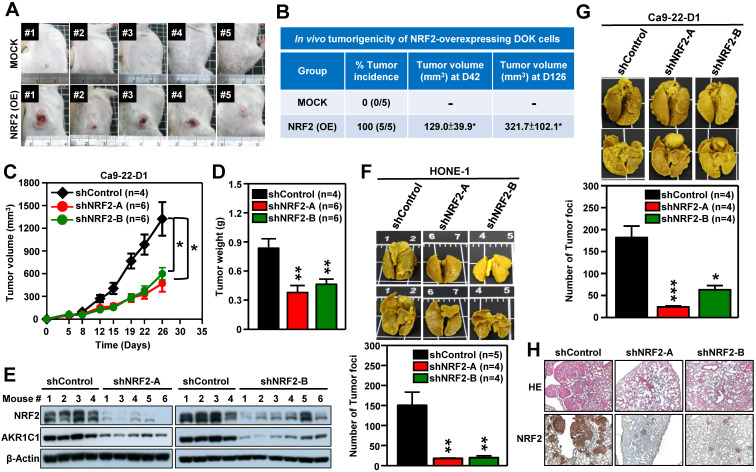
Figure 3.
NRF2 promotes tumorigenesis and malignant progression of HNSCC in vivo. (A, B) In vivo tumorigenicity of NRF2 overexpressing dysplastic oral keratinocytes. DOK cells with stably overexpressed NRF2 (NRF2-OE) or vector-only control (MOCK) were subcutaneously injected into NOD/SCID mice (5×106 cells per mouse), and tumor volume and body weight were measured weekly. (A) Representative photographs of mice with control (upper) and NRF2-overexpressing (lower) xenograft tumors. (B) The tumor incidence was defined as the percentage of mice in each group with a tumor volume that exceeded 50 mm3 in each group. Mean tumor volumes (mm3) of mice at days 42 and 126 were represented as mean ± S.E. (C, D) In vivo tumor growth assay of stable NRF2-knockdown Ca9-22-D1 cells. Tumor growth rate (C) and tumor weight (D) were compared between shControl (black) and two different shNRF2 groups (red and green). Mice were injected subcutaneously with Ca9-22-D1 cells. The tumor growth curves represent tumor volume data measured twice per week throughout the experiment. Mice were sacrificed and tumors were isolated and weighed at Day 26 after cell injection. (E) Expression of NRF2 and AKR1C1 was measured by Western blot assays of excised tumors from Ca9-22-D1 xenograft tumor-bearing mice. (F, G) In vivo experimental pulmonary metastasis assay of stable NRF2-knockdown HNSCC cells. HONE-1 (F) or Ca9-22-D1 (G) cells stably expressing NRF2-shRNA or control vector were delivered via tail vein injection into NOD/SCID mice. After 50 (HONE-1) or 25 (Ca9-22-D1) days, lungs were removed from all mice and fixed in Bouin's solution. The lung nodules were counted, and lung sections were prepared. Upper panel: Representative images of pulmonary metastatic foci produced after intravenous injection. Lower panel: Numbers of detectable tumor foci on the surface of whole lungs were quantified and indicated as mean ± S. E. * p < 0.05; ** p < 0.01; and *** p < 0.001 compared with the control group (shControl). (H) Representative histological photographs showing H&E staining and IHC analyses of lung sections taken under bright-field at 50× magnification using an upright microscope.