Abstract
Knowledge of the vectors of dirofilariasis in the world beside the treatment of infected dog is crucial to establish mosquito vector-based control programs. The current systematic review and meta-analysis were conducted on published studies, documenting the prevalence of Dirofilaria immitis infected/infective mosquitoes from field surveys and laboratory experiments under controlled conditions. Articles up through 2019 from Scopus, PubMed, Embase, Web of Science, Google Scholar were screened systematically. The overall prevalence of D. immitis infected/infective mosquitoes was estimated using a random effect model. Meta-regression was used to identify factors related to high dirofilariasis prevalence in the vectors. In these studies, the detection method was not identified as a heterogeneity and the overall prevalence in both subgroups had overlap (7.9-34.9 and 1.5-48.5). The overall prevalence of infective stage was 2.6 (95% CI: 0.97-4.77 per 1,000) and 84.7 per 1000 (95% CI: 20.5-183.8 per 1,000) for the field survey/laboratory experiment, respectively. The higher overall prevalence of D. immitis infected/infective mosquitoes were reported across studies in which take place in Eastern Mediterranean Region office (EMRO), longitude: 80 to 110, latitude: 20 to 40, annual rainfall: 250 to 1000, sea level: 26 to 100 and <1,000, humidity: 66 to 70, during 2000 to 2005 by dissection methods. Our review determined that mosquito species within the genus Anopheles and to a less extent Culex were the main vectors of dirofilariasis.
Keywords: Meta-analysis, Systematic review, Culicidae, Dirofilariasis, Diagnostic methods
Dirofilariasis is a zoonotic vector-borne disease transmitted by at least 27 species of the genus Dirofilaria (Spirurida, Onchocercidae), especially D. repens and D. immitis (canine or dog heartworm). The disease is widely distributed and the reservoirs are mainly mammals from approximately 111 species including canids. Presently, dirofilariasis in humans is considered to be an emerging disease in some regions (Azari-Hamidian et al., 2007, 2009; Simón et al., 2012). At present, at least 77 mosquito species (Diptera: Culicidae) of the genera Culex, Aedes, Anopheles, Mansonia, Coquillettidia, Psorophora, and Culiseta are presumed to have a role in the transmission of dirofilariasis, while the third-stage infective larvae (L3) have been detected from in a few field-captured dipteral species (Azari‐Hamidian et al., 2009). The mosquitoes can become infected by ingestion of blood meal from microfilaremic host. The ingested microfilaria can develop to infected (L1, L2) and subsequently into the infective larvae (L3) in the Malpighian tubules. The L3 migrate to the mosquito proboscis. The L3’s transmit through mosquito bite and become sexually mature in the pulmonary artery and right ventricles of suitable mammalian final host (Ledesma and Harrington, 2011). Heartworm disease is endemic disease, mainly locate in regions with temperate and tropical climate. The transmission of dirofilariasis from endemic to the new area directly depend on availability of microfilaremic hosts, competent vectors as well as a favored ecological factors for development of larval stages to L3 in the mosquitoes (Sassnau et al., 2014). The role of ecological key factors has been proposed for development of dirofilarial larvae in possible vectors for specific locations (Brown et al., 2012). Most of the field studies only reported the prevalence in mosquitoes. So, it is important to perform a comprehensive study to evaluate these key factors. The monitoring of mosquitoes for the infection was based on dissection method which is the gold standard (Latrofa et al., 2012). The experimental studies allow the evaluation of vector competence of suspected mosquitoes for transmission of dirofilariasis (Nayar and Knight, 1999). The results of such experimental studies have been recorded, but no comprehensive evaluation of vector efficiency has been done in all mosquitoes to date. The current systematic review and meta-analysis were conducted on published studies, documenting the prevalence of D. immitis infected (L1-L2)/infective(L3) mosquitoes from field surveys, examine the effect of different key factors on their overall prevalence and comprehensively assess the vector competence from experimental studies.
Material and methods
Research was conducted in accordance with Systematic Reviews and Meta-Analysis (PRISMA) 2015 Guidelines (Moher et al., 2015).
Search strategy and study design
Original search studies that evaluated the mosquito vectors for D. immitis in field and experiment were screened in PubMed, Embase, Web of Sciences, Scopus, and Google Scholar up to October 10, 2019. The keys terms used for searching in literature were ‘Dirofilaria immitis’, ‘vector’, ‘prevalence’, ‘Culicidae’, and ‘mosquitoes’ with the Boolean operators ‘OR’ and/or ‘AND’. The manually searched in the reference lists of the collected studies was also performed. After removal of duplicate articles, the retrieved papers were screened by title and abstract by two independent authors to exclude any that were irrelevant to our study question (S. R. and R. SM). The full text of the remaining articles was evaluated to identify eligible articles. Studies were considered eligible if they had cross-sectional design. Results were screened initially excluding all studies that did not included D. immitis and vectors. The other exclusion criteria were: non original paper (review, systematic reviews, case reports, letter or editorial articles, thesis), unidentified filarial species, undefined data, and lacking control groups for experimental studies. The experimental vector competence studies as well as the field studies were included.
Data extraction
Data were extracted from published articles, evaluating the prevalence of D. immitis infected/infective mosquitoes from field and experimental studies in an Excel form (Microsoft, Redmond, WA, USA). The data extraction was performed by S. R. and R. SM. and in cases of discrepancy, consensus was carried out by discussion with A. S. Subsequently, an author (S. R) extracted the requisite data, and the others (R. SM. and A. S.) rechecked them. Data analysis was performed by R. SM. For field study, the following items were extract: ‘General information’ including first author, year of implementation (The years for collection of samples), and location; geographical data of sampling site including average temperature, annual rainfall, humidity, WHO regional office, altitude, latitude, longitude; ‘vectorial characteristics’ including number of samples, total number of pools, total number of D. immitis infected/infective mosquitoes. In some studies, mosquitoes were evaluated individually for both head+thorax and abdomen in order to differentiate infective and infected specimens, and in others, different parts of mosquitoes were studied in pools. In order to consistency of calculations, in studies in which mosquitoes were studied individually each mosquito was considered as a one pool. So, pool in this study defines as a number of one or more mosquitoes that were placed in a one cluster and considered as a unit of study. The total number of infective and infected pool of mosquitoes was calculated as a number of pools that have an infective and infected larvae stages, respectively. Minimal infected/infective rate, calculated as the ratio of the number of positive pools of infected/infective mosquitoes to the total pool of tested mosquitoes, assumes that there is only one infected/infective individual in each positive pool. Since the nominator and denominator of fraction were the same, it has little effect on the infected/infective rate. For experiment study, the general information and vectorial characteristics were extracted. To quantitatively evaluate vector competence, overall prevalence of D. immitis infection and infective stage in filed and experiment collected mosquitoes was performed.
Quality assessment
Evaluation of the included study quality was conducted by two authors (R. SM and S. R.) independently using the Newcastle-Ottawa Scale (Stang, 2010). Briefly, maximum of nine scores was defined for the following items including the subject selection criteria (0-4 points), comparability of subjects (0-2 points), and exposure (0-3 points). The papers with a total score of 0 to 3, 4 to 6, and 7 to 9 points were specified as the poor, moderate, and high quality, respectively.
Data synthesis and statistical analysis
The STATA software version 13 was used for meta-analysis (Stata Corp LP, College Station, Texas). The main goals were the evaluation of prevalence of D. immitis in field collected vectors and assessment the competence of suspected vector for D. immitis in experimental studies by dissection and molecular methods. The evaluation of field and experimental studies was performed separately. First, random effects model was used for calculation of overall estimates. Metaprop was used for calculating overall prevalence using a Freeman–Tukey double arcsine transformation and then confidence intervals (CI = 95%) for each survey were assessed (Freeman and Tukey, 1950; Harris et al., 2008; Nyaga et al., 2014). Next, the key factors that could be the source of heterogeneity for the prevalence of infection and infective were assessed using meta-regression and subgroup analyses for some factors such as: data collection years, country, mean temperature, annual rainfall, humidity, WHO regional office, altitude, latitude, detection type, vector genus, and vector species. Heterogeneity among studies was evaluated by statistical tests and graphical (i.e., Cochran’s Q test, I2 statistics, and Galbraith). The range of I2 was between 0 and 100%. I2 > 70% were considered heterogeneous (Higgins, 2008; Riahi and Mokhayeri, 2017). In this study the relationship between exposures and outcome was not studied, so evaluation of publication bias was not logical (Mokhayeri et al., 2018). The significance level of ≤0.05 was considered.
Results
Inclusion of studies and data extraction
A flow chart for identification, screening, exclusion, and finally including of retrieved article was shown in Figure 1. A total of 985 literatures was at first screened that of which 680 were removed as duplicated. After that, initial searching using title and abstract (n = 305 articles) was performed. After screening, 210 articles were excluded and full text of remaining papers (n = 95) reviewed for evaluating of eligibility, which of those 53 record removed (Fig. 1). From 42 included articles, 33 and 9 studies were field studies and laboratory experiments, respectively. The selected articles from field study involved 19 countries from Pan American Health Organization (PAHO) (21%), European Region (EURO) (52.6%), Eastern Mediterranean Regional Office (EMRO) (5.2%), and Western Pacific Regional Office (WPRO) (21%). Studies were published over a 29-year period (1990-2019). Molecular assay was used in 9 out of 33 field study and 3 out of 9 experimental studies to identify the D. immitis larvae in vectors (Tables 1, 2).
Figure 1:
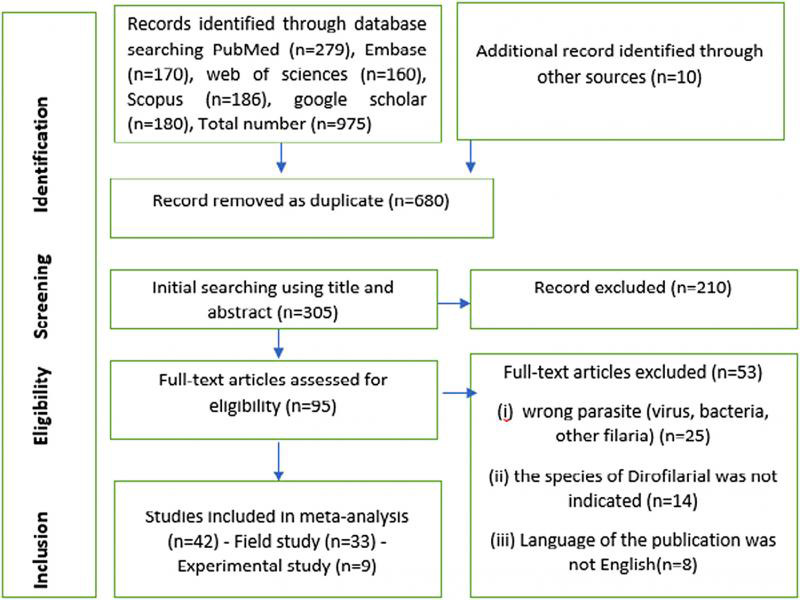
Flow chart detailing the number of studies excluded and included at each step for systematic review of the prevalence of D. immitis infection in Culicidae mosquitoes.
Table 1.
Characteristics of the individual studies included in the meta-analysis based on eligibility criteria.
| Country | Year of implementation | Method | Total sample | Total pool | Total infected pool | Total infective pool | Quality score | References |
|---|---|---|---|---|---|---|---|---|
| Brazil | 2013 | PCR | 3115 | 311 | 1 | 0 | 7 | Ogawa et al. (2013) |
| Mexico | 2015 | PCR | 2618 | 595 | 4 | 0 | 6 | Oswaldo et al. (2018) |
| USA | 2006 | PCR | 1,922 | 390 | 33 | 0 | 7 | Chambers et al. (2009) |
| Mexico | 2007 | Dissection | 272 | 272 | 28 | 21 | 8 | Manrique-Saide et al. (2008) |
| Italy | 2012 | PCR | 40,892 | 955 | 21 | 0 | 7 | Latrofa et al. (2012) |
| Iran | 2005-2011 | PCR | 190 | 190 | 15 | 10 | 6 | Azari-Hamidian et al. (2009) |
| USA | 2009 | PCR | 91,798 | 1,212 | 184 | 54 | 8 | Mckay et al. (2013) |
| Germany | 2011-2013 | PCR | 16,878 | 955 | 2 | 0 | 7 | Kronefeld et al. (2014) |
| Italy | 2000-2002 | PCR | 3,198 | 1,364 | 10 | 4 | 6 | Cancrini et al. (2006) |
| Brazil | 1995-1996 | Dissection | 3,677 | 3,677 | 55 | 8 | 7 | Labarthe et al. (1998) |
| Portugal | 2011-2013 | PCR | 5,866 | 1,815 | 54 | 23 | 8 | Ferreira et al. (2015) |
| Argentina | 2003-2005 | Dissection | 2,380 | 2,380 | 3 | 0 | 7 | Vezzani et al. (2006) |
| Russia | 2007-2017 | PCR | 387 | 78 | 5 | 0 | 8 | Shaikevich et al. (2018) |
| USA | 1994 | Dissection | 188 | 188 | 3 | 0 | 6 | Comiskey and Wesson (1995) |
| Italy | 2002-2003 | PCR | 1,576 | 880 | 6 | 3 | 5 | Cancrini et al. (2007) |
| Italy | 2003 | PCR | 154 | 154 | 2 | 1 | 6 | Cancrini et al. (2003a, 2003b) |
| Italy | 2000-2002 | Dissection | 2,534 | 336 | 81 | 22 | 5 | Cancrini et al. (2003a, 2003b) |
| Spain | 2005-2007 | PCR | 1,219 | 1,219 | 1 | 0 | 7 | Morchón et al. (2011) |
| Argentina | 2007-2008 | PCR | 453 | 82 | 2 | 0 | 6 | Vezzani et al. (2011) |
| Portugal | 2002-2003 | PCR | 554 | 554 | 4 | 2 | 7 | Santa-Ana et al. (2006) |
| Turkey | 2008-2009 | PCR | 6,153 | 559 | 15 | 14 | 8 | Yildirim et al. (2011) |
| Spain | 2012-2013 | PCR | 881 | 881 | 1 | 1 | 6 | Bravo-Barriga et al. (2016) |
| Turkey | 2008 | PCR | 301 | 54 | 2 | 1 | 8 | Bıs¸kın et al. (2010) |
| Slovakia | 2013 | PCR | 10,500 | 105 | 4 | 0 | 8 | Bocková et al. (2015) |
| Spain | 2004-2006 | PCR | 725 | 725 | 2 | 0 | 7 | Morchon et al. (2007) |
| USA | 1985-1987 | Dissection | 9,377 | 9,377 | 123 | 18 | 7 | Parker (1993) |
| South-Korea | 2005 | PCR | 2,059 | 172 | 12 | 0 | 6 | Lee et al. (2007) |
| USA | 2006-2007 | PCR | 1,401 | 206 | 15 | 0 | 8 | Licitra et al. (2010) |
| France | 2015 | Real time PCR | 797 | 797 | 29 | 0 | 8 | Tahir et al. (2017) |
| Italy | 2005 | PCR | 637 | 119 | 1 | 0 | 7 | Masetti et al. (2008) |
| Taiwan | 1997 | Dissection | 4,537 | 4,537 | 146 | 0 | 6 | Lai et al. (2001) |
| Samoa | 1978-1980 | Dissection | 41,980 | 41,980 | 179 | 49 | 7 | Samarawickrema et al. (1992) |
| French Polynesia | 2003-2004 | Dissection | 1,194 | 1,194 | 28 | 4 | 8 | Russell et al. (2005) |
Table 2.
List of published articles included in the systematic review for experiment studies of D. immitis in vectors.
| Country | Year of implementation | Detection method | Total sample | Total pool | Total infected pool | Total infective pool | Quality score | References |
|---|---|---|---|---|---|---|---|---|
| Brazil | 2000 | Dissection | 756 | 756 | 225 | 57 | 8 | Ahid et al. (2000) |
| Italy | 2017 | PCR | 136 | 136 | 66 | 10 | 7 | Silaghi et al. (2017) |
| Italy | 2015 | PCR | 45 | 45 | 37 | 18 | 8 | Montarsi et al. (2015) |
| Iran | 2017 | PCR | 90 | 90 | 58 | 16 | 7 | Solgi et al. (2017) |
| Brazil | 2008 | Dissection | 1,942 | 1,942 | 465 | 0 | 8 | Carvalho et al. (2008) |
| USA | 2005 | Dissection | 750 | 750 | 630 | 0 | 8 | Debboun et al. (2005) |
| Brazil | 1998 | Dissection | 73 | 73 | 43 | 0 | 7 | Macêdo et al. (1998) |
| Australia | 1989 | Dissection | 215 | 215 | 0 | 49 | 6 | Russell et al. (2005) |
| Brazil | 1999 | Dissection | 505 | 505 | 0 | 101 | 8 | Brito et al. (1999) |
Meta-analysis for estimating overall prevalence of Dirofilaria immitis infected/infective stages in the filed collected mosquitoes
Meta-analysis was performed for estimating overall prevalence of D. immitis infected/infective stages in the genera and species of field collected mosquitoes (Table 3). The magnitude of the overall prevalence differed largely across all mosquito genera and species. When reporting overall prevalence of D. immitis infected stages in vectors, it ranged between 6.7 per 1000 (95% CI: 3.48-10.88 per 1000) in Cx. pipiens complex and 49.5 per 1,000 (95% CI: 14.7-101.6 per 1000) in Ae. albopictus. The overall prevalence of D. immitis infected stages across all mosquito species was 21.8 per 1000 (95% CI: 14.06-31.18 per 1,000) and heterogeneity was substantial (I2 = 98.5%). Subgroup analysis for infective rate in vector produced overall prevalence ranging from 0 per 1,000 in Ochlerotatus sollicitans to 19.2 per 1,000 in Cx. theileri (95% CI: 0.06-62.5 per 1,000). The overall prevalence of D. immitis infective stages across all mosquito species was 2.6 per 1,000 (95% CI: 0.97-4.77 per 1,000) and heterogeneity was substantial (I2 = 94.4%).
Table 3.
Subgroup meta-analysis of studies reporting Dirofilaria immitis infected and infective rates in vectors grouped by mosquito genus and species.
| Minimum infected ratea | Minimum infective rateb | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | pool size | Positive pool | Overall prevalence (95% CI) | I2c | Q | Positive pool | Overall prevalence (95% CI) | I2 | Q |
| Total | 124,480 | 1,071 | 21.86 (14.06-31.1) | 98.5 | 2,475.9 | 235 | 2.6 (0.97-4.77) | 94.4 | 622.1 |
| Aedes | 62,924 | 526 | 29.5 (17.21-46.4) | 97.5 | 1,016.7 | 138 | 1.8 (0.03-5.3) | 91.6 | 298.4 |
| Culex | 16,652 | 260 | 12.36 (5.8-20.88) | 92.9 | 241.8 | 42 | 1.28 (0.0-4.04) | 83.3 | 95.6 |
| Anopheles | 4,244 | 157 | 51.37 (5-133.5) | 98.1 | 372.9 | 19 | 3.14 (0.0-12.2) | 75.5 | 28.6 |
| Ochlerotatus | 5,382 | 73 | 43.29 (0.1-140.6) | 96.3 | 54.9 | 24 | 0.97 (0.14-2.27) | 0.0 | – |
| Cx. quinciafasiatus | 4,892 | 168 | 24.7 (7.79-49.4) | 92 | 62.7 | 13 | 2.6 (0.0-12.46) | 87.6 | 40.2 |
| Ae. polynesiensis | 24,041 | 198 | 31.8 (3.5-85.5) | 98 | 100.8 | 53 | 1.8 (0.5-3.86) | 32.7 | 2.9 |
| Oc. taeniorhynchus | 2,517 | 44 | 12.6 (8.42-17.5) | 0.0 | – | 32 | 4.1 (1.73-7.31) | 0.0 | – |
| Oc. sollicitans | 2,539 | 25 | 0 | – | – | 2 | 0 | – | – |
| Cx. pipiens | 5,518 | 43 | 6.7 (3.48-10.88) | 59.7 | 19.8 | 7 | 0.62 (0-2.29) | 48.3 | 15.5 |
| Ae. ochcaspius | 285 | 14 | 48.6 (25.81-77.6) | 0.0 | – | 4 | 10.7 (0.91-27.7) | 0.0 | – |
| Ae. vexans | 13,439 | 77 | 47.1 (1.08-135) | 97.6 | 299.3 | 30 | 6.2 (0.0-38.53) | 94.0 | 117.2 |
| Cx. theileri | 1,574 | 43 | 35.3 (2.06-101.4) | 96.2 | 78.5 | 25 | 19.2 (0.06-62.5) | 94.5 | 54.3 |
| Ae. albopictus | 2,298 | 151 | 49.5 (14.7-101.6) | 95.3 | 169.7 | 25 | 3.2 (0.00-15.30) | 86.3 | 58.6 |
Notes: a,bThe result present as a case per 1,000; cheterogenicity index.
Meta-analysis for evaluating risk factor on overall prevalence of Dirofilaria immitis infected/infective stages in vectors
Different factors were analyzed on overall prevalence of D. immitis infected/infective stages in vectors (Tables 4, 5). The overall prevalence of dirofilariasis decreased with implementation years from 2000 to 2019. Dirofilariasis prevalence differs between countries. The overall prevalence of D. immitis infective stages in vectors ranged from 1.4 per 1,000 (95% CI: 0.3-3 per 1,000) in Brazil to 52.6 per 1,000 (95% CI: 25.5-94.7 per 1,000) in Iran. The greatest overall prevalence of infective rate was reported across studies conducted in the Eastern Mediterranean Region, longitude 80 to 110 (12.5 per 1,000, 95% CI: 0.0-49 per 1,000), latitude 20 to 40 (5.7 per 1,000, 95% CI: 1.3-12.2 per 1,000), annual rainfall 250 to 500 millimeter (5.1 per 1,000, 95% CI: 1.4-10.2 per 1,000), sea level 26 to 100 (11.2 per 1,000, 95% CI: 0.3-34.6 per 1,000) and <1,000 meter (10 per 1,000, 95% CI: 0.0-35.1 per 1,000), humidity 66-70% (7.4 per 1,000, 95% CI: 1.2-18 per 1,000) during 2000 to 2005 (9.6 per 1,000, 95% CI: 0.0-36.4 per 1,000) by dissection methods (4.5 per 1,000, 95% CI: 1.6-8.6 per 1,000). The mean temperatures of collection sites vary from 7 to 28°C. The higher infective rate was report between 15 and 21°C.
Table 4.
Subgroup meta-analysis of field studies reporting pool prevalence Dirofilaria immitis infected/infective rates in vectors grouped by country.
| Minimum infected ratea | Minimum infective rateb | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Pool size | Positive pool | Overall prevalence (95% CI) | I2c | Positive pool | Overall prevalence (95% CI) | I2 |
| Global | 124,480 | 1,071 | 21.9 (14-31.2) | 98.6 | 235 | 2.6 (1-4.8) | 94.4 |
| PAHO | 18,690 | 451 | 32.4 (13.3-59) | 98.2 | 101 | 3.8 (0.1-11.1) | 95.3 |
| Argentina | 2,462 | 5 | 0 (0-1.4) | 99.9 | 0 | 0 (0-0.3) | 99.8 |
| Brazil | 3,988 | 56 | 13.2 (9.8-17.1) | 99.9 | 8 | 1.4 (0.3-3) | 99.8 |
| Mexico | 867 | 32 | 25 (15.4-36.8) | 99.9 | 21 | 9.4 (3.7-17.3) | 99.8 |
| USA | 11,373 | 358 | 57.9 (8.1-146.5) | 99 | 72 | 4 (0-23.4) | 96.8 |
| EURO | 57,717 | 240 | 14.8 (5-28.7) | 97.2 | 71 | 2.2 (0.1-6.2) | 92.9 |
| Italy | 3,808 | 121 | 30.3 (3.1-80.6) | 97.7 | 30 | 6.3 (0-20.8) | 91.9 |
| Portugal | 2,389 | 58 | 23.1 (17.4-29.6) | 99.5 | 25 | 10 (6.3-14.5) | 98.4 |
| Slovakia | 187 | 4 | 14.7 (0.9-39.4) | 99.5 | 0 | 0 (0-10.2) | 98.4 |
| Spain | 2,825 | 4 | 1.3 (0.2-3.2) | 0 | 1 | 0.1 (0-1.3) | 0 |
| Turkey | 613 | 17 | 24.5 (12.7-39.3) | 5.1 | 15 | 21.4 (10.4-35.5) | 34.2 |
| Czech | 237 | 0 | 0 (0-15.4) | 0 | 0 | 0 (0-15.4) | 0 |
| France | 797 | 29 | 36.4 (24.5-51.8) | – | 0 | 0 (0-4.6) | – |
| Germany | 955 | 2 | 2.1 (0.3-7.5) | – | 0 | 0 (0-3.9) | – |
| Russia | 78 | 5 | 64.1 (21.2-143) | – | 0 | 0 (0-46.2) | – |
| Australia | 2,389 | 0 | 0 (0-0.1) | – | 0 | 0 (0-0.1) | – |
| EMRO | 190 | 15 | 78.9 (44.9-126.9) | – | 10 | 52.6 (25.5-94.7) | – |
| Iran | 190 | 15 | 78.9 (44.9-126.9) | – | 10 | 52.6 (25.5-94.7) | – |
| WPRO | 47,883 | 365 | 25.6 (6.1-57.4) | 98.9 | 53 | 0.3 (0-2.0) | 81.2 |
| French Polynesia | 1,194 | 28 | 23.5 (15.6-33.7) | – | 4 | 3.4 (0.9-8.6) | – |
| Samoa | 41,980 | 179 | 4.3 (3.7-4.9) | – | 49 | 1.2 (0.9-1.5) | – |
| Taiwan | 4,537 | 146 | 32.2 (27.2-37.7) | – | 0 | 0 (0.0-0.8) | – |
| South-Korea | 172 | 12 | 69.8 (36.6-118.7) | – | 0 | 0 (0.0-21.2) | – |
Notes: a,bThe result presents as a case per 1,000; cheterogenicity index.
Table 5.
Subgroup meta-analysis of field studies reporting pool prevalence D. immitis infected and infective rates in vectors grouped by risk factor.
| Minimum infected ratea | Minimum infective rateb | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Pool size | Positive pool | Overall prevalence (95% CI) | I2c | Positive pool | Overall prevalence (95% CI) | I2 |
| Latitude | |||||||
| 0-20 | 44,470 | 245 | 16.9 (3.2-40.1) | 96.6 | 53 | 0.5 (0.2-0.9) | 1.8 |
| 20-40 | 25,560 | 609 | 29 (15.1-46.9) | 97.7 | 129 | 5.7 (1.3-12.2) | 95.1 |
| >40 | 54,450 | 217 | 17.4 (4.5-37) | 98.3 | 53 | 1.70 (0-6.1) | 93.1 |
| Longitude | |||||||
| 0-10 | 4,218 | 86 | 13.1 (1.3-36) | 95.7 | 24 | 1.9 (0-9.5) | 90.9 |
| 10-20 | 52,621 | 128 | 12 (1.8-29.6) | 98.1 | 32 | 2.1 (0-6.7) | 93.3 |
| 20-50 | 4,745 | 96 | 29.9 (12.9-52.6) | 81.9 | 33 | 8.4 (0-26-9) | 87.8 |
| 50-80 | 12,150 | 129 | 6.1 (0-18.8) | 93.7 | 18 | 0 (0-1.4) | 66.9 |
| 80-110 | 2,473 | 234 | 58.1 (9.1-143) | 97.7 | 75 | 12.5 (0-49) | 96 |
| >110 | 48,273 | 398 | 34.8 (11.5-69.4) | 98.8 | 53 | 0.3 (0-1.7) | 75.1 |
| Mean temperature °C | |||||||
| 7-14.9 | 52,431 | 190 | 23.3 (7.3-46.9) | 98 | 47 | 2.5 (0-8.1) | 92.3 |
| 15-21.9 | 18,905 | 404 | 19.6 (6-39.9) | 98.1 | 106 | 4.1 (0.4-10.4) | 94.2 |
| 22-28.1 | 53,144 | 477 | 23.9 (10.8-41.5) | 98.1 | 82 | 1.9 (0.1-5.1) | 90.9 |
| Annual rainfall (mm) | |||||||
| <250 | 49,029 | 5 | 0.4 (0-3) | 79.5 | 1 | 0 (0-0.1) | 37.3 |
| 251-500 | 24,280 | 509 | 27.8 (16.6-41.5) | 96 | 100 | 5.1 (1.4-10.2) | 92 |
| 501-1,000 | 8,801 | 345 | 32 (9.8-65.7) | 97.9 | 85 | 3.4 (0-12.7) | 94.9 |
| >1,000 | 42,370 | 212 | 3.9 (3.3-4.6) | 99.7 | 49 | 0.6 (0.3-0.9) | 99.3 |
| Sea level (m) | |||||||
| <25 | 15,367 | 281 | 28.7 (12.4-50.9) | 96.4 | 52 | 3.4 (0.2-9.1) | 87.8 |
| 26-100 | 6,945 | 291 | 36.1 (4.7-93.7) | 98.9 | 98 | 11.2 (0.3-34.6) | 97.6 |
| 101-200 | 95,282 | 379 | 13.3 (4.1-26.8) | 99.2 | 57.9 | 0 (0-1.1) | 93.7 |
| 201-1000 | 4,067 | 58 | 12.3 (1.4-31.7) | 94 | 3 | 0.1 (0-1) | 0 |
| >1,000 | 2,819 | 62 | 27.8 (4.4-67.3) | 95.1 | 25 | 10 (0-35.1) | 93.8 |
| Mean humidity (%) | |||||||
| <65 | 5,171 | 73 | 12.3 (1.5-29.6) | 89.5 | 24 | 5.8 (0-19.6) | 89.6 |
| 66-70 | 15,821 | 413 | 26.9 (6.1-61.1) | 98.8 | 104 | 7.4 (1.2-18) | 95.9 |
| 71-75 | 51,514 | 132 | 24.4 (8.2-48.1) | 97.6 | 31 | 1.8 (0-7.1) | 91 |
| 76-80 | 8,579 | 235 | 22.2 (9.9-38.8) | 93.1 | 27 | 1.2 (0-8.5) | 93.7 |
| >80 | 43,395 | 218 | 14.1 (0.9-39.4) | 95.3 | 0.49 | 0.1 (0-0.3) | 0 |
| Detection method | |||||||
| PCR | 60,539 | 425 | 19.3 (7.9-34.9) | 98.3 | 113 | 2 (0-5.7) | 93.7 |
| Dissection | 63,941 | 646 | 30.5 (1.5-48.5) | 98.8 | 122 | 4.5 (1.6-8.6) | 95.2 |
| Implementation year | |||||||
| 1992-2000 | 55,222 | 360 | 10.5 (4-19.7) | 97.3 | 75 | 0.9 (0.4-1.6) | 40.7 |
| 2000-2005 | 6,221 | 257 | 57.3 (14.5-124.7) | 98 | 27 | 9.6 (0-36.4) | 96.6 |
| 2006-2010 | 8,525 | 132 | 24.4 (10.1-43.9) | 95.3 | 41 | 3.7 (0.1-10.3) | 89.6 |
| 2011-2015 | 6,313 | 283 | 19.9 (2.6-50.2) | 94.4 | 91 | 3.8 (0-14.6) | 93.5 |
| 2016-2018 | 48,199 | 39 | 10.3 (0–35.1) | 97.4 | 1 | 0 (0-0.1) | 45.4 |
Notes: a,bThe result presents as a case per 1,000; cheterogenicity index.
Meta-analysis of experimental studies for overall prevalence of Dirofilaria immitis infected/infective stages in mosquitoes
The total sample size of experimental studies was 4,512. The overall prevalence of D. immitis infected and infective stages in mosquitoes were 397 per 1,000 (95% CI: 154.5-636.5 per 1,000) and 84.7 per 1,000 (95% CI: 20.5-183.8 per 1,000), respectively. The PCR technique was more sensitive than morphological tests. Anopheles spp were accused as a more competent vector than other mosquitoes for transmission of D. immitis (Table 6).
Table 6.
Subgroup meta-analysis of experimental studies reporting Dirofilaria immitis infected and infective rates in vectors grouped by mosquito genus and species.
| Minimum infected ratea | Minimum infective rateb | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Pool size | Positive pool | Overall prevalence (95% CI) | I2 | Positive pool | Overall prevalence (95% CI) | I2 |
| Overall | 4,512 | 1,524 | 379.9 (154.5-636.5) | 89.2 | 251 | 84.7 (20.5-183.8) | 98.8 |
| Method PCR | 271 | 161 | 649.2 (459.5-817.8) | 99.7 | 44 | 194.6 (55-388.8) | 99.4 |
| Method Dissection | 4,241 | 1,363 | 253.7 (40.4-565.7) | 99.6 | 207 | 46.9 (0.9-147) | 99.1 |
| Ae. spp. | 1,506 | 905 | 482.2 (105.2-871.6) | 99.6 | 168 | 132.4 (17.3-324.2) | 98.5 |
| Cx. spp. | 2,893 | 561 | 61.2 (0.5-197) | 98.8 | 65 | 55.9 (2.7-163.1) | 98.2 |
| An. spp. | 90 | 58 | 644.4 (536.5-742.6) | – | 16 | 177.8 (105.2-274.6) | – |
| Oc. spp. | 0 | 0 | 0 | 0 | 0 | ||
Notes: a,bThe result presents as a case per 1,000.
Discussion
Summary of evidence
This is the first study that aimed to summarizing information from field and experiments studies related to overall prevalence of D. immitis infected/infective mosquitoes using a meta-analysis. The potency of mosquito species to become infected and then transmit D. immitis is an important step that led to the accurate measurement the role of vectors in transmission (Morchón et al., 2012). Based on our result, the prevalence of infective stages of D. immitis in field mosquitoes was different according to genus and species. The most infective rate belongs to Anopheles spp., Aedes spp., and Culex spp. with the overall prevalence of 3.1 per 1,000 (95% CI: 0-12.2 per 1,000), 1.8 per 1,000 (95% CI: 0.03-5.3 per 1,000), and 1.2 per 1,000 (95% CI: 0-4.4 per 1,000), respectively. Due to the higher species diversity of Anopheles compare to other studied genera and the high infective rate among species of this genus, it was expected to have the highest rate of infectivity for Anopheles spp. Interestingly, the overall prevalence of the D. immitis infective mosquitoes in experimental studies was the same of field cached mosquitoes in accordance to the genus. D. immitis is sub-periodic, with peak density of microfilaria in a host’s peripheral circulation during the evening, presumably synchronizing with circadian cycles of their potential vectors. So, Culex theileri with feeding behavior of nocturnal is also among the species with the highest overall prevalence of D. immitis infected and infective stages. (Ledesma and Harrington, 2011). The prevalence rate of mosquitoes in field studies were varied in different geographical property, including latitude, longitude, mean temperature, annual rainfall, sea level, and humidity. The factor ‘country’ should be explicate with the many factors such as different national vector control measures and ecological context (Genchi et al., 2009). Diverse ecological and geographical characteristics can also contribute toward biological variation. Climate change together with a shifting weather patterns facilitate the development of mosquitoes vectors of D. immitis and faster larvae development on them (Morchón et al., 2012). Today, it was completely determined that climate pattern can affect vector-borne disease transmission. In this meta-analysis study, it was shown that the high infected and infective rate was registered at the temperature optima of 15 to 21°C that it may be due to a suitable temperature for the development of the infectious stages of D. immitis in the mosquito. In this regard, Iran with average temperature of 17.5 had a highest registered D. immitis infective rate among the world. The extrinsic development is possible above 14°C and it takes e.g. 10 to 12 days at 24 to 26°C (Silaghi et al., 2017). One of the main reasons for decrease of infection rate at higher temperatures despite the appropriateness of the extrinsic period may be due to the high mortality rate of insects (Bayoh and Lindsay, 2004). The other important factors that could influence vector behavior and survival is relative humidity (Brown et al., 2012). Based on the current meta-analysis study, the mean humidity for highest infective rate was 66 to 70%. In this regard, the highest infective rate was belonged to the studies in Iran and Turkey with relative humidity of 72 and 62% at sampling sites. The standard laboratory conditions for transformation of microfilaria to infective larvae on mosquitoes are at a temperature of 25°C and relative humidity of 85% (Silaghi et al., 2017), while it was 15 to 21°C and relative humidity of 66 to 70% for field condition. In the experimental studies, the optimum condition for infection of mosquitoes is provided, so as expected, the overall prevalence of D. immitis infection of mosquitoes in the experimental studies is higher than the field studies.
Strength
To our knowledge, our work is the only study to evaluate an overall prevalence of D. immitis in vectors. The other strength of this study were comprehensive literature searches, rigorous methodology, studies from different part of the world, defined clear inclusion and exclusion criteria.
Limitation
This study has some limitations that are based on the nature of the published studies such as different diagnostic test in included studies. Further, some studies published in local journals may be missed.
Conclusion
Based on finding of this study, among different genera of mosquitoes, the genus of Anopheles were introduced as a potential vectors of Heartworm disease with the highest infective rate. It must be considered that in addition to the infection rate other factor such as host preference; vector feeding behavior; mortality rates of infected vector; vector and host abundance and microfilaremic reservoir and susceptible host encounter rate are important for affecting transmission of mosquito species (Ledesma and Harrington, 2011). This study showed that the WHO region Number, humidity, longitude, latitude, annual rainfall, sea level, the year of implementation and identification methods may affect the overall prevalence of infected and infective rate.
Acknowledgments
This work was supported by the Birjand University of Medical Science, Birjand, Iran [5007]. Conflict of interest: the authors declare they have no conflict of interest. Ethical approval: this study was approved by the Ethical Committee of Birjand University of Medical Sciences, Birjand, Iran (IR.BUMS.REC.1398-396).
References
- Ahid, S. M. M., Vasconcelos, P. S. D. S. and Lourenço-de-Oliveira, R.. 2000. Vector competence of Culex quinquefasciatus Say from different regions of Brazil to Dirofilaria immitis. Memórias do Instituto Oswaldo Cruz 95:769–775. [DOI] [PubMed] [Google Scholar]
- Azari-Hamidian, S., Yaghoobi-Ershadi, M., Javadian, E., Mobedi, I. and Abai, M.. 2007. Review of dirofilariasis in Iran. Journal of Guilan University of Medical Sciences 15:102–114. [Google Scholar]; Azari-Hamidian, S., Yaghoobi-Ershadi, M., Javadian, E., Abai, M., Mobedi, I., Linton, Y. M. and Harbach, R. 2009. Distribution and ecology of mosquitoes in a focus of dirofilariasis in northwestern Iran, with the first finding of filarial larvae in naturally infected local mosquitoes. Medical and Veterinary Entomology 23:111–. [DOI] [PubMed] [Google Scholar]
- Azari‐Hamidian, S., Yaghoobi‐Ershadi, M., Javadian, E., Abai, M., Mobedi, I., Linton, Y. M. and Harbach, R.. 2009. Distribution and ecology of mosquitoes in a focus of dirofilariasis in northwestern Iran, with the first finding of filarial larvae in naturally infected local mosquitoes. Medical and Veterinary Entomology 23:111–121. [DOI] [PubMed] [Google Scholar]
- Bayoh, M. N. and Lindsay, S. W.. 2004. Temperature-related duration of aquatic stages of the Afrotropical malaria vector mosquito Anopheles gambiae in the laboratory. Medical and Veterinary Entomology 18:174–179. [DOI] [PubMed] [Google Scholar]
- Bışkın, Z., Düzlü, O., Yildirim, A. and Incı, A.. 2010. The molecular diagnosis of Dirofilaria immitis in vector mosquitoes in Felahiye district of Kayseri. Turkiye parazitolojii dergisi 34:200–205. [PubMed] [Google Scholar]
- Bocková, E., Iglódyová, A. and Kočišová, A.. 2015. Potential mosquito (Diptera: Culicidae) vector of Dirofilaria repens and Dirofilaria immitis in urban areas of Eastern Slovakia. Parasitology Research 114:4487–4492. [DOI] [PubMed] [Google Scholar]
- Bravo-Barriga, D., Parreira, R., Almeida, A. P., Calado, M., Blanco-Ciudad, J., Serrano-Aguilera, F. J., Pérez-Martín, J. E., Sanchez-Peinado, J., Pinto, J. and Reina, D.. 2016. Culex pipiens as a potential vector for transmission of Dirofilaria immitis and other unclassified Filarioidea in Southwest Spain. Veterinary Parasitology 223:173–180. [DOI] [PubMed] [Google Scholar]
- Brito, A. C., Fontes, G., da Rocha, E. M., Rocha, D. A. and Regis, L.. 1999. Development of Dirofilaria immitis (Leidy) in Aedes aegypti (L.) and Culex quinquefasciatus (say) from Maceio, Alagoas, Brazil. Memórias do Instituto Oswaldo Cruz 94:575–576. [DOI] [PubMed] [Google Scholar]
- Brown, H. E., Harrington, L. C., Kaufman, P. E., McKay, T., Bowman, D. D., Nelson, C. T., Wang, D. and Lund, R.. 2012. Key factors influencing canine heartworm, Dirofilaria immitis, in the United States, BioMed Central.
- Cancrini, G., Di Regalbono, A. F., Ricci, I., Tessarin, C., Gabrielli, S. and Pietrobelli, M.. 2003a. Aedes albopictus is a natural vector of Dirofilaria immitis in Italy. Veterinary Parasitology 118:195–202. [DOI] [PubMed] [Google Scholar]
- Cancrini, G., Romi, R., Gabrielli, S., Toma, L., Di Paolo, M. and Scaramozzino, P.. 2003b. First finding of Dirofilaria repens in a natural population of Aedes albopictus. Medical and Veterinary Entomology 17:448–451. [DOI] [PubMed] [Google Scholar]
- Cancrini, G., Scaramozzino, P., Gabrielli, S., Paolo, M. D., Toma, L. and Romi, R.. 2007. Aedes albopictus and Culex pipiens implicated as natural vectors of Dirofilaria repens in central Italy. Journal of Medical Entomology 44:1064–1066. [DOI] [PubMed] [Google Scholar]
- Cancrini, G., Magi, M., Gabrielli, S., Arispici, M., Tolari, F., Dell’Omodarme, M. and Prati, M.. 2006. Natural vectors of dirofilariasis in rural and urban areas of the Tuscan region, central Italy. Journal of Medical Entomology 43:574–579. [DOI] [PubMed] [Google Scholar]
- Carvalho, G. A. D., Alves, L. C., Maia, R. T., Andrade, C. F. S. D., Ramos, R. A. D. N. and Faustino, M. A. D. G.. 2008. Vector competence of Culex quinquefasciatus Say, 1823 exposed to different densities of microfilariae of Dirofilaria immitis (Leidy, 1856). Revista Brasileira de Entomologia 52:658–662. [Google Scholar]
- Chambers, E. W., McClintock, S. K., Avery, M. F., King, J. D., Bradley, M. H., Schmaedick, M. A., Lammie, P. J. and Burkot, T. R.. 2009. Xenomonitoring of Wuchereria bancrofti and Dirofilaria immitis infections in mosquitoes from American samoa: trapping considerations and a comparison of polymerase chain reaction assays with dissection. American Journal of Tropical Medicine and Hygiene 80:774–781. [PubMed] [Google Scholar]
- Comiskey, N. and Wesson, D. M.. 1995. Dirofilaria (Filarioidea: Onchocercidae) infection in Aedes albopictus (Diptera: Culicidae) collected in Louisiana. Journal of Medical Entomology 32:734–737. [DOI] [PubMed] [Google Scholar]
- Debboun, M., Green, T. J., Rueda, L. M. and Hall, R. D.. 2005. Relative abundance of tree hole-breeding mosquitoes in Boone County, Missouri, USA, with emphasis on the vector potential of Aedes triseriatus for canine heartworm, Dirofilaria immitis (Spirurida: Filariidae). Journal of the American Mosquito Control Association 21:274–278. [DOI] [PubMed] [Google Scholar]
- Ferreira, C. A. C., de Pinho Mixão, V., Novo, M. T. L. M., Calado, M. M. P., Gonçalves, L. A. P., Belo, S. M. D. and De Almeida, A. P. G.. 2015. First molecular identification of mosquito vectors of Dirofilaria immitis in continental Portugal. Parasites & Vectors 8:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman, M. F. and Tukey, J. W.. 1950. Transformations related to the angular and the square root. The Annals of Mathematical Statistics, 21: 607–611. [Google Scholar]
- Genchi, C., Rinaldi, L., Mortarino, M., Genchi, M. and Cringoli, G.. 2009. Climate and Dirofilaria infection in Europe. Veterinary Parasitology 163:286–292. [DOI] [PubMed] [Google Scholar]
- Harris, R., Bradburn, M., Deeks, J., Harbord, R., Altman, D. and Sterne, J.. 2008. Metan: fixed-and random-effects meta-analysis. Stata Journal 8:3. [Google Scholar]
- Higgins, J. 2008. “Assessing risk of bias in included studies”, In Altman, D. G., Higgins, J. P. T. and S. G. (Eds), Cochrane Handbook for Systematic Reviews of Interventions Version 501 the Cochrane Collaboration Wiley-Blackwell, Chichester, pp. 187–. [Google Scholar]
- Kronefeld, M., Kampen, H., Sassnau, R. and Werner, D.. 2014. Molecular detection of Dirofilaria immitis, Dirofilaria repens and Setaria tundra in mosquitoes from Germany. Parasites & Vectors 7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarthe, N., Serrão, M. L., Melo, Y. F., De Oliveira, S. J. and Lourenço-de-Oliveira, R.. 1998. Potential Vectors of Dirofilaria immitis (Leidy, 1856) in Itacoatiara, Oceanic Region of Niterói Municipality, State of Rio de Janeiro, Brazil. Memorias do Instituto Oswaldo Cruz 93:425–432. [DOI] [PubMed] [Google Scholar]
- Lai, C. H., Tung, K. C., Ooi, H. K. and Wang, J. S.. 2001. Susceptibility of mosquitoes in central Taiwan to natural infections of Dirofilaria immitis. Medical and Veterinary Entomology 15:64–67. [DOI] [PubMed] [Google Scholar]
- Latrofa, M. S., Montarsi, F., Ciocchetta, S., Annoscia, G., Dantas-Torres, F., Ravagnan, S., Capelli, G. and Otranto, D.. 2012. Molecular xenomonitoring of Dirofilaria immitis and Dirofilaria repens in mosquitoes from north-eastern Italy by real-time PCR coupled with melting curve analysis. Parasites and Vectors 5:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledesma, N. and Harrington, L.. 2011. Mosquito vectors of dog heartworm in the United States: Vector status and factors influencing transmission efficiency. Topics in Companion Animal Medicine 26:178–185. [DOI] [PubMed] [Google Scholar]
- Lee, S. -E., Kim, H. -C., Chong, S. -T., Klein, T. A. and Lee, W. -J.. 2007. Molecular survey of Dirofilaria immitis and Dirofilaria repens by direct PCR for wild caught mosquitoes in the Republic of Korea. Veterinary Parasitology 148:149–155. [DOI] [PubMed] [Google Scholar]
- Licitra, B., Chambers, E. W., Kelly, R. and Burkot, T. R.. 2010. Detection of dirofilaria immitis (Nematoda: Filarioidea) by polymerase chain reaction in aedes albopictus, anopheles punctipennis, and anopheles crucians (Diptera: Culicidae) from Georgia. Journal of Medical Entomology 47:634–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macêdo, F. C. D., Labarthe, N. and Lourenço-de-Oliveira, R.. 1998. Susceptibility of Aedes scapularis (Rondani, 1848) to Dirofilaria immitis (Leidy, 1856), an emerging zoonosis. Memórias do Instituto Oswaldo Cruz 93:435–437. [DOI] [PubMed] [Google Scholar]
- Manrique-Saide, P., Bolio-González, M., Sauri-Arceo, C., Dzib-Florez, S. and Zapata-Peniche, A.. 2008. Ochlerotatus taeniorhynchus: a probable vector of Dirofilaria immitis in coastal areas of Yucatan, Mexico. Journal of Medical Entomology 45:169–171. [DOI] [PubMed] [Google Scholar]
- Masetti, A., Rivasi, F. and Bellini, R.. 2008. Mosquito-based survey for the detection of flaviviruses and filarial nematodes in Aedes albopictus and other anthropophilic mosquitoes collected in northern Italy. New Microbiologica 31:457–465. [PubMed] [Google Scholar]
- Mckay, T., Bianco, T., Rhodes, L. and Barnett, S.. 2013. Prevalence of Dirofilaria immitis (Nematoda: Filarioidea) in mosquitoes from northeast Arkansas, the United States. Journal of Medical Entomology 50:871–878. [DOI] [PubMed] [Google Scholar]
- Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., Shekelle, P. and Stewart, L. A.. 2015. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews 4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokhayeri, Y., Riahi, S. M., Rahimzadeh, S., Pourhoseingholi, M. A. and Hashemi-Nazari, S. S.. 2018. Metabolic syndrome prevalence in the Iranian adult’s general population and its trend: a systematic review and meta-analysis of observational studies. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 12:441–453. [DOI] [PubMed] [Google Scholar]
- Montarsi, F., Ciocchetta, S., Devine, G., Ravagnan, S., Mutinelli, F., Frangipane Di Regalbono, A., Otranto, D. and Capelli, G.. 2015. Development of Dirofilaria immitis within the mosquito Aedes (Finlaya) koreicus, a new invasive species for Europe. Parasites and Vectors 8: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morchón, R., Carretón, E., González Miguel, J. and Mellado Hernández, I.. 2012. Heartworm disease (Dirofilaria immitis) and their vectors in Europe–new distribution trends. Frontiers in Physiology 3:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morchon, R., Bargues, M. D., Latorre, J. M., Melero-Alcibar, R., Pou-Barreto, C., Mas-Coma, S. and Simon, F.. 2007. Haplotype H1 of Culex pipiens implicated as natural vector of Dirofilaria immitis in an endemic area of Western Spain. Vector-borne and Zoonotic Diseases 7:653–658. [DOI] [PubMed] [Google Scholar]
- Morchón, R., Bargues, M., Latorre-Estivalis, J., Pou-Barreto, C., Melero-Alcibar, R., Moreno, M., Valladares, B., Molina, R., Montoya-Alonso, J. and Mas-Coma, S.. 2011. Molecular Characterization of Culex theileri from Canary Islands, Spain, a potential vector of Dirofilaria immitis. Journal of Clinical and Experimental Pathology 3:001. [Google Scholar]
- Nayar, J. and Knight, J.. 1999. Aedes albopictus (Diptera: Culicidae): an experimental and natural host of Dirofilaria immitis (Filarioidea: Onchocercidae) in Florida, USA. Journal of Medical Entomology 36:441–448. [DOI] [PubMed] [Google Scholar]
- Nyaga, V. N., Arbyn, M. and Aerts, M.. 2014. Metaprop: a Stata command to perform meta-analysis of binomial data. Archives of Public Health 72:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa, G. M., Cruz, E.N.D., Cunha, P. N. A. and Camargo, L. M. A.. 2013. Canine heartworm disease in Porto Velho: first record, distribution map and occurrence of positive mosquitoes. Revista Brasileira de Parasitologia Veterinária 22:559–564. [DOI] [PubMed] [Google Scholar]
- Oswaldo, M. T. -C., Baak-Baak, C. M., Cigarroa-Toledo, N., Blitvich, B. J., Brito-Argaez, L. G., Alvarado-Kantun, Y. N., Zaragoza-Vera, C. V., Arjona-Jimenez, G., Moreno-Perez, L. G. and Medina-Perez, P.. 2018. Molecular detection of Dirofilaria immitis in dogs and mosquitoes in Tabasco, Mexico. Journal of Vector Borne Diseases 55:151. [DOI] [PubMed] [Google Scholar]
- Parker, B. M. 1993. Variation in mosquito (Diptera: Culicidae) relative abundance and Dirofilaria immitis (Nematoda: Filarioidea) vector potential in coastal North Carolina. Journal of Medical Entomology 30:436–442. [DOI] [PubMed] [Google Scholar]
- Riahi, S. M. and Mokhayeri, Y.. 2017. Methodological issues in a meta-analysis. Current Medical Research and Opinion 33:1813–1813. [DOI] [PubMed] [Google Scholar]
- Russell, R. C., Webb, C. E. and Davies, N.. 2005. “Aedes aegypti (L.) and Aedes polynesiensis Marks (Diptera: Culicidae) in Moorea, French Polynesia: a study of adult population structures and pathogen (Wuchereria bancrofti and Dirofilaria immitis) infection rates to indicate regional and seasonal epidemiological risk for dengue and filariasis”, Journal of Medical Entomology 42:1045–1056. [DOI] [PubMed] [Google Scholar]
- Samarawickrema, W. A., Kimura, E., Sones, F., Paulson, G. S. and Cummings, R. F.. 1992. Natural infections of Dirofilaria immitis in Aedes (Stegomyia) polynesiensis and Aedes (Finlaya) samoanus and their implication in human health in Samoa. Transactions of the Royal Society of Tropical Medicine and Hygiene 86:187–188. [DOI] [PubMed] [Google Scholar]
- Santa-Ana, M., Khadem, M. and Capela, R.. 2006. Natural infection of Culex theileri (Diptera: Culicidae) with Dirofilaria immitis (Nematoda: Filarioidea) on Madeira Island, Portugal. Journal of Medical Entomology 43:104–106. [DOI] [PubMed] [Google Scholar]
- Sassnau, R., Daugschies, A., Lendner, M. and Genchi, C.. 2014. Climate suitability for the transmission of Dirofilaria immitis and D. repens in Germany. Veterinary Parasitology 205:239–245. [DOI] [PubMed] [Google Scholar]
- Shaikevich, E. V., Patraman, I. V., Bogacheva, A. S., Rakova, V. M., Zelya, O. P. and Ganushkina, L. A.. 2018. Invasive mosquito species Aedes albopictus and Aedes aegypti on the Black Sea coast of the Caucasus: genetics (COI, ITS2), Wolbachia and Dirofilaria infections. Vavilovskii Zhurnal Genetiki i Selektsii 22:574–585. [Google Scholar]
- Silaghi, C., Beck, R., Capelli, G., Montarsi, F. and Mathis, A.. 2017. Development of Dirofilaria immitis and Dirofilaria repens in Aedes japonicus and Aedes geniculatus. Parasites & Vectors 10:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simón, F., Siles-Lucas, M., Morchón, R., González-Miguel, J., Mellado, I., Carretón, E. and Montoya-Alonso, J. A.. 2012. Human and animal dirofilariasis: the emergence of a zoonotic mosaic. Clinical Microbiology Reviews 25:507–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solgi, R., Sadjjadi, S. M., Mohebali, M., Djadid, N. D., Raz, A., Zakeri, S. and Zarei, Z.. 2017. Susceptibility of Anopheles stephensi (Diptera: Culicidae) to Dirofilaria immitis (Spirurida: Onchocercidae). Russian Journal of Nematology 25:121–127. [Google Scholar]
- Stang, A. 2010. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European Journal of Epidemiology 25:603–605. [DOI] [PubMed] [Google Scholar]
- Tahir, D., Bittar, F., Barré-Cardi, H., Sow, D., Dahmani, M., Mediannikov, O., Raoult, D., Davoust, B. and Parola, P.. 2017. Molecular survey of Dirofilaria immitis and Dirofilaria repens by new real-time TaqMan® PCR assay in dogs and mosquitoes (Diptera: Culicidae) in Corsica (France). Veterinary Parasitology 235:1–7. [DOI] [PubMed] [Google Scholar]
- Vezzani, D., Eiras, D. F. and Wisnivesky, C.. 2006. Dirofilariasis in Argentina: Historical review and first report of Dirofilaria immitis in a natural mosquito population. Veterinary Parasitology 136:259–273. [DOI] [PubMed] [Google Scholar]
- Vezzani, D., Mesplet, M., Eiras, D. F., Fontanarrosa, M. F. and Schnittger, L.. 2011. PCR detection of Dirofilaria immitis in Aedes aegypti and Culex pipiens from urban temperate Argentina. Parasitology Research 108:985–989. [DOI] [PubMed] [Google Scholar]
- Yildirim, A., Inci, A., Duzlu, O., Biskin, Z., Ica, A. and Sahin, I.. 2011. Aedes vexans and Culex pipiens as the potential vectors of Dirofilaria immitis in Central Turkey. Veterinary Parasitology 178:143–147. [DOI] [PubMed] [Google Scholar]


