Abstract
BACKGROUND:
Uterine fibroids are common. Symptoms are debilitating for many, leading to high medical and societal costs. Indirect data suggest that compared to white women, African-Americans develop fibroids at least ten years earlier on average, and their higher health burden has been well documented.
OBJECTIVE:
To directly measure fibroid incidence and growth in a large, community-based cohort of young African-American women.
STUDY DESIGN:
This observational, community-based, prospective study enrolled 1693 African American women, ages 23–35 with no prior diagnosis of fibroids. Standardized transvaginal ultrasound examinations at enrollment and after approximately 18-months were conducted to identify and measure fibroids ≥0.5 cm in diameter. Fibroid growth (change in natural log volume per 18 months) was analyzed with mixed model regression (n = 344 fibroids from 251 women whose baseline ultrasound revealed already existing fibroids).
RESULTS:
Among the 1123 fibroid-free women with follow-up data (88% were followed), incidence was 9.4% (95% confidence interval, CI=7.7,11.2) and increased with age (ptrend=<0.0001), from 6% (CI=3,9) for 23–25 year-olds to 13% (CI=9,17) for 32–35 year-olds. The chance of any new fibroid development was over twice as high for women with existing fibroids compared to women who were fibroid-free at baseline (age-adjusted relative risk = 2.3, (CI=1.7,3.0). The uterine position of most incident fibroids (60%) was intramural corpus. Average fibroid growth was 89% per 18 months (CI=74%,104%), but varied by baseline fibroid size (p<0.0001). Fibroids ≥2 cm in diameter had average growth rates well under 100%. In contrast, small fibroids (<1 cm diameter) had an average growth rate of nearly 200% (188%, CI=145%,238%). However, these small fibroids also had a high estimated rate of disappearance (23%).
CONCLUSIONS:
This is the first study to directly measure age-specific fibroid incidence with a standardized ultrasound protocol and to measure fibroid growth in a large community-based sample. Findings indicate that very small fibroids are very dynamic in their growth, with rapid growth, but a high chance of loss. Larger fibroids grow more slowly. For example, a 2-cm fibroid is likely to take 4–5 years to double its diameter. Detailed data on fibroid incidence confirm an early onset in African American women.
Keywords: epidemiology, incidence, tumor growth, ultrasound, uterine leiomyomata
Introduction
Uterine leiomyomata (fibroids) are common benign tumors of the uterine muscle layer. The condition is a major cause of morbidity for reproductive-age women. Symptoms include pelvic pain, heavy menstrual bleeding with subsequent anemia, urinary incontinence, bleeding during pregnancy and postpartum hemorrhage.1 These tumors are the leading indication for gynecologic in-patient care for premenopausal women in the United States.2 Annual costs are estimated at from $6–$34 billion,3 and high costs are also seen internationally.4 Despite the major health burden, in vivo fibroid development has received limited attention by researchers. More information is needed about age-specific fibroid incidence and growth to develop life-course treatment strategies.
African Americans are at higher risk of fibroid-related health problems than are women of European ancestry.5,6,7 Mathematical models from cross-sectional prevalence data suggest about a 10-year earlier tumor onset in black women compared to white women.8 Yet, once the 10-year offset is considered, the two ethnic groups appear to have a similar rate of increase in risk with increasing age.8 The two ethnic groups may also have similar fibroid growth rates among women <35 years of age.9 It may be that the earliear onset in blacks that results in a longer period of hormonal support for tumor development is the major contributor to the increased health burden experienced by black women.
Despite the health burden of fibroids, no prospectively collected, image-based data on age-specific fibroid incidence exists. Data on fibroid growth are limited, especially for African-Americans. The aim of the current study is to provide such needed data by conducting standardized ultrasound examinations at baseline and after an 18-month follow-up period in a large cohort of African-American women.
Materials and Methods
Study population
The Study of Environment, Lifestyle & Fibroids (SELF) is a community-based, cohort study of fibroid incidence and growth. Detailed methods have been published.10 Eligible participants were self-identified African-American women who lived in the Detroit, Michigan area, 23–34 years of age at recruitment (23–35 at enrollment), with an intact uterus and no prior diagnosis of fibroids. Participants were screened for fibroids at baseline and again after an approximate 18-month follow-up. Of the 1693 participants 1490 (88%) returned for the follow-up visit. After exclusion due to limited ultrasound quality at either visit (n=56) or uterine surgery during follow-up (n=7), 1427 constituted the analysis sample (Supplementary Methods 1.1). The study was approved by the institutional review boards of the National Institute of Environmental Health Sciences and Henry Ford Health Systems. Participants gave informed consent.
Ultrasound assessment of fibroid status
Study sonographers were registered diagnostic medical sonographers and had at least three years of gynecologic ultrasound experience. Additional study-specific training was conducted to assure standardized fibroid detection and data recording for fibroids ≥0.5 cm in diameter.6, 11,12 Ultrasound examinations were conducted transvaginally, with 2-D equipment (Supplementary Methods 1.2). The largest six fibroids were measured in triplicate, i.e., during each of 3 separate passes through the uterus each fibroid’s 3 dimensions were measured. In addition, the total number of fibroids were counted, with “10 or more” recorded as “10”. The position of each fibroid in the uterus was recorded with a similar protocol to that used by Peddada et al. 9 See Supplementary Methods 1.2 for details of the ultrasound examination and data recording.
For analyses, the volume of each fibroid was calculated from the 3 measured diameters using the ellipsoid formula, and the calculated volumes from the 3 replicate measures were averaged. Still and video images were archived from each examination and used for quality control. The head sonographer (TC) reviewed 8% of each sonographer’s examinations each month, or at least one per sonographer (oversampled for those with fibroids).
Statistical analyses
Variables for fibroid size and position in uterus were constructed to have sufficient numbers in each category for analysis. Fibroid size (volume) was categorized into a 5-level variable defined by the diameter equivalent of the volume (Supplementary Methods 1.3). Position in the uterus was categorized as submucosal, intramural fundal, intramural corpus, intramural lower uterine segment, or subserosal/serosally-pedunculated, given the predominance of intramural fibroids.
Analysis of fibroid incidence –
Fibroid incidence was defined as detection of fibroid(s) at follow-up in the 1123 women who were fibroid-free at baseline (Supplementary Methods 1.1). We used logistic regression to assess the association between women’s age and development of incident fibroids. We did not adjust for length of follow-up, because it differed little among participants (90% within 3 months of the mean length), and when tested, it did not influenced incidence. To investigate whether the size and position of incident fibroids differed from size and position of fibroids present at baseline, we compared the two groups of fibroids using logistic regression. Results from an analysis that adjusted for possible correlation among fibroids from the same woman (mixed model, SAS PROC GLIMMIX) showed very similar results, and are not shown.
Analysis of net gain or loss in number of fibroids among those with fibroids at baseline –
The analysis sample included those who had 1–4 fibroids at baseline (n=282 women, 93% of the 304 women with fibroids at baseline) (Supplementary Methods 1.1). Those with ≥5 at baseline were excluded because ultrasound accuracy declines as number of fibroids increase,13 especially when numbers exceed four.14 Net gain (or loss) was calculated by subtracting the number of fibroids at baseline from the number at follow-up. It is possible that some women who had no net change in fibroid number had both lost a fibroid and gained a fibroid. Thus, our net gain and net loss estimates may underestimate true gain and loss.
Logistic regression was used to examine factors associated with net gain (vs no net gain) and net loss (vs no net loss) of fibroids, including baseline age (4-level ordinal variable), number of fibroids (continuous), and size of largest fibroid (5-level ordinal variable). Follow-up interval length was not predictive of net gain or loss, so was not included in the model.
The specific fibroids gained or lost were not identified except for those in the 33 women who lost all their fibroids (n=35 fibroids). Using logistic regression, we compared fibroid size (5-level ordinal variable) and position (5-level class variable) of the lost fibroids with size and position of baseline fibroids from women who still had fibroids at follow-up (N = 249). Results of a mixed-model analyses were essentially identical and not presented. Extrapolating from the 35 lost fibroids in the 33 women who lost all their fibroids, we also estimated the percent of prevalent baseline fibroids in each fibroid size category that were lost (Supplementary Methods 1.4).
Risk of developing new fibroids for women with baseline fibroids compared to risk for women fibroid-free at baseline –
We combined the sample of 1123 fibroid-free women with the 282 women with 1–4 fibroids at baseline. We used log-binomial regression to estimate the relative risk (RR) and 95% CIs of any new fibroids for the women with prevalent fibroids at baseline compared to development of incident fibroids in fibroid-free women.
Analysis of Fibroid growth –
Fibroid growth was evaluated for a set of 344 fibroids from 251 women. This sample of fibroid pairs was identified as the same fibroid at baseline and follow-up based on the position in the uterus and/or by confirmation from examination of the archived scans by the head sonographer (Supplementary Methods 1.1). Growth was calculated as the difference between baseline and follow-up size indexed to 18 months, using the natural logarithm of the volumes and previously described methods.9 We conducted outlier analysis, as described by Peddada et al.9 and identified five rapidly shrinking fibroids (residuals >3 standard deviations from the mean). On examination, the patterns across covariate categories for analyses with and without outliers were similar, and we present results excluding the outliers, as done in the prior literature.9 For ease of interpretation, growth rates were converted to percent change in fibroid volume per 18 months, and fibroid sizes are presented in diameter equivalencies of volume (Supplementary Methods 1.5).
We examined factors potentially affecting fibroid growth using a mixed model (GLIMMIX procedure in SAS 9.4) to account for the potentially correlated growth among fibroids from the same woman. Factors were examined first in univariate models. Then, multivariate models were used to adjust for factors found to be important in univariate models (age and initial fibroid size), after removing outliers. To better describe the association of initial fibroid volume with 18-month growth, we developed a natural cubic spline model with four a priori cut-points (Supplementary Methods 1.6).
Results
Participant characteristics of the 1427 women in the analysis sample are shown in Table 1. Uterine fibroids were detected at baseline for 21%. Most women with baseline fibroids had single small fibroids (Table 1).
TABLE 1.
Characteristics of participants (N = 1427)
| Variable | n | % |
|---|---|---|
| Age at baseline (years) | ||
| 23–25 | 304 | 21 |
| 26–28 | 363 | 25 |
| 29–31 | 392 | 27 |
| 32–35 | 368 | 26 |
| Education | ||
| ≤High school or GED | 300 | 21 |
| Some college | 730 | 51 |
| Bachelors/Masters/PhD | 396 | 28 |
| Missing | 1 | -- |
| Body mass index (kg/m2) | ||
| <25 | 288 | 20 |
| 25-<30 | 295 | 21 |
| 30-<35 | 270 | 19 |
| ≥35 | 574 | 40 |
| Parity | ||
| Never pregnant | 376 | 26 |
| 0 | 178 | 12 |
| 1 | 379 | 27 |
| 2 | 259 | 18 |
| ≥3 | 235 | 16 |
| Smoking | ||
| Never | 1055 | 74 |
| Former | 103 | 7 |
| Current | ||
| <10 cigarettes/day | 195 | 14 |
| ≥10 cigarettes/day | 74 | 5 |
| Length of interval between baseline and follow-up (months) | ||
| 16 | 118 | 8 |
| 17–22 | 1166 | 82 |
| 23–25 | 132 | 9 |
| 26–28 | 11 | 1 |
| Number of fibroids | ||
| 0 | 1123 | 79 |
| 1 | 190 | 13 |
| 2 | 49 | 3 |
| 3 | 30 | 2 |
| 4 | 13 | 1 |
| ≥5 | 22 | 2 |
| Size of largest fibroid (among 304 with fibroids)a | ||
| <0.52 cm3 [<1 cm diameter] | 85 | 28 |
| 0.52 – <4.19 cm3 [1 cm – <2 cm diameter] | 116 | 38 |
| 4.19 – <14.1 cm3 [2 cm – <3 cm diameter] | 48 | 16 |
| 14.1 – <33.5 cm3 [3 cm – <4 cm diameter] | 25 | 8 |
| ≥33.5 cm3 [≥4 cm diameter] | 30 | 10 |
Diameter equivalency of the fibroid volume shown in brackets.
Fibroid incidence
Of the 1123 women without fibroids at baseline, 106 (9.4%, CI=7.7,11.2) developed 133 fibroids during the 18-month follow-up interval. Incidence increased with age from 6% (CI=3,9) for 23–25 year-olds, 8% (CI=5,11) for 26–28 year-olds, 11% (CI=8,15) for 29–31 year-olds, and 13% (CI=9,17) for 32–35 year-olds (ptrend=<0.0001). Nearly all participants with incident fibroids developed either a single fibroid (77%) or two fibroids (21%) during follow-up. The 133 incident fibroids were significantly smaller (p<0.0001) than the fibroids prevalent at baseline with 57% <1 cm in diameter (Table 2). The location of incident fibroids in the uterus was only marginally different from the distribution seen for the baseline fibroids (p=0.08); intramural corpus was the dominant location (Table 2).
TABLE 2.
Fibroid size and position in the uterus for incident fibroids compared to fibroids prevalent at baseline
| Fibroid Characteristic | Incident Fibroidsa | Prevalent Fibroidsb | P-valuec | ||
|---|---|---|---|---|---|
| N=133 | N=430 | ||||
| N | % | N | % | ||
| Fibroid size (cm3)d | <0.0001 | ||||
| <0.52 [<1cm diameter] | 76 | 57 | 144 | 33 | |
| 0.52–<4.19 [1 cm – <2 cm] | 49 | 37 | 173 | 40 | |
| 4.19–<14.1 [2 cm – <3 cm] | 6 | 5 | 62 | 14 | |
| 14.1–33.5 [3 cm – <4cm] | 2 | 2 | 22 | 5 | |
| >33.5 [≥4 cm] | 0 | 0 | 29 | 7 | |
| Position of fibroid | <0.08 | ||||
| Submucosal | 8 | 6 | 23 | 5 | |
| Intramural fundal | 19 | 14 | 102 | 24 | |
| Intramural corpus | 80 | 60 | 201 | 47 | |
| Intramural lower uterine segment | 4 | 3 | 14 | 3 | |
| Subserosal/pedunculated | 22 | 17 | 90 | 21 | |
Incident fibroids seen at follow-up in 106 women (82 with 1 fibroid, 22 with 2, 1 with 3, 1 with 4).
Fibroids seen at baseline in the 282 women with 1–4 baseline fibroids.
P-value from logistic regression with both fibroid size (ordinal variable) and position (class variable) in model; unadjusted p-values for each variable were <0.001 and 0.07, respectively.
Diameter equivalency of the fibroid volume shown in brackets.
Net gain or loss in number of fibroids
Nearly a quarter (N=67) of the 282 women with 1–4 fibroids at baseline had a net gain in fibroid number during follow-up (Table 3). A net loss of fibroids was nearly as common (21%). For most, the gain or loss was a single fibroid. Women with larger fibroids tended to gain (p=0.06), while women with small fibroids tended to lose fibroids (p=0.009). Having a net gain or a net loss in fibroid number did not vary significantly by participant age.
TABLE 3.
Characteristics of participants with net gain or loss of fibroids during follow-up among women with 1–4 fibroids at baseline, n=282
| Characteristic at Baseline | Any Net Gaina | No Net Gain | p-valueb | Any Net Lossa | No Net Loss | p-valueb |
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |||
| Sample | 67 (24) | 215 (76) | 58 (21) | 224 (79) | ||
| Age | 0.67 | 0.49 | ||||
| 23–25 | 4 (6) | 24 (11) | 4 (7) | 24 (11) | ||
| 26–28 | 13 (19) | 47 (22) | 12 (21) | 48 (21) | ||
| 29–31 | 25 (37) | 66 (31) | 19 (33) | 72 (32) | ||
| 31–35 | 25 (37) | 78 (36) | 23 (40) | 80 (36) | ||
| Number of fibroids | 0.04 | 0.003 | ||||
| 1 | 36 (54) | 154 (72) | 31 (53) | 159 (71) | ||
| 2 | 14 (21) | 35 (16) | 16 (28) | 33 (15) | ||
| 3 | 12 (18) | 18 (8) | 6 (10) | 24 (11) | ||
| 4 | 5 (7) | 8 (4) | 5 (9) | 8 (4) | ||
| Size of largest fibroid (cm3)c | 0.06 | 0.009 | ||||
| <0.52 cm3 [<1 cm] | 17 (25) | 67 (31) | 19 (33) | 65 (29) | ||
| 0.52 – <4.19 cm3 [1 cm – <2 cm] | 22 (33) | 86 (40) | 25 (43) | 83 (37) | ||
| 4.19 – <14.1 cm3 [2 cm – <3 cm] | 9 (13) | 34 (16) | 9 (16) | 34 (15) | ||
| 14.1 – <33.5 cm3 [3 cm – <4 cm] | 6 (9) | 14 (7) | 3 (5) | 17 (8) | ||
| ≥33.5 cm3 [≥4 cm] | 13 (19) | 14 (7) | 2 (3) | 25 (11) |
Net gain was 1 fibroid (64%), 2 fibroids (27%), ≥3 fibroids (9%); net loss was 1 fibroid (91%), ≥2 fibroids (9%).
P-value from logistic regression with both fibroid number (continuous) and size of largest (ordinal) in model (not age); unadjusted p-values for each variable with gain were 0.32, 0.005, 0.007, respectively and for each variable with loss were 0.40, 0.03, 0.09, respectively.
Diameter equivalency of the fibroid volume is shown in brackets.
The specific fibroids that were gained or lost were not identified, except for the 35 fibroids in the 33 women who lost all their fibroids, but a comparison of those 35 fibroids with the 249 fibroids in women who did not lose all their fibroids show that position in the uterus does not differ between the two groups (p=0.41) (see Table, Supplementary Results 2.1).
Sixty-five of the 430 prevalent baseline fibroids were lost by follow-up (15%). Extrapolating from rates of loss seen for those who lost all fibroids where fibroid size is known, we estimated that loss rates were high (23%) in the smallest fibroid-size category (fibroid <1 cm diameter) and declined across size categories to 0% in the largest size category (≥4 cm in diameter).
Risk of developing a new fibroid for women with existing fibroids compared to women fibroid-free at baseline
The risk of developing a new fibroid was over twice as high for women who already had fibroids than for fibroid-free women (RRcrude=2.5, 95% CI 1.9, 3.3 and aRRadj=2.3, 95% CI 1.7, 3.0 after age-adjustment).
Fibroid growth
The 344 matched fibroids from 251 women varied in their baseline size from 0.05 cm3 to 380 cm3. Diameter equivalents were 0.5 to 9.0 cm, with median=1.7 cm. Based on calculations from ln-volume change (indexed to 18 months), the average fibroid growth was an 89% increase in volume per 18 months (CI 74%,104%). Figure 1 shows the adjusted fibroid growth per 18 months associated with categories of age, baseline fibroid size, fibroid number, uterine position (retroverted versus anteverted), and fibroid position in the uterus, adjusting for age and baseline size of fibroid. Though age had appeared important in univariate analyses, the four-category age variable was only marginally significant (in the final adjusted model (p=0.09). The 32–35 year-olds had slower growing fibroids than younger women (57% volume increase per 18 months compared to approximately 90% increase in the younger age categories). Baseline fibroid size was strongly related to growth rate (p<0.0001). Overall, small fibroids (<1.0 cm in diameter) tended to grow rapidly (188% increase per 18 months (95% CI 145,238)), while fibroids ≥2 cm diameter generally grew at less than half that rate. Position in the uterus was associated with growth (p=0.04) after adjusting for age and fibroid size. There was rapid growth for submucosal (137%) and intramural fundal (110%), less rapid growth for intramural corpus (77%) serosal (69%) and intramural fibroids in the lower segment (40%), but the sample sizes were small for the lower segment and submucosal groups (Figure 1) (see Supplementary Results 2.2 for unadjusted estimates).
Figure 1.
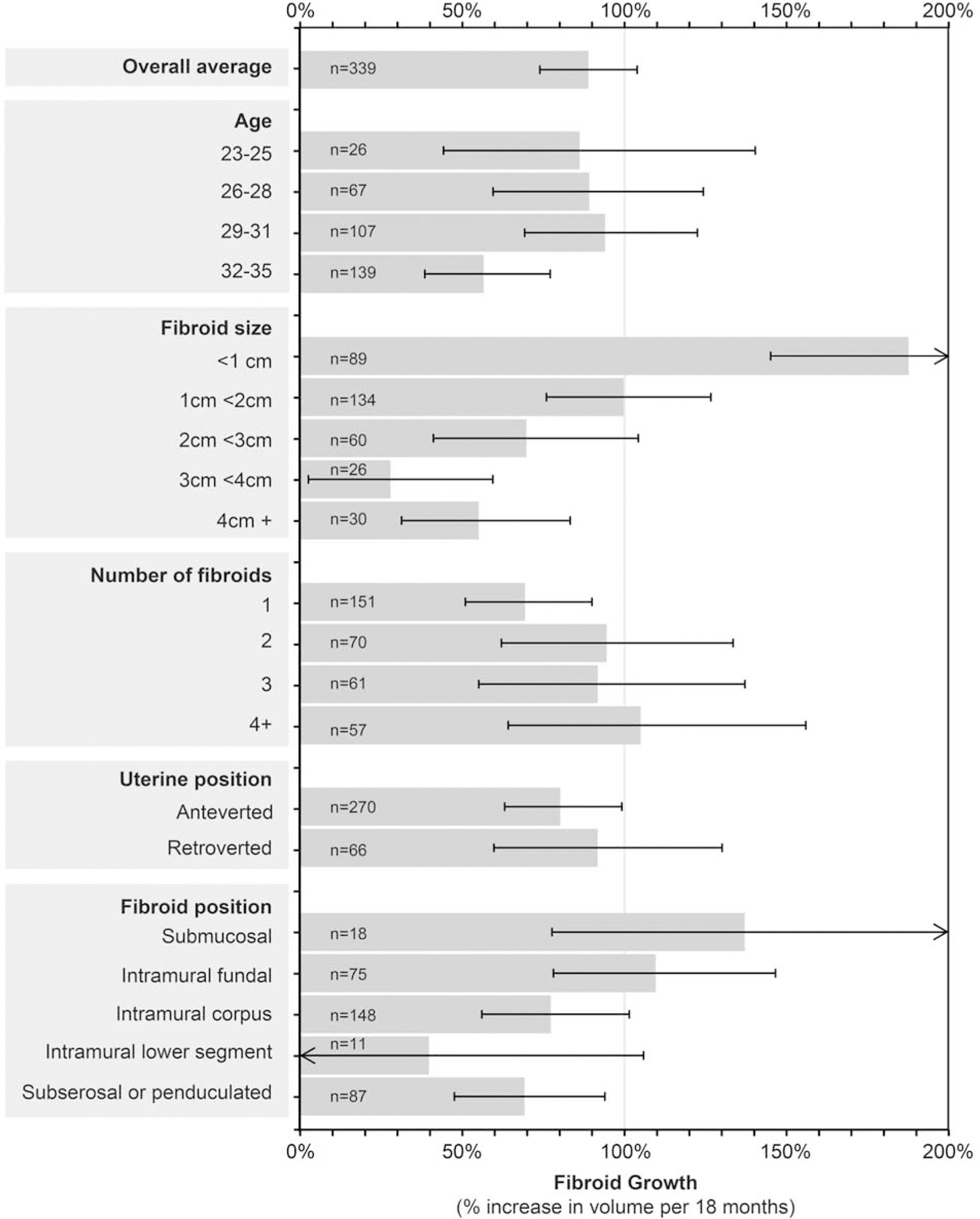
Variation in fibroid growth by woman and fibroid characteristics
Fibroid growth per 18 months associated with categories of baseline characteristics (n = 339 fibroids after excluding 5 outliers). Horizontal lines show 95% CIs. Estimates are adjusted for baseline age and fibroid size and are based on converting model results (change in ln-volume over time) to percent change in volume indexed to 18 months. There was no interaction between age and fibroid size (p=0.70).
The decline in growth rate with increase in baseline fibroid size is shown in Figure 2. A sharp decline is apparent for fibroids <2 cm in diameter. The very smallest fibroids were estimated to grow nearly 300% per 18 months. However, among fibroids ≥2 cm in diameter, the estimated growth rates were all well below 100% per 18 months, with only a small difference in growth rates across baseline fibroid-size categories (p=0.06).
Figure 2.
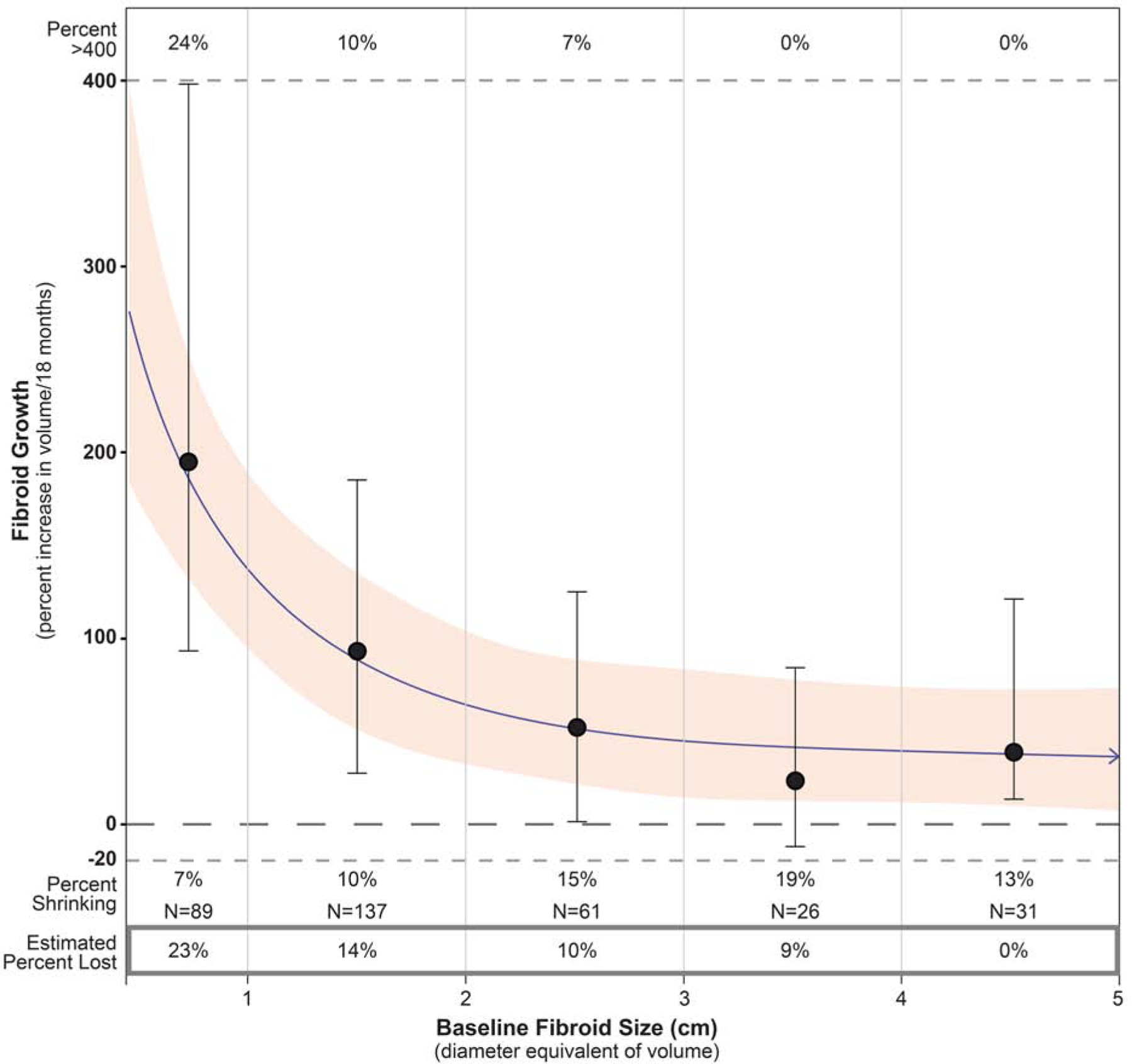
Change in fibroid growth with fibroid size
Change in fibroid growth rate with baseline fibroid size as estimated by cubic spline analyses. Estimates are based on change in ln-volume for 344 fibroids with nodes at diameter equivalencies of 1, 2, 3, and 4 cm, and indexed to women 26–29 years of age. The graph is truncated at 5 cm to better show the drop in fibroid growth rates in the smaller fibroid-size categories. The shaded area represents the 95% confidence limits around the estimated average growth. The vertical lines show the observed median (solid circle on vertical line) and interquartile range of growth rates for the fibroids in each size category. Percent >400 (above the upper dotted line) are the percent of observed fibroids with growth rates that exceed 400 percent per 18 months. Percent shrinking are the percent of observed fibroids with a decrease in volume of 20% or more. The estimated percent of fibroids lost in each size category during the 18-month follow-up is shown in the box below the graph; methods for estimating loss are described in Supplementary Methods 1.3.
Fibroid shrinkage (decrease of ≥20% in fibroid volume) was seen in 11% of the fibroids and occurred in all size categories, varying from 7% to 19% (Figure 2). Shrinkage is underestimated because fibroids that were lost during follow-up are not included. Therefore, Figure 2 includes a separate bottom panel showing the estimated percentage of fibroids lost in each size category (23% loss for <1 cm fibroids, decreasing to 0% loss for the ≥4 cm fibroids).
Our fibroid-size-dependent growth estimates allow us to estimate the average time it takes for a fibroid to grow from some given starting size to some larger size. For example, growth from a 2-cm-sized to a 4-cm-sized fibroid (indexing to women age 26–28) is estimated to take 4.5 years on average (95% CI=3.6–5.3) (Supplementary Methods 1.7).
Comment
During an 18-month follow-up of young African Americans, we found a nearly 10% incidence of fibroids among those who were fibroid-free at baseline. Incidence increased with age. We found that new fibroid development was even more frequent in women who already had fibroids (24%), and new development in this group did not increase with age. Fibroid growth varied by initial size. Small fibroids grew very rapidly on average, but also had a high risk of loss.
The single prior image-based, prospective study of fibroid incidence reported 13% per 31 months among 53 fibroid-free white women. Prior estimates of age-specific fibroid incidence have all been modeled from cross-sectional data.8,15,16, Our study validates the prior estimates showing that tumor onset begins in the 20s for African-American women, about ten years earlier than estimated for white women.
Eight prior studies have examined fibroid growth,9,17,18,19,20,21,22,23 some of which were included in a recent review.24 The studies vary in design, number of women studied (11 to 152), eligibility criteria, size of monitored fibroids, imaging method, length of follow-up, and methods of analyzing fibroid growth (Table 4). The prior studies report average or median growth rates ranging from 18% per year 9,23 to 82% per year,22 and our estimates fall within this range. Our data suggest that much of this variation could be due to the different fibroid-size distributions being studied (see Supplementary Results 2.3 for detailed review).
TABLE 4.
Summary of studies that have measured uterine fibroid growth
| Reference | Study Design | Follow-up | Participants | Fibroid Size | Growth Measure | Findings | ||
|---|---|---|---|---|---|---|---|---|
| Country Age N in study |
(cm) | MRI or Ultrasound | Growth Rate | Shrinkage of ≥20% | Factors Associated with Growth | |||
| Ichimura et al. 199818 | Prospective | 1 year | Japan 24–53 N=31 (31 fibroids) |
Mean=9.1 Range: 4.8–12.4a |
% change in V/yr MRI | Mean=31%/yr Range: −30%–560% |
26% of fibroids | Size: ND Age: ND Biomarkers from baseline biopsy (smooth muscle, ER, PR) |
| Tsuda et al. 199819 | Prospective | Every 3 mos for 1 yr | Japan 30–37 N=70 (101 fibroids) |
Mean=3.9 Range: ND |
Growth = >30% V increase during yr Ultrasound | 27% of fibroids grew >30% in V/yr | ND | Size: no association adj for artery Age: no association adj for artery Fibroids with arteries grew faster |
| DeWaay et al. 200220 | Prospective | 2.4–2.7 yrs | US, whites 33–56 N=11 (18 fibroids) |
Mean=2.2 Range: ND |
Change in diameter Ultrasound | Mean=1.2 cm growth per 2.5 yr Range: −.9, 6.8 cm |
ND | Size: ND Age: ND |
| Peddada et al. 200810 | Prospective | 3, 6, 12 mos | US, whites & blacks 24–54 N=72 (262 fibroids) |
Median=3.2 Range: 1.4–12.8 |
(1) % change in V/6 mos back-calculated from regression with outcome: change in ln(V)/day averaged over 1–3 intervals, indexed to change/6 mos. (2) >20% growth/6 mos defined as rapid growth and analyzed with logistic regression MRI |
Median=9%/6 mos Range: −85–140% |
7% of fibroids | Size: no association Age: younger had faster growth in whites; no difference in blacks Single fibroids grew faster (n=5) |
| Mavrelos et al. 201021 | Retrospective record review | 8–90 mos median =21.5 | Germany 27–45 N=122 (122 fibroids) |
Median=2.7 Range: 0.5–9.3 |
% change in V per yr Ultrasound | Median=35%/yr Range: ND |
3% of fibroids | Size: small grew faster than large Age: interpretation not clearb |
| David et al. 201422 | Retrospective record review | 2–40 mos | Germany 26–53 N=53 (72 fibroids) |
Median=3.8 Range: 0.8–6.9 |
(1) change in V/6 mos (2) % change in V/6 mos Ultrasound |
Mean=30%/6 mos Range: −46–459% |
3% of fibroids | Size: small grew faster than large Age: interpretation not clearb |
| Armbrust et al. 201823 | Retrospective record review | ≥ 6 mos | Germany, whites <30 to >45 N=152 (272 fibroids) |
Not stated 43% were <2 37% were >5 |
% change in V/6 mos Ultrasound | Median=41%/6 mos Range: −61–929% |
ND | Size: small grew faster than large Age: interpretation not clearb |
| Nieuwenhuis et al. 201845 | Prospective | 2–28 mos median =12 |
Netherlands, Europeans & other Mean age=42 N=61 (61 fibroids) |
Not stated Median=4 Range: 1.3–8.0 |
% change in V/yr 3-D Ultrasound with software to estimate V | Mean=9%/yr Range: −96–271% |
ND | Size: ND Age: ND Vascularity associated with faster growth |
Adj: adjusted, Mos: months, ND: no data, V: volume, yr: year
Diameter estimated from volume data in paper.
Percent change can describe growth, but is problematic as an outcome for regression analyses of factors associated with growth because outcome is not normally distributed (negative growth cannot exceed 100% while positive growth is unrestrained).
Our fibroid growth data suggest that women with small fibroids (<2cm in diameter) can be re-assured that such fibroids often shrink, and growth to a size likely to cause symptoms (>4 cm) will usually take several years. Our prospectively confirmed increase in fibroid incidence with age has reproductive health implications. Women are increasingly delaying their childbearing.25 Older women will be more likely to have fibroids, with the associated problems such as the possibility of reduced fertility,26 pain and bleeding during early pregnancy,27 and increased risk of cesarean birth.28
The higher risk of new fibroid development that we observed among women with existing fibroids compared to incidence in fibroid-free women may result from population heterogeneity in fibroid susceptibility. Multiple susceptibilities are likely at play, including genotype at time of conception, along with numerous life-course exposures that could have acute and/or long-term impacts through mutation, epigenetic changes, and immunologic/endocrine effects. The fibroid-free women likely include a full range of susceptibilities, while the women with existing fibroids likely include more highly susceptible individuals. This suggests that women who develop multiple fibroids at a young age constitute a high-risk group, and they may benefit from more frequent follow-up.
Little is known about early fibroid development, but mutation-driven initiation is likely, with MED12 mutations the most common.29,30 However, the high variability we observed in growth of small fibroids, with their frequent loss, suggests that other factors, such as inflammatory or hormonal factors, influence lesion development and survival. Further research on factors associated with fibroid loss that might be leveraged for treatment is needed. Research that separately evaluates fibroid incidence and fibroid growth will be important for planning future clinical trials of new fibroid medications because medications that reduce growth may not reduce incidence.
Our study has limitations. Because we focused exclusively on African Americans, the ethnic group with the highest fibroid health burden in the U.S., our findings may not be directly generalizable to other ethnic groups. Also, we used ultrasound imaging rather than MRI. However, ultrasound is the usual diagnostic procedure for clinical practice, making the findings directly relevant to clinical practice. Though sonographers were trained in distinguishing fibroids from other pathologies, some misclassification is possible. In addition, though fibroid loss has been reported previously,19 all data on fibroid loss is limited by detection limits. “Lost fibroids” were not seen either because they shrank below detection (<0.5 cm diameter) or resolved completely.
The major strengths were the prospective study design and large sample size with low loss to follow-up which allowed us to measure age-specific fibroid incidence directly for the first time. In addition, the detailed analysis of fibroid growth allowed us to estimate time required for a fibroid to grow from a given size to a larger size.
Conclusions
In summary, this is the first study to directly measure age-specific fibroid incidence and the largest study of fibroid growth. Our incidence data suggest that previous estimates of age-specific incidence rates provide reasonable approximations of population-level onset-age. Our use of fibroid growth data to estimate the time required for fibroids to grow a given amount is an approach that will be useful for developing future life-course treatment strategies.
Supplementary Material
AJOG at a Glance:
A. Why was this study conducted?
Age-specific fibroid incidence has been estimated with prevalence data, but not directly. This study prospectively quantified fibroid incidence and growth.
B. Key findings
During an 18-month follow-up, fibroid incidence increased from 6% for 23–25 year-olds to 13% for 32–35 year-olds. The small fibroids (<1 cm diameter) averaged a nearly 200% increase in volume, while fibroids ≥4 cm averaged about a 50% increase. Estimated average time for fibroids to grow from 2 to 4 cm in diameter was 4.5 years.
C. What does this add to what is known?
This is the first study of age-specific fibroid incidence and the first to measure fibroid growth in a large, community-based, high-risk cohort. These data will be useful in designing life-course treatment strategies for this common condition.
Acknowledgements
We are grateful to the superb SELF study staff under the leadership of study managers Christie-Barker Cummings and Thomasina Austin (Social & Scientific Systems, Durham, NC (SSS)) and Karen Bourgeois and Osberita Norman (Henry Ford Health System, Detroit, MI). Special thanks to the sonographers at Henry Ford Health System who conducted hundreds of careful examinations. Shyamal Peddada (University of Pittsburg) consulted on the fibroid growth analysis. Allen Wilcox (NIEHS), Shanshan Zhao (NIEHS), and Lauren Wise (Boston University) provided valuable comments on an earlier draft of the manuscript. David Green (NIEHS), Lois Wyrick (NIEHS), and Michele Justice (SSS) assisted with the figures.
Funding
This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences, NIH, and in part by funds allocated for health research by the American Recovery and Reinvestment Act.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors report no conflict of interest.
Employment Disclaimer: Authors employed by the federal government are D.D.B., Q.E.H., and D.M.U.
Condensation: Fibroid incidence increases with age, and fibroid development begins with very rapid growth that decreases as the fibroids develop.
References
- 1.Al-Hendy A, Myers ER, Stewart E. Uterine Fibroids: Burden and Unmet Medical Need. Seminars in reproductive medicine 2017;35:473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whiteman MK, Kuklina E, Jamieson DJ, Hillis SD, Marchbanks PA. Inpatient hospitalization for gynecologic disorders in the United States. American journal of obstetrics and gynecology 2010;202:541.e1–6. [DOI] [PubMed] [Google Scholar]
- 3.Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. American journal of obstetrics and gynecology 2012;206:211.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soliman AM, Yang H, Du EX, Kelkar SS, Winkel C. The direct and indirect costs of uterine fibroid tumors: a systematic review of the literature between 2000 and 2013. American journal of obstetrics and gynecology 2015;213:141–60. [DOI] [PubMed] [Google Scholar]
- 5.Eltoukhi HM, Modi MN, Weston M, Armstrong AY, Stewart EA. The health disparities of uterine fibroid tumors for African American women: a public health issue. American journal of obstetrics and gynecology 2014;210:194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moshesh M, Peddada SD, Cooper T, Baird D. Intraobserver variability in fibroid size measurements: estimated effects on assessing fibroid growth. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine 2014;33:1217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. American journal of obstetrics and gynecology 2003;188:100–7. [DOI] [PubMed] [Google Scholar]
- 8.Laughlin SK, Schroeder JC, Baird DD. New directions in the epidemiology of uterine fibroids. Seminars in reproductive medicine 2010;28:204–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proceedings of the National Academy of Sciences of the United States of America 2008;105:19887–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baird DD, Harmon QE, Upson K, et al. A Prospective, Ultrasound-Based Study to Evaluate Risk Factors for Uterine Fibroid Incidence and Growth: Methods and Results of Recruitment. Journal of women’s health (2002) 2015;24:907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fedele L, Bianchi S, Dorta M, Zanotti F, Brioschi D, Carinelli S. Transvaginal ultrasonography in the differential diagnosis of adenomyoma versus leiomyoma. American journal of obstetrics and gynecology 1992;167:603–6. [DOI] [PubMed] [Google Scholar]
- 12.Wilde S, Scott-Barrett S. Radiological appearances of uterine fibroids. The Indian journal of radiology & imaging 2009;19:222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levens ED, Wesley R, Premkumar A, Blocker W, Nieman LK. Magnetic resonance imaging and transvaginal ultrasound for determining fibroid burden: implications for research and clinical care. American journal of obstetrics and gynecology 2009;200:537.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Accuracy of magnetic resonance imaging and transvaginal ultrasonography in the diagnosis, mapping, and measurement of uterine myomas. American journal of obstetrics and gynecology 2002;186:409–15. [DOI] [PubMed] [Google Scholar]
- 15.Laughlin SK, Baird DD, Savitz DA, Herring AH, Hartmann KE. Prevalence of uterine leiomyomas in the first trimester of pregnancy: an ultrasound-screening study. Obstetrics and gynecology 2009;113:630–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marsh EE, Ekpo GE, Cardozo ER, Brocks M, Dune T, Cohen LS. Racial differences in fibroid prevalence and ultrasound findings in asymptomatic young women (18–30 years old): a pilot study. Fertility and sterility 2013;99:1951–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ichimura T, Kawamura N, Ito F, et al. Correlation between the growth of uterine leiomyomata and estrogen and progesterone receptor content in needle biopsy specimens. Fertility and sterility 1998;70:967–71. [DOI] [PubMed] [Google Scholar]
- 18.Tsuda H, Kawabata M, Nakamoto O, Yamamoto K. Clinical predictors in the natural history of uterine leiomyoma: preliminary study. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine 1998;17:17–20. [DOI] [PubMed] [Google Scholar]
- 19.Dewaay DJ, Syrop CH, Nygaard IE, Davis WA, Van Voorhis BJ. Natural history of uterine polyps and leiomyomata. Obstetrics and gynecology 2002;100:3–7. [DOI] [PubMed] [Google Scholar]
- 20.Mavrelos D, Ben-Nagi J, Holland T, Hoo W, Naftalin J, Jurkovic D. The natural history of fibroids. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2010;35:238–42. [DOI] [PubMed] [Google Scholar]
- 21.David M, Adams L, Stupin JH. Natural Size Development of Myomata - Ultrasound Observational Study of 55 Premenopausal Patients. Geburtshilfe und Frauenheilkunde 2014;74:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armbrust R, Wernecke KD, Sehouli J, David M. The growth of uterine myomas in untreated women: influence factors and ultrasound monitoring. Archives of gynecology and obstetrics 2018;297:131–37. [DOI] [PubMed] [Google Scholar]
- 23.Nieuwenhuis LL, Keizer AL, Stoelinga B, et al. Fibroid vascularisation assessed with three-dimensional power Doppler ultrasound is a predictor for uterine fibroid growth: a prospective cohort study. BJOG : an international journal of obstetrics and gynaecology 2018;125:577–84. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh S, Naftalin J, Imrie R, Hoo WL. Natural History of Uterine Fibroids: A Radiological Perspective. Current obstetrics and gynecology reports 2018;7:117–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Matthews TJ. Births: final data for 2012. Natl Vital Stat Rep 2013;62:1–68. [PubMed] [Google Scholar]
- 26.Karlsen K, Mogensen O, Humaidana P, Kesmodel US, Ravn P. Uterine fibroids increase time to pregnancy: a cohort study. Eur J Contracept Reprod Health Care 2019:1–6. [DOI] [PubMed]
- 27.Michels KA, Hartmann KE, Archer KR, Ye F, Edwards DRV. The Relationship between Total Fibroid Burden and First Trimester Bleeding and Pain. Paediatr Perinat Epidemiol 2016;30:115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michels KA, Velez Edwards DR, Baird DD, Savitz DA, Hartmann KE. Uterine leiomyomata and cesarean birth risk: a prospective cohort with standardized imaging. Ann Epidemiol 2014;24:122–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart EA, Laughlin-Tommaso SK, Catherino WH, Lalitkumar S, Gupta D, Vollenhoven B. Uterine fibroids. Nature reviews Disease primers 2016;2:16043. [DOI] [PubMed] [Google Scholar]
- 30.Mittal P, Shin YH, Yatsenko SA, Castro CA, Surti U, Rajkovic A. Med12 gain-of-function mutation causes leiomyomas and genomic instability. The Journal of clinical investigation 2015;125:3280–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


