Abstract
Background:
Diesel exhaust is a complex mixture comprised of gases and particulate matter and is a contributor to ambient air pollution. To reduce health risks, recent changes in diesel engine technology have significantly altered the composition of diesel exhaust, primarily by lowering emissions of particulate matter. However, animal toxicological studies continue to report health effects following exposure to whole diesel exhaust from engines employing particulate filters. The cause of these effects remains unclear.
Objective and methods:
To gain an understanding of the role of both particle-filtered and whole diesel exhaust on specific health outcomes, we conducted a systematic review in which we examined animal toxicological and controlled human exposure studies that included a comparison between inhalation of particle-filtered and whole diesel exhaust on any health endpoint.
Results:
We identified 26 studies that met both the inclusion and study evaluation criteria. For most health outcomes, the particle filtration methods employed in the included studies did not appreciably attenuate the health effects associated with exposure to whole diesel exhaust. There were also several health endpoints for which significant effects were associated with exposure to either particle-filtered or whole diesel exhaust, but not to both.
Conclusions:
Overall, the results from this systematic review demonstrate that exposure to different components in diesel exhaust can have distinct and independent health effects. Thus, to better inform human health risk assessments, future studies aimed at elucidating the health effects from diesel exhaust should include exposure to both particle-filtered and whole diesel exhaust.
Keywords: diesel exhaust particles, filtered exhaust, particulate matter, nitrogen dioxide, traffic-related air pollution
Introduction
Diesel exhaust is a major contributor to ambient air pollution in urban areas (Yin et al. 2010). The exhaust emissions from diesel engines have been linked to a variety of adverse health effects, including airway inflammation (Ghio et al. 2012), vascular dysfunction (Mills et al. 2005), developmental toxicity (Ema et al. 2013), neuroinflammation (Levesque et al. 2011; Costa et al. 2017), and respiratory mortality (Atkinson et al. 2016), among others. In addition, diesel engine exhaust is categorized as “carcinogenic to humans” by the International Agency for Research on Cancer (IARC 2014). Interestingly, particulate matter (PM) is often considered the primary driver of the adverse health effects associated with diesel exhaust exposure (Sydbom et al. 2001; Lall et al. 2011; Ristovski et al. 2012). Fine particulate matter (PM2.5; <2.5 μm aerodynamic diameter) is well studied and there are strong causal associations between PM2.5 exposure and cardiovascular and pulmonary diseases (EPA 2009; Brook et al. 2010; Landrigan et al. 2018). Furthermore, there is evidence that ultrafine PM (<100 nm aerodynamic diameter) may have a greater potential for toxicity per-mass than that of PM2.5 given the higher surface area of the particles and greater ability to adsorb organic chemicals and metals (Cassee et al. 2013; Li N et al. 2016; Tyler et al. 2016).
Diesel engine exhaust is a complex mixture, and its composition depends on the fuel type, temperature, humidity, engine technology, pattern of use, and maintenance state (Steiner et al. 2016). The gaseous fraction consists of carbon monoxide, carbon dioxide, nitrogen oxides, sulfur oxides, and volatile organic compounds, such as benzene and formaldehyde. The particulate fraction includes elemental and organic carbon, sulfate, and metals (Steiner et al. 2016). Polycyclic aromatic hydrocarbons and nitroarenes can be distributed over both fractions of the exhaust, depending on operating and environmental conditions (Hesterberg et al. 2011; Steiner et al. 2016). By total mass, the majority of particulate matter in diesel exhaust consists of fine particles (PM2.5), whereas ultrafine particles account for the majority of the total particle number (Hesterberg et al. 2011).
Due to concerns related to the health effects associated with exposure to diesel exhaust, in 2001, the U.S. Environmental Protection Agency (U.S. EPA) adopted new standards for heavy-duty diesel engines (EPA 2001). Following this change, new technology diesel engines were developed, producing a 90% reduction in the total mass emissions of particulate matter from the previous standard, as well as lower emissions of carbon monoxide and nonmethane hydrocarbons (Costantini et al. 2016). Emissions of most chemicals were significantly reduced, though emissions of nitrogen dioxide and sulfates increased (Khalek et al. 2011; McClellan et al. 2012; Costantini et al. 2016). Then, in 2010, to comply with a lower emission standard for oxides of nitrogen, new diesel engines began employing selective catalytic reduction devices, resulting in a 94% reduction in nitrogen dioxide emissions when compared to 2007-compliant diesel engines (Costantini et al. 2016).
Importantly, many vehicles currently on the road do not employ the latest particulate and nitrogen dioxide control technologies. Thus, there continues to be a need to characterize the potential health risks from these older engines, and in particular, to assess whether health effects are specifically associated with exposure to diesel exhaust as a whole, as opposed to the separate gaseous or particulate fractions (Reis et al. 2018). These uncertainties are also important from an air pollution risk assessment standpoint. For example, the U.S. EPA reviews the health effects from particulate matter and oxides of nitrogen separately, thus the ability to delineate which effects arise from gases, such as nitrogen dioxide, and which arise from particles is critical.
To gain an understanding of the role of whole versus fractions of diesel exhaust on specific health outcomes, we conducted a systematic review in which we examined animal inhalation and controlled human exposure studies. These studies used a variety of particle filtration devices to examine the potential for differential health effects following exposure to particle-filtered and whole diesel exhaust, none of which included selective catalytic reduction devices designed to further reduce emissions of nitrogen dioxide. Interestingly, the results of our analysis revealed that there were several health endpoints for which significant effects were associated with exposure to particle-filtered or whole diesel exhaust, but not to both. However, for the majority of endpoints, exposure to both treatments resulted in statistically significant health effects, suggesting the effects are primarily driven by exposure to the unfiltered components of diesel exhaust. These results suggest that future research describing the health effects from exposure to diesel exhaust should include a particle filtered group in order to better delineate the health effects from combustion derived particles from those caused by the particle-filtered fraction likely dominated by gaseous emissions.
Methods
Literature search and screening strategy
A PECO (Population, Exposure, Comparator, Outcome) statement was developed to frame the research question and guide the literature search and subsequent screening. The PECO statement was defined as follows:
Population: The study population of any controlled human exposure or animal toxicological study of mammals at any lifestage.
Exposure: Short- (0–30 days) or long-term (>30 days) inhalation exposure to particle-filtered or whole (particulate matter <5000 μg/m3) diesel exhaust when evaluated in the same analysis.
Comparator: Relative to filtered air controls, or a direct comparison between particle-filtered and whole diesel exhaust.
Outcome: Any health-related endpoints.
A literature search was conducted in Web of Science and PubMed using search strings designed to capture all relevant studies (Supplemental Material). This search was supplemented by direct keyword searches in Google Scholar.
Abstract screening was performed independently by two reviewers using SWIFT Active Screener, a software application that applies machine learning to prioritize articles during the screening process. Initial discrepancies as to the relevance of a given study were settled by re-screening each abstract. References that were retained following abstract screening underwent independent full-text screening by two reviewers. We then conducted study quality evaluations on the final set of studies identified for inclusion following the full-text screening.
Study quality evaluation
Study quality evaluations were conducted in the U.S. EPA version of the Health Assessment Workplace Collaborative (HAWC), an open-source web-based software application that allows for data extraction, synthesis, and interpretation of literature (Shapiro et al. 2018). The following nine domains were assessed: reporting quality; allocation; observational bias/blinding; confounding/variable control; selective reporting and attrition; chemical administration and characterization; exposure timing, frequency, and duration; outcome assessment; and results presentation. Each domain was assigned a rating of either good, adequate, deficient, or critically deficient. If insufficient information was included in the study to assign a rating for a domain, a rating of not reported was assigned and interpreted as either adequate or deficient based on the potential impact on the results. Once all domains were evaluated, an overall confidence rating was assigned for each health endpoint from the study, of either high, medium, low, or uninformative. This was determined by considering the impact of the limitations or uncertainties in reporting and bias. For a rating of high confidence to be assigned, no notable concerns were identified; for medium confidence, some concerns were identified but were expected to have minimal impact on the interpretation of results; a low confidence rating suggested the noted concerns were likely to significantly impact the interpretation of the study. Each study was independently assessed in HAWC by two reviewers. Discrepancies between reviewers were resolved through in-person discussions. For more information on the general considerations employed, see https://hawcprd.epa.gov/assessment/100000039/.
Results
Literature search and screening
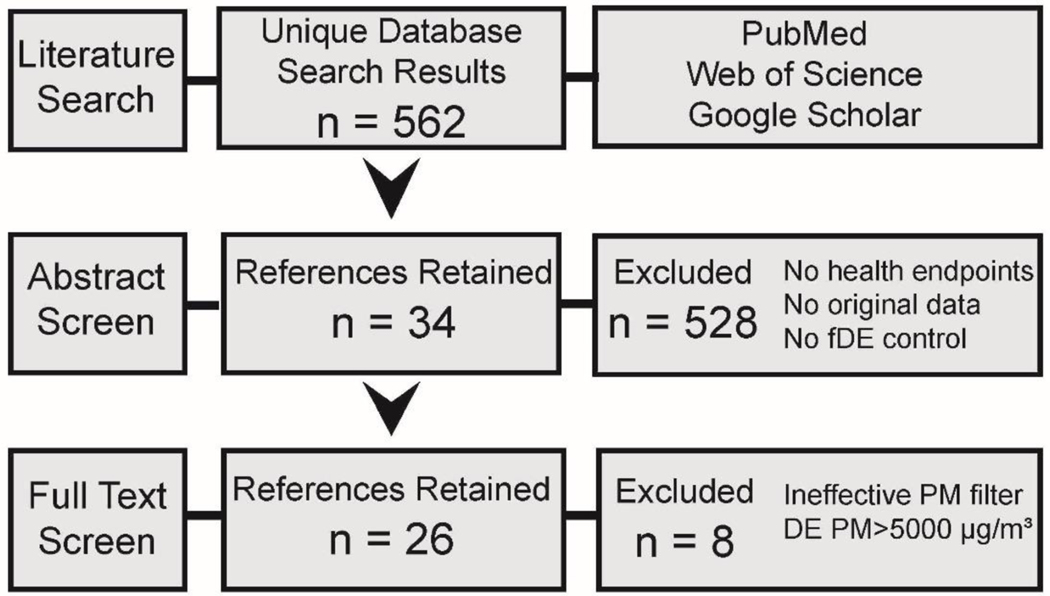
The literature search retrieved a total of 562 references, 347 from PubMed and 336 from Web of Science, with 121 of the 562 references overlapping (Figure 1). The search was conducted October 8, 2018. An additional 4 references resulting from the keyword search in Google Scholar were included in the abstract screen. Within SWIFT Active Screener, 386 abstracts were screened before reaching the 95% inclusion threshold from the 562 total references. From this abstract screen, 34 references were retained. The majority of the 528 references were excluded because they did not include health endpoints (i.e., were solely analyses of diesel exhaust composition). Other references were review articles that did not include original data. Furthermore, a number of studies did not include a particle-filtered comparison group. From the 34 retained references, the full-text screen by two reviewers identified a total of 26 references that were moved forward for study quality evaluation and data extraction. The studies excluded by the full-text screen lacked effective particulate matter filters and/or had very high concentrations of particulate matter (>5000 μg/m3; see Methods) in whole exhaust. Due to the heterogeneity of health endpoints and study designs, we did not conduct any form of meta-analysis on the included studies, but rather report the results in a narrative style (Yost et al. 2019).
Figure 1.
Literature search and screening flow diagram. fDE: filtered diesel exhaust; PM: particulate matter.
Study quality evaluation
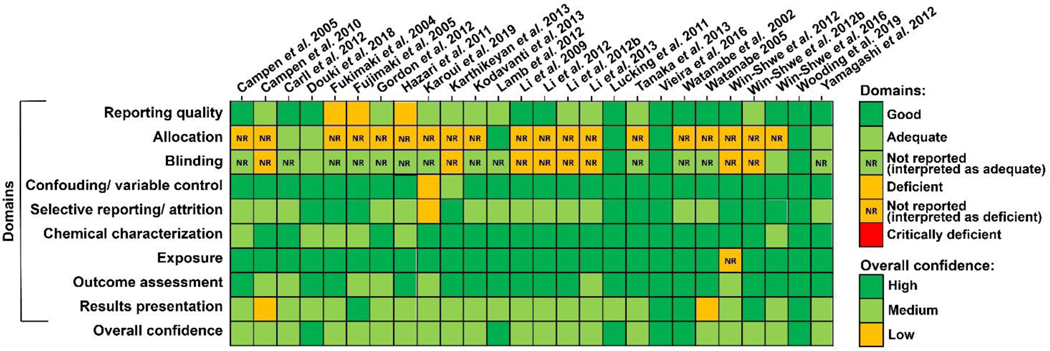
Study evaluation ratings for each domain across all studies are shown as a heatmap in Figure 2. For overall confidence, 6 studies were rated as providing high confidence, 20 studies were rated as providing medium confidence, and no studies were rated as providing low confidence.
Figure 2.
Heatmap showing results from the study quality evaluation for each domain [NR = not reported (interpreted as either adequate or deficient)] and for overall confidence.
Summary of exposure metrics
All 26 studies reported levels of particulate matter in whole exhaust, which ranged from 35.5 to 2500 μg/m3. For filtered exhaust, 22 studies reported levels of particulate matter, which ranged from 0 to 40 μg/m3. In addition to particulate matter concentration, we also report concentrations of NO2 (the most consistently measured and reported gas), particle diameter, and estimates of total particle counts. We include these values for study interpretation and characterization; however, given the inconsistency in reporting, further analysis of the contribution of these measurements to health effects is limited. For NO2 levels, 19 studies reported the levels for both filtered and whole diesel exhaust. These ranged from 0.1 to 15.7 ppm for filtered exhaust, and from 0.052 to 4.2 ppm in whole exhaust. In 10 of the 19 studies where NO2 levels were reported, NO2 is higher in the filtered exhaust group by a factor of 2 or more. Of the 26 total studies, 16 reported measures of particle diameter, and 13 reported estimates of total particles (Supplemental Material). For most studies, the mode diameter of particles in whole exhaust was in the range of 22 to 70 nm. One study, Fujimaki et al. 2005, reported a particle diameter of 400 nm, and two studies, Watanabe 2005 and Watanabe et al. 2002, reported that over 90% of particles were smaller than 500 nm. The total particle numbers in whole exhaust ranged from 3.2×105 to 4.4×106 per cm3. High-efficiency particulate air (HEPA) filters were the most commonly employed filtration method (Supplemental Material).
Respiratory system
One controlled human exposure and four animal toxicological studies allowed for a delineation of respiratory effects from particle-filtered and whole diesel exhaust (Table 1). With the exception of one animal toxicological study, particle filtration did not attenuate effects associated with diesel exhaust exposure.
Table 1.
Significant effects on the respiratory system resulting from exposure to particle filtered diesel exhaust (fDE) or whole diesel exhaust (wDE) relative to filtered air exposure (arrows indicate direction of effect and statistical significance at α < 0.05; dashes indicate no statistically significant effect observed). NR = not reported; BALF = bronchoalveolar lavage fluid; GGT = gamma-glutamyl transpeptidase.
| Respiratory System | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PM μg/m3 | NO2 (ppm) | Species | Sex | Duration | Effect | fDE | wDE | Author | Year | ||
| fDE | wDE | fDE | wDE | ||||||||
| 18.9 | 292.2 | 0.15 | 0.052 | Human | Male and female | 2 hr | FEV1 (allergen co-exposure) | ↓ | ↓ | Wooding et al. | 2019 |
| Airway responsiveness (allergen co-exposure) | ↓ | ↓ | |||||||||
| 1.7 | 269 | 15.7 | 4.2 | F344/DuCrl rats | Male | 4 hr/day for 1 day (single); 4hr/day for 3 days (repeated) | BALF protein (single) | ↑ | ↑ | Karthikeyan et al. | 2013 |
| BALF neutrophils (single) | ↑ | ↑ | |||||||||
| BALF macrophages (repeated) | ↑ | ↑ | |||||||||
| cytokine production (single) | ↑ | ↑ | |||||||||
| BALF neutrophils (repeated) | ↓ | - | |||||||||
| NR | 1900 | NR | NR | Wistar-Kyoto rats | Male | 5 hr/day for 2 days; 5 hr/day, 5 day/week for 4 weeks | BALF neutrophils | ↑ | ↑ | Gordon et al. | 2012 |
| BALF GGT activity | ↑ | ↑ | |||||||||
| BALF GGT activity (2-d, 4-w) | ↑ | ↑ | |||||||||
| NR | 1220 (2-d); 1494 (4-w) | 1.0 (2-d); 0.8 (4-w) | 0.9 (2-d); 0.7 (4-w) | Wistar-Kyoto rats; SH rats | Male | 4 hr/day for 2 days; 4 hr/day, 5 day/week for 4 weeks | BALF neutrophils (WKY rats) | ↑ | ↑ | Kodavanti et al. | 2013 |
| BALF GGT activity (2-d, 4-w) | ↑ | ↑ | |||||||||
| 15 (low), 21 (high) | 168 (low), 425 (high) | <0.5 | <0.5 | Wistar-Kyoto rats; SH rats | Male | 4 hr | Susceptibility to apnea after capsaicin | - | ↑ | Lamb et al. | 2012 |
In human adults that received an inhaled allergen co-exposure, particle-filtered exhaust resulted in a greater impairment in forced expiratory volume (FEV1) at 30 minutes after exposure compared to whole diesel exhaust (Wooding et al. 2019). Furthermore, co-exposure to an allergen resulted in significantly increased airway responsiveness in normally responsive adults following exposure to both particle-filtered and whole diesel exhaust (Wooding et al. 2019).
For animal toxicology, three studies found that effects on markers of inflammation and injury in the respiratory system stem mostly from exposure to both particle-filtered and whole diesel exhaust. Both short-term (0–30 days) and long-term (>30 days) exposures to filtered and whole exhaust increased levels of bronchoalveolar lavage fluid (BALF) neutrophils (Gordon et al. 2012; Karthikeyan et al. 2013; Kodavanti et al. 2013). In addition, increased BALF gamma-glutamyl transpeptidase (GGT) activity, a marker of injury, was measured following 2-day and 4-week exposures to both treatments (Gordon et al. 2012; Kodavanti et al. 2013). A single exposure to filtered and whole diesel exhaust increased BALF protein levels (an index of lung injury) and cytokine production, while repeated exposure to both treatments increased levels of BALF macrophages (Karthikeyan et al. 2013). Repeated exposure to particle-filtered, but not whole, exhaust resulted in decreased levels of BALF neutrophils (Karthikeyan et al. 2013). In contrast, Lamb et al. found that 4-hour exposure to whole diesel exhaust, but not particle-filtered, resulted in increased susceptibility to apnea after a capsaicin challenge (Lamb et al. 2012).
Nervous system
Three animal toxicological studies examined effects from particle-filtered and whole diesel exhaust on the nervous system, two of which employed three-month exposures in adults, and one of which involved gestational and postnatal exposure (Table 2). In Win-Shwe et al., only whole diesel exhaust exposure led to an increased escape latency in mice following training in a Morris water maze, suggesting a negative effect on spatial learning from the particulate fraction (Win-Shwe, Yamamoto, et al. 2012). In a second study, mice exposed to both filtered and whole exhaust showed impaired novel object discrimination, represented by no change in the time spent exploring a novel versus familiar object (Win-Shwe, Fujimaki, et al. 2012). Finally, in male offspring that were exposed from gestational day 14 to postnatal day 21, both particle-filtered and whole diesel exhaust impaired sociability, as assessed by time spent with a social vs asocial stimulus, and impaired social novelty preference, as assessed by time spent with a novel vs familiar mouse (Win-Shwe et al. 2016). In contrast, exposure to whole diesel exhaust but not particle-filtered exhaust reduced time spent with a juvenile and resulted in increased levels of the excitatory neurotransmitter glutamate in the hypothalamus (Win-Shwe et al. 2016).
Table 2.
Significant effects on the nervous system resulting from exposure to particle filtered diesel exhaust (fDE) or whole diesel exhaust (wDE) relative to filtered air exposure (arrows indicate direction of effect and statistical significance at α < 0.05; dashes indicate no statistically significant effect observed).
| Nervous System | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PM μg/m3 | NO2 (ppm) | Species | Sex | Duration | Effect | fDE | wDE | Author | Year | ||
| fDE | wDE | fDE | wDE | ||||||||
| 5.5 | 35.5 (med), 122 (high) | 0.47 | 0.16 (med), 0.48 (high) | BALB/c mice | Female | 5 hr/day, 5 day/week for 3 months | Impaired spatial learning – increased escape latency from maze (high) | - | ↑ | Win-Shwe et al. | 2012a |
| 8.5 | 47.8 (med), 129 (high) | 0.46 | 0.18 (med), 0.47 (high) | BALB/c mice | Female | 5 hr/day, 5 day/week for 3 months | Impaired novel object recognition | ↑ | ↑ | Win-Shwe et al. | 2012b |
| 13.3 | 113.9 | 0.99 | 0.43 | BALB/c mice | Male offspring | 5 hr/day, 5 day/week from gestational day 14 to postnatal day 21 | Impaired sociability | ↑ | ↑ | Win-Shwe et al. | 2016 |
| Impaired social novelty preference | ↑ | ↑ | |||||||||
| Impaired social interaction with juvenile | - | ↑ | |||||||||
| Hypothalamic glutamate | - | ↑ | |||||||||
Reproductive/Developmental system
Six animal toxicological studies assessed effects from particle-filtered and whole diesel exhaust on the reproductive/developmental system, five of which were conducted in rats and one in mice (Table 3). Five of these studies assessed endpoints in males (Watanabe and Ohsawa 2002; Watanabe 2005; Li C et al. 2009; Li C, Li, Jigami, et al. 2012; Yamagishi et al. 2012), while one assessed endpoints in females (Li C et al. 2013). Four studies included whole-body gestational exposure and showed health effects from exposure to both particle-filtered and whole diesel exhaust (Watanabe and Ohsawa 2002; Watanabe 2005; Li C et al. 2009; Li C et al. 2013). In contrast, two studies of long-term exposure in adults on reproductive hormones reported most effects from exposure to only whole diesel exhaust (Li C, Li, Suzuki, et al. 2012; Yamagishi et al. 2012).
Table 3.
Significant effects on the reproductive/development system resulting from exposure to particle filtered diesel exhaust (fDE) or whole diesel exhaust (wDE) relative to filtered air exposure (arrows indicate direction of effect and statistical significance at α < 0.05; dashes indicate no statistically significant effect observed). NR = not reported; FSH = follicle stimulating hormone; 3β-HSD =3β-hydroxysteroid dehydrogenase; StAR = steroidogenic acute regulatory protein; hCG = human chorionic gonadotropin.
| Reproductive/Developmental System | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PM μg/m3 | NO2 (ppm) | Species | Sex | Duration | Effect | fDE | wDE | Author | Year | ||
| fDE | wDE | fDE | wDE | ||||||||
| 3.1 | 148.86 | 0.51 | 0.53 | Fischer rats | Male (in utero exposure) | 5 hr/day on days 1–19 of gestation | Serum testosterone | ↓ | ↓ | Li et al. | 2009 |
| Serum progesterone and FSH | ↓ | ↓ | |||||||||
| Inhibin | ↑ | ↑ | |||||||||
| Gene expression of 3β-HSD and StAR in testes | ↓ | ↓ | |||||||||
| Gene expression of FSH receptor in testes | - | ↑ | |||||||||
| Relative weight of seminal vesicle and prostate | ↓ | ↓ | |||||||||
| NR | 1730 | NR | 0.79 | Fischer rats | Male (in utero exposure) | 6 hr/day from day 7 of gestation until delivery | Serum testosterone | ↑ | ↑ | Watanabe & Ohsawa | 2002 |
| Relative spleen and thymus weight | ↓ | ↓ | |||||||||
| 0.69 | 41.7 (low), 152 (high) | 0.53 | 0.16 (low), 0.54 (high) | C57BL/Jcl mice | Male | 5 hr/day, 5 day/week, for 8 weeks | Testosterone in serum and hCG-stimulated Leydig cells | - | ↑ | Li et al. | 2012 |
| Upregulated testosterone biosynthesis genes | - | ↑ | |||||||||
| 3.1 | 38 (low), 149 (high) | 0.51 | 0.17 (low), 0.53 (high) | Fischer rats | Male | 5 hr/day, 5 day/week, for 1, 2, or 3 months | Testosterone in testes (3-m) | ↓ | - | Yamagishi et al. | 2012 |
| Testosterone in plasma and testes (1-m) | - | ↑ | |||||||||
| Androstenedione (1-m) | - | ↑ | |||||||||
| 3.1 | 148.9 | NR | NR | Fischer rats | Female | 5 hr/day on days 1–19 of gestation | Maternal progesterone | ↓ | ↓ | Li et al. | 2013 |
| Maternal luteinizing hormone and corticosterone | ↑ | ↑ | |||||||||
| Estradiol | ↑ | - | |||||||||
| Corpus luteum gene expression | → | → | |||||||||
| Fetal body weight and length | → | → | |||||||||
| Maternal relative spleen and liver weight | ↓ | ↓ | |||||||||
| NR | 170 (low), 1710 (high) | 0.1 (low), 0.8 (high) | 0.1 (low), 0.79 (high) | Fischer rats | Male (in utero exposure) | 6 hr/day from day 7 of gestation until delivery | FSH levels | ↑ | ↑ | Watanabe | 2005 |
| Sperm production, spermatids, Sertoli cells | ↓ | ↓ | |||||||||
| Spermatogonia | ↓ | - | |||||||||
Among these reproductive and development studies, changes to testosterone (T) levels were consistently reported. In utero exposure to both particle-filtered and whole diesel exhaust led to decreases in serum levels of T in male offspring (Li C et al. 2009), and increases in serum T levels following exposure during the suckling period (Watanabe and Ohsawa 2002). In adult male mice, only exposure to whole exhaust resulted in increased serum T and upregulation of T biosynthesis genes in the testes (Li C, Li, Jigami, et al. 2012). Similarly, in adult male rats one month of exposure to whole diesel exhaust, but not particle-filtered, resulted in increased serum T and hippocampal androstenedione levels (Yamagishi et al. 2012).
Similar to T, other reproductive hormones and their signaling pathways were also dysregulated following exposure to diesel exhaust. In Li et al. 2013, pregnant dams exposed to both whole, and particle filtered exhaust had lower serum progesterone (P) and higher serum levels of luteinizing hormone (LH). Maternal gene expression in the corpus luteum at gestation day 20 was also affected by exposure to both filtered and whole exhaust, with reduced expression of the LH receptor, cytochrome P450 side-chain cleavage enzyme, and 3β-hydroxysteroid dehydrogenase (HSD) (Li C et al. 2013). In male offspring, in utero exposure to filtered and whole exhaust led to decreased serum levels of P, as well as decreases in expression of 3β-HSD and steroidogenic acute regulatory protein (StAR) in the testes (Li C et al. 2009).
Two studies reported conflicting effects from diesel exhaust exposure on serum levels of follicle stimulating hormone (FSH) following in utero exposure. Although Li et al. observed decreases in FSH levels following exposure to either particle-filtered or whole exhaust, Watanabe observed increases in FSH (Watanabe 2005; Li C et al. 2009). Li et al. also examined expression of the FSH receptor in the testes and reported increases in receptor levels following exposure to only whole diesel exhaust; this endpoint was not examined in Watanabe (Watanabe 2005; Li C et al. 2009).
A couple of studies also showed that in utero exposure to diesel exhaust affected male reproductive potential, with exposure to whole and particle-filtered exhaust resulting in decreased relative weights of the seminal vesicle and prostate (Li C et al. 2009), reduced sperm production, and a lower number of spermatids and Sertoli cells (Watanabe 2005). Exposure to filtered but not whole exhaust resulted in a reduced number of spermatogonia (Watanabe 2005). In Li et al. 2013, pregnant dams exposed to filtered and whole exhaust had lower relative spleen and liver weights (Li C et al. 2013), while exposure to filtered but not whole diesel exhaust resulted in higher levels of the hormone 17β-estradiol (Li C et al. 2013). Exposure to both treatments resulted in changes in the offspring, including changed fetal body weight and length (Li C et al. 2013) and reductions in relative spleen and thymus weight (Watanabe and Ohsawa 2002).
Cardiovascular system
We identified two controlled human exposure and eight animal toxicological studies that were conducted to differentiate the effects of particle-filtered from whole diesel exhaust exposure on the cardiovascular system (Table 4). In both human studies, cardiovascular effects were generally reported following exposure to whole diesel exhaust with an attenuation in effect associated with particle filtration. However, in animal studies, cardiovascular effects occurred following exposure to both treatments.
Table 4.
Significant effects on the cardiovascular system resulting from exposure to particle filtered diesel exhaust (fDE) or whole diesel exhaust (wDE) relative to filtered air exposure (arrows indicate direction of effect and statistical significance at α < 0.05; dashes indicate no statistically significant effect observed). NR = not reported; SH = spontaneously hypertensive; WKY = Wistar Kyoto; SHHF = spontaneously hypertensive heart failure; LV = left ventricular; QA, PR, QRS, QTc = electrocardiography metrics (see text for details).
| Cardiovascular System | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PM μg/m3 | NO2 (ppm) | Species | Sex | Duration | Effect | fDE | wDE | Author | Year | ||
| fDE | wDE | fDE | wDE | ||||||||
| 7.2 | 320 | 3.44 | 0.69 | Humans | Male | 1 hr (alternating 15 min moderate exercise and rest) | Vasodilation | - | ↓ | Lucking et al. | 2011 |
| Thrombus formation | - | ↑ | |||||||||
| 25 | 325 | 0.0001 | 0.0001 | Humans (patients with heart failure, controls) | Mixed | 21 min (15 min rest, 6 min moderate exercise) | 6-min walking distance | ↓ | ↓ | Vieira et al. | 2016 |
| Arterial stiffness | ↓ | ↓ | |||||||||
| Reactive hyperemia response (heart failure patients) | - | ↓ | |||||||||
| B-type natriuretic peptide (heart failure patients) | - | ↑ | |||||||||
| 0 (low, high) | 153.4 (low), 512.4 (high) | NR | NR | SH rats | Male | 4 hr | QRS duration (low) | - | ↑ | Hazari et al. | 2011 |
| LF/LH ratio (high) | ↑ | - | |||||||||
| Dose aconitine necessary to trigger ventricular fibrillation and cardiac arrest (low) | ↓ | - | |||||||||
| Dose aconitine necessary to trigger ventricular tachycardia (low) | ↓ | ↓ | |||||||||
| Dose aconitine necessary to trigger ventricular fibrillation (high) | ↓ | - | |||||||||
| NR | 1900 | NR | NR | Wistar-Kyoto rats | Male | 5 hr/day, 5 days/week for 4 weeks | Heart rate | ↓ | ↓ | Gordon et al. | 2012 |
| QA interval (4-w) | - | ↑ | |||||||||
| NR | 1220 (2-d); 1494 (4-w) | 1.0 (2-d); 0.8 (4-w) | 0.9 (2-d); 0.7 (4-w) | Wistar-Kyoto rats; SH rats | Male | 4 hr/day for 2 days; 4 hr/day, 5 day/week for 4 weeks | Changes in aortic gene expression | → | → | Kodavanti et al. | 2013 |
| 27.5 | 109 (low), 304.8 (med), 1012.3 (high) | NR | NR | ApoE−/− mice | Male | 6 hr/day for 50 days | Lipid-rich aortic regions | ↓ | ↓ | Campen et al. | 2010 |
| Lipid peroxidases | ↑ | ↑ | |||||||||
| Changes in aortic gene expression | → | → | |||||||||
| 15 (low), 21 (high) | 168 (low), 425 (high) | <0.5 | <0.5 | Wistar-Kyoto rats; SH rats | Male | 4 hr | Heart rate (SH) | ↓ | - | Lamb et al. | 2012 |
| Heart rate (WKY; high) | - | ↓ | |||||||||
| Change in ST amplitude (low) | ↑ | - | |||||||||
| PR interval (high) | ↑ | - | |||||||||
| NR | 2500 | 3 | 3 | Wistar rats | Male | 3 hr/day, 5 day/week for 3 weeks; 3 hours | LV systolic and diastolic diameters | ↑ | ↑ | Karoui et al. | 2019 |
| 6 (low), 770 (high) | 512 (low), 3634 (high) | NR | NR | ApoE−/− mice; C57BL/6J mice | Male | 6 hr/day for 3 days | Heart rate (ApoE−/−) | ↓ | ↓ | Campen et al. | 2005 |
| T-wave area (high) | ↓ | ↓ | |||||||||
| 3 | 472 | 0.4 | 0.3 | SHHF rats | Male | 4 hr | Heart rate | ↓ | - | Carll et al. | 2012 |
| PR interval | ↑ | - | |||||||||
| Blood pressure | ↓ | - | |||||||||
| QTc | ↑ | - | |||||||||
In healthy adults, Lucking et al. demonstrated that a 1-hour exposure to whole, but not particle-filtered, exhaust reduced vasodilation and increased ex vivo thrombus formation (Lucking et al. 2011). In Vieira et al., patients with heart failure exposed to whole, but not particle-filtered exhaust for 21-minutes showed a decrease in the reactive hyperemia index and an increase in B-type natriuretic peptide, a potential molecular marker for heart failure (Vieira et al. 2016). All participants exposed to both filtered and whole exhaust showed decreases in the 6-minute walking distance and in arterial stiffness (Vieira et al. 2016).
In addition to studies in humans, animal toxicological studies examined the potential for diesel exhaust exposure to result in a variety of cardiovascular effects using electrocardiography (ECG). Gordon et al., estimated cardiac contractility using the QA interval and reported that long-term exposure to whole, but not particle-filtered exhaust resulted in an increase in the QA interval (Gordon et al. 2012). In Hazari et al., low dose 4-hour exposure to whole but not particle-filtered diesel exhaust increased QRS duration, indicating a prolongation of ventricular depolarization (Hazari et al. 2011). In contrast, two studies using 4-hour exposures reported ECG measures changed in response to exposure to particle-filtered, but not whole diesel exhaust, including increased QTc (indicator of potential arrythmia) (Carll et al. 2012), increased negative ST amplitude (indicator of potential ischemia) (Lamb et al. 2012), and increased PR interval (an indicator of atrial block) (Carll et al. 2012; Lamb et al. 2012). Meanwhile, exposure to both treatments was reported to decrease the T-wave area (indicator of impaired ventricle repolarization) in a long-term study (Campen et al. 2005) and increase the LF/LH ratio (high dose; estimate of balance between sympathetic and vagal activity) following short-term exposure (Hazari et al. 2011).
In addition to ECG endpoints, animal toxicological studies also examined a variety of other endpoints, with most endpoints changing in response to both particle-filtered and whole diesel exhaust (Table 4). Two long-term studies reported changes in aortic gene expression related to markers of vascular toxicity following exposure to both filtered and whole exhaust (Campen et al. 2010; Kodavanti et al. 2013). In hypertensive rats, 4-hour exposure to both particle-filtered and low-dose whole exhaust decreased the dose of aconitine necessary to trigger ventricular tachycardia (Hazari et al. 2011). In Campen et al. 2010, long-term exposure to both particle-filtered and whole diesel exhaust resulted in a significant reduction in total lipids in the aortic leaflet region, as well as an increase in aortic lipid peroxidases (Campen et al. 2010). Furthermore, Karoui et al., demonstrated that acute and repeated exposure to filtered and whole exhaust resulted in increases in left ventricular diastolic and systolic diameter (Karoui et al. 2019). In contrast, only particle-filtered diesel exhaust decreased the dose of aconitine necessary to trigger ventricular fibrillation and cardiac arrest (Hazari et al. 2011). Similarly, only exposure to particle-filtered exhaust decreased blood pressure in spontaneously hypertensive heart failure rats (Carll et al. 2012).
Heart rate showed significant changes in four studies. In both a short-term and a long-term exposure study, heart rate decreased in response to exposure to both particle-filtered and whole exhaust (Campen et al. 2005; Gordon et al. 2012). However, in two studies of 4-hr exposure with spontaneously hypertensive rats, heart rate decreased following exposure to particle-filtered, but not whole, diesel exhaust (Carll et al. 2012; Lamb et al. 2012). In contrast, in Wistar-Kyoto rats, only exposure to whole diesel exhaust resulted in decreased heart rate (Lamb et al. 2012).
Immune System
One controlled human exposure and four animal toxicological studies examined effects from exposure to particle-filtered and whole diesel exhaust on the immune system (Table 5).
Table 5.
Significant effects on the immune system resulting from exposure to particle filtered diesel exhaust (fDE) or whole diesel exhaust (wDE) relative to filtered air exposure (arrows indicate direction of effect and statistical significance at α < 0.05; dashes indicate no statistically significant effect observed). NR = not reported; IgG = immunoglobulin G; IgE = immunoglobulin E.
| Immune System | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PM μg/m3 | NO2 (ppm) | Species | Sex | Duration | Allergen co-exposure | Effect | fDE | wDE | Author | Year | ||
| fDE | wDE | fDE | wDE | |||||||||
| 18.9 | 292.2 | 0.15 | 0.052 | Human | Male and female | 2 hr | House dust mite; grass; birch | White blood cell count (24 h) | ↑ | ↑ | Wooding et al. | 2019 |
| Neutrophil count (24 h) | - | ↑ | ||||||||||
| Eosinophil count (48 h) | - | ↑ | ||||||||||
| 40 | 1010 | 2.93 | 1.99 | BABL/c mice | Male | 12 hr/day for 5 weeks | Sugi basic protein (allergen of Japanese cedar pollen) | Chemokine production | ↑ | ↑ | Fujimaki et al. | 2004 |
| 40 | 1010 | NR | NR | C57BL/6 mice | Male | 12 hr/day for 5 weeks | Sugi basic protein (allergen of Japanese cedar pollen) | T lymphocytes | ↓ | - | Fujimaki et al. | 2005 |
| Chemokine production | ↑ | - | ||||||||||
| IgG1 titers | ↓ | ↓ | ||||||||||
| IgG2a titers | ↑ | ↑ | ||||||||||
| 0 | 36 (low), 169 (high) | 0.51 | 0.15 (low), 0.51 (high) | ICR mice | Female | 5 hr day, 5 day/wk for 8 weeks | Ovalbumin | Ovalbumin-induced eosinophilic airway inflammation | ↑ | ↑ | Tanaka et al. | 2013 |
| Cytokine and chemokine production | ↑ | ↑ | ||||||||||
| Production/release of myeloperoxidase into alveolar space | ↑ | ↑ | ||||||||||
| NR | 1730 | NR | 0.79 | Fischer rats | Male (in utero exposure) | 6 hr/day for 19 days during gestation | Crude Japanese cedar pollen | Serum testosterone | ↑ | ↑ | Watanabe & Ohsawa | 2002 |
| IgE titers | ↑ | ↑ | ||||||||||
In adult humans that were co-exposed to an inhaled allergen, white blood cell counts increased at 24 h following exposure to both particle-filtered and whole diesel exhaust (Wooding et al. 2019). In contrast, only exposure to whole diesel exhaust resulted in increased neutrophil and eosinophil counts at 24 and 48 h post-exposure, respectively (Wooding et al. 2019).
Of the animal toxicological studies, three involved long-term exposure (Fujimaki and Kurokawa 2004; Fujimaki et al. 2005; Tanaka et al. 2013), while one involved exposure through gestation (Watanabe and Ohsawa 2002). Fujimaki and Kurokawa found that filtered and whole exhaust exposure increased chemokine production in cervical lymph node cells from mice treated with sugi basic protein (SBP), a major allergen of Japanese cedar pollen (Fujimaki and Kurokawa 2004). Fujimaki et al. found that exposure to filtered and whole exhaust affected levels of anti-SBP antibodies, with decreases in anti-SBP IgG1 and increases in anti-SBP IgG2a (Fujimaki et al. 2005). In Tanaka et al., filtered and whole exhaust-exposed mice treated with ovalbumin showed increased cytokine and chemokine production, as well as increased release of myeloperoxidase into the alveolar space (Tanaka et al. 2013). Similarly, exposure to both fractions during the fetal or suckling periods resulted in elevated serum immunoglobin E in rats immunized with Japanese cedar pollen (Watanabe and Ohsawa 2002). In contrast to these studies, Fujimaki et al. reported that exposure to filtered but not whole diesel exhaust increased chemokine production and decreased T lymphocyte populations in mice treated with SBP (Fujimaki et al. 2005).
Other
Three additional animal toxicological studies were identified that allow for the delineation of effects from the two fractions of diesel exhaust, one that examined genotoxicity and two on effects on the endocrine system (Table 6). With respect to genotoxic stress, when male rats were exposed long-term to filtered, but not whole diesel exhaust, Douki et al. reported an increase in genotoxic stress, as measured by assessing the phosphorylation of H2AX in lung samples (Douki et al. 2018). These authors also reported that exposure to filtered exhaust led to an increased fold change in lung tissue gene expression, relating to cell cycle and DNA repair pathways. In regard to the endocrine system, Li et al. 2012(b), reported that the adrenocorticotropic hormone-stimulated release of corticosterone (Cort) and P was blocked in male mice adrenal cells following long-term exposure to filtered and whole exhaust (Li C, Li, Suzuki, et al. 2012). Similarly, in utero exposure to both fractions led to decreased serum levels of corticosterone (Li C et al. 2009). Effects on reproductive hormones are discussed in the Reproductive/Developmental system section.
Table 6.
Significant effects on the other systems resulting from exposure to particle filtered diesel exhaust (fDE) or whole diesel exhaust (wDE) relative to filtered air exposure (arrows indicate direction of effect and statistical significance at α < 0.05; dashes indicate no statistically significant effect observed). NR = not reported.
| Other | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PM μg/m3 | NO2 (ppm) | Species | Sex | Duration | Effect | fDE | wDE | Author | Year | ||
| fDE | wDE | fDE | wDE | ||||||||
| <1 | 2300 | NR | NR | Wistar rat | Male | 3 hr/day, 5 day/week for 3 weeks | γH2AX | ↑ | - | Douki et al. | 2018 |
| Fold change in lung tissue gene expression of cell cycle and DNA repair pathways | ↑ | - | |||||||||
| 0.69 | 41.7 (low), 152 (high) | 0.53 | 0.16 (low), 0.54 (high) | C57BL/Jcl mice | Male | 5 hr/day, 5 days/week for 8 weeks | Adrenocorticotropic hormone -stimulated hormone release from adrenal cells | ↓ | ↓ | Li et al. | 2012b |
| 3.1 | 148.86 | 0.51 | 0.53 | Fischer rats | Male (in utero exposure) | 5 hr/day during days 1–19 of gestation | Corticosterone | ↓ | ↓ | Li et al. | 2009 |
Discussion
In the United States, most commercial transportation vehicles rely on diesel engines, and in other parts of the world, particularly Europe and India, diesel vehicles are also common in the private transportation sector. Given that diesel is a significant contributor to urban air pollution, it is important to examine how recent changes in diesel engine technology, primarily resulting in a reduction in particulate matter mass, may impact public health. Our systematic review of the literature revealed 26 relevant studies that included treatment groups of filtered and unfiltered (whole) diesel exhaust, allowing for a delineation of statistically significant health effects from particle-filtered and whole diesel exhaust. We found that the majority of statistically significant effects on health-related endpoints were in response to exposure to both filtered and whole diesel exhaust. Given that the primary difference between these treatment groups is the filtering of particulate matter, the gaseous fraction of diesel exhaust may be the primary driver of the observed effects. However, given the variation across studies in the amount and size of particulate matter removed, it remains possible that particles not removed by filtration may be responsible for observed effects. Specifically, we found that all respiratory and immune system endpoints, as well as all studies that involved gestational exposure, showed significant effects associated with filtered-diesel exhaust exposure. Studies on cardiovascular, nervous system, and endocrine endpoints showed specific effects from both particle-filtered and whole diesel exhaust. It is important to note, however, that many of the studies we examined likely lacked the power to detect statistically significant differences between treatment groups, and our focus only on significant differences may unintentionally distort the complexity of some of the study results.
The toxicity of particulate matter is well characterized. While the reduction in particulate matter from newer technology diesel engines and the addition of filtration has very likely reduced the overall toxicity of diesel exhaust (McDonald et al. 2015; Reis et al. 2018), our evaluation suggests that particulate-filtered exhaust can still result in impaired physiological function. An important limitation to this result is that none of the studies that met our inclusion criteria (i.e., studies that directly compared the health effects of filtered to whole diesel exhaust) reflect 2010-compliant diesel engines with manufacturer built-in catalyzed filters, which greatly reduce emissions of oxides of nitrogen. In addition, filtering methods and other operating conditions employed in the laboratory can result in a different exhaust composition compared to on-road aftertreatment approaches (Lee et al. 2015). In laboratory combustion atmospheres, the ratio of gases to particulate matter may be higher than in the environment, depending on the dilution factors.
Still, certain gaseous components of exhaust common across combustion sources result in known toxicity. In a meta-analysis that examined the effects of individual chemical components in diesel and gasoline exhaust, coal emissions, and wood smoke on vascular responses in mice, filtration of particles was shown to have little effect, with the most pronounced health effects from oxides of nitrogen, sulfur dioxide, ammonia, and carbon monoxide (Seilkop et al. 2012). Several studies have suggested that exposure to NO2, an oxidant gas, may be responsible for the toxicity associated with filtered diesel exhaust, as well as with exhaust from newer technology diesel engines. For example, the inflammatory responses observed in Karthikeyan et al. were consistent with the inflammatory potential of NO2 (Karthikeyan et al. 2013). Furthermore, in the Advanced Collaborative Emissions Study (ACES), the lung pathology that resulted from exposure to diesel exhaust was similar to that from studies in which rodents were exposed to NO2 alone (McDonald et al. 2015). Importantly, the engines employed in the study were 2007-, not 2010-compliant, suggesting that exposure to exhaust from newer diesel engines may result in substantially reduced pathology, though additional research is needed (McDonald et al. 2015). Moreover, while 2010-compliant engines are designed to produce lower levels of NO2 (Khalek et al. 2015), selective catalytic reduction devices do not operate optimally under all driving conditions, such as under cold-start and stop-and-go driving (Misra et al. 2013; Costantini et al. 2016). With respect to the studies that met our study inclusion criteria and measured NO2, nearly half of them reported that levels were higher in the filtered exhaust by a factor of 2 or more. Finally, while there are many other gases present in diesel exhaust that may result in adverse health effects (e.g., acetaldehyde), these were not consistently measured or reported in the included studies, thus we are unable to draw further conclusions.
For eighteen health endpoints from nine different studies that met our inclusion criteria (affecting respiratory, reproductive, cardiovascular, and immune systems), treatment with filtered diesel exhaust resulted in more overt responses compared to whole diesel exhaust (i.e., significant effects were only observed in the particle-filtered treatment groups). More potent effects from filtered exhaust relative to whole exhaust could be the result of several potential mechanisms. If the components remain the same, physical and chemical interactions between gases and particles may inhibit the adverse effects of either component in whole exhaust, or the gas and particles could each result in competing autonomic effects (Carll et al. 2012). Alternatively, there is evidence that filtration of particles can increase the levels of select chemicals. In a recent study, application of a particle filter reduced levels of alkanes and polycyclic aromatic hydrocarbons, but increased levels of carbonyl compounds, acetaldehyde, and formaldehyde (Karoui et al. 2019). As mentioned earlier, some particle filtration technologies can also increase NO2 levels, which may account for the more overt responses to filtered exhaust that were observed in Karthikeyan et al, where NO2 levels were 3-fold higher. This again suggests that exposure to emissions from 2010-compliant diesel engines may result in reduced health effects. Finally, some particle filtration technologies can increase levels of ultrafine particles (Karthikeyan et al. 2013), which may have greater toxicity relative to larger particles per-mass (Chen et al. 2016). Unfortunately, there is an insufficient amount of data on particle number and size across studies, particularly for cardiovascular endpoints, to draw associations between particle measurements and health effects.
The studies identified by our systematic review included exposure conditions that were highly variable. The concentrations of particulate matter varied widely between studies, from 35.5 to 2500 μg/m3 (n=26 studies) in whole diesel exhaust, and from 0 to 40 μg/m3 (n=22 studies) in filtered diesel exhaust. Notably, for studies in which there were significant effects from whole exhaust exposure alone, the concentrations of particulate matter were on the lower scale, from 122 to 472 μg/m3 (n=8 studies). Most studies reported only total particle mass, rather than particle number. It is possible that the primary driver of effects in these studies was a high concentration of ultrafine particles which was not captured by measuring mass alone. It would be helpful for risk identification if particle number were reliably and consistently reported. Further, the duration of exposure ranged from a single 21-minute exposure to repeated exposure for 3 months. Two human studies were the shortest in duration (21 minutes and 1 hour) and did not identify changes to heart rate. In contrast, four animal toxicological studies, ranging from 4 hours to 2 days, reported heart rate changes, though the results were mixed from exposure to particle-filtered and whole diesel exhaust across studies. There appears to be little association between exposure time and the exhaust fraction responsible for the observed effects. Given that our review spanned multiple health effects, it is likely that there are diverse mechanisms at play and that there are too few studies on a single health endpoint to allow an examination of associations between exposure dose, duration, and effect.
The design of new diesel engine technology is critically dependent on an understanding of which components of diesel exhaust are the most harmful to human health. Importantly, our review of the literature found that there were a relatively limited number of studies that directly compared the health effects from whole and filtered diesel exhaust. In the studies we did identify, most of the statistically significant health effects from whole diesel exhaust were not appreciably attenuated by the particle filtration methods employed. This was particularly true for respiratory and immune system endpoints. However, interestingly, there were also several health endpoints for which significant effects resulted from exposure to particle-filtered, but not whole diesel exhaust. The addition of future studies may aid in further revealing differences in organ system susceptibility to the different components of exhaust. Finally, a more complete understanding of the contributions of all individual components within diesel exhaust will be important for human risk assessment.
Supplementary Material
Acknowledgements
We thank George Woodall, Stephen Graham, Chad Bailey, Steve Dutton, and John Vandenberg for their review of the manuscript. This manuscript has been reviewed by the U.S. EPA and approved for publication. Approval does not necessarily signify that the contents reflect the views or opinions of the U.S. EPA, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Footnotes
Declaration of Interest Statement
The authors report no conflict of interest.
References
- Atkinson RW, Analitis A, Samoli E, Fuller GW, Green DC, Mudway IS, Anderson HR, Kelly FJ. 2016. Short-term exposure to traffic-related air pollution and daily mortality in London, UK. J Expo Sci Env Epid. 26(2):125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA et al. 2010. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 121(21):2331–2378. [DOI] [PubMed] [Google Scholar]
- Campen MJ, Babu NS, Helms GA, Pett S, Wernly J, Mehran R, McDonald JD. 2005. Nonparticulate components of diesel exhaust promote constriction in coronary arteries from ApoE−/− mice. Toxicological sciences : an official journal of the Society of Toxicology. 88(1):95–102. [DOI] [PubMed] [Google Scholar]
- Campen MJ, Lund AK, Knuckles TL, Conklin DJ, Bishop B, Young D, Seilkop S, Seagrave J, Reed MD, McDonald JD. 2010. Inhaled diesel emissions alter atherosclerotic plaque composition in ApoE(−/−) mice. Toxicol Appl Pharmacol. 242(3):310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carll AP, Hazari MS, Perez CM, Krantz QT, King CJ, Winsett DW, Costa DL, Farraj AK. 2012. Whole and particle-free diesel exhausts differentially affect cardiac electrophysiology, blood pressure, and autonomic balance in heart failure-prone rats. Toxicological sciences : an official journal of the Society of Toxicology. 128(2):490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassee FR, Heroux ME, Gerlofs-Nijland ME, Kelly FJ. 2013. Particulate matter beyond mass: recent health evidence on the role of fractions, chemical constituents and sources of emission. Inhalation toxicology. 25(14):802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Hu B, Liu Y, Xu JX, Yang GS, Xu DD, Chen CY. 2016. Beyond PM2.5: The role of ultrafine particles on adverse health effects of air pollution. Bba-Gen Subjects. 1860(12):2844–2855. English. [DOI] [PubMed] [Google Scholar]
- Costa LG, Cole TB, Coburn J, Chang YC, Dao K, Roque PJ. 2017. Neurotoxicity of traffic-related air pollution. Neurotoxicology. 59:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini MG, Khalek I, McDonald JD, van Erp AM. 2016. The advanced collaborative emissions study (ACES) of 2007-and 2010-emissions compliant heavy-duty diesel engines: characterization of emissions and health effects. Emission Control Science and Technology. 2(4):215–227. [Google Scholar]
- Douki T, Corbiere C, Preterre D, Martin PJ, Lecureur V, Andre V, Landkocz Y, Pottier I, Keravec V, Fardel O et al. 2018. Comparative study of diesel and biodiesel exhausts on lung oxidative stress and genotoxicity in rats. Environ Pollut. 235:514–524. [DOI] [PubMed] [Google Scholar]
- Ema M, Naya M, Horimoto M, Kato H. 2013. Developmental toxicity of diesel exhaust: a review of studies in experimental animals. Reproductive toxicology. 42:1–17. [DOI] [PubMed] [Google Scholar]
- EPA US. 2001. Control of Air Pollution From New Motor Vehicles: Heavy-Duty Engine and Vehicle Standards and Highway Diesel Fuel Sulfur Control Requirements; Final Rule. Federal Register. 66(12):5002–5193. [Google Scholar]
- EPA US. 2009. Integrated Science Assessment (ISA) for Particulate Matter (Final Report, Dec 2009). Washington, DC: U.S. Environmental Protection Agency. [Google Scholar]
- Fujimaki H, Kurokawa Y. 2004. Diesel exhaust-associated gas components enhance chemokine production by cervical lymph-node cells from mice immunized with sugi basic proteins. Inhal Toxicol. 16(1):61–65. [DOI] [PubMed] [Google Scholar]
- Fujimaki H, Yamamoto S, Kurokawa Y. 2005. Effect of diesel exhaust on immune responses in C57BL/6 mice intranasally immunized with pollen antigen. J UOEH. 27(1):11–24. [DOI] [PubMed] [Google Scholar]
- Ghio AJ, Smith CB, Madden MC. 2012. Diesel exhaust particles and airway inflammation. Current opinion in pulmonary medicine. 18(2):144–150. [DOI] [PubMed] [Google Scholar]
- Gordon CJ, Schladweiler MC, Krantz T, King C, Kodavanti UP. 2012. Cardiovascular and thermoregulatory responses of unrestrained rats exposed to filtered or unfiltered diesel exhaust. Inhalation toxicology. 24(5):296–309. [DOI] [PubMed] [Google Scholar]
- Hazari MS, Haykal-Coates N, Winsett DW, Krantz QT, King C, Costa DL, Farraj AK. 2011. TRPA1 and sympathetic activation contribute to increased risk of triggered cardiac arrhythmias in hypertensive rats exposed to diesel exhaust. Environmental health perspectives. 119(7):951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesterberg TW, Long CM, Sax SN, Lapin CA, McClellan RO, Bunn WB, Valberg PA. 2011. Particulate matter in new technology diesel exhaust (NTDE) is quantitatively and qualitatively very different from that found in traditional diesel exhaust (TDE). Journal of the Air & Waste Management Association. 61(9):894–913. [DOI] [PubMed] [Google Scholar]
- IARC. 2014. Diesel and Gasoline Engine Exhausts and Some Nitroarenes. Iarc Monographs on the Evaluation of Carcinogenic Risks to Humans. IARC monographs on the evaluation of carcinogenic risks to humans. 105:9–699. [PMC free article] [PubMed] [Google Scholar]
- Karoui A, Crochemore C, Mulder P, Preterre D, Cazier F, Dewaele D, Corbiere C, Mekki M, Vendeville C, Richard V et al. 2019. An integrated functional and transcriptomic analysis reveals that repeated exposure to diesel exhaust induces sustained mitochondrial and cardiac dysfunctions. Environmental pollution. 246:518–526. [DOI] [PubMed] [Google Scholar]
- Karthikeyan S, Thomson EM, Kumarathasan P, Guenette J, Rosenblatt D, Chan T, Rideout G, Vincent R. 2013. Nitrogen Dioxide and Ultrafine Particles Dominate the Biological Effects of Inhaled Diesel Exhaust Treated by a Catalyzed Diesel Particulate Filter. Toxicological Sciences. 135(2):437–450. [DOI] [PubMed] [Google Scholar]
- Khalek IA, Blanks MG, Merritt PM, Zielinska B. 2015. Regulated and unregulated emissions from modern 2010 emissions-compliant heavy-duty on-highway diesel engines. Journal of the Air & Waste Management Association. 65(8):987–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalek IA, Bougher TL, Merritt PM, Zielinska B. 2011. Regulated and unregulated emissions from highway heavy-duty diesel engines complying with U.S. Environmental Protection Agency 2007 emissions standards. Journal of the Air & Waste Management Association. 61(4):427–442. [DOI] [PubMed] [Google Scholar]
- Kodavanti UP, Thomas RF, Ledbetter AD, Schladweiler MC, Bass V, Krantz QT, King C, Nyska A, Richards JE, Andrews D et al. 2013. Diesel exhaust induced pulmonary and cardiovascular impairment: the role of hypertension intervention. Toxicol Appl Pharmacol. 268(2):232–240. [DOI] [PubMed] [Google Scholar]
- Lall R, Ito K, Thurston GD. 2011. Distributed Lag Analyses of Daily Hospital Admissions and Source-Apportioned Fine Particle Air Pollution. Environmental health perspectives. 119(4):455–460. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb CM, Hazari MS, Haykal-Coates N, Carll AP, Krantz QT, King C, Winsett DW, Cascio WE, Costa DL, Farraj AK. 2012. Divergent electrocardiographic responses to whole and particle-free diesel exhaust inhalation in spontaneously hypertensive rats. Toxicological sciences : an official journal of the Society of Toxicology. 125(2):558–568. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Fuller R, Acosta NJR, Adeyi O, Arnold R, Basu NN, Balde AB, Bertollini R, Bose-O’Reilly S, Boufford JI et al. 2018. The Lancet Commission on pollution and health. Lancet. 391(10119):462–512. [DOI] [PubMed] [Google Scholar]
- Lee SH, Kwak JH, Lee SY, Lee JH. 2015. On-road chasing and laboratory measurements of exhaust particle emissions of diesel vehicles equipped with aftertreatment technologies (DPF, urea-SCR). Int J Auto Tech-Kor. 16(4):551–559. [Google Scholar]
- Levesque S, Taetzsch T, Lull ME, Kodavanti U, Stadler K, Wagner A, Johnson JA, Duke L, Kodavanti P, Surace MJ et al. 2011. Diesel exhaust activates and primes microglia: air pollution, neuroinflammation, and regulation of dopaminergic neurotoxicity. Environmental health perspectives. 119(8):1149–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Li X, Jigami J, Hasegawa C, Suzuki AK, Zhang Y, Fujitani Y, Nagaoka K, Watanabe G, Taya K. 2012. Effect of nanoparticle-rich diesel exhaust on testosterone biosynthesis in adult male mice. Inhalation toxicology. 24(9):599–608. [DOI] [PubMed] [Google Scholar]
- Li C, Li X, Suzuki AK, Fujitani Y, Jigami J, Nagaoka K, Watanabe G, Taya K. 2012. Effects of exposure to nanoparticle-rich diesel exhaust on adrenocortical function in adult male mice. Toxicology letters. 209(3):277–281. [DOI] [PubMed] [Google Scholar]
- Li C, Li X, Suzuki AK, Zhang Y, Fujitani Y, Nagaoka K, Watanabe G, Taya K. 2013. Effects of exposure to nanoparticle-rich diesel exhaust on pregnancy in rats. The Journal of reproduction and development. 59(2):145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Taneda S, Taya K, Watanabe G, Li X, Fujitani Y, Nakajima T, Suzuki AK. 2009. Effects of in utero exposure to nanoparticle-rich diesel exhaust on testicular function in immature male rats. Toxicology letters. 185(1):1–8. [DOI] [PubMed] [Google Scholar]
- Li N, Georas S, Alexis N, Fritz P, Xia T, Williams MA, Horner E, Nel A. 2016. A work group report on ultrafine particles (American Academy of Allergy, Asthma & Immunology): Why ambient ultrafine and engineered nanoparticles should receive special attention for possible adverse health outcomes in human subjects. J Allergy Clin Immun. 138(2):386–396. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucking AJ, Lundback M, Barath SL, Mills NL, Sidhu MK, Langrish JP, Boon NA, Pourazar J, Badimon JJ, Gerlofs-Nijland ME et al. 2011. Particle traps prevent adverse vascular and prothrombotic effects of diesel engine exhaust inhalation in men. Circulation. 123(16):1721–1728. [DOI] [PubMed] [Google Scholar]
- McClellan RO, Hesterberg TW, Wall JC. 2012. Evaluation of carcinogenic hazard of diesel engine exhaust needs to consider revolutionary changes in diesel technology. Regulatory toxicology and pharmacology : RTP. 63(2):225–258. [DOI] [PubMed] [Google Scholar]
- McDonald JD, Doyle-Eisele M, Seagrave J, Gigliotti AP, Chow J, Zielinska B, Mauderly JL, Seilkop SK, Miller RA, Committee HEIHR. 2015. Part 1. Assessment of carcinogenicity and biologic responses in rats after lifetime inhalation of new-technology diesel exhaust in the ACES bioassay. Research report.(184):9–44; discussion 141–171. [PubMed] [Google Scholar]
- Mills NL, Tornqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W, Boon NA, Donaldson K, Blomberg A, Sandstrom T et al. 2005. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 112(25):3930–3936. [DOI] [PubMed] [Google Scholar]
- Misra C, Collins JF, Herner JD, Sax T, Krishnamurthy M, Sobieralski W, Burntizki M, Chernich D. 2013. In-Use NOx Emissions from Model Year 2010 and 2011 Heavy-Duty Diesel Engines Equipped with Aftertreatment Devices. Environmental science & technology. 47(14):7892–7898. English. [DOI] [PubMed] [Google Scholar]
- Reis H, Reis C, Sharip A, Reis W, Zhao Y, Sinclair R, Beeson L. 2018. Diesel exhaust exposure, its multi-system effects, and the effect of new technology diesel exhaust. Environ Int. 114:252–265. English. [DOI] [PubMed] [Google Scholar]
- Ristovski ZD, Miljevic B, Surawski NC, Morawska L, Fong KM, Goh F, Yang IA. 2012. Respiratory health effects of diesel particulate matter. Respirology. 17(2):201–212. English. [DOI] [PubMed] [Google Scholar]
- Seilkop SK, Campen MJ, Lund AK, McDonald JD, Mauderly JL. 2012. Identification of chemical components of combustion emissions that affect pro-atherosclerotic vascular responses in mice. Inhalation toxicology. 24(5):270–287. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro AJ, Antoni S, Guyton KZ, Lunn RM, Loomis D, Rusyn I, Jahnke GD, Schwingl PJ, Mehta SS, Addington J et al. 2018. Software Tools to FacilitateSystematic Review Used for Cancer Hazard Identification. Environmental health perspectives. 126(10):104501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner S, Bisig C, Petri-Fink A, Rothen-Rutishauser B. 2016. Diesel exhaust: current knowledge of adverse effects and underlying cellular mechanisms. Archives of toxicology. 90(7):1541–1553. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydbom A, Blomberg A, Parnia S, Stenfors N, Sandstrom T, Dahlen SE. 2001. Health effects of diesel exhaust emissions. The European respiratory journal. 17(4):733–746. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Aoki Y, Takano H, Fujitani Y, Hirano S, Nakamura R, Sone Y, Kiyono M, Ichinose T, Itoh T et al. 2013. Effects of exposure to nanoparticle-rich or -depleted diesel exhaust on allergic pathophysiology in the murine lung. J Toxicol Sci. 38(1):35–48. [DOI] [PubMed] [Google Scholar]
- Tyler CR, Zychowski KE, Sanchez BN, Rivero V, Lucas S, Herbert G, Liu J, Irshad H, McDonald JD, Bleske BE et al. 2016. Surface area-dependence of gas-particle interactions influences pulmonary and neuroinflammatory outcomes. Part Fibre Toxicol. 13. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira JL, Guimaraes GV, de Andre PA, Cruz FD, Saldiva PH, Bocchi EA. 2016. Respiratory Filter Reduces the Cardiovascular Effects Associated With Diesel Exhaust Exposure: A Randomized, Prospective, Double-Blind, Controlled Study of Heart Failure: The FILTER-HF Trial. JACC Heart failure. 4(1):55–64. [DOI] [PubMed] [Google Scholar]
- Watanabe N. 2005. Decreased number of sperms and Sertoli cells in mature rats exposed to diesel exhaust as fetuses. Toxicology letters. 155(1):51–58. English. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Ohsawa M. 2002. Elevated serum immunoglobulin E to Cryptomeria japonica pollen in rats exposed to diesel exhaust during fetal and neonatal periods. BMC pregnancy and childbirth. 2(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win-Shwe TT, Fujimaki H, Fujitani Y, Hirano S. 2012. Novel object recognition ability in female mice following exposure to nanoparticle-rich diesel exhaust. Toxicol Appl Pharm. 262(3):355–362. English. [DOI] [PubMed] [Google Scholar]
- Win-Shwe TT, Kyi-Tha-Thu C, Moe Y, Fujitani Y, Tsukahara S, Hirano S. 2016. Exposure of BALB/c Mice to Diesel Engine Exhaust Origin Secondary Organic Aerosol (DE-SOA) during the Developmental Stages Impairs the Social Behavior in Adult Life of the Males. Frontiers in neuroscience. 9:524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win-Shwe TT, Yamamoto S, Fujitani Y, Hirano S, Fujimaki H. 2012. Nanoparticle-rich diesel exhaust affects hippocampal-dependent spatial learning and NMDA receptor subunit expression in female mice. Nanotoxicology. 6(5):543–553. English. [DOI] [PubMed] [Google Scholar]
- Wooding DJ, Ryu MH, Huls A, Lee AD, Lin DTS, Rider CF, Yuen ACY, Carlsten C. 2019. Particle Depletion Does Not Remediate Acute Effects of Traffic-related Air Pollution and Allergen. A Randomized, Double-Blind Crossover Study. American journal of respiratory and critical care medicine. 200(5):565–574. [DOI] [PubMed] [Google Scholar]
- Yamagishi N, Ito Y, Ramdhan DH, Yanagiba Y, Hayashi Y, Wang D, Li CM, Taneda S, Suzuki AK, Taya K et al. 2012. Effect of nanoparticle-rich diesel exhaust on testicular and hippocampus steroidogenesis in male rats. Inhalation toxicology. 24(8):459–467. English. [DOI] [PubMed] [Google Scholar]
- Yin JX, Harrison RM, Chen Q, Rutter A, Schauer JJ. 2010. Source apportionment of fine particles at urban background and rural sites in the UK atmosphere. Atmos Environ. 44(6):841–851. English. [Google Scholar]
- Yost EE, Euling SY, Weaver JA, Beverly BEJ, Keshava N, Mudipalli A, Arzuaga X, Blessinger T, Dishaw L, Hotchkiss A et al. 2019. Hazards of diisobutyl phthalate (DIBP) exposure: A systematic review of animal toxicology studies. Environ Int. 125:579–594. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.