Abstract
Purpose of Review:
The development of the skeleton is controlled by cellular decisions determined by the coordinated activation of multiple transcription factors. Recent evidence suggests that the transcriptional regulator proteins, Yes-associated protein (YAP) and transcriptional co-activator with PDZ-binding motif (TAZ), could have important roles in directing the activity of these transcriptional programs. However, in vitro evidence for the roles of YAP and TAZ in skeletal cells has been hopelessly contradictory. The goal of this review is to provide a cross-sectional view on the state of the field and to synthesize the available data toward a unified perspective.
Recent Findings:
YAP and TAZ are regulated by diverse upstream signals and interact downstream with multiple transcription factors involved in skeletal development, positioning YAP and TAZ as important signal integration nodes in an hourglass-shaped signaling pathway. Here, we provide a survey of putative transcriptional co-effectors for YAP and TAZ in skeletal cells. Synthesizing the in vitro data, we conclude that TAZ is consistently pro-osteogenic in function, while YAP can exhibit either pro- or anti-osteogenic activity depending on cell type and context. Synthesizing the in vivo data, we conclude that YAP and TAZ combinatorially promote developmental bone formation, bone matrix homeostasis, and endochondral fracture repair by regulating a variety of transcriptional programs depending on developmental stage.
Summary:
Here, we discuss the current understanding of the roles of the transcriptional regulators, YAP and TAZ in skeletal development, and provide recommendations for continued study of molecular mechanisms, mechanotransduction, and therapeutic implications for skeletal disease.
Introduction:
Édouard-Alfred Martel (1859–1938) is perhaps the most famous cave explorer of all time. He was obsessed with cartography, specifically underground cartography, i.e., the mapping of caves (Hunt 2018). Martel’s innovation was to divide a cave into distinct cross-sections, or “coupés” (Figure 1).
Figure 1.
Coupé of “Bridge Cave”, on the island of Mallorca, drawn by Édouard-Alfred Martel in 1901. Uncovering the mechanisms that control the development of the skeleton is like exploring a cave.
In 1889, Martel became the first explorer to reach the bottom of the Gouffre de Padirac, a 100m-deep chasm in southwest France. Of the experience, he wrote: “The unknown draws us irresistibly forward. No man has gone before us in these depths, no one knows where we go nor what we see, nothing so strangely beautiful was ever presented to us, and spontaneously we ask each other the same question: are we not dreaming?” (Chevalier 1951).
In many ways, being a scientist is like being a 19th c. cave explorer: you walk into the dark with a backpack full of candles and begin to illuminate one cavern after another. But you also spend the vast majority of your time stumbling in the dark, stubbing your toes, making wrong turns. Once a fissure is illuminated, you don’t set up camp in the light, but rather continue straight for the darkest corner with a cold draught and leave the light behind.
In this review article, we seek to provide a coupé of the cave system that represents the developmental biology of the skeleton, with specific focus on the transcriptional regulators, Yes-associated protein (YAP) and transcriptional co-activator with PDZ-binding motif (TAZ). This is a story of numerous discoveries and some wrong turns and dead ends, but ultimately of new depths to be plumbed and caverns to be explored.
Development of the skeleton
The development of the skeletal elements occurs through two types of bone formation: intramembranous or endochondral ossification. Intramembranous ossification occurs by direct differentiation of mesenchymal cells into bone-depositing osteoblasts (Karsenty 2008), and is the dominant mechanism of bone formation in the craniofacial bones and the clavicles. The embryonic bone collar that forms the early cortical bone, in the long bones also occurs through intramembranous ossification of perichondrium-resident mesenchymal progenitors. In contrast, endochondral ossification (i.e., “through cartilage”) occurs first through differentiation of condensed mesenchymal cells into chondrocytes, which form a cartilage anlage, or template, that will be remodeled and replaced by bone. The transition from cartilage to bone initiates with growth plate chondrocyte hypertrophy. The fate of these hypertrophic chondrocytes has been long debated (Shapiro et al. 2005). Evidence since the 1970s has pointed to the possibility of hypertrophic chondrocytes to transform into bone cells (Kahn and Simmons 1977; Roach 1992). Recent reports using inducible Cre-based lineage-tracing have shown direct chondrocyte transformation into bone cells during development (L. Yang et al. 2014; G. Yang et al. 2014; Zhou et al. 2014; Ono et al. 2014; Jing et al. 2015) and fracture repair (C. Bahney et al. 2014; C. S. Bahney et al. 2014; Hu et al. 2017). The process of bone development is therefore an exquisitely coordinated sequence of cellular decisions: proliferation, (trans-)differentiation, mobilization, etc.
Each of these cellular decisions is controlled by the coordinated activation of specific transcriptional programs. Thus, transcription factors are the master regulators of cell identity and decision making (Takahashi et al. 2007). The variety of transcription factors involved in skeletal development have been reviewed in detail elsewhere (Karsenty 2008), but new insights continue to emerge. A mechanistic understanding of how transcription factors regulate skeletal development is critical to understand the biology of the musculoskeletal system and to enable the development of therapeutic interventions for musculoskeletal disease. Careful work over the past decades has enhanced our understanding of the mechanisms by which transcription factors control skeletogenesis, yet many questions remain.
A key question is: how is the symphony of these transcription factors conducted during bone development? It is clear that different cells express different transcription factors to different degrees, but beyond expression, the regulation of transcriptional activity is critical and poorly understood. For example, chromatin organization and epigenetic mechanisms are important mediators of transcription factor activity in bone development, reviewed in (Wijnen and Westendorf 2019). However, transcription factors can also be regulated by transcriptional co-activators/co-repressors, which are proteins that bind to and form complexes with transcription factors and directly regulate their activity.
Yes-associated protein (YAP, also known as YAP1 and YAP65) and transcriptional co-activator with PDZ-binding motif (TAZ, also known as WWTR1) are transcriptional regulators whose primary function is to bind to other proteins, including many transcription factors (Sudol 1994). Orthologs of the Drosophila protein, Yorkie, YAP and TAZ share ~42% homology at the amino acid level (Kaan et al. 2017). Thus, YAP and TAZ can exhibit either convergent or divergent function, depending on context. For example, mice harboring a global deletion of YAP die early in embryogenesis (Morin-Kensicki et al. 2006), while TAZ knockout mice live to maturity, despite phenotypic deformities in various organ systems (Hossain et al. 2007). Both YAP and TAZ lack DNA-binding domains and cannot induce gene expression by themselves but require binding to co-effector transcription factors to drive or repress gene expression. The TEAD family of transcription factors (TEAD1–4) are the canonical transcriptional partners for YAP and TAZ. Complementarily, the TEAD proteins possess DNA-binding domains, but lack transcription activation domains, providing specificity for YAP/TAZ-TEAD (Vassilev et al. 2001).
YAP and TAZ also co-regulate many transcription factors that are involved in bone development, homeostasis, and repair. Here we have collected a list of transcription factors known to be involved in bone development or the function of skeletal cells and tabulated their known or putative regulation by YAP and/or TAZ (Table 1). The table is broken into two categories: I) transcription factors for which co-regulation by YAP/TAZ has been demonstrated in skeletal cells, II) transcription factors with known roles in skeletal cells for which YAP/TAZ binding has been studied in non-skeletal cell types.
Table 1:
Putative YAP/TAZ-interacting transcription factors
| Transcription factor | Cells | YAP binding activity | Role in skeletogenesis | |
|---|---|---|---|---|
| Skeletogenesis | RUNX2 | Osteoblasts | PPxY motif in Runx proteins binds to WW domain of YAP11. | Master regulator of osteogenesis required for osteoblast proliferation2. |
| Snail/Slug | Skeletal stem/stromal cells | N terminal domain of Snail/Slug (SNAG domain) binds to WW domain of YAP13. | Promote skeletal stem cell renewal3. | |
| β-catenin | Osteoblast lineage cells/BMSCs | Exact mechanism of interaction is unknown4. | Involved in orchestrating endochondral or intramembranous ossification by specifying chondrogenic versus osteogenic fate5. | |
| TEAD | C terminal region of TEAD binds to TEAD binding domain of YAP16. | YAP/TAZ TEAD complex promotes osteogenic and collagen-related gene expression7. | ||
| Involved in other cells | CREB | Neuroblastoma cells | CREB binds to N terminal of YAP18. | CREB family of activators is involved in regulating chondrocyte proliferation in developing bone9. |
| HIF1-α | Hepatocellular carcinoma cells | Exact mechanism of interaction is unknown10,11. | Regulates chondrogenesis by regulating Sox9 expression in hypoxic prechondrogenic cells12. | |
| SOX5 | Non-small cell lung cancer cells | Exact mechanism of interaction is unknown13. | In combination with Sox6, Sox5 is required for proper development of endochondral bones14. | |
| Notch | Mouse aortic smooth muscle cells | Notch intracellular domain binds to first WW domain of YAP115. | Notch signaling is essential for normal progression of hypertrophic chondrocyte differentiation into bone16. | |
| RUNX3 | Mammary epithelial cells | PPxY motif in Runx proteins binds to WW domain of YAP117. | Essential for chondrocyte maturation18. | |
| Pax3 | Neural crest derived cells or HEK293 cells | Pax3 homeodomain is involved in binding activity19. | Downregulation of Pax3 is required for normal differentiation of cranial neural crest cells20. | |
| SMAD1/5/8 | HEK293 cells | Phosphorylation by CDK8/9 at S206 facilitates binding of SMAD1 to YAP21. | SMAD signaling is suppressed by YAP to impair chondrogenesis22. | |
| SMAD2/3 | HEK293 cells | SMAD2 MH1 domain binds to TAZ coiled-coil domain23. | SMAD2 is indespensible during early development and SMAD3 is required for articular chondrocyte homeostasis24. | |
| SMAD7 | COS-7 cells | SMAD7 PY motif interacts with WW domain of YAP125. | SMAD7 inhibits chondrocyte differentiation during endochondral ossification at different steps26. | |
| p73 | Mouse embryonic carcinoma (P19) cells | WW domain of YAP1 interacts with the PPPPY motif of p73 protein27. | p73 is essential for vitamin D mediated osteoblast differentiation28. | |
| ErbB-4 | HEK293 cells | WW domain of YAP1 interacts with the PPxY motif closest to the C-terminal of ErbB-4 receptor29,30. | ErbB signaling is involved in chondrocyte maturation and periosteal osteoblast differentiation31. | |
| EGR1 | Prostate cancer cells | WW domain of YAP1 interacts with the PPXY motif of EGR132. | EGR1 is involved in maintaining the chondrocyte extracellular matrix32,33. | |
| C/EBPα | Liver cancer cells | WW domain of YAP1 interacts with the PPXY motif of C/EBPα34. | C/EBPα is involved in terminal differentiation of osteoclasts35. | |
| TBX5 | WW domain of TAZ interacts with the C-terminal region of TBX536. | TBX5 is involved in forelimb bud formation and outgrowth37. |
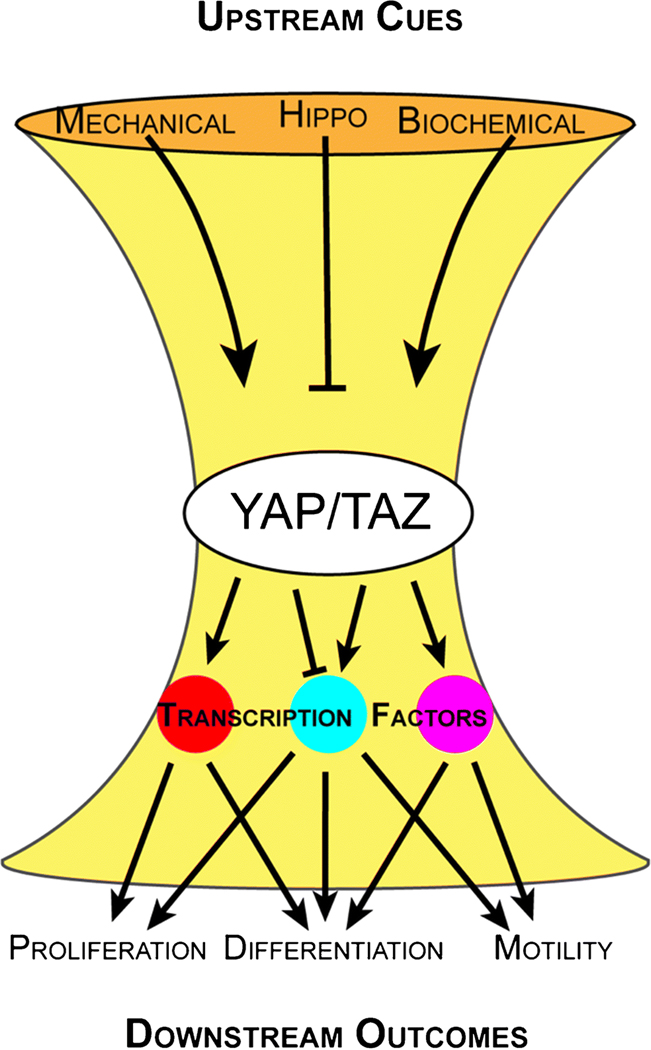
The YAP/TAZ hourglass.
Multiple upstream cues converge on YAP/TAZ activation to regulate downstream outcomes. Therefore, the cellular role of YAP and TAZ is highly context-dependent and determined by the integrated upstream cues and the available transcriptional co-effectors. This mode of regulation positions YAP/TAZ at the bottleneck of a biological signaling “hourglass,” where collective upstream inputs can generate different downstream outcomes via YAP/TAZ activation and transcription factor binding (Figure 2).
Figure 2. The YAP/TAZ hourglass: a schematic of YAP/TAZ regulation and function.
YAP and TAZ are controlled by three primary inputs: mechanical cues, the Hippo pathway, and biochemical cues. Upon activation, YAP/TAZ bind to a variety of transcription factors in the nucleus to regulate diverse downstream outcomes.
YAP/TAZ activity is controlled by their subcellular location: either inside or outside the nucleus. To function as transcriptional regulators, YAP and TAZ must bind transcription factors in the nucleus. Cytosolic retention therefore de-activates YAP/TAZ-driven transcriptional activity. YAP/TAZ subcellular localization is controlled by three primary, interconnected mechanisms: the Hippo pathway, mechanical cues, and biochemical cues. Collectively, these three upstream drivers of YAP/TAZ subcellular localization and the downstream transcriptional co-effectors regulate the function of YAP and TAZ within a cell.
Regulation of YAP/TAZ activity by the Hippo Pathway:
YAP and TAZ are the terminal effectors of the Hippo pathway (Huang J et al 2005) (Huang et al. 2005), a kinase cascade that inhibits YAP and TAZ by sequential Serine phosphorylation. The majority of the core components of the Hippo pathway are conserved across species and can be categorized into core components, upstream regulators and downstream effectors (B. Zhao, Li, and Guan 2010). Briefly, the Hippo pathway initiates with the Sterile 20 (Ste20) family protein kinases MST1 and MST2, which become activated upon binding to and phosphorylation of Salvador. MST1/2 then phosphorylate LATS1 and/or LATS2, which are a part of the nuclear Dbf2-related (NDR) family of protein kinases. LATS kinases are also activated when phosphorylated by MPS One Binder Kinase activator-like 1A and 1B (MOB1A and MOB1B) proteins. LATS kinases phosphorylate YAP and TAZ and inhibit their activity as transcriptional coactivators. Phosphorylation of YAP by LATS 1/2 at Ser127 (or TAZ at S87) initiates a progressive phosphorylation of multiple Serine residues and facilitates binding to 14–3-3 proteins to sequester YAP/TAZ in the cytoplasm (B. Zhao, Li, and Guan 2010) and initiate ubiquitin-mediated proteasomal degradation by the E3 ubiquitin ligase β-TRCP. YAP can also be phosphorylated by other proteins that are not a part of the core Hippo pathway, which can lead to its degradation or stabilization depending on which residue has been phosphorylated (B. Zhao, Li, and Guan 2010). Non-phosphorylated YAP/TAZ translocate to the nucleus and form a complex with their co-effector transcription factors, bind to gene promoters or enhancers, and activate or repress the expression of target genes.
Regulation of YAP/TAZ by Growth Factors
YAP/TAZ activity is also orchestrated by growth factor signaling in both Hippo-dependent and -independent manners. For example, lysophosphatidic acid (LPA) and sphingosine 1-phosphosphate act through G12/13PCRs to inhibit LATS1/2 and promote nuclear translocation of YAP (Moya and Halder 2019). Independent of the Hippo pathway, YAP/TAZ activity can be regulated by alternative Wnt signaling via Frizzled (FZD) receptors (Park et al. 2015). Likewise, platelet-derived growth factor (PDGF) signaling can directly signal to YAP via Src Family kinases (SFK) that catalyze activating tyrosine phosphorylation of YAP (Smoot et al. 2018). Interaction of YAP/TAZ with growth factors is contextual, and different growth factors have been found to regulate YAP/TAZ/Hippo activity differentially depending on cell type and experimental or physiologic context. Discretion is recommended when applying prior findings to a new cell type or context.
Mechanoregulation of YAP and TAZ
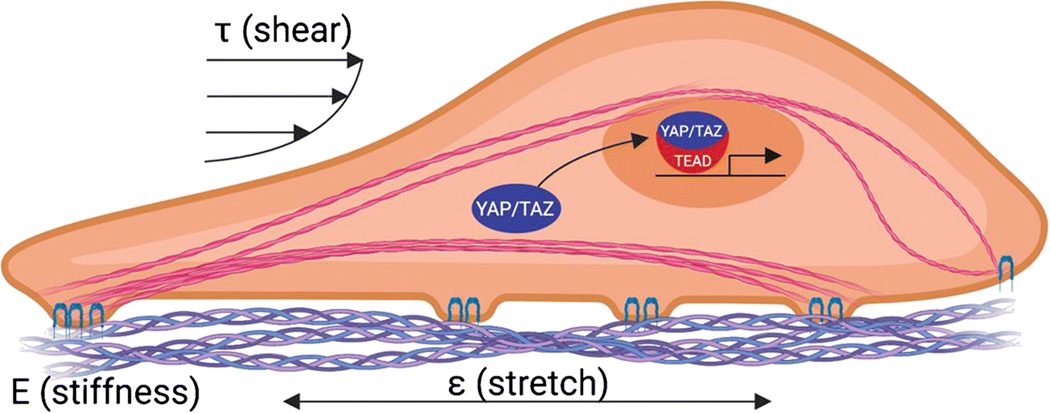
YAP and TAZ are also activated by mechanical cues. In 2006, Engler et al. demonstrated that mechanical properties of the extracellular matrix influence progenitor cell fate (Engler et al. 2006). Five years later, two independent groups linked these matrix stiffness-dependent cell lineage decisions to the mechanosensitive nuclear localization of YAP and TAZ (Dupont et al. 2011)(Wada et al. 2011). Both papers showed that YAP and TAZ translocate to the nucleus in response to matrix rigidity, but are sequestered in the cytosol in soft ECM environments (Dupont et al. 2011)(Wada et al. 2011). The Dupont et al. paper showed that this mechanoactivation of YAP/TAZ was necessary for the matrix stiffness-dependent switch between adipogenic and osteogenic differentiation of bone marrow stromal cells (Dupont et al. 2011). In this section, we will briefly summarize the current state of knowledge on the mechanisms by which YAP and TAZ are controlled by mechanical cues, including mechanosensation at the plasma membrane that leads to cytoskeletal remodeling and cytosolic signal transduction to effect YAP/TAZ nuclear localization (Figure 3).
Figure 3. Illustration of YAP/TAZ activation in response to mechanical cues.
YAP and TAZ translocate to the nucleus in response to cytoskeletal tension induced by various mechanical stimuli, including matrix stiffness (E), stretch (ɛ), and fluid shear stress (τ), among others.
Mechanosensation
YAP/TAZ-activating mechanical cues first reach the cell at the plasma membrane through cell-matrix, cell-cell, and cell-environment interactions. Forces between cells and their extracellular matrix are transduced in part by integrins, which are transmembrane adhesion molecules that couple the ECM to the cytoskeleton at focal adhesions (Sun, Guo, and Fässler 2016). Force production by the actomyosin cytoskeleton via talin-bound integrins (Sun, Guo, and Fässler 2016) induces conformational changes in nascent integrin engagements, promoting integrin clustering and increasing affinity for intracellular ligand binding (Sun, Guo, and Fässler 2016), (Horton et al. 2016). Recruitment of focal adhesion-stabilizing proteins, including vinculin, and paxillin (Martino et al. 2018) in turn initiate intracellular signaling cascades including Rho/ROCK and FAK/Src to promote further actomyosin contractility and subsequent YAP/TAZ nuclear localization. Forces between cells are transduced in part by cadherins, a family of transmembrane adhesion receptors that form adherens junctions, coupling the actomyosin cytoskeleton and transcription factor activation to intercellular mechanical force transduction (Cosgrove et al. 2016). Other forces exerted on cells by their environment, such as fluid shear stress, can be transduced in part by mechanosensitive ion channels, such as the Piezo channels. These mechanically gated, stretch-activated channels modulate the influx of ions, such as Ca2+, into the cytosol (Pathak et al. 2014). Pathak and colleagues first demonstrated that YAP is activated downstream of Piezo activation in neural stem cells (Pathak et al. 2014), and recent data by Ellefson et al. show that myosin II activation produces membrane tension at focal adhesions to activate local Piezo1 channels, providing a direct mechanosensory link between actomyosin tension, Piezo-mediated Ca2+ flux, and YAP/TAZ activation (Ellefsen et al. 2019).
Mechanotransduction
Inside the cell, these mechanical cues are transduced by both physical and biochemical means to control the phosphorylation status and localization of YAP and TAZ (Dupont 2016). Here, we will briefly discuss the actomyosin cytoskeleton and the prototypical mechano-activated Rho/ROCK and Src signaling pathways.
The actin-myosin cytoskeleton provides support, structure, and protection, and is the primary effector of motion in the cell. The actin cytoskeleton assembles from monomeric globular (G)-actin, which polymerizes to form filamentous (F)-actin (Aragona et al. 2013; Driscoll et al. 2015; Dupont et al. 2011). F-actin-bound non-muscle myosin II generates tensile forces in the actin cytoskeleton to regulate a variety of cell processes including polarity, cytokinesis, differentiation, and motility. In 2011, Sansores-Garcia and colleagues observed that altering F-actin dynamics in both drosphila and mamallian cells influenced Yorkie/YAP nuclear localization, such that increased F-actin organization promoted Yorkie/YAP activation (Sansores-Garcia et al. 2011). Actin dynamics are regulated in part by the Rho/ROCK pathway. Forces generated at the ECM activate the Rho GTPases, to activate ROCK, which phosphorylates and inactivates myosin light chain (MLC) phosphatase, promoting MLC activation (Maekawa et al. 1999; Aragona et al. 2013). Myosin activation induces tension generation, stress fiber formation, and recruitment of stabilizing proteins to the connections between the cell and the extracellular matrix at focal adhesions (Oakes et al. 2012). Remarkably, increased cytoskeletal tension causes YAP/TAZ nuclear localization (Aragona et al. 2013; Dupont et al. 2011).
The Src pathway is both a direct and indirect regulator of YAP/TAZ phosphorylation and activation. Src acts upstream of the Hippo pathway kinase, merlin, to promote LATS1/2-mediated YAP/TAZ phosphorylation and inactivation (Sabra et al. 2017), but can also directly phosphorylate YAP1 on three separate tyrosine residues (Y341/357/394) in its transcription activation domain, independent of the Hippo pathway (P. Li et al. 2016)(Elbediwy et al. 2018). In addition to responding to cytoskeletal tension, YAP/TAZ can also regulate the cytoskeleton in a feedback loop. Our recent data implicate YAP and TAZ in transcriptional feedback regulation of the cytoskeleton (Mason et al. 2019).
The mechanisms that control the nuclear shuttling of YAP/TAZ continue to emerge, but YAP/TAZ localization appears to be an equilibrium process that occurs without physical binding to a fixed component in either the nucleus or the cytoplasm (Ege et al. 2018). In addition to phosphorylation status, evidence suggests that physical deformation of the nucleus through the LINC complex is required for YAP/TAZ translocation (Driscoll et al. 2015), and recent data suggest that physical stretching of the nuclear membrane is necessary to open the nuclear pore complex to allow YAP translocation (Elosegui-Artola et al. 2017). However, protein shuttling through the nuclear pore complex may also be guided by importins and exportins which act through nuclear localization and nuclear export sequences (NLS; NES) (S. Wang et al. 2016; Ege et al. 2018). It was long thought that YAP/TAZ lacked defined NLS, but recent studies by Kofler, et al. identify an NLS in the transcription activation domain of TAZ, overlapping with the LATS phosphorylation site and a NES in the TEAD-binding domain (Kofler et al. 2018). Taken together, these observations establish YAP and TAZ as important transcriptional regulators that respond dynamically to mechanical cues and form a mechanistic link between physical stimuli and cell behavior.
Different types of mechanical stimuli in bone
Importantly, bone cells experience a variety of mechanical stimuli through development, disease, and repair. In the embryo, fetal movement and muscle forces are critical for the proper development of the skeleton (Hogg and Hosseini 1992). In adulthood, bone-lining osteoblasts are exposed to matrix strain (Martin et al. 2015) and bone marrow shear stress (Curtis et al. 2018). Within the osteocyte lacunar-canalicular system, osteocytes likewise respond to mechanical forces, predominantly as a consequence of fluid flow through the lacunar/canalicular system, as reviewed elsewhere (Weinbaum 2009), (Jenneke Klein-Nulend et al. 2013), (Schaffler et al. 2014). Whether YAP/TAZ mechanosignaling is necessary for bone physiology in vivo has not yet been studied.
Current understanding of the roles of YAP and TAZ in bone
Insights from in vitro studies
Although identified as critical regulators of mesenchymal progenitor cell differentiation, the evidence for positive vs. negative roles for YAP and/or TAZ during osteogenic differentiation in vitro is complicated. Studies have demonstrated both pro- and anti-osteogenic functions of both YAP and TAZ, depending on the context. Differences in experimental and cellular context may partially explain the conflicting evidence, but further study is necessary. Here, we discuss the existing evidence for both YAP and/or TAZ in both promoting and inhibiting in vitro osteogenic differentiation in model and primary skeletal cells.
A majority of the evidence for YAP and/or TAZ in inhibiting in vitro osteogenic differentiation is focused on YAP. YAP was first reported to suppress osteoblastic differentiation through sequestration and transcriptional repression of RUNX2 in ROS17/2.8 rat osteosarcoma cells (Zaidi et al. 2004). Sen and colleagues found that, in mouse bone marrow stromal cells (BM-MSCs), nuclear YAP inhibited RUNX2-mediated initiation of osteogenic differentiation while YAP nuclear export enhanced osteogenic differentiation (Sen et al. 2015). More recently, activator protein 2a (AP2a) was shown to recruit YAP and release the inhibition of RUNX2 by forming a YAP-AP2a protein complex, resulting in elevated osteogenic differentiation (Lin et al. 2019). Similarly, Basu-Roy and colleagues observed that SOX2 antagonized YAP expression to reduce osteogenic differentiation and maintain stemness in mOS-482 mouse osteosarcoma cells while YAP overexpression in primary mouse osteoblasts inhibited alkaline phosphatase activity and osteogenic differentiation (Basu-Roy et al. 2015). Seo and colleagues identified YAP as a target of SOX2 that antagonized activation of WNT/β-catenin target genes to inhibit osteogenic differentiation in both a model stem cell line (C3H10T1/2) and primary bone marrow stromal cells (Seo et al. 2013). With respect to TAZ, Park and colleagues implicated both YAP and/or TAZ as mediators of alternative WNT signaling via antagonizing WNT/β-catenin signaling, and found that either YAP or TAZ overexpression inhibited WNT/β-catenin signaling and osteogenesis (Park et al. 2015).
In contrast, YAP has also been found to promote osteogenic differentiation in vitro. YAP overexpression enhanced, while YAP depletion inhibited, osteogenic differentiation in MC3T3-E1 cells (B. Yang et al. 2019). In BM-MSCs, over-expression of a constitutively-active YAP mutant (YAP5SA) promoted osteogenic differentiation even under conditions more favorable for adipogenesis (Dupont et al. 2011). Further, enhanced YAP activation by cytoskeletal contractility in differentiating BM-MSCs promoted the osteogenic capacity both in the context of topographical cues (X. Liu et al. 2019) and mechanical stimulation (Xue et al. 2017). Both pharmacological treatment and RNAi-depletion of YAP inhibited topographyinduced osteogenic differentiation in BM-MSCs (H. Pan et al. 2017). In addition to topographical cues, reductions in extracellular pH inhibited osteogenic differentiation by suppressing YAP in BM-MSCs (Tao et al. 2016). Finally, olfactomedin-like protein (OLFML1) negatively regulated mineralization in primary calvarial osteoblasts by inhibiting YAP nuclear translocation, consistent with a role for YAP promoting osteogenic differentiation in vitro (Murakami et al. 2018).
In contrast to YAP, evidence for TAZ is largely consistent and indicates a role for TAZ in promoting in vitro osteogenic differentiation. TAZ was first identified as a RUNX2 co-activator and inhibitor of the adipogenic nuclear receptor, PPARγ, in C2C12 cells (Hong et al. 2005; Hong and Yaffe 2006). More recent evidence in both C2C12 and C3H10T1/2 cells further found that TAZ promoted osteogenic differentiation through both RUNX2- (J. Feng et al. 2015; Mi Ran Byun, Sung, et al. 2014) and β-catenin- (M R Byun et al. 2014) dependent transcription. Similar work by Byun and colleagues observed that TAZ activation downstream of FGF2 and ERK mediated RUNX2-related osteogenic gene expression (Mi Ran Byun, Kim, et al. 2014). Similar to YAP, both topographical cues and mechanical stimulation affected TAZ-dependent in vitro osteogenic differentiation in BM-MSCs. For example, both nano-topographical surfaces (Qian et al. 2017; Hwang et al. 2017) and extracellular matrix stiffness (Hwang et al. 2015) promoted osteogenic differentiation through nuclear TAZ activation. Furthermore, simulated microgravity depolymerized F-actin and reduced TAZ nuclear translocation, which hindered osteogenic differentiation in BM-MSCs (Chen et al. 2016). Conversely, fluid shear stress stimulated TAZ nuclear localization and increased osteogenic differentiation (Kim et al. 2014). Lastly, pharmacological activation of TAZ enhanced osteogenic differentiation in adipose-derived stem cells (Zhu et al. 2018) while BM-MSCS from mice with heterozygous global deletion of TAZ exhibited defective in vitro osteogenic differentiation (Xiao et al. 2018).
In addition to their individual roles, a few studies have modulated both YAP and TAZ during in vitro osteogenic differentiation. For example, Park and colleagues found that RNAi-mediated depletion of YAP/TAZ in BM-MSCs reduced alkaline phosphatase activity and mineral deposition (Park et al. 2015). Similarly, dual RNAi-depletion of YAP and TAZ in BM-MSCs inhibited alkaline phosphatase activity under conditions favorable for osteogenesis (Dupont et al. 2011). Finally, heterozygous deletion of both YAP and TAZ in BM-MSCs inhibited osteogenic differentiation with reduced mineral deposition and downstream osteogenic gene expression (Tang et al. 2016).
Synthesizing these studies, we postulate that TAZ primarily promotes osteogenic differentiation in vitro, while YAP can either promote or inhibit osteogenesis, depending on the cellular and experimental context. Despite the emerging important roles of YAP and TAZ during osteogenic differentiation in vitro, continued careful and thorough interpretation of experiments modulating either YAP and/or TAZ is warranted. For example, the limitations of overexpression approaches that non-physiologically express otherwise tightly-regulated transcriptional co-effectors should be taken into consideration. Further, dissecting the individual roles of YAP versus TAZ during osteogenic differentiation is necessary as current evidence suggests the potential for both divergent and convergent functions of YAP versus TAZ. Lastly, we caution against the use of YAP and/or TAZ expression or subcellular localization as markers or indicators of osteogenic differentiation, as these are insufficient to determine lineage commitment.
We further recommend that, while powerful and important for dissecting molecular mechanisms, in vitro studies must be supported and validated by in vivo approaches that enable the study of YAP/TAZ function in a physiologic context.
Insights from in vivo studies
A definitive understanding of YAP/TAZ function in bone will necessarily come from in vivo approaches. Because YAP and TAZ cannot bind DNA directly, and are capable of co-regulating multiple transcription factors, the transcriptional consequences of YAP and/or TAZ manipulation will depend on the transcription factor milieu present in a given cell. Thus, YAP and TAZ may have fundamentally distinct roles in one cell type compared to another. Here, we review the current literature on the in vivo roles of YAP and TAZ in mesenchymal progenitors, chondrocytes, osteoblasts, osteocytes, and osteoclasts. Other extra-skeletal cell types also contribute to the developmental niche and may likewise depend on YAP/TAZ signaling, but these are beyond the scope of this review.
Limb mesenchyme progenitors.
YAP/TAZ have important, but potentially divergent roles in the mesenchymal progenitors of the embryonic limb bud. Prx1-Cre targets these mesenchymal progenitors, and homozygous conditional ablation of both YAP and TAZ in Prx1-Cre mice produced embryonic lethality. However, mice with haploinsufficiency of YAP and homozygous TAZ deletion survived with increased postnatal bone mass (Xiong, Almeida, and O’Brien 2018). In contrast, Prx1-Cre deletion of YAP resulted reduced bone mass (Deng et al. 2016). Dermo1-Cre also targets limb mesenchymal progenitors, and Dermo1-conditional deletion of the Hippo kinases, MST1 and MST2, resulted in a mild developmental phenotype, but caused a significant defect in callus formation during fracture repair (Deng et al. 2016). Continued research will be necessary to dissect the mechanistic and combinatorial roles of YAP and TAZ in early skeletal development.
Chondroprogenitors
YAP and TAZ negatively regulate chondrogenesis in vivo. YAP-overexpression in Col2-expressing cells produced mice with a smaller skeleton and decreased bone volume due to delayed chondrocyte hypertrophy and reduced chondrocyte maturation (Deng et al. 2016). Conversely, Col2-conditional YAP deletion caused elongated growth plates and increased bone volume (Deng et al. 2016). Through complementary in vitro assays, Deng et. al. found that YAP positively regulated early chondrocyte proliferation by TEAD-dependent Sox6 expression and negatively regulated chondrocyte maturation via Runx2-dependent Col10a1 expression. Notably, YAP overexpression in Col2-expressing cells had more severe effects on fracture repair than development (Deng et al. 2016). Complementarily, conditional deletion of Mob1a/b in Col2-expressing cells resulted in chondrodysplasia from impaired chondrocyte maturation (Goto et al. 2018). Mob1a/b is a core component of the Hippo pathway whose deletion results in hyper-activation of YAP/TAZ. However, Mob1a/b deletion-induced YAP/TAZ hyper-activity reduced early chondrocyte proliferation through transcriptional repression of Sox9 (Goto et al. 2018). Notably, this phenotype was largely rescued by the additional deletion of either YAP or TAZ. These data suggest that YAP and TAZ may be mutually compensatory in chondrogenesis, but explicit in vivo combinatorial gain- and loss-of-function experiments in chondrocytes will be required.
Osteoprogenitors
Though initially contradictory, the emerging evidence converges on positive roles for both YAP and TAZ in promoting osteoblast-lineage progression. YAP and TAZ most prominently immunolocalize in hypertrophic chondrocytes, osteoprogenitors, and osteoblasts during developmental bone formation (Kegelman et al. 2018). These expression patterns coincide with the localization of the transcription factor, Osterix/Sp7, which is critical to osteoblastogenesis (Rodda and McMahon 2006), motivating the use of Osterix-Cre for conditional YAP/TAZ deletion (Kegelman et al. 2018). We found that constitutive homozygous deletion of both YAP and TAZ from Osterix-expressing cells caused perinatal lethality due to asphyxiation, secondary to rib cage malformation (Kegelman et al. 2018). Importantly, mice with a single allele of YAP or a single allele of TAZ in Osterix-expressing cells survived, indicating mutual, but partial, compensation. Mice expressing only a single allele of either gene exhibited severe skeletal defects including spontaneous neonatal fractures, defects in collagen content and organization, and altered osteoblast/osteoclast-mediated bone remodeling (Kegelman et al. 2018). These data implicate both YAP and TAZ in functional bone development. Interestingly, post-natal deletion of both YAP and TAZ (doxycycline induced deletion at 3 weeks of age, and assayed at 12 weeks of age) exhibited only a modest bone phenotype, with increased osteoblast numbers and mineralizing surface percentage (Xiong, Almeida, and O’Brien 2018). Recent data provide insight into these discrepant phenotypes. Using post-natal fracture healing as a model to study YAP/TAZ roles in endochondral ossification, we found that constitutive deletion of YAP and/or TAZ from Osterix-expressing cells caused defects in both cartilage callus formation and callus mineralization due to a developmental defect in periosteal progenitor cell supply (Kegelman et al. 2020). However, inducible deletion after skeletal maturity impaired periosteal osteoprogenitor amplification and subsequent osteogenesis (Kegelman et al. 2020). Mechanical loading also promotes YAP/TAZ activation and endochondral bone regeneration through development-mimetic mechanisms (McDermott et al. 2019). Together, these data suggest that YAP/TAZ signaling in osterix-expressing cells has particularly important roles in bone development and in processes that partially reactivate developmental programs, such as fracture repair. Orthogonally, Li and colleagues observed that Osterix-conditional genetic deletion of MST1/2, the upstream Hippo kinases, inhibited bone accrual, formation and remodeling while stabilizing the key glucose transporter, Glut1, independent of YAP/TAZ regulation (W. Li et al. 2018).
Osteoblasts
Evidence for the roles of YAP and TAZ in mature osteoblasts is largely convergent. During osteoblastogenesis, immature, yet committed osteoblasts begin to express collagen I. Two established Collagen 1-dependent Cre drivers, Col1(3.6kb) (immature osteoblasts) and Col1(2.3kb) (committed osteoblasts) exist (Kalajzic et al. 2002; F. Liu et al. 2004), but neither has yet been used to assess osteoblast lineage-conditional loss-of-function of YAP and/or TAZ. However, Col-1(2.3kb)-conditional over-expression of TAZ promoted bone formation, suggesting a similar role for TAZ in promoting osteoblasts as in osteoprogenitors (J.-Y. Yang et al. 2013). Although not specific to skeletal lineage cells, in vivo lentiviral delivery of TAZ alleviated osteoporotic symptoms in ovariectomized rats, further supporting a role for TAZ in promoting bone formation in vivo (Y. Zhang et al. 2016). As committed osteoblasts mature, osteocalcin expression increases, enabling Cre-mediated targeting in mature osteoblasts (M. Zhang et al. 2002). YAP deletion from Osteocalcin-expressing cells significantly reduced bone formation, impairing osteoblast proliferation induced by YAP co-activation of β-catenin (J.-X. Pan et al. 2018), supporting a role for YAP in promoting osteogenesis in vivo. In contrast, dual deletion of the upstream regulator MST1/2 from Osteocalcin-expressing cells inhibited bone accrual and formation consistent with a negative role for YAP in bone formation (W. Li et al. 2018). Nonetheless, deletion of both downstream YAP/TAZ target genes, CTGF and CYR61, from Osteocalcin expressing-cells resulted in reduced bone mass phenotypes (G. Zhao et al. 2018; Canalis et al. 2010), consistent with the evidence of osteoblast-specific genetic manipulations of YAP and TAZ.
Osteoclasts
Although the roles of YAP and TAZ in osteoclasts using cell-specific loss-of-function approaches has not been investigated directly, the Hippo pathway intersects with multiple signaling pathways that regulate osteoclastogenesis and osteoclast function (W. Yang et al. 2018). YAP/TAZ signaling in osteoblasts and osteocytes regulates the crosstalk to osteoclasts. For example, both deletion of YAP and/or TAZ in skeletal lineage cells and deletion of CYR61 from Osteocalcin-expressing cells increased osteoclast activity (Kegelman et al. 2018; G. Zhao et al. 2018). Similarly, DMP-1-conditional YAP/TAZ ablation promotes osteoclast activation (Xiong, Almeida, and O’Brien 2018; Kegelman et al. 2019), likely via paracrine signaling (Kegelman et al. 2019). Consistently, dual deletion of MST1/2 from Osteocalcin expressing-cells inhibited osteoclast formation (W. Li et al. 2018). However, deletion of YAP from Osteocalcin expressing-cells did not significantly impact osteoclast remodeling, potentially due to the compensatory effects of TAZ and/or the Cre model used (J.-X. Pan et al. 2018). As the roles for YAP and TAZ in regulating both osteoblastogenesis and osteoclastogenesis are beginning to emerge, a complete mechanistic understanding of their role in osteoblast/osteoclast-mediated bone remodeling during skeletal development remains incomplete.
Osteocytes
Similar to their role in osteoblasts, the evidence for the roles of YAP and TAZ in late stage osteoblasts and osteocytes is consistent. Late stage osteoblasts and early stage osteocytes express dentin matrix protein (DMP1). Both 10kb and 8kb DMP1-Cre models have been used to target gene deletion from osteocytes, but the osteocyte specificity depends on the sensitivity of the floxed alleles (O’Brien et al. 2008; Xiong et al. 2015; Rhee et al. 2011; Bivi et al. 2012). Regardless, dual YAP/TAZ deletion using either the 10kb-DMP1-Cre or the 8kb-DMP1-Cre reduced bone formation in vivo, with decreased osteoblast numbers and increased osteoclast activity (Kegelman et al. 2019; Xiong, Almeida, and O’Brien 2018). We further showed that dual deletion of YAP and TAZ using 8kb-DMP1-Cre impaired perilacunar/canalicular remodeling by regulating the expression of perilacunar/canalicular matrix remodeling enzymes including Ctsk, MMP13, and MMP14 as well as Collagen 1a1, resulting in skeletal fragility due to impaired collagen organization in the bone matrix (Kegelman et al. 2019). Therefore, YAP/TAZ in late stage osteoblasts and osteocytes promote bone function in vivo. Since osteocytes are known as the primary mechanosensory cell in bone and YAP/TAZ are critical for mechanotransduction, the role of YAP and/or TAZ in osteocyte-mediated bone adaptation in the context of skeletal loading is an exciting emerging area, but has not yet been studied in vivo.
Directions for future research
YAP/TAZ signaling in bone mechanotransduction
Mechanical loading is extremely important in bone development and maintenance. Mechanical loading mediates many different pathways implicated as mechanistic mediators in bone cells. The focus of this review is YAP/TAZ but other mechanisms have been reviewed in (Tian, Wang, and Bikle 2017; Thompson, Rubin, and Rubin 2012; Regard et al. 2012; J Klein-Nulend, Bacabac, and Bakker 2012). YAP/TAZ can be activated by multiple cues in skeletal cells (Papachroni et al. 2009), including ECM stiffness (Dupont et al. 2011), strain or stretch (Codelia, Sun, and Irvine 2014; Cui et al. 2015), and fluid shear stress (Kim et al. 2014). Most evidence indicates that YAP/TAZ activation by mechanical cues promotes osteogenic differentiation (Khetan et al. 2013; Panciera et al. 2017; Low et al. 2014). Further, fluid shear stress in osteocytes activates Piezo1 calcium channels which were shown to act upstream of YAP/TAZ signaling in vitro (X. Li et al. 2019) and that in osteoblast stimulation by fluid shear caused YAP/TAZ activation in an integrin-dependent manner (Kaneko et al. 2014). Similarly, Piezo1 knockout mice exhibit decreased bone mass and mechanical load adaptation, while in vitro data show that Piezo1 signaling in osteocyte-like cells exposed to fluid shear stress requires YAP/TAZ (X. Li et al. 2019). However, the roles of YAP and TAZ in the mechanical load adaptation of bone has not yet been studied.
Implications for metabolic and developmental bone diseases
YAP and TAZ are known to drive aberrant cellular function in many diseases including atherosclerosis (K.-C. Wang et al. 2016), cancer (Zanconato, Cordenonsi, and Piccolo 2016), fibrosis (F. Liu et al. 2015; Mannaerts et al. 2015), cardiac hypertrophy (Xin et al. 2013), and muscular dystrophy (Iyer et al. 2019; Bertrand et al. 2014), but beyond their neoplastic activity in osteosarcoma (Fullenkamp et al. 2016), the role of YAP and TAZ in bone disease is unknown. Due to the altered mechanical environment in disease pathogenesis, the mechanotransductive effects of YAP and TAZ are implicated in driving abnormal cellular function (Panciera et al. 2017). Further synthesis of the upstream signals and the downstream targets of YAP/TAZ signaling in bone is therapeutically important to understand the disease pathology and potential therapeutic interventions. Accordingly, the emerging roles of YAP and TAZ in skeletal lineage cells implicate YAP and TAZ in both developmental and metabolic skeletal diseases.
Dysfunctional YAP and/or TAZ signaling in mice mimicked characteristics of human cases of developmental bone diseases such as skeletal dysplasia (Lemyre et al. 1999) and osteogenesis imperfecta (OI) (Forlino and Marini 2016; van Dijk et al. 2011; Rauch and Glorieux 2004; Kegelman et al. 2018). As a heterogeneous group of inheritable diseases, the severity of OI ranges from mildly increased fracture risk to perinatal lethality (Forlino and Marini 2016). OI is characterized by increased bone fragility and deformity as well as collagen matrix disorganization (van Dijk et al. 2011). Osterix-conditional YAP/TAZ knockout mice also mimicked several established mouse models of OI (Khillan et al. 1991; Pereira et al. 1993; Chipman et al. 1993) with spontaneous fractures, disorganized collagen, and altered osteoblast/osteoclast-mediated remodeling. More recently, a novel transgenic mouse model of OI type I with mutations in the COL1A1 gene demonstrated downregulation of YAP expression in bone, potentially implicating a feedback loop between upstream matrix activation and downstream transcriptional regulation (Y. Liu et al. 2019). Although the emerging evidence of YAP/TAZ in skeletal lineage cells resembles OI pathogenesis, loss of function mutations directly in either YAP or TAZ are unlikely to play a casual role in human OI. Nonetheless, YAP/TAZ coordinate multiple signaling axes, including TGFβ (Varelas et al. 2008, 2010) and WNT-β-catenin (Heallen et al. 2011; Azzolin et al. 2014), with known roles in bone disease.
In addition to developmental disease, the emerging roles of skeletal cell YAP and/or TAZ in regulating bone remodeling implicate a potential link to metabolic skeletal diseases, specifically related to the coordination of osteoblast/osteoclast and osteocyte-intrinsic remodeling. Metabolic skeletal disorders and diseases primarily related to abnormal bone remodeling include Paget’s disease and osteoporosis (X. Feng and McDonald 2011). In the context of both diseases, aberrant cellular function in osteoblasts, osteoclasts, and osteocytes causes altered bone remodeling, resulting in low bone mass, structural deterioration and/or deformities (X. Feng and McDonald 2011). YAP/TAZ deletion in mature osteoblasts and/or osteocytes resulted in low bone mass with increased osteoclastic remodeling, similar to these diseases (Kegelman et al. 2019; Xiong, Almeida, and O’Brien 2018). While targeting osteoblast/osteoclast-mediated bone remodeling is under clinical investigation, therapies targeting bone quality to treat metabolic bone remodeling disease are currently emerging. Treating bone quality to improve bone strength relates improving the integrity of the osteocyte lacunar/canalicular network. Both increased age (Vashishth et al. 2000) and reduced TGFβ signaling (Dole et al. 2017) cause defects in osteocyte lacunar/canalicular network associated with skeletal fragility. Correspondingly, YAP/TAZ deletion from osteocytes affected both bone quantity and quality via defects in perilacunar/canalicular remodeling, resulting in bone fragility (Kegelman et al. 2019). Evidence for YAP/TAZ signaling in both regulating osteoprogenitor cell function in skeletal development and coordinating osteoblast-osteoclast as well as osteocyte-mediated bone remodeling suggests a more mechanistic understanding of how YAP/TAZ signaling affects bone function could contribute new insights into the heterogeneity and/or etiology of both metabolic and developmental skeletal diseases.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Aragona Mariaceleste, Panciera Tito, Manfrin Andrea, Giulitti Stefano, Michielin Federica, Elvassore Nicola, Dupont Sirio, and Piccolo Stefano. 2013. “A Mechanical Checkpoint Controls Multicellular Growth through YAP/TAZ Regulation by Actin-Processing Factors.” Cell 154 (5): 1047–59. 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- Azzolin Luca, Panciera Tito, Soligo Sandra, Enzo Elena, Bicciato Silvio, Dupont Sirio, Bresolin Silvia, et al. 2014. “YAP/TAZ Incorporation in the β-Catenin Destruction Complex Orchestrates the Wnt Response.” Cell 158 (1): 157–70. 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Bahney Chelsea, Hu Diane, Ferro Federico, Taylor Aaron, Miclau Ted, and Marcucio Ralph. 2014. “Transdifferentiation of Hypertrophic Chondrocytes during Endochondral Bone Repair by Activation of Pluripotent Stem Cell Programs (216.1).” The FASEB Journal 28 (1 Supplement): 216.1. [Google Scholar]
- Bahney Chelsea S, Hu Diane P, Taylor Aaron J, Federico Ferro, Britz Hayley M, Benedikt Hallgrimsson, Brian Johnstone, Theodore Miclau, and Marcucio Ralph S. 2014. “Stem Cell–Derived Endochondral Cartilage Stimulates Bone Healing by Tissue Transformation.” Journal of Bone and Mineral Research 29 (5): 1269–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu-Roy Upal, Bayin N. Sumru, Rattanakorn Kirk, Han Eugenia, Placantonakis Dimitris G., Mansukhani Alka, and Basilico Claudio. 2015. “Sox2 Antagonizes the Hippo Pathway to Maintain Stemness in Cancer Cells.” Nature Communications 6 (1): 6411. 10.1038/ncomms7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand Anne T, Simindokht Ziaei, Camille Ehret, Hélène Duchemin, Kamel Mamchaoui, Anne Bigot, Michèle Mayer, et al. 2014. “Cellular Microenvironments Reveal Defective Mechanosensing Responses and Elevated YAP Signaling in LMNA-Mutated Muscle Precursors.” Journal of Cell Science 127 (Pt 13): 2873–84. 10.1242/jcs.144907. [DOI] [PubMed] [Google Scholar]
- Bivi Nicoletta, Condon Keith W., Allen Matthew R., Farlow Nathan, Passeri Giovanni, Brun Lucas R., Rhee Yumie, Bellido Teresita, and Plotkin Lilian I. 2012. “Cell Autonomous Requirement of Connexin 43 for Osteocyte Survival: Consequences for Endocortical Resorption and Periosteal Bone Formation.” Journal of Bone and Mineral Research 27 (2): 374–89. 10.1002/jbmr.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun MR, Hwang J-H, Kim AR, Kim KM, Hwang ES, Yaffe MB, and Hong J-H. 2014. “Canonical Wnt Signalling Activates TAZ through PP1A during Osteogenic Differentiation.” Cell Death and Differentiation 21 (6): 854–63. 10.1038/cdd.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun Mi Ran, Kim A Rum, Hwang Jun-Ha, Kyung Min Kim, Eun Sook Hwang, and Hong Jeong-Ho. 2014. “FGF2 Stimulates Osteogenic Differentiation through ERK Induced TAZ Expression.” Bone 58 (January): 72–80. 10.1016/j.bone.2013.09.024. [DOI] [PubMed] [Google Scholar]
- Byun Mi Ran, Mi Kyung Sung, Kim A Rum, Cham Han Lee, Eun Jung Jang, Mi Gyeong Jeong, Minsoo Noh, Eun Sook Hwang, and Hong Jeong-Ho. 2014. “(−)-Epicatechin Gallate (ECG) Stimulates Osteoblast Differentiation via Runt-Related Transcription Factor 2 (RUNX2) and Transcriptional Coactivator with PDZ-Binding Motif (TAZ)-Mediated Transcriptional Activation.” The Journal of Biological Chemistry 289 (14): 9926–35. 10.1074/jbc.M113.522870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canalis Ernesto, Zanotti Stefano, Beamer Wesley G., Economides Aris N., and Anna Smerdel-Ramoya. 2010. “Connective Tissue Growth Factor Is Required for Skeletal Development and Postnatal Skeletal Homeostasis in Male Mice.” Endocrinology 151 (8): 3490–3501. 10.1210/en.2010-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Zhe, Luo Qing, Lin Chuanchuan, Kuang Dongdong, and Song Guanbin. 2016. “Simulated Microgravity Inhibits Osteogenic Differentiation of Mesenchymal Stem Cells via Depolymerizing F-Actin to Impede TAZ Nuclear Translocation.” Scientific Reports 6 (1): 30322. 10.1038/srep30322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier Pierre. 1951. Subterranean Climbers: Twelve Years in the World’s Deepest Chasm. First. London: Faber & Faber. [Google Scholar]
- Chipman SD, Sweet HO, McBride DJ, Davisson MT, Marks SC, Shuldiner AR, Wenstrup RJ, Rowe DW, and Shapiro JR. 1993. “Defective pro Alpha 2(I) Collagen Synthesis in a Recessive Mutation in Mice: A Model of Human Osteogenesis Imperfecta.” Proceedings of the National Academy of Sciences of the United States of America 90 (5): 1701–5. 10.1073/PNAS.90.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codelia Veronica A., Sun Gongping, and Irvine Kenneth D. 2014. “Regulation of YAP by Mechanical Strain through Jnk and Hippo Signaling.” Current Biology 24 (17): 2012–17. 10.1016/j.cub.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove Brian D, Mui Keeley L, Driscoll Tristan P, Caliari Steven R, Mehta Kush D, Assoian Richard K, Burdick Jason A, and Mauck Robert L. 2016. “N-Cadherin Adhesive Interactions Modulate Matrix Mechanosensing and Fate Commitment of Mesenchymal Stem Cells.” Nature Materials 15 (12): 1297–1306. 10.1038/nmat4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Yidan, Hameed Feroz M., Yang Bo, Lee Kyunghee, Catherine Qiurong Pan, Sungsu Park, and Sheetz Michael. 2015. “Cyclic Stretching of Soft Substrates Induces Spreading and Growth.” Nature Communications 6: 1–8. 10.1038/ncomms7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis KJ, Coughlin TR, Mason DE, Boerckel JD, and Niebur GL 2018. “Bone Marrow Mechanotransduction in Porcine Explants Alters Kinase Activation and Enhances Trabecular Bone Formation in the Absence of Osteocyte Signaling.” Bone 107. 10.1016/j.bone.2017.11.007. [DOI] [PubMed] [Google Scholar]
- Deng Yujie, Wu Ailing, Li Pikshan, Li Gang, Qin Ling, Song Hai, and Kinglun Kingston Mak. 2016. “Yap1 Regulates Multiple Steps of Chondrocyte Differentiation during Skeletal Development and Bone Repair.” Cell Reports 14 (9): 2224–37. 10.1016/j.celrep.2016.02.021. [DOI] [PubMed] [Google Scholar]
- Dijk F S van, Cobben JM, Kariminejad A, Maugeri A, Nikkels PGJ, van Rijn RR, and Pals G. 2011. “Osteogenesis Imperfecta: A Review with Clinical Examples.” Molecular Syndromology 2 (1): 1–20. https://doi.org/000332228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dole Neha S, Mazur Courtney M, Claire Acevedo, Lopez Justin P, Monteiro David A, Fowler Tristan W, Bernd Gludovatz, et al. 2017. “Osteocyte-Intrinsic TGF-β Signaling Regulates Bone Quality through Perilacunar/Canalicular Remodeling.” Cell Reports 21 (9): 2585–96. 10.1016/j.celrep.2017.10.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll Tristan P., Cosgrove Brian D., Su Jin Heo, Shurden Zach E., and Mauck Robert L. 2015. “Cytoskeletal to Nuclear Strain Transfer Regulates YAP Signaling in Mesenchymal Stem Cells.” Biophysical Journal 108 (12): 2783–93. 10.1016/j.bpj.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont Sirio. 2016. “Role of YAP/TAZ in Cell-Matrix Adhesion-Mediated Signalling and Mechanotransduction.” Experimental Cell Research 343 (1): 42–53. 10.1016/j.yexcr.2015.10.034. [DOI] [PubMed] [Google Scholar]
- Dupont Sirio, Morsut Leonardo, Aragona Mariaceleste, Enzo Elena, Giulitti Stefano, Cordenonsi Michelangelo, Zanconato Francesca, et al. 2011. “Role of YAP/TAZ in Mechanotransduction.” Nature 474 (7350): 179–83. 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Ege Nil, Dowbaj Anna M., Jiang Ming, Howell Michael, Hooper Steven, Foster Charles, Jenkins Robert P., and Sahai Erik. 2018. “Quantitative Analysis Reveals That Actin and Src-Family Kinases Regulate Nuclear YAP1 and Its Export.” Cell Systems 6 (6): 692–708.e13. 10.1016/j.cels.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbediwy Ahmed, Vanyai Hannah, Maria Del Carmen Diaz-De-La-Loza, David Frith, Snijders Ambrosius P., and Thompson Barry J. 2018. “Enigma Proteins Regulate YAP Mechanotransduction.” Journal of Cell Science 131 (22). 10.1242/jcs.221788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellefsen Kyle L., Holt Jesse R., Chang Alice C., Nourse Jamison L., Arulmoli Janahan, Mekhdjian Armen H., Abuwarda Hamid, et al. 2019. “Myosin-II Mediated Traction Forces Evoke Localized Piezo1-Dependent Ca2+ Flickers.” Communications Biology 2 (1): 1–13. 10.1038/s42003-019-0514-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elosegui-Artola Alberto, Andreu Ion, Beedle Amy E.M., Ainhoa Lezamiz, Marina Uroz, Kosmalska Anita J., Roger Oria, et al. 2017. “Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores.” Cell 171 (6): 1397–1410.e14. 10.1016/j.cell.2017.10.008. [DOI] [PubMed] [Google Scholar]
- Engler Adam J, Shamik Sen, Sweeney H Lee, and Discher Dennis E. 2006. “Matrix Elasticity Directs Stem Cell Lineage Specification.” Cell 126 (4): 677–89. 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Feng Jie, Sun Qinyan, Liu Lei, and Xing Da. 2015. “Photoactivation of TAZ via Akt/GSK3β Signaling Pathway Promotes Osteogenic Differentiation.” The International Journal of Biochemistry & Cell Biology 66 (September): 59–68. 10.1016/J.BIOCEL.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Feng Xu, and McDonald Jay M. 2011. “Disorders of Bone Remodeling.” Annual Review of Pathology 6: 121–45. 10.1146/annurev-pathol-011110-130203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlino Antonella, and Marini Joan C. 2016. “Osteogenesis Imperfecta.” The Lancet 387 (10028): 1657–71. 10.1016/S0140-6736(15)00728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullenkamp Colleen A, Hall Sarah L, Jaber Omar I, Pakalniskis Brittany L, Savage Erica C, Savage Johanna M, Ofori-Amanfo Georgina K, et al. 2016. “TAZ and YAP Are Frequently Activated Oncoproteins in Sarcomas.” Oncotarget 7 (21): 30094–108. 10.18632/oncotarget.8979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Hiroki, Nishio Miki, To Yoko, Oishi Tatsuya, Miyachi Yosuke, Maehama Tomohiko, Nishina Hiroshi, et al. 2018. “Loss of Mob1a/b in Mice Results in Chondrodysplasia Due to YAP1/TAZ-TEAD-Dependent Repression of SOX9.” Development (Cambridge, England) 145 (6): dev.159244. 10.1242/dev.159244. [DOI] [PubMed] [Google Scholar]
- Heallen Todd, Zhang Min, Wang Jun, Margarita Bonilla-Claudio, Ela Klysik, Johnson Randy L, and Martin James F. 2011. “Hippo Pathway Inhibits Wnt Signaling to Restrain Cardiomyocyte Proliferation and Heart Size.” Science (New York, N.Y.) 332 (6028): 458–61. 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg DA, and Hosseini A 1992. “The Effects of Paralysis on Skeletal Development in the Chick Embryo.” Comparative Biochemistry and Physiology -- Part A: Physiology 103 (1): 25–28. 10.1016/0300-9629(92)90237-K. [DOI] [PubMed] [Google Scholar]
- Hong Jeong-Ho, Eun Sook Hwang, McManus Michael T, Adam Amsterdam, Yu Tian, Ralitsa Kalmukova, Elisabetta Mueller, et al. 2005. “TAZ, a Transcriptional Modulator of Mesenchymal Stem Cell Differentiation.” Science (New York, N.Y.) 309 (5737): 1074–78. 10.1126/science.1110955. [DOI] [PubMed] [Google Scholar]
- Hong Jeong-Ho, and Yaffe Michael B. 2006. “TAZ: A Β-Catenin-like Molecule That Regulates Mesenchymal Stem Cell Differentiation.” Cell Cycle 5 (2): 176–79. 10.4161/cc.5.2.2362. [DOI] [PubMed] [Google Scholar]
- Horton Edward R., Astudillo Pablo, Humphries Martin J., and Humphries Jonathan D. 2016. “Mechanosensitivity of Integrin Adhesion Complexes: Role of the Consensus Adhesome.” Experimental Cell Research 343 (1): 7–13. 10.1016/j.yexcr.2015.10.025. [DOI] [PubMed] [Google Scholar]
- Hossain Zakir, Safiah Mohamed Ali, Hui Ling Ko, Xu Jianliang, Chee Peng Ng, Ke Guo, Qi Zeng, Ponniah Sathivel, Hong Wanjin, and Hunziker Walter. 2007. “Glomerulocystic Kidney Disease in Mice with a Targeted Inactivation of Wwtr1.” Proceedings of the National Academy of Sciences of the United States of America 104 (5): 1631–36. 10.1073/pnas.0605266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Diane P., Ferro Federico, Yang Frank, Taylor Aaron J., Chang Wenhan, Miclau Theodore, Marcucio Ralph S., and Bahney Chelsea S. 2017. “Cartilage to Bone Transformation during Fracture Healing Is Coordinated by the Invading Vasculature and Induction of the Core Pluripotency Genes.” Development 144 (2). 10.1242/dev.130807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Jianbin, Wu Shian, Barrera Jose, Matthews Krista, and Pan Duojia. 2005. “The Hippo Signaling Pathway Coordinately Regulates Cell Proliferation and Apoptosis by Inactivating Yorkie, the Drosophila Homolog of YAP.” Cell 122 (3): 421–34. 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Hunt Will (Urban adventurer). 2018. Underground : A Human History of the Worlds beneath Our Feet. First edit. Spiegel & Grau. [Google Scholar]
- Hwang Jun-Ha, Mi Ran Byun, Kim A. Rum, Kyung Min Kim, Hang Jun Cho, Yo Han Lee, Juwon Kim, Mi Gyeong Jeong, Eun Sook Hwang, and Hong Jeong-Ho. 2015. “Extracellular Matrix Stiffness Regulates Osteogenic Differentiation through MAPK Activation.” Edited by Adam J. Engler. PLOS ONE 10 (8): e0135519. 10.1371/journal.pone.0135519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang Jun-Ha, Lee Dong-Hyun, Mi Ran Byun, Kim A. Rum, Kyung Min Kim, Park Jung Il, Ho Taek Oh, Eun Sook Hwang, Kyu Back Lee, and Hong Jeong-Ho. 2017. “Nanotopological Plate Stimulates Osteogenic Differentiation through TAZ Activation.” Scientific Reports 7 (1): 3632. 10.1038/s41598-017-03815-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer Shama R., Shah Sameer B., Ward Christopher W., Stains Joseph P., Spangenburg Espen E., Folker Eric S., and Lovering Richard M. 2019. “Differential YAP Nuclear Signaling in Healthy and Dystrophic Skeletal Muscle.” American Journal of Physiology-Cell Physiology 317 (1): C48–57. 10.1152/ajpcell.00432.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Y, Zhou X, Han X, Jing J, von der Mark K, Wang J, de Crombrugghe B, Hinton RJ, and Feng JQ. 2015. “Chondrocytes Directly Transform into Bone Cells in Mandibular Condyle Growth.” Journal of Dental Research 94 (12): 1668–75. 10.1177/0022034515598135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaan Hung Yi Kristal, Siew Wee Chan, Siew Kim Joyce Tan, Guo Fusheng, Chun Jye Lim, Wanjin Hong, and Song Haiwei. 2017. “Crystal Structure of TAZ-TEAD Complex Reveals a Distinct Interaction Mode from That of YAP-TEAD Complex.” Scientific Reports 7 (1): 2035. 10.1038/s41598-017-02219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn AJ, and Simmons DJ. 1977. “Chondrocyte-to-Osteocyte Transformation in Grafts of Perichondrium-Free Epiphyseal Cartilage.” Clinical Orthopaedics and Related Research 129 (129): 299–304. 10.1097/00003086-197711000-00042. [DOI] [PubMed] [Google Scholar]
- Kalajzic I, Kalajzic Z, Kaliterna M, Gronowicz G, Clark SH, Lichtler AC, and Rowe D 2002. “Use of Type I Collagen Green Fluorescent Protein Transgenes to Identify Subpopulations of Cells at Different Stages of the Osteoblast Lineage.” Journal of Bone and Mineral Research 17 (1): 15–25. 10.1359/jbmr.2002.17.1.15. [DOI] [PubMed] [Google Scholar]
- Kaneko Keiko, Ito Masako, Naoe Yoshinori, Adam Lacy-Hulbert, and Kyoji Ikeda. 2014. “Integrin Αv in the Mechanical Response of Osteoblast Lineage Cells.” Biochemical and Biophysical Research Communications 447 (2): 352–57. 10.1016/j.bbrc.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenty Gerard. 2008. “Transcriptional Control of Skeletogenesis.” Annual Review of Genomics and Human Genetics 9 (1): 183–96. 10.1146/annurev.genom.9.081307.164437. [DOI] [PubMed] [Google Scholar]
- Kegelman Christopher D., Mason Devon E., Dawahare James H., Horan Daniel J., Vigil Genevieve D., Howard Scott S., Robling Alexander G., Bellido Teresita M., and Boerckel Joel D. 2018. “Skeletal Cell YAP and TAZ Combinatorially Promote Bone Development.” The FASEB Journal, January, fj.201700872R. 10.1096/fj.201700872R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegelman Christopher D., Nijsure Madhura P., Moharrer Yasaman, Pearson Hope B., Dawahare James H., Jordan Kelsey M., Qin Ling, and Boerckel Joel D. 2020. “YAP and TAZ Promote Periosteal Osteoblast Precursor Expansion and Differentiation for Fracture Repair.” BioRxiv, March, 2020.03.17.995761. 10.1101/2020.03.17.995761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegelman Christopher D, Coulombe Jennifer C, Jordan Kelsey M, Horan Daniel J, Qin Ling, Robling Alexander G, Ferguson Virginia L, Bellido Teresita M, and Boerckel Joel D. 2019. “YAP and TAZ Mediate Osteocyte Perilacunar/Canalicular Remodeling.” Journal of Bone and Mineral Research, October, jbmr.3876. 10.1002/jbmr.3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khetan Sudhir, Guvendiren Murat, Legant Wesley R., Cohen Daniel M., Chen Christopher S., and Burdick Jason A. 2013. “Degradation-Mediated Cellular Traction Directs Stem Cell Fate in Covalently Crosslinked Three-Dimensional Hydrogels.” Nature Materials 12 (5): 458–65. 10.1038/nmat3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khillan JS, Olsen AS, Kontusaari S, Sokolov B, and Prockop DJ. 1991. “Transgenic Mice That Express a Mini-Gene Version of the Human Gene for Type I Procollagen (COL1A1) Develop a Phenotype Resembling a Lethal Form of Osteogenesis Imperfecta.” The Journal of Biological Chemistry 266 (34): 23373–79. [PubMed] [Google Scholar]
- Kim Kyung Min, Yoon Jung Choi, Hwang Jun-Ha, Kim A. Rum, Hang Jun Cho, Eun Sook Hwang, Joong Yull Park, Lee Sang-Hoon, and Hong Jeong-Ho. 2014. “Shear Stress Induced by an Interstitial Level of Slow Flow Increases the Osteogenic Differentiation of Mesenchymal Stem Cells through TAZ Activation.” Edited by David T. Eddington. PLoS ONE 9 (3): e92427. 10.1371/journal.pone.0092427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Nulend J, Bacabac RG, and Bakker AD. 2012. “Mechanical Loading and How It Affects Bone Cells: The Role of the Osteocyte Cytoskeleton in Maintaining Our Skeleton.” European Cells & Materials 24 (January): 278–91. http://www.ncbi.nlm.nih.gov/pubmed/23007912. [DOI] [PubMed] [Google Scholar]
- Klein-Nulend Jenneke, Bakker Astrid D, Bacabac Rommel G, Aviral Vatsa, and Sheldon Weinbaum. 2013. “Mechanosensation and Transduction in Osteocytes.” Bone 54 (2): 182–90. 10.1016/j.bone.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Kofler Michael, Speight Pam, Little Darby, Caterina Di Ciano-Oliveira, Katalin Szászi, and Kapus András. 2018. “Mediated Nuclear Import and Export of TAZ and the Underlying Molecular Requirements.” Nature Communications 9 (1). 10.1038/s41467018-07450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemyre E, Azouz EM, Teebi AS, Glanc P, and Chen MF. 1999. “Bone Dysplasia Series. Achondroplasia, Hypochondroplasia and Thanatophoric Dysplasia: Review and Update.” Canadian Association of Radiologists Journal = Journal l’Association Canadienne Des Radiologistes 50 (3): 185–97. [PubMed] [Google Scholar]
- Li Peng, Silvis Mark R., Honaker Yuchi, Wen Hui Lien, Arron Sarah T., and Vasioukhin Valeri. 2016. “ΑE-Catenin Inhibits a Src–YAP1 Oncogenic Module That Couples Tyrosine Kinases and the Effector of Hippo Signaling Pathway.” Genes and Development 30 (7): 798–811. 10.1101/gad.274951.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Wenling, Deng Yujie, Feng Bo, and Kingston King-Lun Mak. 2018. “Mst1/2 Kinases Modulate Glucose Uptake for Osteoblast Differentiation and Bone Formation.” Journal of Bone and Mineral Research 33 (6): 1183–95. 10.1002/jbmr.3413. [DOI] [PubMed] [Google Scholar]
- Li Xuehua, Han Li, Nookaew Intawat, Mannen Erin, Silva Matthew J., Almeida Maria, and Xiong Jinhu. 2019. “Stimulation of Piezo1 by Mechanical Signals Promotes Bone Anabolism.” ELife 8: 1–22. 10.7554/eLife.49631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Xiao, Yang Haoqing, Wang Lijun, Li Wenzhi, Diao Shu, Du Juan, Wang Songlin, Dong Rui, Li Jun, and Fan Zhipeng. 2019. “AP2a Enhanced the Osteogenic Differentiation of Mesenchymal Stem Cells by Inhibiting the Formation of YAP/RUNX2 Complex and BARX1 Transcription.” Cell Proliferation 52 (1): e12522. 10.1111/cpr.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Fei, Lagares David, Kyoung Moo Choi, Lauren Stopfer, Aleksandar Marinković, Vladimir Vrbanac, Probst Clemens K., et al. 2015. “Mechanosignaling through YAP and TAZ Drives Fibroblast Activation and Fibrosis.” American Journal of Physiology - Lung Cellular and Molecular Physiology 308 (4): L344–57. 10.1152/ajplung.00300.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Fei, Woitge Henning W., Braut Alen, Kronenberg Mark S., Lichtler Alexander C., Mina Mina, and Kream Barbara E. 2004. “Expression and Activity of Osteoblast-Targeted Cre Recombinase Transgenes in Murine Skeletal Tissues.” The International Journal of Developmental Biology 48 (7): 645–53. 10.1387/ijdb.041816fl. [DOI] [PubMed] [Google Scholar]
- Liu Xuan, Hou Wenqing, He Lei, Han Fangping, Lu Mengjie, Lu Xiaobo, Duan Ke, Guo Tailin, and Weng Jie. 2019. “AMOT130/YAP Pathway in Topography-Induced BMSC Osteoblastic Differentiation.” Colloids and Surfaces B: Biointerfaces 182 (October): 110332. 10.1016/J.COLSURFB.2019.06.061. [DOI] [PubMed] [Google Scholar]
- Liu Yi, Wang Jianhai, Liu Shuo, Kuang Mingjie, Jing Yaqing, Zhao Yuxia, Wang Zihan, and Li Guang. 2019. “A Novel Transgenic Murine Model with Persistently Brittle Bones Simulating Osteogenesis Imperfecta Type I.” Bone 127 (October): 646–55. 10.1016/J.BONE.2019.07.021. [DOI] [PubMed] [Google Scholar]
- Low Boon Chuan, Catherine Qiurong Pan, Shivashankar GV, Alexander Bershadsky, Marius Sudol, and Michael Sheetz. 2014. “YAP/TAZ as Mechanosensors and Mechanotransducers in Regulating Organ Size and Tumor Growth.” FEBS Letters 588 (16): 2663–70. 10.1016/j.febslet.2014.04.012. [DOI] [PubMed] [Google Scholar]
- Maekawa Midori, Ishizaki Toshimasa, Boku Shuken, Watanabe Naoki, Fujita Akiko, Iwamatsu Akihiro, Obinata Takashi, Ohashi Kazumasa, Mizuno Kensaku, and Narumiya Shuh. 1999. “Signaling from Rho to the Actin Cytoskeleton through Protein Kinases ROCK and LIM-Kinase.” Science 285 (5429): 895–98. 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- Mannaerts Inge, Sofia Batista Leite, Stefaan Verhulst, Claerhout Sofie, Eysackers Nathalie, Thoen Lien F.R., Anne Hoorens, Hendrik Reynaert, Georg Halder, and van Grunsven Leo A. 2015. “The Hippo Pathway Effector YAP Controls Mouse Hepatic Stellate Cell Activation.” Journal of Hepatology 63 (3): 679–88. 10.1016/j.jhep.2015.04.011. [DOI] [PubMed] [Google Scholar]
- Martin R. Bruce, Burr David B., Sharkey Neil A., and Fyhrie David P. 2015. Skeletal Tissue Mechanics. Skeletal Tissue Mechanics. 10.1007/978-1-4939-3002-9. [DOI] [Google Scholar]
- Martino Fabiana, Perestrelo Ana R., Vladimír Vinarský, Stefania Pagliari, and Forte Giancarlo. 2018. “Cellular Mechanotransduction: From Tension to Function.” Frontiers in Physiology 9 (July): 1–21. 10.3389/fphys.2018.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason Devon E., Collins Joseph M., Dawahare James H., Trung Dung Nguyen Yang Lin, Voytik-Harbin Sherry L., Pinar Zorlutuna, Yoder Mervin C., and Boerckel Joel D. 2019. “YAP and TAZ Limit Cytoskeletal and Focal Adhesion Maturation to Enable Persistent Cell Motility.” J Cell Biol, February, jcb.201806065. 10.1083/JCB.201806065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott Anna M., Herberg Samuel, Mason Devon E., Collins Joseph M., Pearson Hope B., Dawahare James H., Tang Rui, et al. 2019. “Recapitulating Bone Development through Engineered Mesenchymal Condensations and Mechanical Cues for Tissue Regeneration.” Science Translational Medicine 11 (495): eaav7756. 10.1126/scitranslmed.aav7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin-Kensicki Elizabeth M, Boone Brian N, Howell Michael, Stonebraker Jaclyn R, Jeremy Teed, Alb James G, Magnuson Terry R, O’Neal Wanda, and Milgram Sharon L. 2006. “Defects in Yolk Sac Vasculogenesis, Chorioallantoic Fusion, and Embryonic Axis Elongation in Mice with Targeted Disruption of Yap65.” Molecular and Cellular Biology 26 (1): 77–87. 10.1128/MCB.26.1.77-87.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya Iván M., and Halder Georg. 2019. “Hippo–YAP/TAZ Signalling in Organ Regeneration and Regenerative Medicine.” Nature Reviews Molecular Cell Biology. 10.1038/s41580-018-0086-y. [DOI] [PubMed] [Google Scholar]
- Murakami Kohei, Kikugawa Shingo, Kobayashi Yasuhiro, Uehara Shunsuke, Suzuki Takako, Kato Hiroyuki, Udagawa Nobuyuki, and Nakamura Yukio. 2018. “Olfactomedin-like Protein OLFML1 Inhibits Hippo Signaling and Mineralization in Osteoblasts.” Biochemical and Biophysical Research Communications 505 (2): 419–25. 10.1016/J.BBRC.2018.09.112. [DOI] [PubMed] [Google Scholar]
- O’Brien Charles A., Plotkin Lilian I., Galli Carlo, Goellner Joseph J., Gortazar Arancha R., Allen Matthew R., Robling Alexander G., et al. 2008. “Control of Bone Mass and Remodeling by PTH Receptor Signaling in Osteocytes.” Edited by Dong-Yan Jin. PLoS ONE 3 (8): e2942. 10.1371/journal.pone.0002942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes Patrick W, Yvonne Beckham, Jonathan Stricker, and Gardel Margaret L. 2012. “Tension Is Required but Not Sufficient for Focal Adhesion Maturation without a Stress Fiber Template.” The Journal of Cell Biology 196 (3): 363–74. 10.1083/jcb.201107042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Noriaki, Ono Wanida, Nagasawa Takashi, and Kronenberg Henry M. 2014. “A Subset of Chondrogenic Cells Provides Early Mesenchymal Progenitors in Growing Bones.” Nature Cell Biology 16 (12): 1157–67. 10.1038/ncb3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Houhua, Xie Youtao, Zhang Zequan, Li Kai, Hu Dandan, Zheng Xuebin, Fan Qiming, and Tang Tingting. 2017. “YAP-Mediated Mechanotransduction Regulates Osteogenic and Adipogenic Differentiation of BMSCs on Hierarchical Structure.” Colloids and Surfaces B: Biointerfaces 152 (April): 344–53. 10.1016/J.COLSURFB.2017.01.039. [DOI] [PubMed] [Google Scholar]
- Pan Jin-Xiu, Xiong Lei, Zhao Kai, Zeng Peng, Wang Bo, Tang Fu-Lei, Sun Dong, et al. 2018. “YAP Promotes Osteogenesis and Suppresses Adipogenic Differentiation by Regulating β-Catenin Signaling.” Bone Research 6 (1): 18. 10.1038/s41413-018-0018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panciera Tito, Azzolin Luca, Cordenonsi Michelangelo, and Piccolo Stefano. 2017. “Mechanobiology of YAP and TAZ in Physiology and Disease.” Nature Reviews Molecular Cell Biology 18 (12): 758–70. 10.1038/nrm.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papachroni Katerina K., Karatzas Demetrios N., Papavassiliou Kostas A., Basdra Efthimia K., and Papavassiliou Athanasios G. 2009. “Mechanotransduction in Osteoblast Regulation and Bone Disease.” Trends in Molecular Medicine 15 (5): 208–16. 10.1016/j.molmed.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Park Hyun Woo, Young Chul Kim Bo Yu, Moroishi Toshiro, Mo Jung-Soon, Plouffe Steven W., Meng Zhipeng, et al. 2015. “Alternative Wnt Signaling Activates YAP/TAZ.” Cell 162 (4): 780–94. 10.1016/j.cell.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak Medha M., Nourse Jamison L., Tran Truc, Hwe Jennifer, Arulmoli Janahan, Le Dai Trang T., Elena Bernardis, Flanagan Lisa A., and Francesco Tombola. 2014. “Stretch-Activated Ion Channel Piezo1 Directs Lineage Choice in Human Neural Stem Cells.” Proceedings of the National Academy of Sciences of the United States of America 111 (45): 16148–53. 10.1073/pnas.1409802111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira R, Khillan JS, Helminen HJ, Hume EL, and Prockop DJ. 1993. “Transgenic Mice Expressing a Partially Deleted Gene for Type I Procollagen (COL1A1). A Breeding Line with a Phenotype of Spontaneous Fractures and Decreased Bone Collagen and Mineral.” The Journal of Clinical Investigation 91 (2): 709–16. 10.1172/JCI116252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Weiyi, Gong Lanqi, Cui Xin, Zhang Zijing, Bajpai Apratim, Liu Chao, Castillo Alesha B., Teo Jeremy C. M., and Chen Weiqiang. 2017. “Nanotopographic Regulation of Human Mesenchymal Stem Cell Osteogenesis.” ACS Applied Materials & Interfaces 9 (48): 41794–806. 10.1021/acsami.7b16314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch Frank, and Glorieux Francis H. 2004. “Osteogenesis Imperfecta.” The Lancet 363 (9418): 1377–85. 10.1016/S0140-6736(04)16051-0. [DOI] [PubMed] [Google Scholar]
- Regard Jean B., Zhong Zhendong, Williams Bart O., and Yang Yingzi. 2012. “Wnt Signaling in Bone Development and Disease: Making Stronger Bone with Wnts.” Cold Spring Harbor Perspectives in Biology 4 (12): 1–17. 10.1101/cshperspect.a007997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee Yumie, Allen Matthew R, Keith Condon, Lezcano Virginia, Ronda Ana C, Carlo Galli, Olivos Naomi, et al. 2011. “PTH Receptor Signaling in Osteocytes Governs Periosteal Bone Formation and Intracortical Remodeling.” Journal of Bone and Mineral Research 26 (5): 1035–46. 10.1002/jbmr.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach HI 1992. “Trans-Differentiation of Hypertrophic Chondrocytes into Cells Capable of Producing a Mineralized Bone Matrix.” Bone and Mineral 19 (1): 1–20. 10.1016/0169-6009(92)90840-A. [DOI] [PubMed] [Google Scholar]
- Rodda Stephen J, and McMahon Andrew P. 2006. “Distinct Roles for Hedgehog and Canonical Wnt Signaling in Specification, Differentiation and Maintenance of Osteoblast Progenitors.” Development (Cambridge, England) 133 (16): 3231–44. 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- Sabra Hiba, Brunner Molly, Mandati Vinay, Bernhard Wehrle-Haller, Dominique Lallemand, Anne Sophie Ribba, Genevieve Chevalier, Guardiola Philippe, Block Marc R., and Bouvard Daniel. 2017. “Β1 Integrin–Dependent Rac/Group I PAK Signaling Mediates YAP Activation of Yes-Associated Protein 1 (YAP1) via NF2/Merlin.” Journal of Biological Chemistry 292 (47): 19179–97. 10.1074/jbc.M117.808063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansores-Garcia Leticia, Bossuyt Wouter, Ken Ichi Wada, Shigenobu Yonemura, Tao Chunyao, Sasaki Hiroshi, and Halder Georg. 2011. “Modulating F-Actin Organization Induces Organ Growth by Affecting the Hippo Pathway.” EMBO Journal 30 (12): 2325–35. 10.1038/emboj.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffler Mitchell B, Wing-Yee Cheung, Robert Majeska, and Oran Kennedy. 2014. “Osteocytes: Master Orchestrators of Bone.” Calcified Tissue International 94 (1): 5–24. 10.1007/s00223-013-9790-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen Buer, Xie Zhihui, Uzer Gunes, Thompson William R., Styner Maya, Wu Xin, and Rubin Janet. 2015. “Intranuclear Actin Regulates Osteogenesis.” STEM CELLS 33 (10): 3065–76. 10.1002/stem.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo Eunjeong, Upal Basu-Roy, Gunaratne Preethi H, Cristian Coarfa, Dae-Sik Lim, Claudio Basilico, and Alka Mansukhani. 2013. “SOX2 Regulates YAP1 to Maintain Stemness and Determine Cell Fate in the Osteo-Adipo Lineage.” Cell Reports 3 (6): 2075–87. 10.1016/j.celrep.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro Irving M., Adams Christopher S., Freeman Theresa, and Srinivas Vickram. 2005. “Fate of the Hypertrophic Chondrocyte: Microenvironmental Perspectives on Apoptosis and Survival in the Epiphyseal Growth Plate.” Birth Defects Research Part C - Embryo Today: Reviews 75 (4): 330–39. 10.1002/bdrc.20057. [DOI] [PubMed] [Google Scholar]
- Smoot Rory L., Werneburg Nathan W., Sugihara Takaaki, Hernandez Matthew C., Yang Lin, Mehner Christine, Graham Rondell P., Bronk Steven F., Truty Mark J., and Gores Gregory J. 2018. “Platelet-Derived Growth Factor Regulates YAP Transcriptional Activity via Src Family Kinase Dependent Tyrosine Phosphorylation.” Journal of Cellular Biochemistry. 10.1002/jcb.26246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudol M 1994. “Yes-Associated Protein (YAP65) Is a Proline-Rich Phosphoprotein That Binds to the SH3 Domain of the Yes Proto-Oncogene Product.” Oncogene 9 (8): 2145–52. http://www.ncbi.nlm.nih.gov/pubmed/8035999. [PubMed] [Google Scholar]
- Sun Zhiqi, Guo Shengzhen S, and Reinhard Fässler. 2016. “Integrin-Mediated Mechanotransduction” 215 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Kazutoshi, Tanabe Koji, Ohnuki Mari, Narita Megumi, Ichisaka Tomoko, Tomoda Kiichiro, and Yamanaka Shinya. 2007. “Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors.” Cell 131 (5): 861–72. 10.1016/J.CELL.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tang Yi, Feinberg Tamar, Keller Evan T., Li Xiao-Yan, and Weiss Stephen J. 2016. “Snail/Slug Binding Interactions with YAP/TAZ Control Skeletal Stem Cell Self-Renewal and Differentiation.” Nature Cell Biology 18 (9): 917–29. 10.1038/ncb3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Shi-Cong, Gao You-Shui, Zhu Hong-Yi, Yin Jun-Hui, Chen Yi-Xuan, Zhang Yue-Lei, Guo Shang-Chun, and Zhang Chang-Qing. 2016. “Decreased Extracellular PH Inhibits Osteogenesis through Proton-Sensing GPR4-Mediated Suppression of Yes-Associated Protein.” Scientific Reports 6 (1): 26835. 10.1038/srep26835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson William R, Rubin Clinton T, and Janet Rubin. 2012. “Mechanical Regulation of Signaling Pathways in Bone.” Gene, May. 10.1016/j.gene.2012.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Faming, Wang Yongmei, and Bikle Daniel D. 2017. “IGF-1 Signaling Mediated Cell-Specific Skeletal Mechano-Transduction.” Journal of Orthopaedic Research, November. 10.1002/jor.23767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varelas Xaralabos, Sakuma Rui, Payman Samavarchi-Tehrani, Raheem Peerani, Rao Balaji M, Joanna Dembowy, Yaffe Michael B, Zandstra Peter W, and Wrana Jeffrey L. 2008. “TAZ Controls Smad Nucleocytoplasmic Shuttling and Regulates Human Embryonic Stem-Cell Self-Renewal.” Nature Cell Biology 10 (7): 837–48. 10.1038/ncb1748. [DOI] [PubMed] [Google Scholar]
- Varelas Xaralabos, Payman Samavarchi-Tehrani, Masahiro Narimatsu, Alexander Weiss, Cockburn Katie, Larsen Brett G., Rossant Janet, and Wrana Jeffrey L. 2010. “The Crumbs Complex Couples Cell Density Sensing to Hippo-Dependent Control of the TGF-β-SMAD Pathway.” Developmental Cell 19 (6): 831–44. 10.1016/j.devcel.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Vashishth D, Verborgt O, Divine G, Schaffler MB, and Fyhrie DP 2000. “Decline in Osteocyte Lacunar Density in Human Cortical Bone Is Associated with Accumulation of Microcracks with Age.” Bone 26 (4): 375–80. 10.1016/S87563282(00)00236-2. [DOI] [PubMed] [Google Scholar]
- Vassilev A, Kaneko KJ, Shu H, Zhao Y, and DePamphilis ML. 2001. “TEAD/TEF Transcription Factors Utilize the Activation Domain of YAP65, a Src/Yes-Associated Protein Localized in the Cytoplasm.” Genes & Development 15 (10): 1229–41. 10.1101/gad.888601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada Ken-Ichi, Itoga Kazuyoshi, Okano Teruo, Yonemura Shigenobu, and Sasaki Hiroshi. 2011. “Hippo Pathway Regulation by Cell Morphology and Stress Fibers.” Development (Cambridge, England) 138 (18): 3907–14. 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- Wang Kuei-Chun, Yeh Yi-Ting, Nguyen Phu, Limqueco Elaine, Lopez Jocelyn, Thorossian Satenick, Guan Kun-Liang, Yi-Shuan J Li, and Shu Chien. 2016. “Flow-Dependent YAP/TAZ Activities Regulate Endothelial Phenotypes and Atherosclerosis.” Proceedings of the National Academy of Sciences of the United States of America 113 (41): 11525–30. 10.1073/pnas.1613121113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Shimin, Lu Yi, Meng Xin Yin, Chao Wang, Wu Wei, Li Jinhui, Wu Wenqing, et al. 2016. “Importin Α1 Mediates Yorkie Nuclear Import via an N-Terminal Non-Canonical Nuclear Localization Signal.” Journal of Biological Chemistry 291 (15): 7926–37. 10.1074/jbc.M115.700823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbaum S. Fritton and S. 2009. “Fluid_and_Solute_Transport_in_Bone_Flow_Induced.Pdf,” no. 1991: 347–74. 10.1146/annurev.fluid.010908.165136.Fluid. [DOI] [Google Scholar]
- Wijnen Andre J., and Westendorf Jennifer J. 2019. “Epigenetics as a New Frontier in Orthopedic Regenerative Medicine and Oncology.” Journal of Orthopaedic Research 37 (7): 1465–74. 10.1002/jor.24305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Zhousheng, Baudry Jerome, Cao Li, Huang Jinsong, Chen Hao, Yates Charles R., Li Wei, et al. 2018. “Polycystin-1 Interacts with TAZ to Stimulate Osteoblastogenesis and Inhibit Adipogenesis.” The Journal of Clinical Investigation 128 (1): 157–74. 10.1172/JCI93725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Mei, Kim Yuri, Sutherland Lillian B, Masao Murakami, Qi Xiaoxia, John McAnally, Porrello Enzo R, et al. 2013. “Hippo Pathway Effector Yap Promotes Cardiac Regeneration.” Proceedings of the National Academy of Sciences of the United States of America 110 (34): 13839–44. 10.1073/pnas.1313192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Jinhu, Almeida Maria, and O’Brien Charles A. 2018. “The YAP/TAZ Transcriptional Co-Activators Have Opposing Effects at Different Stages of Osteoblast Differentiation.” Bone 112 (July): 1–9. 10.1016/J.BONE.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Jinhu, Piemontese Marilina, Onal Melda, Campbell Josh, Goellner Joseph J., Dusevich Vladimir, Bonewald Lynda, Manolagas Stavros C., and O’Brien Charles A. 2015. “Osteocytes, Not Osteoblasts or Lining Cells, Are the Main Source of the RANKL Required for Osteoclast Formation in Remodeling Bone.” Edited by Dominique Heymann. PLOS ONE 10 (9): e0138189. 10.1371/journal.pone.0138189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Xufeng, Hong Xiaowei, Li Zida, Deng Cheri X., and Fu Jianping. 2017. “Acoustic Tweezing Cytometry Enhances Osteogenesis of Human Mesenchymal Stem Cells through Cytoskeletal Contractility and YAP Activation.” Biomaterials 134 (July): 22–30. 10.1016/J.BIOMATERIALS.2017.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Beining, Sun Hualing, Chen Peiyu, Fan Nana, Zhong Heli, Liu Xiayi, Wu Yanru, and Wang Jiawei. 2019. “YAP1 Influences Differentiation of Osteoblastic MC3T3‐E1 Cells through the Regulation of ID1.” Journal of Cellular Physiology 234 (8): 14007–18. 10.1002/jcp.28088. [DOI] [PubMed] [Google Scholar]
- Yang Guan, Zhu Liang, Hou Ning, Lan Yu, Wu Xi-Mei, Zhou Bin, Teng Yan, and Yang Xiao. 2014. “Osteogenic Fate of Hypertrophic Chondrocytes.” Cell Research 24 (10): 1266–69. 10.1038/cr.2014.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Jae-Yeon, Sun Wook Cho, Jee Hyun An, Ju Yeon Jung, Sang Wan Kim, Seong Yeon Kim, Jung Eun Kim, and Chan Soo Shin. 2013. “Osteoblast-Targeted Overexpression of TAZ Increases Bone Mass in Vivo.” PloS One 8 (2): e56585. 10.1371/journal.pone.0056585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Tsang KY, Tang HC, Chan D, and Cheah KS. 2014. “Hypertrophic Chondrocytes Can Become Osteoblasts and Osteocytes in Endochondral Bone Formation.” Proceedings of the National Academy of Sciences of the United States of America 111 (33): 12097–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Wanlei, Han Weiqi, Qin An, Wang Ziyi, Xu Jiake, and Qian Yu. 2018. “The Emerging Role of Hippo Signaling Pathway in Regulating Osteoclast Formation.” Journal of Cellular Physiology 233 (6): 4606–17. 10.1002/jcp.26372. [DOI] [PubMed] [Google Scholar]
- Zaidi, Sayyed K, Sullivan Andrew J, Ricardo Medina, Yoshiaki Ito, van Wijnen Andre J, Stein Janet L, Lian Jane B, and Stein Gary S. 2004. “Tyrosine Phosphorylation Controls Runx2-Mediated Subnuclear Targeting of YAP to Repress Transcription.” The EMBO Journal 23 (4): 790–99. 10.1038/sj.emboj.7600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanconato Francesca, Cordenonsi Michelangelo, and Piccolo Stefano. 2016. “YAP/TAZ at the Roots of Cancer.” Cancer Cell 29 (6): 783–803. 10.1016/j.ccell.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Mei, Xuan Shouhong, Bouxsein Mary L., von Stechow Dietrich, Nagako Akeno, Marie Claude Faugere, Hartmut Malluche, et al. 2002. “Osteoblast-Specific Knockout of the Insulin-like Growth Factor (IGF) Receptor Gene Reveals an Essential Role of IGF Signaling in Bone Matrix Mineralization.” Journal of Biological Chemistry 277 (46): 44005–12. 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
- Zhang Yan, Wang Zheyang, Ding Lin, Damaolar Ainiwaerjiang, Li Zhiqiang, Qiu Yusheng, and Yin Zhanhai. 2016. “Lentivirus-TAZ Administration Alleviates Osteoporotic Phenotypes in the Femoral Neck of Ovariectomized Rats.” Cellular Physiology and Biochemistry : International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology 38 (1): 283–94. 10.1159/000438629. [DOI] [PubMed] [Google Scholar]
- Zhao Bin, Li Li, and Kun Liang Guan. 2010. “Hippo Signaling at a Glance.” Journal of Cell Science. 10.1242/jcs.069070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Gexin, Huang Bau-Lin, Rigueur Diana, Wang Weiguang, Bhoot Chimay, Charles Kemberly R, Jongseung Baek, Mohan Subburaman, Jiang Jie, and Lyons Karen M. 2018. “CYR61/CCN1 Regulates Sclerostin Levels and Bone Maintenance.” Journal of Bone and Mineral Research, March. 10.1002/jbmr.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Xin, von der Mark Klaus, Stephen Henry, William Norton, Henry Adams, and de Crombrugghe Benoit. 2014. “Chondrocytes Transdifferentiate into Osteoblasts in Endochondral Bone during Development, Postnatal Growth and Fracture Healing in Mice.” PLoS Genetics 10 (12): e1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Yumin, Wu Yaping, Cheng Jie, Wang Qiong, Li Zhongwu, Wang Yanling, Wang Dongmiao, et al. 2018. “Pharmacological Activation of TAZ Enhances Osteogenic Differentiation and Bone Formation of Adipose-Derived Stem Cells.” Stem Cell Research & Therapy 9 (1): 53. 10.1186/s13287-018-0799-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Table 1 References:
- 1.Zaidi SK et al. Tyrosine phosphorylation controls Runx2-mediated subnuclear targeting of YAP to repress transcription. EMBO J. (2004). doi: 10.1038/sj.emboj.7600073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawane T et al. Runx2 is required for the proliferation of osteoblast progenitors and induces proliferation by regulating Fgfr2 and Fgfr3. Sci. Rep. (2018). doi: 10.1038/s41598-018-31853-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang Y, Feinberg T, Keller ET, Li XY & Weiss SJ Snail/Slug binding interactions with YAP/TAZ control skeletal stem cell self-renewal and differentiation. Nat. Cell Biol. (2016). doi: 10.1038/ncb3394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan JX et al. YAP promotes osteogenesis and suppresses adipogenic differentiation by regulating β-catenin signaling. Bone Res. (2018). doi: 10.1038/s41413-018-0018-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krishnan V, Bryant HU & MacDougald OA Regulation of bone mass by Wnt signaling. Journal of Clinical Investigation (2006). doi: 10.1172/JCI28551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vassilev A, Kaneko KJ, Shu H, Zhao Y & DePamphilis ML TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev. (2001). doi: 10.1101/gad.888601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kegelman CD et al. Skeletal cell YAP and TAZ combinatorially promote bone development. FASEB J. (2018). doi: 10.1096/fj.201700872R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L et al. cAMP response element-binding protein and Yes-associated protein form a feedback loop that promotes neurite outgrowth. J. Cell. Mol. Med. (2018). doi: 10.1111/jcmm.13324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long F, Schipani E, Asahara H, Kronenberg H & Montminy M The CREB family of activators is required for endochondral bone development. Development (2001). [DOI] [PubMed] [Google Scholar]
- 10.Zhang X et al. Yes-associated protein (YAP) binds to HIF-1α and sustains HIF-1α protein stability to promote hepatocellular carcinoma cell glycolysis under hypoxic stress. J. Exp. Clin. Cancer Res. (2018). doi: 10.1186/s13046-018-0892-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sivaraj KK et al. YAP1 and TAZ negatively control bone angiogenesis by limiting hypoxia-inducible factor signaling in endothelial cells. Elife (2020). doi: 10.7554/eLife.50770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amarilio R et al. HIF1α regulation of Sox9 in necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development (2007). doi: 10.1242/dev.008441 [DOI] [PubMed] [Google Scholar]
- 13.Zou H et al. SOX5 interacts with YAP1 to drive malignant potential of non-small cell lung cancer cells. Am. J. Cancer Res. (2018). [PMC free article] [PubMed] [Google Scholar]
- 14.Smits P, Dy P, Mitra S & Lefebvre V Sox5 and Sox6 are needed to develop and maintain source, columnar, and hypertrophic chondrocytes in the cartilage growth plate. J. Cell Biol. (2004). doi: 10.1083/jcb.200312045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manderfield LJ et al. Hippo signaling is required for Notch-dependent smooth muscle differentiation of neural crest. Dev. (2015). doi: 10.1242/dev.125807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mead TJ & Yutzey KE Notch pathway regulation of chondrocyte differentiation and proliferation during appendicular and axial skeleton development. Proc. Natl. Acad. Sci. U. S. A. (2009). doi: 10.1073/pnas.0902306106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kulkarni M et al. RUNX1 and RUNX3 protect against YAP-mediated EMT, stemness and shorter survival outcomes in breast cancer. Oncotarget (2018). doi: 10.18632/oncotarget.24419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida CA et al. Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog. Genes Dev. (2004). doi: 10.1101/gad.1174704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manderfield LJ et al. Pax3 and Hippo Signaling Coordinate Melanocyte Gene Expression in Neural Crest. Cell Rep. (2014). doi: 10.1016/j.celrep.2014.10.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu M et al. Persistent expression of Pax3 in the neural crest causes cleft palate and defective osteogenesis in mice. J. Clin. Invest. (2008). doi: 10.1172/JCI33715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aragón E et al. A smad action turnover switch operated by WW domain readers of a phosphoserine code. Genes Dev. (2011). doi: 10.1101/gad.2060811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karystinou A et al. Yes-associated protein (YAP) is a negative regulator of chondrogenesis in mesenchymal stem cells. Arthritis Res. Ther. (2015). doi: 10.1186/s13075-015-0639-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varelas X et al. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat. Cell Biol. (2008). doi: 10.1038/ncb1748 [DOI] [PubMed] [Google Scholar]
- 24.Song B, Estrada KD & Lyons KM Smad signaling in skeletal development and regeneration. Cytokine Growth Factor Rev. (2009). doi: 10.1016/j.cytogfr.2009.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrigno O et al. Yes-associated protein (YAP65) interacts with Smad7 and potentiates its inhibitory activity against TGF-β/Smad signaling. Oncogene (2002). doi: 10.1038/sj.onc.1205623 [DOI] [PubMed] [Google Scholar]
- 26.Iwai T, Murai J, Yoshikawa H & Tsumaki N Smad7 inhibits chondrocyte differentiation at multiple steps during endochondral bone formation and down-regulates p38 MAPK pathways. J. Biol. Chem. (2008). doi: 10.1074/jbc.M801175200 [DOI] [PubMed] [Google Scholar]
- 27.Strano S et al. Physical Interaction with Yes-associated Protein Enhances p73 Transcriptional Activity. J. Biol. Chem. (2001). doi: 10.1074/jbc.M010484200 [DOI] [PubMed] [Google Scholar]
- 28.Kommagani R, Whitlatch A, Leonard MK & Kadakia MP P73 is essential for vitamin D-mediated osteoblastic differentiation. Cell Death Differ. (2010). doi: 10.1038/cdd.2009.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Komuro A, Nagai M, Navin NE & Sudol M WW domain-containing protein YAP associates with ErbB-4 and acts as a co-transcriptional activator for the carboxyl-terminal fragment of ErbB-4 that translocates to the nucleus. J. Biol. Chem. (2003). doi: 10.1074/jbc.M305597200 [DOI] [PubMed] [Google Scholar]
- 30.Schuchardt BJ et al. Molecular basis of the binding of YAP transcriptional regulator to the ErbB4 receptor tyrosine kinase. Biochimie (2014). doi: 10.1016/j.biochi.2014.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisher MC, Clinton GM, Maihle NJ & Dealy CN Requirement for ErbB2/ErbB signaling in developing cartilage and bone. Dev. Growth Differ. (2007). doi: 10.1111/j.1440-169X.2007.00941.x [DOI] [PubMed] [Google Scholar]
- 32.Zagurovskaya M et al. EGR-1 forms a complex with YAP-1 and upregulates Bax expression in irradiated prostate carcinoma cells. Oncogene (2009). doi: 10.1038/onc.2008.461 [DOI] [PubMed] [Google Scholar]
- 33.Reumann MK et al. Early growth response gene 1 regulates bone properties in mice. Calcif. Tissue Int. (2011). doi: 10.1007/s00223-011-9486-0 [DOI] [PubMed] [Google Scholar]
- 34.Wang J et al. TRIB2 acts downstream of Wnt/TCF in liver cancer cells to regulate YAP and C/EBPα function. Mol. Cell (2013). doi: 10.1016/j.molcel.2013.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen W, Zhu G, Tang J, Zhou H. De & Li YP C/ebpα controls osteoclast terminal differentiation, activation, function, and postnatal bone homeostasis through direct regulation of Nfatc1. J. Pathol. (2018). doi: 10.1002/path.5001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murakami M, Nakagawa M, Olson EN & Nakagawa O A WW domain protein TAZ is a critical coactivator for TBX5, a transcription factor implicated in Holt-Oram syndrome. Proc. Natl. Acad. Sci. U. S. A. (2005). doi: 10.1073/pnas.0509109102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rallis C et al. Tbx5 is required for forelimb bud formation and continued outgrowth. Development (2003). doi: 10.1242/dev.00473 [DOI] [PubMed] [Google Scholar]