiting valve, it is possible to provide some degree of positive pressure ventilation. At the time of writing, the device is still not available in the market.
Oxygen providing bite blocks
Respa oxygen delivery bite block (Fig. 3) is the latest addition to the Endoscopy’s family of bite blocks in the United States [12]. It was built to hold the nasal cannula in place during procedures. Anyone practicing sedation for upper GI endoscopy would have observed the practical difficulties in both placing and more importantly retaining the oxygen cannula. No studies have documented its effectiveness and possible adverse effects. The OxyShieldTM (Fig. 4) is another endoscopic bite block with similar capabilities [13]. By providing constant guaranteed supplemental oxygen, these devices can reduce the incidence of hypoxemia without dramatic adaption or change in the practice.
Fig. 3.

Respa O2 delivery bite block.
Fig. 4.

OxyShieldTM.
FACE MASKS
Procedural Oxygen Mask®
Procedural Oxygen Mask® (POM®) (https://proceduraloxygenmask.com/) (Fig. 5) improves oxygenation by increasing the oxygen concentration at the laryngeal inlet. It is similar to the non-rebreathing face mask commonly used in the hospitals except that it has a dedicated self-sealing central aperture through which the endoscopist inserts the endoscope [14]. In addition, it has an opening that accepts the standard gas sampling tubing female connector and thereby allows monitoring of the end tidal carbon dioxide. At the Hospital of the University of Pennsylvania, an improvisation of the standard rebreathing mask allows to achieve the same objective at a fraction of the cost (Fig. 6).
Fig. 5.

Procedural Oxygen Mask® (POM®).
Fig. 6.
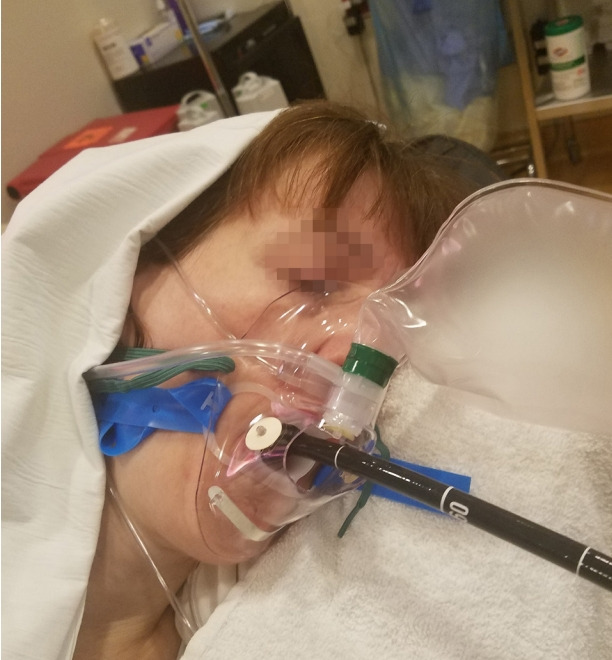
Improvised nonbreathing face mask.
Endoscopy face mask
One of the great challenges of providing adequate oxygenation in patients undergoing upper GI endoscopy is the inability to provide any degree of positive pressure ventilation. Manufacturers have tried to address this by reconfiguring the simple face mask by introducing an intubation port in addition to the ventilation port. An optional carbon dioxide sampling port is available in some of these modifications. The face mask is secured with a series of hooks and harnesses. Both GI endoscopy and bronchoscopy including fibrotic endotracheal intubation can be achieved with these devices.
Apart from being cumbersome, they are impractical to use. Although its use could be straightforward in patients with easy airway, those with airway problems such as patients with obstructive sleep apnea pose challenges. Unfortunately, these patients are prone to hypoventilation and hypoxemia. Examples of these endoscopy masks are DEAS endoscopic mask [15] and VBM Endoscopy Mask (Figs. 7, 8) [16].
Fig. 7.
DEAS endoscopic mask.
Fig. 8.
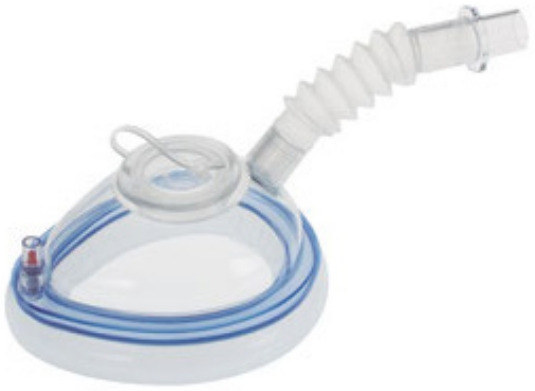
VBM Endoscopy Mask.
Endoscopic nasal mask
Endoscopic nasal mask is a multifunctional device designed to provide oxygen via positive pressure, if necessary. As can be deciphered from the photos, it has a respiratory interface with a soft cushion, a nasal aperture, and a flexible connector, all assembled with a breathing system. Clearly, they are complicated devices that require the anesthesia provider’s patience and dedication to succeed.
In a randomized controlled trial, Gedeon et al. evaluated one such device (Fig. 9A) [17]. In this study, 56 patients with a body mass index of 40−60 were randomized into the treatment, nasal mask capable of providing intermittent positive pressure ventilation (IPPV), and control, nasal cannula, nasal intermittent positive pressure ventilation (NIPPV) for rescue groups (n=28 treatment and n=28 control) [17]. Oxygen desaturation events were defined as less than 94% and those events less than 90% required intervention. In addition, any need for NIPPV as a rescue maneuver was also documented. A statistically significant difference was observed in desaturation events between groups, and those with NIPPV have a lower risk of desaturation. In fact, every patient in the control group developed desaturation necessitating NIPPV for rescue purposes. Similar to the HFNC study mentioned above [11], the authors did not actively intervene until the SpO2 was less than or equal to 94%, which is unacceptable in clinical practice. In fact, the first rescue maneuver was initiated if the SpO2 was less than 90%, which might be considered negligent especially in morbidly obese patients. However, the authors concluded that an adjunct, noninvasive positive pressure ventilation (using bilevel PAP) results in decreased incidence of desaturation events in severely obese patients undergoing upper GI endoscopy. The type and description of the device used to provide NIPPV is not provided by the authors. The following pressure settings were used: inspiratory PAP of 12-cm H2O and expiratory PAP of 6-cm H2O with FiO2 1.00. The pressure was adjusted by 1-to 2-cm H2O to achieve a tidal volume of 450 to 500 mL, up to a maximum inspiratory PAP of 18-cm H2O and expiratory PAP of 8-cm H2O.
Fig. 9.
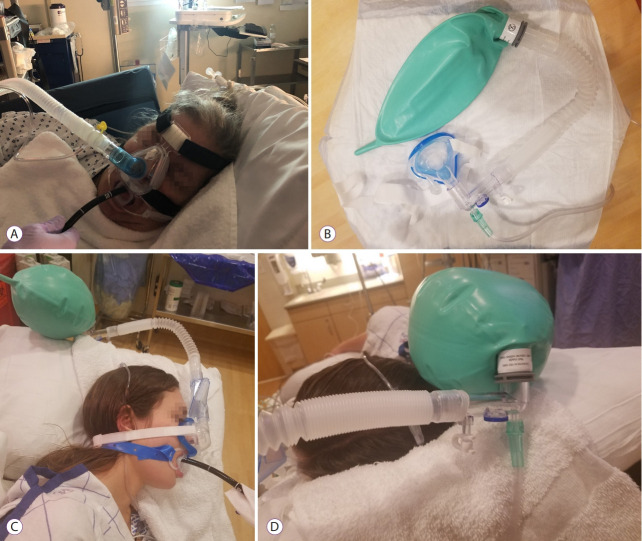
(A) Endoscopic nasal mask (Arilite), (B) SuperNO2VA nasal positive airway pressure (PAP) ventilation system, (C) SuperNO2VA nasal PAP ventilation system, and (D) SuperNO2VA nasal PAP ventilation system.
A second device, also providing nasal IPPV, is the SuperNO2VA nasal PAP ventilation system made by CareFusion (Fig. 9B-D). This device has been investigated in at least two studies, one involving colonoscopy and the other involving upper GI endoscopy. The device looks similar to a pediatric face mask. Instead of an inflatable seal, it has a self-sealing rigid cushion that can potentially cause trauma, especially when used long term. Compression of both nasolacrimal duct and eyes is a distinct possibility. Additionally, it has some design flaws. The oxygen flowing into the mask can create a Venturi effect and prevent the reservoir bag from filling with oxygen. As a result, positive pressure ventilation will be impossible and higher flows will further deplete the bag. In Fig. 9B, flows of 30 L/min have failed to inflate the bag. In spite of these design drawbacks, the mask is found to reduce the frequency of hypoxemia during upper GI endoscopy in patients planning to undergo bariatric surgery [18]. Many of these patients had obstructive sleep apnea. However, the intervention in the control group was not comparable as they were given conventional nasal cannula oxygen. Similarly, the SuperNO2VATM nasal oxygen mask at a target CPAP of 10 cm H2O improved ventilation and decreased the frequency and severity of hypoxemia in spontaneously breathing obese patients during colonoscopy [19]. The device’s mechanism of protection against hypoxemia is similar to that of several devices employed in GI endoscopy. It provides 100% oxygen, some degree of CPAP, increases safe apnea time, and allows some degree of apneic insufflation. In Fig. 9C and D, to eliminate the Venturi effect depleting the bag, the connections are modified to allow oxygen flow into the bag instead of the nose. The heat moisture exchanger port also permits monitoring of end tidal carbon dioxide.
AIRWAY DEVICES
Nasopharyngeal airway
A simple flexible nasopharyngeal airway used appropriately is the best insurance against hypoventilation-associated hypoxemia. At the Hospital of the University of Pennsylvania, we have extensive experience in the use of this technique in thousands of patients for more than a decade. The modification is elegantly demonstrated in the picture (Figs. 10-12). After adequate preoxygenation, we administered appropriate dose of propofol, typically preceded with a small dose of fentanyl especially in patients undergoing advanced endoscopic procedures. If the appropriate depth of anesthesia is achieved, an appropriate-sized nasopharyngeal airway is inserted into the predetermined side without applying undue pressure. Some of our colleagues pretreat the nose with a vasoconstrictive agent, but this is not necessary. More importantly, anesthesia providers must be cognizant of the force used during insertion. If resistance is high, the second nostril should be used. Generous lubrication is necessary to obtain a high degree of success and safety.
Fig. 10.
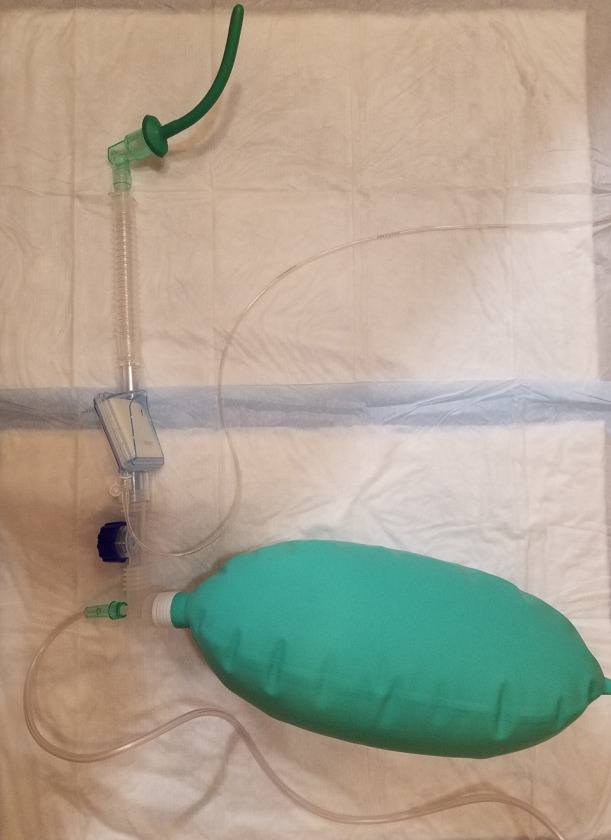
Assembly of nasopharyngeal airway with an endotracheal tube connector, bag, and Mapelson breathing system assembly.
Fig. 11.
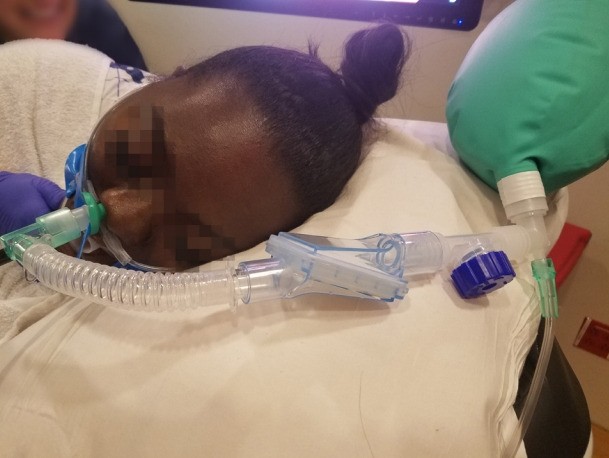
Nasopharyngeal assembly demonstrated in Fig. 9. and used in an obese patient with severe sleep apnea.
Fig. 12.
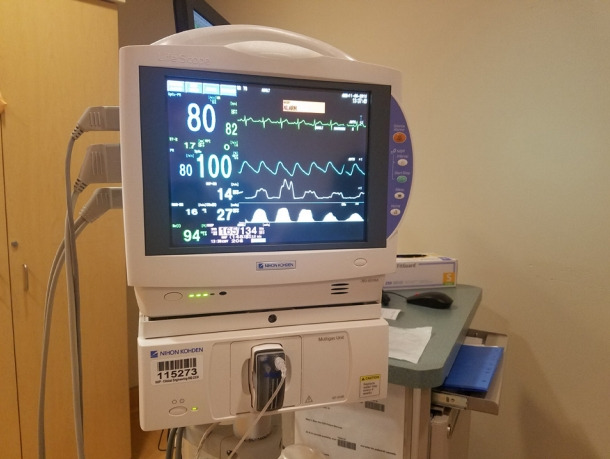
End-tidal carbon dioxide tracing of the patient in Fig. 11.
The nasopharyngeal airway should be attached to an endotracheal tube (ET) connector prior to insertion. A 28−30-Fr tube (attached to the 8.0 ET tube connector) should be used in adult women and a 32−34-Fr tube in adult men (connected to a 9.0 ET tube connector). After insertion, the ET tube connector of the nasopharyngeal airway is connected to a Mapleson breathing system as demonstrated in the photo. An oxygen flow of 8−12 liters with an open expiratory valve is provided. If necessary, the oxygen inflow can be increased to 30−35 liters. It is important to observe the bag all the time. If the distal end of the tube is obstructed or the proximal end of the nasopharyngeal airway is kinked, the bag will expand, sometimes dangerously.
This modification serves many purposes that are performed by expensive modern devices. It provides high concentration of oxygen at the laryngeal inlet. It also provides CPAP, especially if the oral exit is not fully open. Even with an open oral exit, depending on oxygen flow rate, a degree of positive pressure will be noticeable as can be appreciated by the shape of the breathing bag. A combination of apneic insufflation and positive pressure can delay the hypoxemia despite the presence of hypoventilation. Patients typically start self-ventilating after insertion of the endoscope, and appropriate depth of anesthesia is maintained from this point. It is possible to provide a small degree of positive pressure ventilation, especially if the second naris is closed and a hand is applied around the endoscope to close the mouth. Fresh gas flows should be increased appropriately to compensate for the loss of gas due to leakage. At the Hospital of the University of Pennsylvania, we have enormous success with this technique in a variety of patients scheduled to undergo various procedures including an array of advanced procedures [20-22].
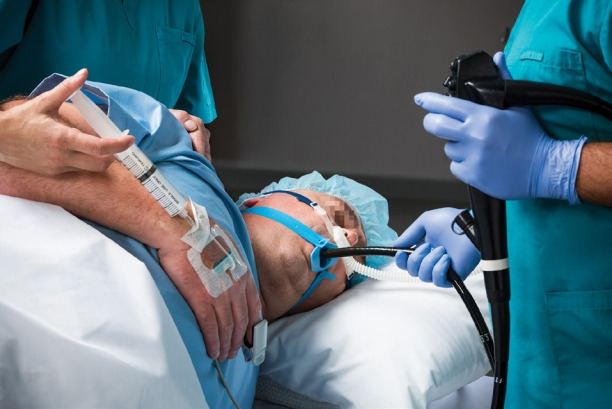
Wei nasal jet tube
This device (Fig. 13) looks like a standard nasopharyngeal airway except that it has two smaller additional channels embedded into the wall with their own external outlets. One of these channels is for insertion of a jet ventilation catheter, while the other is meant for gas sampling for monitoring end tidal carbon dioxide. In a multicenter clinical trial, Qin et al. found that the use of supraglottic jet ventilation via Wei nasal jet tube reduced the incidence of hypoxia in patients who were sedated with propofol during upper GI endoscopy procedures [23]. A total of 1,781 patients were studied in the time period, from March 2015 to July 2016. Of them, one-third received supraglottic jet ventilation, another one-third were administered with oxygen via the nasal tube, and the remaining one-third were administered with oxygen via nasal cannula. Use of Wei nasal tube was associated with a reduction in hypoxemia irrespective of the use of supraglottic jet ventilation. Even in this subgroup, the incidence of hypoxia reduced from 9% to 3% (p<0.0001) in the jet ventilation group compared with the Wei tube without jet ventilation group [23].
Fig. 13.
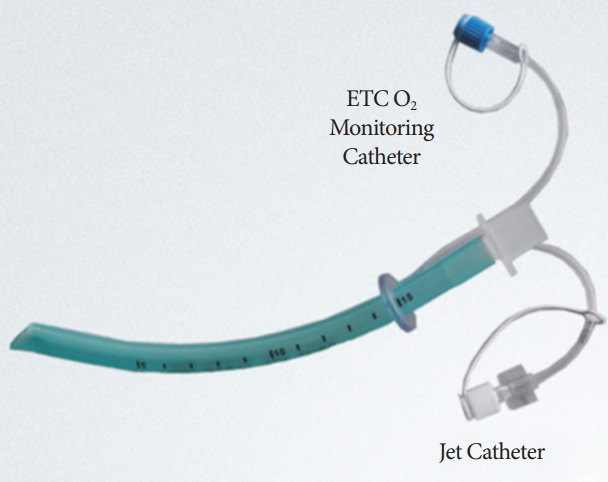
Wei nasal jet tube.
The use of supraglottic jet ventilation is considered unnecessary in most upper GI endoscopic procedures. However, it can be used in patients undergoing advanced procedures. The technique is not free of side effects. There was an increase incidence of ventilation-related minor adverse events 1min after the procedure, which eventually decreased 30min later. At best, the technique could be described as experimental and might be safe in the hands of enthusiasts. Jet ventilation can also be administered with a standard nasopharyngeal airway.
Gastro-Laryngeal Tube
The Gastro-Laryngeal Tube (G-LT, VBM Medizintechnik GmbH, Sulz, Germany; Fig. 14) [24] is a modified laryngeal tube placed in the supraglottic space. It is designed to obtain and retain control of airway patency during GI endoscopic procedures under deep sedation or general anesthesia while maintaining spontaneous or assisted ventilation. Similar to LMA®GastroTM Airway, it has a dedicated channel where the endoscope can be inserted. A preliminary study by Gaitini et al. evaluated the effectiveness of airway management with a G-LT and the possibility of performing procedures using the endoscopic channel [25]. They found that the G-LT is an effective and safe device for patients undergoing endoscopic retrograde cholangiopancreatography (ERCP) [25]. However, in a case series involving 22 patients undergoing interventional endoscopic biliopancreatic procedures, Fabbri reported the occurrence of sore throat in two patients after the procedure, asymptomatic erosion of the upper esophageal mucosa in two patients, Mallory−Weiss syndrome in one patient, and pancreatitis after ERCP in one patient [26]. By any standard of care, this finding suggest the extremely high incidence of device-related complications. The increased risk of complications can be attributed to size and complexity of the device. The necessity of general anesthesia is another drawback. However, Davis et al. observed a significantly lesser degree of stress response in comparison to endotracheal intubation in patients undergoing ERCP [27].
Fig. 14.
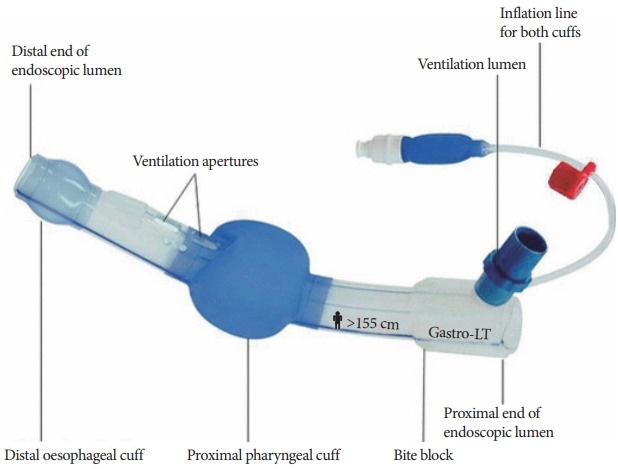
Gastro-Laryngeal Tube.
The majority of ERCPs in North America are performed under deep sedation without any additional airway or relatively less invasive airways [20,28]. As a result, the administration of general anesthesia with all its attendant complications to facilitate the insertion of G-LT seemed to be excessive.
LMA®GastroTM
LMA®GastroTM Airway (Teleflex Medical, Athlone, Ireland; Fig. 15) is a dual channel airway whose method of insertion and removal is similar to that of a standard laryngeal mask airway. As a result, deep general anesthesia is required to facilitate its insertion. In addition to the standard channel to provide pulmonary ventilation, the device has a second channel behind it that allows insertion of a gastroscope. It is supposed to provide esophageal intubation while providing an unobstructed airway and positive pressure lung ventilation with clinically satisfactory seal pressures [29].
Fig. 15.
LMA® GastroTM Airway.
In the first human, open label, prospective, observational study of the device, Terblanche et al. reported that LMA® GastroTM Airway is safe and effective [29]. The study included American Society of Anesthesiology (ASA) physical status classification 1 and 2 patients at low risk of pulmonary aspiration undergoing upper GI endoscopy. The sedation protocol involved 2–3 mg kg−1 IV propofol. Premedication (0.03 mg kg−1 midazolam, 1–2 μg kg−1 fentanyl, and 5–10 μg kg−1 alfentanil) was allowed. Sufficient propofol to maintain general anesthesia was administered during the entire procedure. Clearly, the depth of anesthesia required needed to be sufficient for the insertion of LMA®GastroTM Airway. The dedicated endoscopy channel has an internal diameter of 16 mm, which might be sufficient for insertion of most gastroscopes. The outer diameters of even dual channel and therapeutic gastroscopes are significantly smaller than 16 mm [30]. The majority of the procedures performed in this study were diagnostic upper GI endoscopy with or without colonoscopy.
CONCLUSIONS
Apart from LMA®GastroTM Airway and G-LT, all devices, improvised or not, rely in their ability to increase oxygen concentrations at the laryngeal inlet to nearly 100% and/or provide a degree of positive end expiratory pressure. Dependence on apneic insufflation and increased safe apnea time are the major aspects of their functioning. The majority of upper GI endoscopies are diagnostic and do not last more than 5−10 minutes. Some of the ERCP procedures such as removal of stents are quickly performed. As a result, LMA®GastroTM Airway, which necessarily requires the administration of general anesthesia, to facilitate insertion and retention, would be considered excessive. Similarly, the use of HFNC are prohibited in the majority of patients except those prone to rapid desaturation. Examples include patients with COPD, pulmonary fibrosis, and morbid obesity. However, general anesthesia with endotracheal intubation might be safer and more appropriate in such patients. There are two devices that can provide positive pressure ventilation. While Wei nasal airway relies on jet ventilation, Goudra’s bite block requires establishing a proper seal to accomplish this.
In the end, caution should still be observed. Providing safe sedation for endoscopy is both an art and science. An understanding of the pulmonary physiology and careful evaluation of the patient’s airway are essential. A low threshold, especially in patients scheduled for advanced endoscopic procedures, is beneficial during intubation. The majority of patients who developed cardiac arrests and died after undergoing endoscopy are due hypoventilation and hypoxemia. Having a core group of anesthesia providers will go a long way in increasing the safety [31].
It is not possible to describe every device that is available or invented. However, an effort is made to provide the pros and cons of using some of these devices. Table 1 provides a comparison of the airway devices employed in GI endoscopy.
Table 1.
Comparison of Airway Devices Employed in Gastrointestinal Endoscopy
| Device | Principle of action | Advantages/benefits | Limitations |
|---|---|---|---|
| High-flow nasal cannula supportive oxygen therapy | Creation of CPAP | Decreased incidence of oxygen desaturation | Cost |
| Administration of 100% oxygen | Needs humidification | ||
| Prolongation of apnea time | Ability to increase CPAP by increasing oxygen flow rates | Xeromycteria, rhinalgia, pharyn- galgia, headache, and barotrauma | |
| Apneic insufflation of oxygen | |||
| Goudra’s bite block | Creation of CPAP | Decreased desaturation | Yet to be made available for commercial use |
| Administration of 100% oxygen | Incorporation of airway into the bite block | ||
| Prolongation of apnea time | Connection to the Mapleson breathing systems | ||
| Apneic insufflation of oxygen | Ability to provide IPPV | ||
| Procedural Oxygen Mask® (POM®) | Administration of near 100% oxygen | Prolongation of safe apnea time | Does not provide CPAP |
| Low risk of droplet transmission | Cost | ||
| Endoscopy face mask (DEAS endoscopic mask and VBM Endoscopy Mask) | A facemask with a provision for introduction of an upper gastrointestinal endoscope | Reduced incidence of desaturation | Cost |
| Allows IPPV and CPAP | Cumbersome to use | ||
| Endoscopic nasal mask (e.g., SuperNO2VA nasal PAP ventilation) | Allows IPPV during the performance of the procedure | Decreased incidence of desaturation | The self-sealing rigid cushion can potentially cause trauma, especially when used long term |
| Nasopharyngeal airway | Creation of CPAP | Easily available, the assembly of nasal airway and the Mapleson breathing systems can be easily mastered and implemented | Nasal bleed, occasional difficulty in the insertion of nasopharyn- geal airway |
| Administration of 100% oxygen | |||
| Prolongation of apnea time | |||
| Apneic insufflation of oxygen | |||
| Allows IPPV to a limited extent | |||
| Wei nasal jet tube | A special triple lumen nasal airway that allows jet ventilation, connection to a Mapleson breathing system and sampling of respiratory gas | Decreased incidence of desaturation | Cost and cumbersome to use; nasal bleeding |
| Gastro-Laryngeal Tube | A modified laryngeal tube placed in the supraglottic space; has a dedicated channel for the insertion of an endoscope | Reduced incidence of oxygen desaturation | Requires GA for insertion; higher stress response and trauma |
| LMA®GastroTM | Dual-channel laryngeal mask airway | Has a separate channel for introduction of endoscope including larger scopes for ERCP and endoscopic ultrasound | Requires GA for insertion; higher stress response and trauma |
| Reduced incidence of oxygen desaturation |
CPAP, continuous positive airway pressure; ERCP, endoscopic retrograde cholangiopancreatography; GA, general anesthesia; IPPV, intermittent positive pressure ventilation.
Footnotes
Conflicts of Interest: Basavana Goudra is director of endoscopy anesthesia services at the Penn Presbyterian Medical Center; FDA member for Anesthetic and Analgesic Drug Products Advisory Committee. The other authors have no potential conflicts of interest.
Funding: None.
REFERENCES
- 1.Goudra B, Nuzat A, Singh PM, Gouda GB, Carlin A, Manjunath AK. Cardiac arrests in patients undergoing gastrointestinal endoscopy: a retrospective analysis of 73,029 procedures. Saudi J Gastroenterol. 2015;21:400–411. doi: 10.4103/1319-3767.164202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goudra B, Nuzat A, Singh PM, Borle A, Carlin A, Gouda G. Association between type of sedation and the adverse events associated with gastrointestinal endoscopy: an analysis of 5 years’ data from a tertiary center in the USA. Clin Endosc. 2017;50:161–169. doi: 10.5946/ce.2016.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gleason JM, Christian BR, Barton ED. Nasal cannula apneic oxygenation prevents desaturation during endotracheal intubation: an integrative literature review. West J Emerg Med. 2018;19:403–411. doi: 10.5811/westjem.2017.12.34699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bielawska B, Hookey LC, Sutradhar R, et al. Anesthesia assistance in outpatient colonoscopy and risk of aspiration pneumonia, bowel perforation, and splenic injury. Gastroenterology. 2018;154:77–85.e3. doi: 10.1053/j.gastro.2017.08.043. [DOI] [PubMed] [Google Scholar]
- 5.Wernli KJ, Brenner AT, Rutter CM, Inadomi JM. Risks associated with anesthesia services during colonoscopy. Gastroenterology. 2016;150:888–894. doi: 10.1053/j.gastro.2015.12.018. quiz e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goudra B, Singh PM, Gouda G, Borle A, Carlin A, Yadwad A. Propofol and non-propofol based sedation for outpatient colonoscopy-prospective comparison of depth of sedation using an EEG based SEDLine monitor. J Clin Monit Comput. 2016;30:551–557. doi: 10.1007/s10877-015-9769-5. [DOI] [PubMed] [Google Scholar]
- 7.Sahay N, Sharma S, Bhadani UK, Sinha C, Kumar A, Ranjan A. Effect of nasal oxygen supplementation during apnoea of intubation on arterial oxygen levels: a prospective randomised controlled trial. Indian J Anaesth. 2017;61:897–902. doi: 10.4103/ija.IJA_232_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishimura M. High-flow nasal cannula oxygen therapy devices. Respir Care. 2019;64:735–742. doi: 10.4187/respcare.06718. [DOI] [PubMed] [Google Scholar]
- 9.Zhu Y, Yin H, Zhang R, Ye X, Wei J. High-flow nasal cannula oxygen therapy versus conventional oxygen therapy in patients after planned extubation: a systematic review and meta-analysis. Crit Care. 2019;23:180. doi: 10.1186/s13054-019-2465-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parke RL, Eccleston ML, McGuinness SP. The effects of flow on airway pressure during nasal high-flow oxygen therapy. Respir Care. 2011;56:1151–1155. doi: 10.4187/respcare.01106. [DOI] [PubMed] [Google Scholar]
- 11.Lin Y, Zhang X, Li L, et al. High-flow nasal cannula oxygen therapy and hypoxia during gastroscopy with propofol sedation: a randomized multicenter clinical trial. Gastrointest Endosc. 2019;90:591–601. doi: 10.1016/j.gie.2019.06.033. [DOI] [PubMed] [Google Scholar]
- 12.Mentor (OH): STERIS; Respa O2 Delivery Bite Block [Internet] c2019 [cited 2019 Sep 14]. Available from: https://www.steris.com/healthcare/products/endoscopy-devices/gi-procedure-products/bite-blocks/respa-o2-bite-block. [Google Scholar]
- 13.Achenmühle: OxyshieldTM; Using OxyshieldTM [Internet] c2019 [cited 2019 Sep 14]. Available from: http://www.oxy-shield.com/UsingOxyshield. [Google Scholar]
- 14.Simi Valley (CA): POM Medical; Procedural Oxygen Mask [Internet] c2019 [cited 2019 Sep 13]. Available from: https://proceduraloxygenmask.com/ [Google Scholar]
- 15.Castel Bolognese: DEAS; Endoscopic Mask [Internet] c2019 [cited 2019 Sep 13]. Available from: https://www.deasnet.it/product-catalogue/airway-management/endoscopic-mask/ [Google Scholar]
- 16.Einsteinstrasse: VBM Medizintechnik GmbH; Endoscopy Mask, Bronchoscope Airway [Internet] c2019 [cited 2019 Sep 13]. Available from: https://www.vbm-medical.com/products/airway-management/endoscopy-mask/ [Google Scholar]
- 17.Gedeon M, Gomes S, Roy K, Duclos-Miller P, Rose JS. Use of noninvasive positive pressure ventilation in patients with severe obesity undergoing esophagogastroduodenoscopy: a randomized controlled trial. Surg Obes Relat Dis. 2019;15:1589–1594. doi: 10.1016/j.soard.2019.06.027. [DOI] [PubMed] [Google Scholar]
- 18.Dimou F, Huynh S, Dakin G, et al. Nasal positive pressure with the SuperNO2VATM device decreases sedation-related hypoxemia during pre-bariatric surgery EGD. Surg Endosc. 2019;33:3828–3832. doi: 10.1007/s00464-019-06721-1. [DOI] [PubMed] [Google Scholar]
- 19.Bai Y, Xu Z, Chandrashekar M, et al. Comparison of a simplified nasal continuous positive airways pressure device with nasal cannula in obese patients undergoing colonoscopy during deep sedation: a randomised clinical trial. Eur J Anaesthesiol. 2019;36:633–640. doi: 10.1097/EJA.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 20.Goudra BG, Singh PM, Sinha AC. Outpatient endoscopic retrograde cholangiopancreatography: safety and efficacy of anesthetic management with a natural airway in 653 consecutive procedures. Saudi J Anaesth. 2013;7:259–265. doi: 10.4103/1658-354X.115334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goudra BG, Singh PM, Penugonda LC, Speck RM, Sinha AC. Significantly reduced hypoxemic events in morbidly obese patients undergoing gastrointestinal endoscopy: predictors and practice effect. J Anaesthesiol Clin Pharmacol. 2014;30:71–77. doi: 10.4103/0970-9185.125707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goudra BG, Penugonda LC, Sinha A. A novel way of anesthetizing and maintaining airway/ventilation in an ultra-morbidly obese patient presenting for upper GI endoscopy. J Clin Anesth. 2012;24:604–605. doi: 10.1016/j.jclinane.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Qin Y, Li LZ, Zhang XQ, et al. Supraglottic jet oxygenation and ventilation enhances oxygenation during upper gastrointestinal endoscopy in patients sedated with propofol: a randomized multicentre clinical trial. Br J Anaesth. 2017;119:158–166. doi: 10.1093/bja/aex091. [DOI] [PubMed] [Google Scholar]
- 24.Einsteinstrasse: VBM Medizintechnik GmbH; Gastro-Laryngeal Tube (G-LT) [Internet] c2019 [cited 2019 Sep 13]. Available from: http://carestreammedical.com/wp-content/uploads/Carestream-VBM-Gastro-Laryngeal-Tubes.pdf. [Google Scholar]
- 25.Gaitini LA, Lavi A, Stermer E, Charco Mora P, Pott LM, Vaida SJ. Gastro-laryngeal tube for endoscopic retrograde cholangiopancreatography: a preliminary report. Anaesthesia. 2010;65:1114–1118. doi: 10.1111/j.1365-2044.2010.06510.x. [DOI] [PubMed] [Google Scholar]
- 26.Fabbri C, Luigiano C, Cennamo V, et al. The Gastro-laryngeal tube for interventional endoscopic biliopancreatic procedures in anesthetized patients. Endoscopy. 2012;44:1051–1054. doi: 10.1055/s-0032-1310159. [DOI] [PubMed] [Google Scholar]
- 27.Davis J, Sreevastava DK, Dwivedi D, Gadgi S, Sud S, Dudeja P. A comparison of stress response between insertion of gastro-laryngeal tube and endotracheal intubation in patients undergoing upper gastrointestinal endoscopic procedures for endoscopic retrograde cholangiopancreatography. Anesth Essays Res. 2019;13:13–18. doi: 10.4103/aer.AER_9_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnett SR, Berzin T, Sanaka S, Pleskow D, Sawhney M, Chuttani R. Deep sedation without intubation for ERCP is appropriate in healthier, non-obese patients. Dig Dis Sci. 2013;58:3287–3292. doi: 10.1007/s10620-013-2783-x. [DOI] [PubMed] [Google Scholar]
- 29.Terblanche NCS, Middleton C, Choi-Lundberg DL, Skinner M. Efficacy of a new dual channel laryngeal mask airway, the LMA® GastroTM Airway, for upper gastrointestinal endoscopy: a prospective observational study. Br J Anaesth. 2018;120:353–360. doi: 10.1016/j.bja.2017.11.075. [DOI] [PubMed] [Google Scholar]
- 30.Valhalla (NY): Fujifilm USA; ELUXEO® 700 Series Gastroscopes [Internet] c2019 [cited 2019 Sep 11]. Available from: https://www.fujifilmu-sa.com/products/medical/endoscopy/endoscopes/gastroscopes/index.html#dualChannel. [Google Scholar]
- 31.Goudra BG, Singh PM, Sinha AC. Anesthesia for ERCP: impact of anesthesiologist’s experience on outcome and cost. Anesthesiol Res Pract. 2013;2013:570518. doi: 10.1155/2013/570518. [DOI] [PMC free article] [PubMed] [Google Scholar]