To the Editor:
Under normal conditions, and despite being an organizational challenge, the vast majority of allogeneic hematopoietic progenitor cell (HPC) grafts collected from related donors (RD) or unrelated donors (URD) are freshly infused within hours of collection, while cryopreservation is restricted to exceptional conditions related to donor unreliability/unavailability [1]. However, since the beginning of the global COVID-19 pandemic, recommendations from professional societies (AABB, EBMT, ASTCT, WMDA, and FACT) were issued to cryopreserve allogeneic HPC grafts. The rationale behind is to minimize the risk of harvesting a cell product from a SARS-CoV-2 positive donor (by physical examination and RT-PCR testing of a nasopharyngeal swab) as well as to exclude potential transport setbacks (due to closed national borders or other reasons) before initiation of conditioning regimen. Recent studies reported on the minimal impact cryopreservation of allogeneic grafts has on hematopoietic recoveries, risks for acute graft-versus-host disease, non-relapse mortality or overall survival of patients with hematological malignancies, despite the heterogeneity of these assessments [2, 3].
At our institution, cryopreserved HPC grafts (autologous and occasionally allogeneic) are thawed and washed before infusion, according to standardized, and mostly automated procedures, generating cell products with high CD34+ cell recovery and viability [4]. We hereby report our single-center experience of cryopreserved HPC allogeneic graft manipulation, from both RD and URD, in the peculiar context of the global COVID-19 pandemic.
Data from our cell processing facility were compiled for 42 allogeneic rHuG-CSF-mobilized peripheral blood HPC products (28 RD and 14 URD) processed between March and July 2020. RD HPC grafts were collected at our apheresis unit, while URD HPC grafts were collected in centers in Europe and subsequently temporarily stored and transported at +4–10 °C. Upon reception, systematic platelet depletion/volume reduction, suspension in 6% HES 10% DMSO at 100 ml per bag, and cryopreservation in a controlled-rate freezer were performed, before storage in vapor phase of nitrogen containers. All products tested negative for microbiological contamination (aerobic and anaerobic cultures on both fresh and thawed/washed cell products). Dry-thawing was performed on Smart-Max (Cytiva Europe GmbH), followed by automated washing using Sepax-2 (Cytiva Europe GmbH).
Viable CD34+ and CD45+ cell counts were determined by single-platform flow cytometry assay using Stem-Kit (Beckman Coulter) according to the modified International Society of Hemotherapy and Graft Engineering (ISHAGE, now ISCT) protocol which includes the use of 7-AAD and Flowcount Fluorospheres (Beckman Coulter) for absolute viable CD34+ cells counting of a sample from the washed bag immediately before infusion [5]. As a validated test for potency, colony-forming unit (CFU) assay was performed on all washed products followed by automated counting on STEMVision (Stemcell technologies) at the end of the 14-day incubation period, as previously reported by our group [6]. Data analysis was performed using GraphPad Prism 5 (GraphPad, La Jolla, CA).
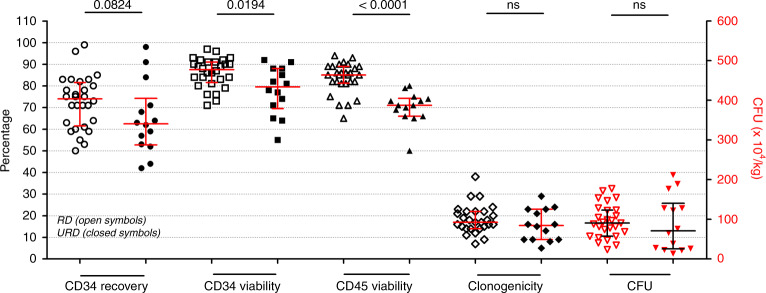
Median CD34+ cell recovery and CD45+ cell viability were 71% (range: 42–99%) and 82% (range: 50–94%), respectively, for all donors (n = 42), yet URD grafts exhibited lower cell recoveries, viabilities and wider variations, longer transit times being probably a key contributing factor (transit time = time between the end of apheresis collection and cryopreservation; median of 2.1 h for RD versus 45.9 h for URD). Storage length in vapor phase of nitrogen containers (17 days for RD, range 9–178 days versus 15 days, range 11–40 days for URD), CD34+ cell dose pre-cryopreservation (9.1 versus 9.2 × 106/kg, respectively), and CD34+ cell dose after thawing and washing (6.1 versus 5.1 × 106/kg, respectively) were similar. Viable CD34+ cell recovery for URD (62%, Fig. 1) was lower than that reported by Berens et al. and Fisher et al. (93 and 98%, respectively) yet CD45+ cell viability (71%, Fig. 1) was higher (58 and 64%, respectively) [7, 8]. Reasons could be related to differences in transit times, techniques for washing thawed cells before infusion and differences in CD34+ cell quantification method.
Fig. 1. CD34+ cell recovery and viability, CD45+ cell viability and potency assay assessment of HPC grafts of both RD and URD after thawing and washing.
Single-platform flow cytometry-based analysis was used for viable CD34+ and CD45+ cells enumeration and measurement of viability, while a 14-day culture and automated cell counting were performed to report CFU counts and clonogenicity. CD34+ cell recovery was calculated as the ratio of absolute count of post-wash viable CD34+ cells to pre-cryopreservation absolute counts of viable CD34+ cells. Clonogenicity was calculated as the percentage of CFU count (×104) to post-wash viable CD34+ cells absolute count (×106). Data are presented as median ± interquartile ranges. Recovery, viabilities and clonogenicity are reported as percentages using the left y-axis. CFU is reported as count (×104/kg) using the right y-axis. Open symbols represent RD, closed symbols represent URD. p values are reported above the groups using Mann–Whitney test for null hypothesis testing.
URD grafts exhibited lower CD34+ and CD45+ cells viabilities than RD grafts, with transit time negatively correlated with CD34+ cells viability at infusion: r2 = 0.198, regression coefficient −0.205 (95% CI: −0,341 to −0,07), p = 0.004. This observation is in line with the recent report by Purtill et al. [9], despite significant differences: nine Australian cell-processing laboratories using heterogeneous cryopreservation and QC methods, without DMSO-removing manipulations prior to infusion. However, in terms of CFU counts as an in vitro test for potency of HPC, no statistically significant difference was detected between URD and RD infused HPC grafts (Fig. 1), ruling out effects of long transit times on the quality of lineage-specific progenitor cells; our CFU counts for thawed and washed RD and URD grafts are similar to those reported by two other groups [10, 11]. The reduced variability in viable CD34+ cell recovery in our assessment (42–99%, Fig. 1, compared to 6–122% for the recent report by Purtill et al.) speaks for the efficacy of standardized and automated post-cryopreservation processing, despite the limitation of being a relatively small series of 42 allogeneic products acquired over a short period of 4 months.
All patients achieved neutrophil recovery at a median of 21 days after infusion for RD (range 13-37 days) versus 22 days for URD grafts (range 16–33 days). Post-infusion grade I adverse events occurred in 5 of the 28 RD and in none of the URD transplanted patients. Of the 44 cryopreserved HPC grafts over this 4 month-period, 2 RD grafts were not infused due to worsening of the patient’s health status (4.5%), thus corroborating the ethical issue prompted by cryopreservation of URD allogeneic grafts [12].
In conclusion, cryopreservation of allogeneic HPC grafts is a reasonable option that might be implemented after benefit-risk assessment to ensure both safety of the collected HPC graft during exceptional conditions such as the COVID-19 pandemic or anticipated challenges in relation to cell procurement or transportation. While CD34+ cell loss remains unavoidable, inter-individual variability can be mitigated by robust, standardized, and automated post-collection processing. Since CD34+ cell recovery tends to be lower for URD grafts (63 versus 74% for RD grafts, Fig. 1), we therefore suggest to systematically reduce transit times when feasible and to request a slightly higher dose of CD34+ cells to be collected by donor centers for URD.
Acknowledgements
We would like to thank the transplant coordinators responsible for RD and URD identification and recruitment as well as the donors for their time and commitment.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Frey NV, Lazarus HM, Goldstein SC. Has allogeneic stem cell cryopreservation been given the ‘cold shoulder’? An analysis of the pros and cons of using frozen versus fresh stem cell products in allogeneic stem cell transplantation. Bone Marrow Transplant. 2006;38:399–405. doi: 10.1038/sj.bmt.1705462. [DOI] [PubMed] [Google Scholar]
- 2.Alotaibi AS, Prem S, Chen S, Lipton JH, Kim DD, Viswabandya A et al. Fresh versus frozen allogeneic peripheral blood stem cell grafts: a successful timely option. Am J Hematol. 2020. 10.1002/ajh.26033 [DOI] [PubMed]
- 3.Hamadani M, Zhang MJ, Tang XY, Fei M, Brunstein C, Chhabra S, et al. Graft cryopreservation does not impact overall survival after allogeneic hematopoietic cell transplantation using post-transplantation cyclophosphamide for graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant. 2020;26:1312–7. doi: 10.1016/j.bbmt.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calmels B, Drezet A, Huynh C, Autret A, Stoppa AM, Bouabdallah R, et al. Automated washing of autologous hematopoietic stem cell grafts after thawing does not impair engraftment. Bone Marrow Transplant. 2014;49:1127–8. doi: 10.1038/bmt.2014.111. [DOI] [PubMed] [Google Scholar]
- 5.Brocklebank AM, Sparrow RL. Enumeration of CD34+ cells in cord blood: a variation on a single-platform flow cytometric method based on the ISHAGE gating strategy. Cytometry. 2001;46:254–61. doi: 10.1002/cyto.1136. [DOI] [PubMed] [Google Scholar]
- 6.Velier M, Chateau AL, Malenfant C, Ouffai S, Calmels B, Chabannon C, et al. Validation of a semi automatic device to standardize quantification of Colony-Forming Unit (CFU) on hematopoietic stem cell products. Cytotherapy. 2019;21:820–3. doi: 10.1016/j.jcyt.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Berens C, Heine A, Müller J, Held SA, Mayer K, Brossart P, et al. Variable resistance to freezing and thawing of CD34-positive stem cells and lymphocyte subpopulations in leukapheresis products. Cytotherapy. 2016;18:1325–31. doi: 10.1016/j.jcyt.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Fisher V, Khuu H, David-Ocampo V, Byrne K, Pavletic S, Bishop M, et al. Analysis of the recovery of cryopreserved and thawed CD34+ and CD3+ cells collected for hematopoietic transplantation. Transfusion. 2014;54:1088–92. doi: 10.1111/trf.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Purtill D, Antonenas V, Chiappini P, Tong D, O’Flaherty E, Bajel A, et al. Variable CD34+ recovery of cryopreserved allogeneic HPC products: transplant implications during the COVID-19 pandemic. Blood Adv. 2020;4:4147–50. doi: 10.1182/bloodadvances.2020002431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim DH, Jamal N, Saragosa R, Loach D, Wright J, Gupta V, et al. Similar outcomes of cryopreserved allogeneic peripheral stem cell transplants (PBSCT) compared to fresh allografts. Biol Blood Marrow Transplant. 2007;13:1233–43. doi: 10.1016/j.bbmt.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Lioznov M, Dellbrügger C, Sputtek A, Fehse B, Kröger N, Zander AR. Transportation and cryopreservation may impair haematopoietic stem cell function and engraftment of allogeneic PBSCs, but not BM. Bone Marrow Transplant. 2008;42:121–8. doi: 10.1038/bmt.2008.93. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt AH, Buk D, Platz A, van den Brink MRM. Cryopreservation for all is no option in unrelated stem cell transplantation. comment on Dholaria B, et al. securing the graft during pandemic: are we ready for cryopreservation for All? Biol Blood Marrow Transplant. 2020;26:e145–e146. Biol Blood Marrow Transplant. 2020;26:e298–e299. doi: 10.1016/j.bbmt.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]