Abstract
Objectives:
To identify plasma markers associated with an increased risk of radiographic knee osteoarthritis (OA) progression using a metabolomics approach.
Methods:
Study participants were from the Multicenter Osteoarthritis Study (MOST) and were categorized into two groups based on the presence of baseline radiographic OA. Subjects in group 1 had unilateral knee OA and subjects in group 2 had bilateral knee OA. Progression was defined as ≥ a half-grade worsening in joint space width at 30-month follow-up. For group 1, a participant progressed when their OA knee showed radiographic progression and the contralateral knee developed OA; for group 2, a participant progressed when both knees with OA showed radiographic progression. Metabolomic profiling was performed on plasma samples collected at baseline and logistic regression was performed to test the association between each metabolite and knee OA progression after adjustment for age, sex, BMI, and clinic site. Significance was defined as p≤0.0003 in the combined analysis.
Results:
234 progressors (57 in group 1 and 177 in group 2) and 322 non-progressors (206 in group 1 and 116 in group 2) were included in analyses. Among 157 metabolites studied, we found that odds of progression were 1.46 times higher per standard deviation (SD) increase of phenylalanine level (95% CI: 1.20–1.77, p=0.0001) in the combined analysis. Sex specific analysis showed that an association was seen in women (p=0.0002) but not in men.
Conclusions:
Our data suggest that phenylalanine might be a novel plasma marker for higher risk of bilateral radiographic knee OA progression in women.
Keywords: Knee Osteoarthritis, Progression, Metabolomics, Plasma Biomarkers, Radiograph
Introduction
Osteoarthritis (OA) is among the most common causes of disability in the older population worldwide (1) and the knee is one of the most common and disabling sites affected (2). OA development and progression are often insidious, and its evolution can be slow and span many years, leading in some to the need for total joint replacement.
The rate of radiographic knee OA progression has been the subject of a number of studies. The Framingham Osteoarthritis Study with a mean 8-year follow-up reported the radiographic progression rate of knee OA defined as Kellgren-Lawrence (KL) grade 2 disease at baseline showing grade ≥ 3 disease on follow-up to be 3.0% and 3.9% per year for men and women respectively (3). A similar progression rate was reported from the Chingford Study with more than 14-year follow-up (4). The low progression rate in addition to disease heterogeneity may contribute to the failure of clinical trials to detect any treatment induced slowing of radiographic progression. In large trials of potential disease modifying drugs, most patients showed little radiographic progression or had changes within the measurement error of the technique (5, 6). This suggests that to detect effects of treatments in trials within a reasonable time, it might be necessary to select a population that is more likely to progress. Thus, identifying factors that predict rapid progression might increase the success rate of clinical trials and facilitate the development of disease modifying drugs.
However, the pathogenesis of knee OA progression remains elusive. Recent application of metabolomics to OA research has generated promising results(7, 8) and identified several metabolomic markers associated with OA risk(9–12), but data on metabolomics of OA progression is still limited(9). We hypothesized that markers for high risk of knee OA progression can be detected by metabolic alterations and undertook the current study to identify plasma marker(s) associated with radiographic disease progression in patients with radiographic knee OA KL grade 2 and 3 using a targeted metabolomics approach.
Participants and Methods
Study participants
Study participants were drawn from the Multicenter Osteoarthritis Study (MOST), a longitudinal, prospective, observational study of knee OA in older Americans with OA or at increased risk of developing it (13). It enrolled 3,026 study participants age 50–79 years in 2003–5 and we focused on the baseline and 30-month follow-up examinations both of which included acquisition of fixed flexion posteroanterior radiographs. Study participants were recruited and examined at clinical centers at the University of Alabama at Birmingham and University of Iowa under local Institutional Review Board (IRB; reference numbers FWA00005960 and FWA00003007) approval and with informed consent given prior to inclusion in the study.
Radiographic knee OA progression assessment:
Knee OA was defined as KL ≥2 and KL<4 at baseline. Progression was at least a half-grade worsening in joint space width in any compartment at follow-up for baseline OA-affected knees as previously defined (14). We used this approach to define knee OA progression because it is a comprehensive approach and more sensitive to change than other methods(14). Participants with unilateral knee OA as group 1 and bilateral knee OA as group 2 at baseline were eligible for the current study. Separation of two groups was arbitrary but represented the reality where either one knee or both knees were affected at baseline. Progression was assessed at 30 months follow-up. For group 1, participants with progression had radiographic progression in the knee with baseline OA and the contralateral knee developed OA defined by KL >=2; for group 2, a participant progressed when both knees with OA showed progression. We defined control subjects as those in groups 1 and 2 in whom neither knee progressed nor developed OA. We used progression/incidence groups that experienced worsening in both knees (not just one knee) as we expected that systemic factors affecting progression would be more likely to be detected in those who had bilateral worsening. Unilateral worsening could arise from an injury to one knee. Such study design has been used in genetic case control studies, comparing genetic backgrounds of using two extreme groups (15).
Covariates:
In addition to information on age, sex and self-reported race, subjects at baseline had weight and height assessed using previously described methods (16) and body mass index was calculated as weight in kilogram divided by squared height in meters.
Metabolic profiling:
Baseline overnight fasting plasma samples were retrieved from the MOST biospecimen repository and sent to St. John’s, Newfoundland for metabolic profiling. Metabolic profiling was performed using Biocrates AbsoluteIDQ® p180 kit, which assesses 186 metabolites including acylcarnitines (n=40), amino acids (n=22), biogenic amines (n=18), hexoses (sum of hexoses) (n=1), and phospho- and sphingolipids (n=105). The details of the 186 metabolites are listed in the Supplementary Table 1. The profiling was done using an API4000 Qtrap® tandem mass spectrometry instrument (Applied Biosystems/MDS Analytical Technologies, Foster City, CA) equipped with Agilent 1100 HPLC system at The Metabolomics Innovation Centre (https://www.metabolomicscentre.ca). The complete analytical process (e.g., the targeted metabolite concentration) was performed using the MetIQ software package, which is an integral part of the AbsoluteIDQ® kit with concentrations reported in μM. The complete metabolic profiling method using this kit was as described (17).
Statistical analysis:
The following quality control (QC) procedures were applied to the metabolomics data. Metabolites were excluded from subsequent analysis if more than 10% of the samples had values below the limit of detection (LOD). For those metabolites with fewer than 10% of samples below LOD, subjects with missing values were excluded in the corresponding association tests. Principal component analysis (PCA) demonstrated that we did not have any batch effect in our experiment; therefore, no correction for batch effects was performed. Among the 186 metabolites, 157 passed the QC procedure and were included in the analysis. The 157 metabolite concentrations were log-transformed to approximate normality and then standardized using a Z-score for use in the subsequent analysis. Z-scores were preferred in the analysis because they were in standard deviation units, making it easier to compare the effect size among metabolites. Z-score was calculated by the following formula:
Where χ is individual observation, μ is the population mean of the entire study cohort, and σ is the standard deviation of the entire study cohort.
Logistic regression model was utilized to test the association between knee progression and 1 SD difference in each of the metabolites with adjustment for covariates including age, sex, BMI, and clinic site which was an indicator for clinic centers where the study participants were recruited. Race was not included in the model due to the collinearity with clinic site because all the African Americans were from one site. The analysis was done in groups 1 and group 2 separately, and then combined analysis of the two groups with adjustment for group as a covariate. The association was considered significant only when the metabolites were associated with knee progression in both groups (p<0.05) and p values were less than 0.0003 in the combined analysis. This took into account performing 186 statistical tests using a Bonferroni correction method (0.05/157=0.00032). Receiver operating characteristic (ROC) curve analysis was performed on the identified metabolite to assess its/their discriminatory ability measured by the area under the curve (AUC).
Results
Among 3026 subjects recruited at baseline in 2003–2005, 600 individuals had one knee affected by radiographic OA with the contralateral knee unaffected; these persons were eligible for knee OA progression defined in group 1. At 30-month follow-up, 63 progressed. 220 of them did not progress in the affected knee and did not develop incident OA in the non-affected knee and were therefore classified as controls. Of these, 57 progression cases and 206 controls had baseline plasma available and were included in the study. In group 2, there were 530 individuals eligible for progression. 196 of them had progression in both knees at 30 months and 177 had baseline plasma available and were included in the study as knee OA progression cases; 122 were classified as controls and, of these, 116 had baseline plasma available and were included in the study.
Baseline age, female sex, race, and clinic sites were not associated with knee OA progression, but baseline BMI was significantly higher in progressors in group 1 as well as in the combined samples than non-progressors (Table 1).
Table 1.
Descriptive statistics of the study population
| Variables | Group 1 | Group 2 | Combined | |||
|---|---|---|---|---|---|---|
| Progressors (n=57) | Non-progressors (n=206) | Progressors (n=177) | Non-progressors (n=116) | Progressors (n=234) | Non-progressors (n=322) | |
| Age (years) | 62.6±7.6 | 61.8±7.4 | 63.5±7.6 | 63.9±8.2 | 63.3±7.6 | 62.6±7.8 |
| Sex (% female) | 56.1 | 56.8 | 66.1 | 71.6 | 63.7 | 62.1 |
| BMI (kg/m2) | 32.1±5.3* | 30.0±5.8 | 33.1±6.7 | 32.8±5.9 | 32.8±6.4* | 31.0±6.0 |
| Races (% white) | 98.3 | 99.0 | 98.3 | 99.1 | 98.3 | 99.1 |
| Clinic sites (%) | 54.4 | 51.5 | 46.3 | 56.0 | 48.3 | 53.1 |
| Group (%) | - | - | - | - | 75.6* | 36.0 |
Statistical testing was done with Chi-squared or Student’s T-test wherever appropriate. BMI at baseline was significantly higher in cases than controls in group 1 with p=0.01 and combined with p=0.0005. Group 2 had significant higher number of cases in the combined analysis with p<0.0001. The percentage was for group 2 and one of the two clinic sites, for example, 36.0% non-progressors were from group 2 in the combined analysis, and 54.4% of the subjects in group 1 were recruited at the clinic center of University of Iowa.
For group 1, six metabolites were associated with knee OA progression at p<0.05. Those metabolites include phenylalanine, arginine, leucine, isoleucine, carnitine, and valerylcarnitine. For group 2, seventeen metabolites were associated with knee OA progression including phenylalanine, serine, tryptophan, histidine, lysine, ornithine, asparagine, and a number of phosphatidylcholines with different number of carbons and double bonds. The complete results for all the metabolites are provided in the Supplementary Table 2.
Phenylalanine was the only metabolite associated with knee progression in each of groups 1 and 2. The plasma concentrations of phenylalanine were statistically significant different between progressors and non-progressors in all groups (Table 2). After adjustment for age, sex, BMI, and clinic sites, log-transformed plasma phenylalanine concentration was associated with progression with an odds ratio (OR) of 1.66 risk for radiographic knee OA progression in group 1 (p=0.004) and an OR of 1.37 in group 2 (p=0.009). When combining group 1 and 2, the OR was 1.46 (p=0.0001). Sex specific analyses showed that higher phenylalanine plasma levels were strongly associated with knee progression in women but not in men (Figure 1). No interaction between sex and phenylalanine for knee OA progression was found when tested.
Table 2.
Plasma phenylalanine concentrations between knee OA progressors and non-progressors
| Progressors | Non-progressors | P values | |
|---|---|---|---|
| Group 1 | 72.13±2.08 | 65.48±0.76 | 0.0003 |
| Group 2 | 68.00±0.96 | 64.28±1.12 | 0.01 |
| Combined | 69.00±0.89 | 65.04±0.63 | 0.0002 |
Figures are mean±SD and Student’s t-test was used in the statistical testing.
Figure 1.
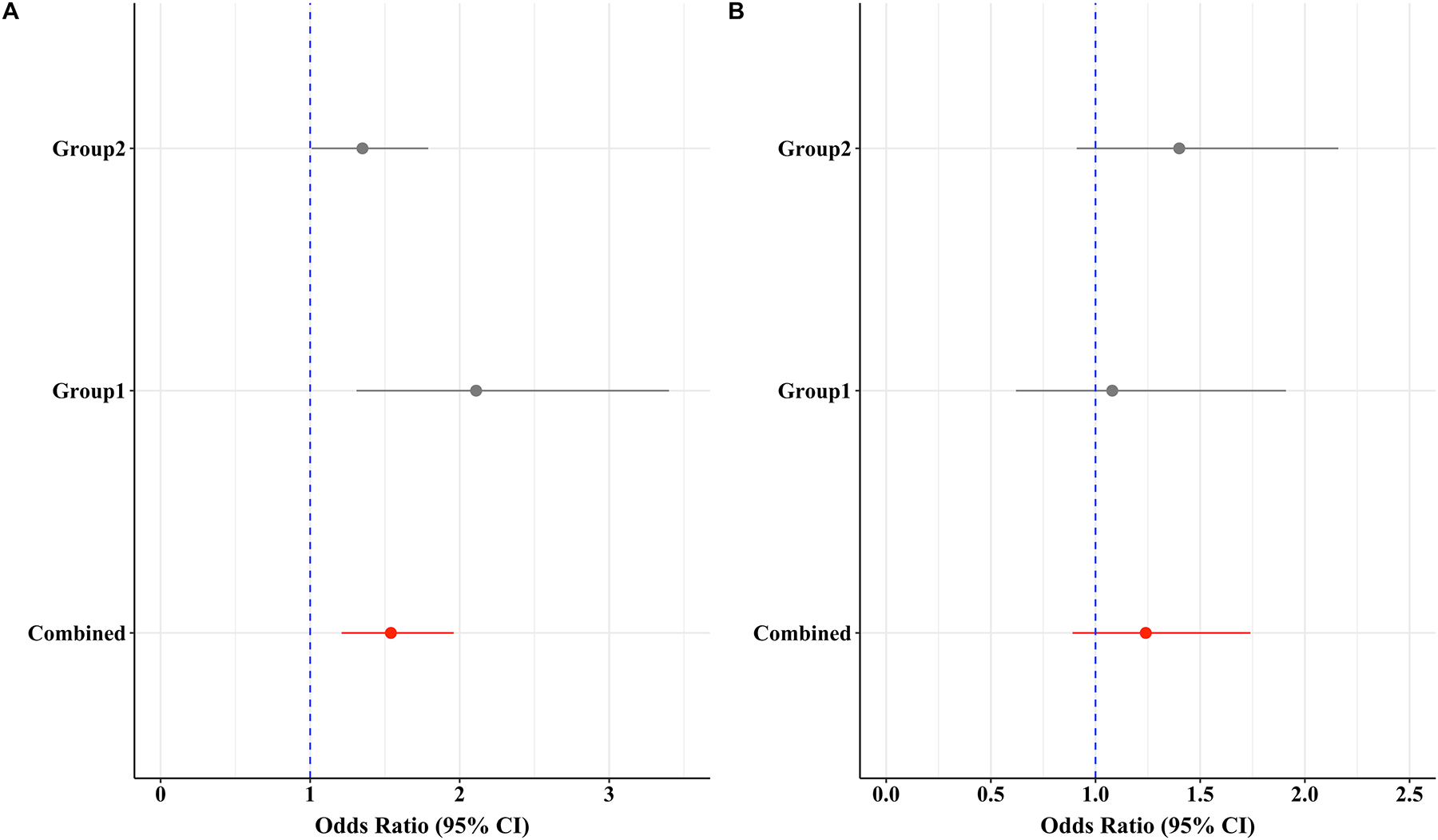
Odds ratios for plasma phenylalanine to the risk of the radiographic knee OA progression in 30 months for women (left) and men (right).
*Group 1 was unilateral knee OA and Group 2 was bilateral knee OA at baseline, the combined was the group 1 and 2 combined. Odds ratios were obtained from logistic regression model with adjustment for age, BMI, and clinic sites and expressed as per standard deviation (SD) of the log-transformed plasma phenylalanine concentration. P values were 0.002, 0.04, and 0.0002 for Group 1, 2, and the combined in women, respectively. P values were 0.30, 0.15, 0.20 for men. The group as a covariate was also adjusted in the combined analysis.
ROC analysis showed that phenylalanine alone has an AUC of 0.69 and 0.59 for women and men in group 1, respectively, to discriminate knee progression cases from controls. It was 0.59 and 0.52 for women and men in group 2, respectively. No covariates were included in the ROC analysis because they were not associated with knee OA progression in the logistic regression analysis. In the combined analysis with including group as a covariate which was significantly associated with knee OA progression in the logistic regression model, the AUC was 0.74 in both women and men. By using R package OptimalCutpoints, we estimated the decision point was 4.2 on the log transformed unit or 66.7 μmol/L on the original concentration unit.
To further assess how phenylalanine behaves as a marker, we examined the plasma phenylalanine concentration who had unilateral but not bilateral knee OA worsening, those who were ‘in-between’ the extreme groups of no progression and bilateral progression. Due to limited funding, we only assess a small number of the eligible subjects to serve the purpose. Specifically, we studied 45 subjects in group 1 who had their baseline OA-affected knee progressed, but contralateral knee did not develop incident OA at the follow-up, and 18 subjects in group 2 who had only one knee progressed at the follow-up. We found that the phenylalanine levels in these subjects were higher than the non-progressors but lower than the progressors although the difference between the ‘in-betweeners’ were not statistically significant different from the nonprogressors (Supplementary Table 3).
Patient-reported Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain scores were collected previously in the study participants at both baseline and follow-up. We found no correlation between phenylalanine concentration and WOMAC pain scores at either baseline or follow-up (correlation coefficients ranged from 0.004 to 0.01, p values ranged from 0.54 to 0.88).
Discussion
We have carried out one of the first longitudinal studies examining metabolic risk factors for radiographic knee OA progression. Our results suggest that plasma phenylalanine level is associated with radiographic knee OA progression, especially in women. To the best of our knowledge, this was the first study that demonstrated that a high plasma phenylalanine level was associated with knee OA progression.
Our data suggests that plasma phenylalanine as a marker behaves well with linear increase from non-progressor, in-betweeners, and progressors (Supplementary Table 3). Our failure to find an association with the WOMAC pain score suggests the phenylalanine is more likely to be associated with structural progression than with clinical symptoms.
The strength of the study was that we used a strict definition of the radiographic disease progression in a well-established longitudinal study. We examined both knees with two scenarios. One scenario was both knees affected at baseline and the other was one knee affected and one knee at risk for incident disease. The positive findings for phenylalanine and OA radiographic progression were present in both scenarios. The findings have potential in direct clinical translation because the established method for measuring blood phenylalanine concentration is readily available at any hospital clinical chemistry lab and OA patients should be advised to avoid any food/drinks containing large amount of aspartame which could raise blood phenylalanine levels.
Phenylalanine is an essential amino acid that cannot be synthesized within the body but has to be obtained from diet. In addition to serving as a building block for various proteins, phenylalanine can be degraded to tyrosine and other metabolites which play a significant role in several rare genetic disorders. Alkaptonuria was the first inborn error of metabolism caused by the aberrant phenylalanine/tyrosine degradation due to mutations in the HGD gene, leading to the accumulation of homogentisic acid (HGA) in the connective tissues. People affected by alkaptonuria develop severe OA in their 50s because of the accumulation of HGA in the articular cartilage, leading to the stiffness of the cartilage matrix, resulting in the aberrant transmission of loading to underlying subchondral bone (18). While our study participants were not affected by alkaptonuria, our findings raise the possibility that a high phenylalanine level could result in an overproduction of HGA some of which may be deposited in articular cartilage before being broken down by the HGD, thus leading to disease progression. Xu et al. recently found that elevated phenylalanine in cartilage was correlated with osteophyte formation in knee OA (19), supporting our hypothesis. Alternatively, type II collagen, which is the major component of the extracellular matrix, is composed mainly of proline, hydroxyproline, and phenylalanine, and breakdown of the cartilage could lead to the release of these amino acids, thus an elevated phenylalanine level. Plasma proline concentration but not hydroxyproline was also higher in the progression cases in the group 1 (p=0.04) in the current study (Supplementary Table 2), suggesting this possibility. Furthermore, elevated phenylalanine could lead to an adequate availability of tyrosine for synthesis of receptor tyrosine kinases (RTKs), most of which could promote cartilage destruction(20, 21). Further studies are needed to reveal the exact mechanism for the observed association.
Previously, an exploratory study of the urinary metabolome with a small sample size reported that baseline urinary glycolate, hippurate, and trigonelline were the most likely metabolites to discriminate knee OA progressors from non-progressors (22). The assay we used did not cover these metabolites, therefore, we cannot confirm these results. However, these metabolites are common constituents of urine, suggesting that studying the metabolome in different body fluids could provide additional tools for predicting knee OA progression.
A number of metabolomic studies have reported several metabolic markers for OA prevalence including arginine deficiency, branched chain amino acid to histidine ratio, and lysophosphatidylcholine to phosphatidylcholine ratio (8). We measured these metabolites in the current study but did not find an association of these metabolites with radiographic knee OA progression (Supplementary Table 2), suggesting different metabolic alterations in radiographic knee OA progression.
Low coverage of the metabolome is a limitation of the study. A large number of metabolites in human blood have been detected and we covered only a fraction of them; thus, we might have missed other important metabolites that could be associated with radiographic knee OA progression. The ROC analysis showed that plasma phenylalanine had a moderate discriminatory ability to predict knee OA progression and there may be other metabolites or factors that need to be identified. The strong association was seen in women but not in men. Phenylalanine has been reported to be associated with telomere shortening in men but not in women(23). Thus, our results may suggest that the association is sex-specific. However, small sample size in men in the current study might also be the blame, and further studies with sufficient study power are needed to confirm. Dietary intake could have an influence on plasma phenylalanine levels, and we do not have diet history information on study participants. However, plasma phenylalanine concentration is stable in fasting state and we measured it on the fasting blood, suggesting it may not an issue. Lastly, we used a strict case definition of both knees with radiographic changes, thus our findings may not be generalizable to the progression defined as a person with a radiographic change in either knee. However, the in-betweeners presented in Supplementary Table 3 were in fact patients with only one knee progressed. When pooling all the in-betweeners together with all the progressors to create a group of progressors defined by either knee progressed, we found that the newly defined progressors still had a significantly higher phenylalanine level than non-progressors (68.68±0.76 vs. 65.05±0.63; p=0.0002).
In conclusion, our data suggest that phenylalanine might be considered as a novel plasma marker for predicting knee radiographic OA progression, especially in women. The findings provide new insights into the pathogenesis of knee OA progression and have potential clinical applications.
Supplementary Material
Acknowledgements:
We thank all the study participants who made this study possible. We also thank all the staff involved in the MOST study.
The sources of support:
The current study was supported by Canadian Institutes of Health Research (FRN153298) and the original study of the MOST was supported by National Institutes of Health and National Institute on Aging (Felson - U01 AG18820; Lewis - U01 AG18947; Nevitt - U01 AG19069; Torner - U01 AG18832).
Footnotes
Conflicts of interest: Both AG and FR are shareholders of Boston Imaging Core Lab (BIC), LLC. All other authors have no conflict of interest to declare.
References
- 1.Collaborators GBDRF, Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: A systematic analysis for the global burden of disease study 2013. Lancet 2015;386:2287–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallace IJ, Worthington S, Felson DT, Jurmain RD, Wren KT, Maijanen H, et al. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc Natl Acad Sci U S A 2017;114:9332–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felson DT, Zhang Y, Hannan MT, Naimark A, Weissman BN, Aliabadi P, et al. The incidence and natural history of knee osteoarthritis in the elderly. The framingham osteoarthritis study. Arthritis Rheum 1995;38:1500–5. [DOI] [PubMed] [Google Scholar]
- 4.Leyland KM, Hart DJ, Javaid MK, Judge A, Kiran A, Soni A, et al. The natural history of radiographic knee osteoarthritis: A fourteen-year population-based cohort study. Arthritis Rheum 2012;64:2243–51. [DOI] [PubMed] [Google Scholar]
- 5.Bingham CO 3rd, Buckland-Wright JC, Garnero P, Cohen SB, Dougados M, Adami S, et al. Risedronate decreases biochemical markers of cartilage degradation but does not decrease symptoms or slow radiographic progression in patients with medial compartment osteoarthritis of the knee: Results of the two-year multinational knee osteoarthritis structural arthritis study. Arthritis Rheum 2006;54:3494–507. [DOI] [PubMed] [Google Scholar]
- 6.Hellio le Graverand MP, Clemmer RS, Redifer P, Brunell RM, Hayes CW, Brandt KD, et al. A 2-year randomised, double-blind, placebo-controlled, multicentre study of oral selective inos inhibitor, cindunistat (sd-6010), in patients with symptomatic osteoarthritis of the knee. Ann Rheum Dis 2013;72:187–95. [DOI] [PubMed] [Google Scholar]
- 7.Zhai G. Alteration of metabolic pathways in osteoarthritis. Metabolites 2019;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhai G, Randell EW, Rahman P. Metabolomics of osteoarthritis: Emerging novel markers and their potential clinical utility. Rheumatology (Oxford) 2018;57:2087–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhai G, Pelletier JP, Liu M, Aitken D, Randell E, Rahman P, et al. Activation of the phosphatidylcholine to lysophosphatidylcholine pathway is associated with osteoarthritis knee cartilage volume loss over time. Sci Rep 2019;9:9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang W, Sun G, Aitken D, Likhodii S, Liu M, Martin G, et al. Lysophosphatidylcholines to phosphatidylcholines ratio predicts advanced knee osteoarthritis. Rheumatology (Oxford) 2016;55:1566–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang W, Sun G, Likhodii S, Aref-Eshghi E, Harper PE, Randell E, et al. Metabolomic analysis of human synovial fluid and plasma reveals that phosphatidylcholine metabolism is associated with both osteoarthritis and diabetes mellitus Metabolomics 2015;12:24. [Google Scholar]
- 12.Zhang W, Sun G, Likhodii S, Liu M, Aref-Eshghi E, Harper PE, et al. Metabolomic analysis of human plasma reveals that arginine is depleted in knee osteoarthritis patients. Osteoarthritis Cartilage 2016;24:827–34. [DOI] [PubMed] [Google Scholar]
- 13.Segal NA, Nevitt MC, Gross KD, Hietpas J, Glass NA, Lewis CE, et al. The multicenter osteoarthritis study: Opportunities for rehabilitation research. PM R 2013;5:647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felson DT, Nevitt MC, Yang M, Clancy M, Niu J, Torner JC, et al. A new approach yields high rates of radiographic progression in knee osteoarthritis. J Rheumatol 2008;35:2047–54. [PMC free article] [PubMed] [Google Scholar]
- 15.Bjornland T, Bye A, Ryeng E, Wisloff U, Langaas M. Powerful extreme phenotype sampling designs and score tests for genetic association studies. Stat Med 2018;37:4234–51. [DOI] [PubMed] [Google Scholar]
- 16.Niu J, Clancy M, Aliabadi P, Vasan R, Felson DT. Metabolic syndrome, its components, and knee osteoarthritis: The framingham osteoarthritis study. Arthritis Rheumatol 2017;69:1194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhai G, Wang-Sattler R, Hart DJ, Arden NK, Hakim AJ, Illig T, et al. Serum branched-chain amino acid to histidine ratio: A novel metabolomic biomarker of knee osteoarthritis. Ann Rheum Dis 2010;69:1227–31. [DOI] [PubMed] [Google Scholar]
- 18.Gallagher JA, Dillon JP, Sireau N, Timmis O, Ranganath LR. Alkaptonuria: An example of a “fundamental disease”--a rare disease with important lessons for more common disorders. Semin Cell Dev Biol 2016;52:53–7. [DOI] [PubMed] [Google Scholar]
- 19.Xu Z, Chen T, Luo J, Ding S, Gao S, Zhang J. Cartilaginous metabolomic study reveals potential mechanisms of osteophyte formation in osteoarthritis. J Proteome Res 2017;16:1425–35. [DOI] [PubMed] [Google Scholar]
- 20.Swanson CD, Akama-Garren EH, Stein EA, Petralia JD, Ruiz PJ, Edalati A, et al. Inhibition of epidermal growth factor receptor tyrosine kinase ameliorates collagen-induced arthritis. J Immunol 2012;188:3513–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watt FE, Ismail HM, Didangelos A, Peirce M, Vincent TL, Wait R, et al. Src and fibroblast growth factor 2 independently regulate signaling and gene expression induced by experimental injury to intact articular cartilage. Arthritis Rheum 2013;65:397–407. [DOI] [PubMed] [Google Scholar]
- 22.Loeser RF, Pathmasiri W, Sumner SJ, McRitchie S, Beavers D, Saxena P, et al. Association of urinary metabolites with radiographic progression of knee osteoarthritis in overweight and obese adults: An exploratory study. Osteoarthritis Cartilage 2016;24:1479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eriksson JG, Guzzardi MA, Iozzo P, Kajantie E, Kautiainen H, Salonen MK. Higher serum phenylalanine concentration is associated with more rapid telomere shortening in men. Am J Clin Nutr 2017;105:144–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


